Abstract
Recent evidence has suggested that truncation of tau protein at the caspase cleavage site D421 precedes hyperphosphorylation and may be necessary for the assembly of tau into filaments in Alzheimer’s disease and other tauopathies. Here we have investigated the time course of the appearance of phosphorylated and truncated tau in the brain and spinal cord of mice transgenic for mutant human P301S tau protein. This mouse line recapitulates the essential molecular and cellular features of the human tauopathies, including tau hyperphosphorylation, tau filament formation, and neurodegeneration. Soluble tau was strongly phosphorylated at 1 to 6 months of age. Low levels of phosphorylated, sarkosyl-insoluble tau were detected at 2 months, with a steady increase up to 6 months of age. Tau truncated at D421 was detected at low levels in Tris-soluble and detergent-soluble tau at 3 to 6 months of age. By immunoblotting, it was not detected in sarkosyl-insoluble tau. However, by immunoelectron microscopy, a small percentage of tau in filaments from brain and spinal cord of transgenic mice was truncated at D421. Similar findings were obtained using dispersed filaments from Alzheimer’s disease and FTDP-17 brains. The late appearance and low abundance of tau ending at D421 indicate that it is unlikely that truncation at this site is necessary for the assembly of tau into filaments.
Intraneuronal inclusions made of hyperphosphorylated microtubule-associated protein tau and extracellular deposits made of β-amyloid protein Aβ are the defining neuropathological characteristics of Alzheimer’s disease (AD).1 Tau inclusions, in the absence of extracellular deposits, are characteristic of a number of other neurodegenerative diseases, including progressive supranuclear palsy, corticobasal degeneration, Pick’s disease, and inherited frontotemporal dementia and parkinsonism linked to chromosome 17 (FTDP-17T).2 The identification of mutations in Tau in FTDP-17T has established that dysfunction of tau protein is central to the neurodegenerative process.3,4,5 In all of the above diseases, a fraction of tau is hyperphosphorylated and in an abnormal filamentous form. Phosphorylation at some sites, such as S422 and the triple phosphorylation at T212, S214, and T217, is abnormal because it is not present in tau from normal adult human brain.6,7 Other sites are phosphorylated in normal brain also, albeit at lower levels than in tau filaments. A pathological pathway leading from normal soluble to filamentous insoluble tau protein is probably at the heart of neurodegeneration in these diseases. At present, the mechanisms underlying the abnormal assembly of tau into filaments are only incompletely understood.
Hyperphosphorylation of tau at many sites appears to precede assembly into filaments, based on findings in mouse lines expressing human tau with FTDP-17T mutations.8,9 Moreover, an increase in the phosphorylation of soluble tau resulted in increased filament formation, suggesting that phosphorylation may drive filament assembly.10 In contrast, other studies have reported that phosphorylation of tau at some sites inhibits filament assembly and that its abnormal phosphorylation is not sufficient to cause filament formation in cellular models.11,12
Besides hyperphosphorylation, another mechanism that has been proposed to cause filament formation is truncation of tau.13 It is well established that a proportion of filamentous tau from human brain is truncated.14,15,16,17 In recent years, caspase-mediated truncation of tau at aspartic acid residue 421 (D421, in the numbering of the longest human brain tau isoform) has been reported to be an early event that may precede hyperphosphorylation and filament formation in AD, Pick’s disease, and progressive supranuclear palsy.18,19,20,21,22 Removal of the last 20 amino acids has also been shown to result in an increased propensity of tau to form filaments in vitro, and it has been suggested that tau truncated in this manner may seed the assembly of full-length tau in human brain.23,24 In addition, caspase 3-mediated cleavage of tau at D421 has been described,18 and it has been suggested that tau truncated in this manner becomes an effector of apoptosis.25,26
Much of the above work has relied on tissue culture studies, in which tau did not assemble into filaments, and on postmortem studies of human brain from end-stage disease. Less use has been made of transgenic mouse models that replicate the essential molecular and cellular features of the human tauopathies, such as the formation of filaments made of hyperphosphorylated human tau and neurodegeneration. Here we have used a previously described mouse line transgenic for mutant human P301S tau protein9 to study the appearance of phosphorylation of tau, truncation at D421, and filament formation. Phosphorylation of tau, but not truncation at D421, was found to be an early event.
Materials and Methods
Animals and Antibodies
Mice transgenic for human P301S tau protein9 and C57BL/6J control mice aged 1 to 6 months were used. We made use of the phosphorylation-dependent anti-tau antibodies AT8 (Innogenetics, Ghent, Belgium), AT100 (Innogenetics), AT180 (Autogen Bioclear, Calne, UK), AT270 (Autogen Bioclear), and P-S422 (Biosource Europe, Nivelle, Belgium), as well as of the phosphorylation-independent anti-tau antibodies BR134, BR135, Tau14 (Invitrogen, Paisley, UK), and Tau-C3 (Chemicon, Temecula, CA). AT8 recognizes tau protein phosphorylated at S202 and T205,27 AT100 requires phosphorylation of T212, S214, and T217,7 AT180 recognizes tau phosphorylated at T231,28 AT270 requires phosphorylation of T18128 and P-S422 recognizes tau protein phosphorylated at S422. BR134 (directed against the C terminus of tau) and BR135 (directed against the microtubule-binding repeat region) recognize murine and human tau isoforms, irrespective of phosphorylation,29 whereas T14 is specific for human tau (recognizing residues 141 to 14930). Tau-C3 is specific for tau protein truncated at D421.31 The antibodies and their epitopes are listed in Table 1. To control for equal loading, anti-β-actin antibody AC-74 (Sigma-Aldrich, Dorset, UK) was used.
Table 1.
Anti-Tau Antibodies Used in This Study
| Antibody | Epitope |
|---|---|
| BR134 | 428-441 |
| BR135 | 323-335 |
| T14 | 141-149 |
| Tau-C3 | ΔD421 |
| AT8 | pS202/pT205 |
| AT100 | pT212/pS214/pT217 |
| AT180 | pT231 |
| P-S422 | pS422 |
The amino acids are numbered according to the 441-amino acid isoform of human brain tau.
Tau Constructs
The 383-amino acid isoform of human brain tau was expressed in Escherichia coli from cDNA clone htau43 and expressed as described.32 Site-directed mutagenesis (QuikChange; Stratagene, La Jolla, CA) was used to generate tau protein truncated at D421 (using the numbering of the 441 amino acid brain tau isoform). All constructs were verified by DNA sequencing. Heparin was used to induce the assembly of full-length tau and tau truncated at D421 into filaments, as described.33
Tissue Extraction
Brains and spinal cords from transgenic and control mice were extracted using either the sarkosyl or the RIPA method. For the sarkosyl extraction, tissues were homogenized in 3 vol of cold extraction buffer (25 mmol/L Tris-HCl, pH 7.4, 150 mmol/L NaCl, 1 mmol/L EDTA, 1 mmol/L EGTA, 5 mmol/L sodium pyrophosphate, 10 mmol/L β-glycerophosphate, 30 mmol/L sodium fluoride, 2 mmol/L sodium vanadate, 1 mmol/L phenylmethyl sulfonyl fluoride, and 10 μg/ml leupeptin, aprotinin, and pepstatin). The homogenates were spun for 15 minutes at 80,000 × g and the supernatants used for the analysis of soluble tau. Protein concentrations were determined using the BCA kit (Pierce, Rockford, IL), and 10 mg of protein was analyzed on 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). To prepare sarkosyl-insoluble tau, the remaining pellets were homogenized in A68 extraction buffer (10 mmol/L Tris-HCl, pH 7.4, 0.8 mol/L NaCl, 10% sucrose, 1 mmol/L EGTA, 1 mmol/L phenylmethyl sulfonyl fluoride, and 10 μg/ml leupeptin, aprotinin, and pepstatin) and spun at 4000 × g for 20 minutes. Sarkosyl was then added to 1% to the supernatants, which were left for 1.5 hours at room temperature. After a 30-minute centrifugation at 80,000 × g, the supernatants were discarded and the pellets resuspended in 50 mmol/L Tris-HCl, pH 7.4. The samples were analyzed on 12% SDS-PAGE. For the RIPA extraction, tissues were homogenized in 3 vol of RAB-HS buffer (0.1 mol/L MES, 0.75 mol/L NaCl, 1 mmol/L EGTA, 0.5 mmol/L MgSO4, 20 mmol/L sodium fluoride, 1 mmol/L phenylmethyl sulfonyl fluoride, and 10 μg/ml leupeptin, aprotinin, and pepstatin), followed by a 40-minute spin at 40,000 × g. The pellets were homogenized in RAB-HS buffer supplemented with 1 mol/L sucrose. After a 20-minute centrifugation at 40,000 × g, the pellets were resuspended in RIPA buffer (50 mmol/L Tris-HCl, pH 7.4, 150 mmol/L NaCl, 0.1% SDS, 5% sodium deoxycholate, 1% Nonidet P-40, 5 mmol/L EDTA, 1 mmol/L phenylmethyl sulfonyl fluoride, 1 mmol/L sodium fluoride, 1 mmol/L sodium orthovanadate, and 10 μg/ml leupeptin, aprotinin, and pepstatin). After a 30-minute spin at 40,000 × g, the supernatants were collected. Protein concentrations were measured using the BCA kit and 10 mg of protein was analyzed on 12% SDS-PAGE. For analysis of human temporal cortex from control and AD cases and frontal cortex from a case of FTDP-17T with the P301L mutation in Tau, only the sarkosyl extraction method was used.
Immunoblot Analysis
After SDS-PAGE, the gels were blotted for 1 hour at room temperature onto polyvinylidene difluoride membranes (Pierce). Membranes were blocked for 30 minutes at room temperature in 0.1 mol/L phosphate buffer, pH 7.4, containing 5% milk and 0.1% Tween 20, followed by incubation for 1 hour at room temperature or overnight at 4°C with the primary antibody in blocking buffer. Membranes were then washed in phosphate buffer containing Tween 20 and incubated for 30 minutes at room temperature in peroxidase-conjugated secondary antibody (Pierce) in blocking buffer. After washing, the blots were developed using enhanced chemiluminescence (Amersham Biosciences, Arlington Heights, IL).
Immunohistochemistry
Mice were perfused transcardially with 4% paraformaldehyde in 0.1 mol/L phosphate buffer, pH 7.4. Brains and spinal cords were removed, postfixed overnight at 4°C, and cryoprotected in 30% sucrose in phosphate buffer for at least 24 hours. Sagittal brain sections and transverse spinal cord sections (30 μm) were cut on a Leica SM2400 microtome (Leica Microsystems, Bucks, UK) and stored at 4°C in 0.1 mol/L phosphate buffer containing 0.1% sodium azide. For single or double labeling, sections were permeabilized and blocked for 3 hours at room temperature in phosphate buffer containing 3% bovine serum albumin and 0.1% Triton X-100. This was followed by an overnight incubation at 4°C with the primary antibodies in blocking solution. After washing, the sections were incubated for 3 hours at room temperature in Alexa Fluor 488 (Molecular Probes, Leiden, The Netherlands) or Cy5 (Abcam, Cambridge, UK) secondary antibodies in blocking solution. After 4,6-diamidino-2-phenylindole staining, the sections were mounted using Vectashield mounting medium (Vector Laboratories, Burlingame, CA). For absorption control experiments, sarkosyl-insoluble tau was extracted from brain and spinal cord of 5-month-old P301S tau transgenic mice and the tau filaments solubilized as described.34 The dialyzed material (50 μl) was incubated overnight at 4°C with the anti-tau antibodies, before addition to the tissue sections. In separate control experiments, recombinant full-length tau and tau truncated at D421 (1 to 25 μg) were incubated overnight with anti-tau antibodies, followed by addition to tissue sections. Immunohistochemistry was also performed on tissue sections from two cases of AD (70 and 86 years old, Braak stages V to VI and IV to V, respectively) and one FTDP-17T case with the P301L mutation in Tau, as described.34,35 After immunohistochemistry, images were recorded using a Radiance 2100 confocal microscope (Bio-Rad, Hercules, CA) with a ×10/0.45 numerical aperture (n.a.) or with ×40/1.30 n.a. and ×100/1.40 n.a. oil immersion objectives. Images were taken using a Sharp 2000 laser (Zeiss Bio-Rad, London, UK) and were typically 512 × 512 pixels. For z analysis, images were analyzed with the oil immersion objectives, and the scanning step size was 0.5-μm intervals. For cellular localization of the signal, cells were analyzed from top to bottom and the z projection images analyzed.
Electron Microscopy
Aliquots of the sarkosyl-insoluble dispersed filament preparations and of synthetic tau filament assemblies were processed for immunoelectron microscopy, as described.16 In some experiments, the filaments were partially digested by floating the grid on a drop of 0.4 mg/ml of pronase for 5 minutes at room temperature before the first blocking step.16 As a control, synthetic filaments assembled from recombinant tau protein truncated at D421 were used. Micrographs were recorded on a Philips (Eindhoven, the Netherlands) EM208S microscope at a nominal magnification of ×40,000. Antibodies BR134 and Tau-C3 were used at a dilution of 1:100.
Results
Time Course of Hyperphosphorylation and Truncation of Tau: Immunoblotting
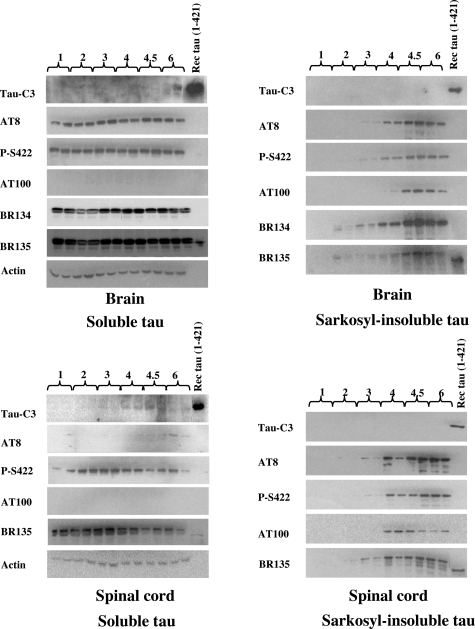
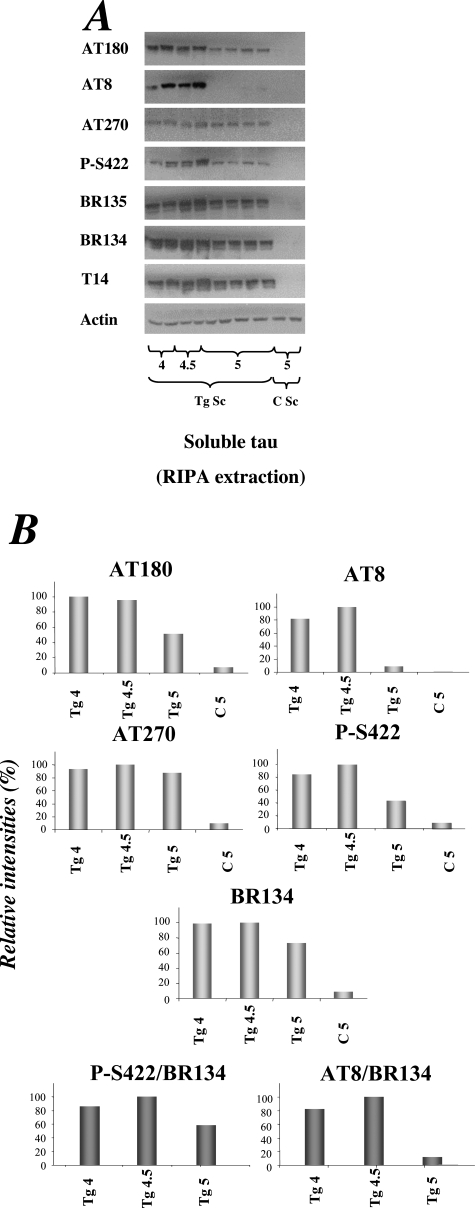
Soluble and sarkosyl-insoluble tau were prepared from brain and spinal cord of human P301S tau transgenic mice 1 to 6 months of age and immunoblotted with phosphorylation-dependent anti-tau antibodies AT8, P-S422, and AT100, as well as antibody Tau-C3 specific for tau truncated at D421 and the phosphorylation-independent anti-tau antibodies BR134 and BR135 (Figure 1). An anti-actin antibody was used to ensure equal loading. In the soluble fraction from brain, strong immunoreactivity with AT8 and P-S422 was already present at 1 month and persisted until 6 months of age. The same was also true of immunoreactivity with P-S422, but not AT8, in the soluble fraction from spinal cord. In contrast to this strong early reactivity for phosphorylated tau, only weak staining for tau truncated at D421 was observed in brain and spinal cord of transgenic mice 4 to 6 months of age (Figure 1). Soluble tau from brain and spinal cord was not labeled by antibody AT100. Sarkosyl-insoluble tau was not detectable in brain or spinal cord from 1-month-old transgenic mice using either phosphorylation-dependent or phosphorylation-independent anti-tau antibodies. Low levels of sarkosyl-insoluble tau were detected at 2 months, with levels increasing steadily up to 6 months of age (Figure 1). AT100 labeled sarkosyl-insoluble tau from 4 months onwards. Tau protein truncated at D421 was not detected in the sarkosyl-insoluble fraction from brain or spinal cord at any age studied. When spinal cords from human P301S tau transgenic mice were extracted using the RIPA method, immunoreactivity for tau truncated at D421 was only detected in small amounts, even at late time points (data not shown). Immunoreactivity for tau phosphorylated at S422 was already present at 1 month of age. It peaked at 4.5 months but was weaker at 6 months of age. The unexpected reduction in phosphorylated, RIPA-soluble tau throughout time was further investigated in spinal cord from 4- to 5-month-old transgenic mice (Figure 2). RIPA-soluble human tau protein was present at all time points, as judged by staining with BR134, BR135, and T14. The same was also true of tau phosphorylated at T181, based on staining with AT270. By contrast, staining with AT180, AT8, and P-S422 was much stronger at 4 and 4.5 than at 5 months. The reduction at 5 months was particularly marked with AT8.
Figure 1.
Time course of tau phosphorylation and truncation at D421 in soluble and sarkosyl-insoluble fractions from brains and spinal cords of human P301S tau transgenic mice. Tris-soluble and sarkosyl-insoluble fractions from 1-, 2-, 3-, 4-, 4.5-, and 6-month-old mice were prepared (two mice per time point), run on SDS-PAGE, and immunoblotted with anti-tau antibodies Tau-C3, AT8, P-S422, AT100, BR134, and BR135. An anti-actin antibody was used to ensure equal loading. Recombinant human tau truncated at D421 was used as a control.
Figure 2.
Tau phosphorylation in the RIPA-extracted fraction from spinal cords of human P301S tau transgenic mice. A: Detergent-soluble (RIPA) fractions from 4-, 4.5-, and 5-month-old transgenic mice (Tg Sc) and 5-month-old control mice (C Sc) were prepared (two to four mice per time point), run on SDS-PAGE, and immunoblotted with anti-tau antibodies AT180, AT8, AT270, P-S422, BR135, BR134, and T14. An anti-actin antibody was used to ensure equal loading. B: Quantitative analysis of the immunoblots shown in A. The results were normalized relative to the actin signal and are expressed as percentage of the largest signal for a given antibody (taken as 100%, light gray bars). The findings obtained with antibodies P-S422 and AT8 were also expressed relative to those with total tau detected with antibody BR134 (gray bars).
Time Course of Hyperphosphorylation and Truncation of Tau: Immunohistochemistry
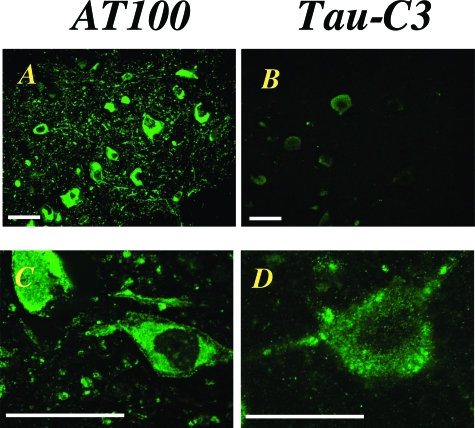
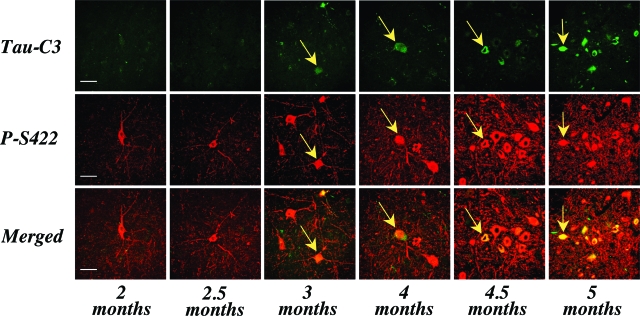
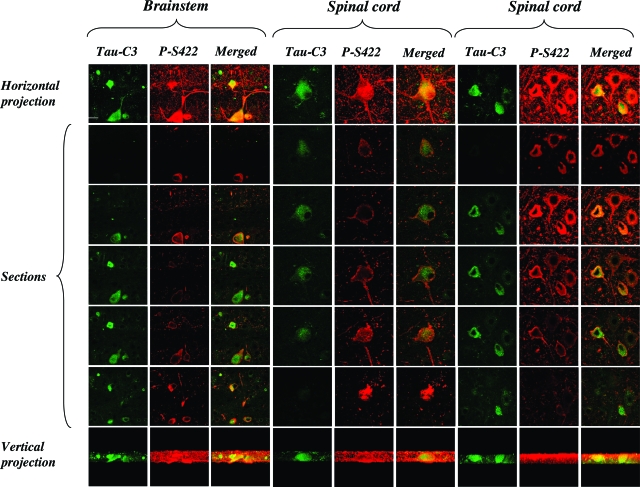
Immunohistochemistry of spinal cord from 5-month-old transgenic mice showed strong staining of many nerve cells and their processes with anti-tau antibody AT100 (Figure 3A). By contrast, only a small number of nerve cells was weakly labeled with Tau-C3 (Figure 3B). The AT100 staining was compact, with the D421 tau staining appearing more diffuse and granular (Figure 3, C and D). Staining with Tau-C3 was abolished after preincubation with recombinant tau truncated at D421. It was not reduced when the antibody was preincubated with recombinant full-length tau or with sarkosyl-insoluble tau from brain and spinal cord of human P301S tau transgenic mice. Preincubation with the latter abolished AT100 staining. Time course analysis in spinal cord using antibodies P-S422 and Tau-C3 showed low levels of immunoreactivity for P-S422 at 2 months, which increased progressively up to 5 months, the longest time point studied (Figure 4). Low levels of staining with Tau-C3 were first observed at 3 months of age, with a steady increase up to 5 months. The merged images showed that staining for tau protein phosphorylated at S422 was more extensive than that for protein truncated at D421 (Figure 4). The distribution of tau phosphorylated at S422 or truncated at D421 within nerve cells was investigated further in spinal cord and brainstem from 5-month-old human P301S tau transgenic mice using confocal microscopy (Figure 5). Projections onto the horizontal showed that some nerve cells were double-labeled for phosphorylated and truncated tau. Analysis of 0.5-μm optical sections through a double-labeled cell showed that staining for truncated tau was present diffusely throughout the nerve cell body, whereas tau labeled by antibody P-S422 had an aggregated appearance, which tended to concentrate toward the periphery of the cell.
Figure 3.
Immunofluorescence staining of phosphorylated tau (antibody AT100) and tau truncated at D421 (antibody Tau-C3) in the spinal cords of 5-month-old human P301S tau transgenic mice. A and C: AT100; B and D: Tau-C3. Note the strong labeling of many nerve cells in A and the weak labeling of a small number of nerve cells in B. In D, the intensity of recording for Tau-C3 was increased by threefold to fivefold, to have a signal similar to that for AT100 in C. Scale bars = 60 μm.
Figure 4.
Time course of immunofluorescence staining of phosphorylated tau (antibody P-S422) and tau truncated at D421 (antibody Tau-C3) in spinal cords of human P301S tau transgenic mice. From 2 months onwards, increasing numbers of nerve cells became strongly immunoreactive with P-S422. From 3 months onwards, some nerve cells were weakly immunoreactive for Tau-C3 (the intensity of recording for Tau-C3 was increased threefold to fivefold). The merged pictures show some co-localization of phosphorylated and truncated tau. Arrows point to double-labeled cells. Scale bars = 60 μm.
Figure 5.
Immunofluorescence staining of phosphorylated tau (antibody P-S422) and truncated tau (antibody Tau-C3) in brainstems and spinal cords of 5-month-old human P301S tau transgenic mice. Projections onto the horizontal and vertical were visualized, and 0.5-μm confocal optical sections were collected. The images showed only a partial co-localization of hyperphosphorylated tau and tau truncated at D421. The sections suggested an aggregated appearance of phosphorylated tau, with a more diffuse distribution of truncated tau within nerve cells. The intensity of recording for Tau-C3 was increased threefold to fivefold. Scale bar = 60 μm.
Immunoelectron Microscopy of Tau Filaments
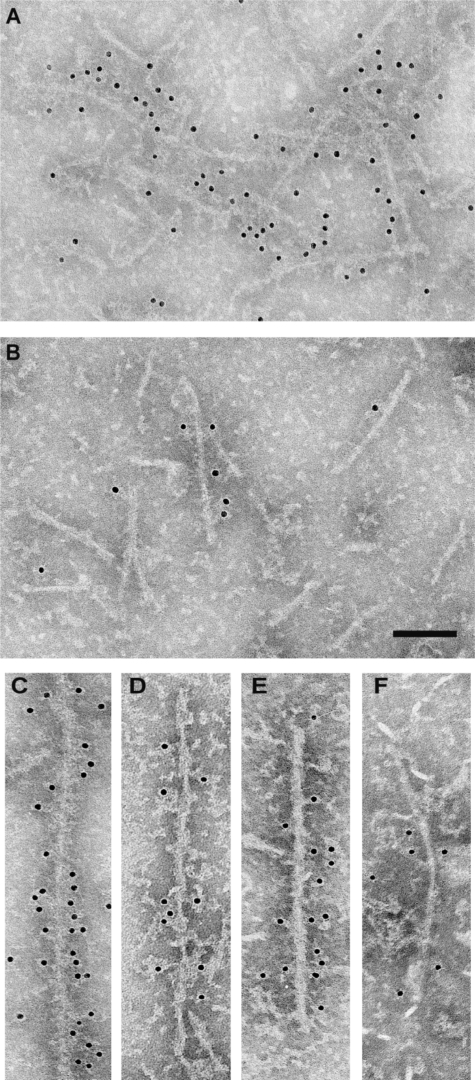
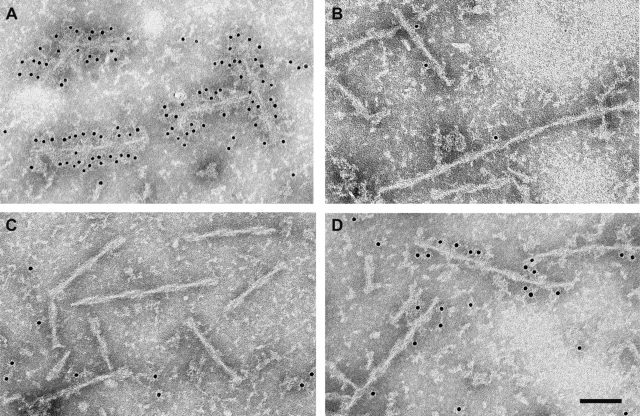
Immunoelectron microscopy of isolated filaments from spinal cords of 5-month-old human P301S tau transgenic mice was performed using BR134 and Tau-C3 (Figure 6, A and B). Whereas more than 95% of filaments were labeled by BR134, only 2 to 3% were decorated by Tau-C3. The latter can label tau filaments, as shown by the fact that synthetic filaments made from recombinant tau protein truncated at D421 were strongly immunopositive (Figure 6C). To relate these findings to the human tauopathies, we immunolabeled tau filaments from AD brain and from a case of FTDP-17T with the P301L mutation in Tau using Tau-C3. We found that fewer than 5% of filaments were decorated (Figure 6, D–F). By contrast, BR134 decorated more than 95% of filaments from the sarkosyl-insoluble fraction from AD and FTDP-17T (not shown). To determine whether truncation of tau could occur after assembly, we labeled filaments from AD brain using BR134 and Tau-C3 before and after mild treatment with pronase (Figure 7). Before pronase treatment, many filaments were strongly decorated by BR134. This was no longer the case after pronase treatment, indicating removal of the C terminus of tau. Labeling with Tau-C3 contrasted with BR134 labeling, in that more filaments were decorated after pronase treatment than before, even though pronase does not specifically cut after D421.
Figure 6.
Immunoelectron microscopy of dispersed tau filaments with antibodies BR134 and Tau-C3. A and B: Tau filaments from the spinal cords of 5-month-old human P301S tau transgenic mice were decorated with BR134 (A) and Tau-C3 (B). C–F: Decoration by Tau-C3 of a filament assembled from recombinant tau(1-421) (C), a paired helical filament (D), and a straight filament (E) from AD brain, as well as a ribbon-like filament (F) from a case of FTDP-17T with the P301L mutation in Tau. Scale bar = 100 nm.
Figure 7.
Immunoelectron microscopy of dispersed tau filaments with antibodies BR134 and Tau-C3 before and after pronase treatment. A and B: Tau filaments from AD brain labeled with BR134 before (A) and after (B) pronase treatment, which greatly reduces the level of decoration. C and D: Tau filaments labeled with Tau-C3 before (C) and after (D) pronase treatment, which increases the level of decoration. Scale bar = 100 nm.
Discussion
We have used a mouse line transgenic for human P301S tau protein that exhibits the essential features of human tauopathies to investigate the temporal relationships between tau phosphorylation, filament formation, and truncation at D421. The latter has been reported to be mediated by caspases and to be critical for tau filament formation in AD, Pick’s disease, progressive supranuclear palsy, and corticobasal degeneration.18,19,20,21,22 In soluble tau, phosphorylation of several sites, such as S202, T205, and S422, was present in 1-month-old mice and remained constant until 6 months of age. When detergent-soluble tau was extracted using RIPA buffer, phosphorylated tau was also present at early time points. However, at later time points, the levels of RIPA-soluble tau phosphorylated at some sites, such as S202, T205, T231, and S422, decreased in mice 5 to 6 months of age. This was not true of total RIPA-soluble tau and of tau phosphorylated at T181. In contrast to soluble tau, sarkosyl-insoluble tau only became detectable at 2 to 3 months of age, and its levels increased steadily up to 6 months. These findings indicate that hyperphosphorylation of soluble tau preceded the formation of sarkosyl-insoluble, phosphorylated tau filaments and that, as the levels of sarkosyl-insoluble tau increased throughout time, all of the soluble tau phosphorylated at some sites became detergent insoluble.
Unlike phosphorylation, truncation of tau at D421 was not detected at early time points. Only low levels of truncated tau were present even at late time points in soluble, but not in sarkosyl-insoluble, tau indicating that the vast majority of tau in filaments had an intact C terminus. This was shown directly by immunoelectron microscopy, in which antibody Tau-C3 was found to decorate only 2 to 3% of filaments extracted from spinal cord. It follows that truncation at D421 is not the trigger for filament assembly in human P301S tau transgenic mice. These results are at odds with a previous report20 that identified truncated tau by immunohistochemistry at an early stage of tangle formation in a triple-transgenic mouse model of AD.36 From the data presented there,20 it is difficult to relate the appearance of truncation at D421 to tau phosphorylation and filament formation. Furthermore, although the antibody used in the present study is a monoclonal of known specificity,19,31 the previous study20 used a less well characterized polyclonal antibody.22 It remains to be seen whether the process leading to tau pathology differs between mice transgenic for human P301S tau and the triple transgenic mice. Similarly, future work will have to establish whether the mechanisms leading to tau dysfunction are the same in FTDP-17T and AD, and animal models thereof. A close correspondence is suggested by recent findings showing that phosphorylation of S422 precedes truncation at D421 in AD.37
Tau filaments are known to be made of a core region that comprises the microtubule-binding repeats and an unstructured fuzzy coat consisting of the N- and C-terminal regions.14,15 In tau filaments from AD brain, it is well established that truncation in the fuzzy coat, in particular at E391, occurs to a limited extent after filament assembly.16 Consistent with this finding, we found that the vast majority of sarkosyl-insoluble tau in filaments from AD brain and from a case of FTDP-17T with a P301L mutation in Tau has an intact C terminus, with truncation at D421 being present in only a small percentage of filaments. After brief pronase treatment, more filaments were labeled by antibody Tau-C3, which is specific for tau truncated at D421. Similar findings were previously reported with antibody 6-423,16 which recognizes tau truncated at E391.17
In the human P301S tau transgenic mice, low levels of tau truncated at D421 were present in both Tris-soluble and detergent-soluble fractions. In the spinal cord, truncated tau was first detected by immunoblotting and immunohistochemistry in 3-month-old mice. In whole brain, it was only detected in 6-month-old animals. Nerve cells were weakly immunoreactive for truncated tau, which showed a diffuse cytoplasmic distribution. By contrast, hyperphosphorylation of tau at most sites was already present in 1-month-old mice, with the first tau inclusions becoming detectable at ∼2 months of age. It is clear, therefore, that hyperphosphorylation preceded truncation in both soluble and insoluble fractions and that truncation at D421 occurred after filament assembly. Truncation was observed in a small percentage of tau filaments from both human and mouse brains by electron microscopy and this percentage could be increased by mild pronase treatment, consistent with previous evidence showing that truncation of tau can occur after filament assembly. In addition, truncation of a small fraction of soluble tau was also observed. The mechanisms underlying truncation of tau remain to be identified. They could be related to the activation of caspases and apoptosis.18,25,26 However, previous work on transgenic mouse models of FTDP-17T has failed to detect apoptotic nerve cell death.9,38 Based on its late appearance and low abundance, we conclude that truncation at D421 is not necessary for the assembly of tau into filaments. Future work will test this directly through the production of mouse lines transgenic for mutant tau resistant to cleavage at D421.
Footnotes
Address reprint requests to M. Goedert, Medical Research Council Laboratory of Molecular Biology, Hills Road, Cambridge CB2 OQH, UK. E-mail: mg@mrc-lmb.cam.ac.uk.
Supported by The European Molecular Biology Organization (fellowship to P.D.), the United Kingdom Medical Research Council, the Alzheimer’s Research Trust, and the United States Public Health Service (grant P30AG10133).
References
- Goedert M, Spillantini MG. A century of Alzheimer’s disease. Science. 2006;314:777–781. doi: 10.1126/science.1132814. [DOI] [PubMed] [Google Scholar]
- Lee VMY, Goedert M, Trojanowski JQ. Neurodegenerative tauopathies. Annu Rev Neurosci. 2001;24:1121–1159. doi: 10.1146/annurev.neuro.24.1.1121. [DOI] [PubMed] [Google Scholar]
- Poorkaj P, Bird TD, Wijsman E, Garruto RM, Anderson L, Andreadis A, Wiederholt WC, Raskind M, Schellenberg GD. Tau is a candidate gene for chromosome 17 frontotemporal dementia. Ann Neurol. 1998;43:815–825. doi: 10.1002/ana.410430617. [DOI] [PubMed] [Google Scholar]
- Hutton M, Lendon CL, Rizzu P, Baker M, Froelich S, Houlden H, Pickering-Brown S, Chakraverty S, Isaacs A, Grover A, Hackett J, Adamson J, Lincoln S, Dickson D, Davies P, Petersen RC, Stevens M, de Graaff E, Wauters E, van Baren J, Hillebrand M, Joosse M, Kwon JM, Nowotny P, Che LK, Norton J, Morris JC, Reed LA, Trojanowski JQ, Basun H, Lannfelt L, Neystat M, Fahn S, Dark F, Tannenberg T, Dodd PR, Hayward N, Kwok JBJ, Schofield PR, Andreadis A, Snowden J, Craufurd D, Neary D, Owen F, Oostra BA, Hardy J, Goate A, van Swieten J, Mann D, Lynch T, Heutink P. Association of missense and 5′-splice-site-mutations in tau with the inherited dementia FTDP-17. Nature. 1998;393:702–705. doi: 10.1038/31508. [DOI] [PubMed] [Google Scholar]
- Spillantini MG, Murrell JR, Goedert M, Farlow MR, Klug A, Ghetti B. Mutation in the tau gene in familial multiple system tauopathy with presenile dementia. Proc Natl Acad Sci USA. 1998;95:7737–7741. doi: 10.1073/pnas.95.13.7737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa M, Jakes R, Crowther RA, Lee VMY, Ihara Y, Goedert M. Characterization of mAb AP422, a novel phosphorylation-dependent monoclonal antibody against tau protein. FEBS Lett. 1996;384:25–30. doi: 10.1016/0014-5793(96)00271-2. [DOI] [PubMed] [Google Scholar]
- Yoshida H, Goedert M. Sequential phosphorylation of tau protein by cAMP-dependent protein kinase and SAPK4/p38δ or JNK2 in the presence of heparin generates the AT100 epitope. J Neurochem. 2006;99:154–164. doi: 10.1111/j.1471-4159.2006.04052.x. [DOI] [PubMed] [Google Scholar]
- Lewis J, McGowan E, Rockwood J, Melrose H, Nacharaju P, van Slegtenhorst M, Gwinn-Hardy K, Murphy MP, Baker M, Yu X, Duff K, Hardy J, Corral A, Lin WL, Yen SH, Dickson DW, Davies P, Hutton M. Neurofibrillary tangles, amyotrophy and progressive motor disturbance in mice expressing mutant (P301L) tau protein. Nat Genet. 2000;25:402–405. doi: 10.1038/78078. [DOI] [PubMed] [Google Scholar]
- Allen B, Ingram E, Takao M, Smith MJ, Jakes R, Virdee K, Yoshida H, Holzer M, Craxton M, Emson PC, Atzori C, Migheli A, Crowther RA, Ghetti B, Spillantini MG, Goedert M. Abundant tau filaments and nonapoptotic neurodegeneration in transgenic mice expressing human P301S tau protein. J Neurosci. 2002;22:9340–9351. doi: 10.1523/JNEUROSCI.22-21-09340.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble W, Olm V, Takata T, Casey E, Mary O, Meyerson J, Gaynor K, La Francois J, Wang L, Kondo T, Davies P, Burns M, Veeranna M, Nixon R, Dickson D, Matsuoka Y, Ahlijanian M, Lau LF, Duff K. Cdk5 is a key factor in tau aggregation and tangle formation in vivo. Neuron. 2003;38:555–565. doi: 10.1016/s0896-6273(03)00259-9. [DOI] [PubMed] [Google Scholar]
- Schneider A, Biernat J, von Bergen M, Mandelkow E, Mandelkow EM. Phosphorylation that detaches tau protein from microtubules (Ser262, Ser214) also protects it against aggregation into Alzheimer paired helical filaments. Biochemistry. 1999;38:3549–3558. doi: 10.1021/bi981874p. [DOI] [PubMed] [Google Scholar]
- Delobel P, Flament S, Hamdane M, Mailliot C, Sambo AV, Begard S, Sergeant N, Delacourte A, Vilain JP, Buée L. Abnormal tau phosphorylation of the Alzheimer type also occurs during mitosis. J Neurochem. 2002;83:412–420. doi: 10.1046/j.1471-4159.2002.01143.x. [DOI] [PubMed] [Google Scholar]
- Cotman CW, Poon WW, Rissman RA, Blurton-Jones M. The role of caspase cleavage of tau in Alzheimer disease neuropathology. J Neuropathol Exp Neurol. 2005;64:104–112. doi: 10.1093/jnen/64.2.104. [DOI] [PubMed] [Google Scholar]
- Wischik CM, Novak M, Thøgersen HC, Edwards PC, Runswick MJ, Jakes R, Walker JE, Milstein C, Roth M, Klug A. Isolation of a fragment of tau derived from the core of the paired helical filament of Alzheimer disease. Proc Natl Acad Sci USA. 1988;85:4506–4510. doi: 10.1073/pnas.85.12.4506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wischik CM, Novak M, Edwards PC, Klug A, Tichelaar W, Crowther RA. Structural characterization of the core of the paired helical filament of Alzheimer disease. Proc Natl Acad Sci USA. 1988;85:4884–4888. doi: 10.1073/pnas.85.13.4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedert M, Spillantini MG, Cairns NJ, Crowther RA. Tau proteins of Alzheimer paired helical filaments: abnormal phosphorylation of all six brain isoforms. Neuron. 1992;8:159–168. doi: 10.1016/0896-6273(92)90117-v. [DOI] [PubMed] [Google Scholar]
- Novak M, Kabat J, Wischik CM. Molecular characterization of the minimal protease resistant tau unit of the Alzheimer’s disease paired helical filament. EMBO J. 1993;12:365–370. doi: 10.1002/j.1460-2075.1993.tb05665.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canu N, Dus L, Barbato C, Ciotti MT, Brancolini C, Rinaldi AM, Novak M, Cattaneo A, Bradbury A, Calissano P. Tau cleavage and dephosphorylation in cerebellar granule neurons undergoing apoptosis. J Neurosci. 1998;18:7061–7074. doi: 10.1523/JNEUROSCI.18-18-07061.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamblin TC, Chen F, Zanbrabo A, Abraha A, Lagalwar S, Guillozet AL, Lu M, Fu Y, Garcia-Sierra F, LaPointe N, Miller R, Berry RW, Binder LI, Cryns VL. Caspase cleavage of tau: linking amyloid and neurofibrillary tangles in Alzheimer’s disease. Proc Natl Acad Sci USA. 2003;100:10032–10037. doi: 10.1073/pnas.1630428100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rissman RA, Poon WW, Blurton-Jones M, Oddo S, Torp R, Vitek MP, LaFerla FM, Rohn TT, Cotman CW. Caspase-cleavage of tau is an early event in Alzheimer disease tangle pathology. J Clin Invest. 2004;114:121–130. doi: 10.1172/JCI20640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman J, Rissman RA, Sarsoza F, Kim RC, Dick M, Bennett DA, Cotman CW, Rohn TT, Head E. Caspase-cleaved tau accumulation in neurodegenerative diseases associated with tau and α-synuclein pathology. Acta Neuropathol. 2005;110:135–144. doi: 10.1007/s00401-005-1027-3. [DOI] [PubMed] [Google Scholar]
- Guillozet-Bongaarts AL, Glajch KE, Libson EG, Cahill ME, Bigio E, Berry RW, Binder LI. Phosphorylation and cleavage of tau in non-AD tauopathies. Acta Neuropathol. 2007;113:513–520. doi: 10.1007/s00401-007-0209-6. [DOI] [PubMed] [Google Scholar]
- Abraha A, Ghoshal N, Gamblin TC, Cryns V, Berry RW, Kuret J, Binder LI. C-terminal inhibition of tau assembly in vitro and in Alzheimer’s disease. J Cell Sci. 2000;113:3737–3745. doi: 10.1242/jcs.113.21.3737. [DOI] [PubMed] [Google Scholar]
- Berry RW, Abraha A, Lagalwar S, La Pomte N, Gamblin TC, Cryns VL, Binder LI. Inhibition of tau polymerisation by its carboxy-terminal caspase cleavage fragment. Biochemistry. 2003;42:8325–8331. doi: 10.1021/bi027348m. [DOI] [PubMed] [Google Scholar]
- Fasulo L, Ugolini G, Visintin M, Bradbury A, Brancolini C, Verzillo V, Novak M, Cattaneo A. The neuronal microtubule-associated protein is a substrate for caspase-3 and an effector of apoptosis. J Neurochem. 2000;75:624–633. doi: 10.1046/j.1471-4159.2000.0750624.x. [DOI] [PubMed] [Google Scholar]
- Chung CW, Song YH, Kim IK, Yoon WJ, Ryu BR, Jo DG, Woo HN, Kwon YK, Kim HH, Gwag BJ, Mook-Jung IH, Jung YK. Proapoptotic effects of tau cleavage product generated by caspase-3. Neurobiol Dis. 2001;8:162–172. doi: 10.1006/nbdi.2000.0335. [DOI] [PubMed] [Google Scholar]
- Goedert M, Jakes R, Vanmechelen E. Monoclonal antibody AT8 recognises tau protein phosphorylated at both serine 202 and threonine 205. Neurosci Lett. 1995;189:167–170. doi: 10.1016/0304-3940(95)11484-e. [DOI] [PubMed] [Google Scholar]
- Goedert M, Jakes R, Crowther RA, Cohen P, Vanmechelen E, Vandermeeren M, Cras P. Epitope mapping of monoclonal antibodies to the paired helical filaments of Alzheimer’s disease: identification of phosphorylation sites in tau protein. Biochem J. 1994;301:871–877. doi: 10.1042/bj3010871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedert M, Spillantini MG, Jakes R, Rutherford D, Crowther RA. Multiple isoforms of human microtubule-associated protein tau: sequences and localization in neurofibrillary tangles of Alzheimer’s disease. Neuron. 1989;3:519–526. doi: 10.1016/0896-6273(89)90210-9. [DOI] [PubMed] [Google Scholar]
- Kosik KS, Orecchio LD, Binder LI, Trojanowski JQ, Lee VMY, Lee G. Epitopes that span the tau molecule are shared with paired helical filaments. Neuron. 1988;1:817–825. doi: 10.1016/0896-6273(88)90129-8. [DOI] [PubMed] [Google Scholar]
- Guillozet-Bongaarts AL, Garcia-Sierra F, Reynolds MR, Horowitz PM, Fu Y, Wang T, Cahill ME, Bigio EH, Berry RW, Binder LI. Tau truncation during neurofibrillary evolution in Alzheimer’s disease. Neurobiol Aging. 2005;26:1015–1022. doi: 10.1016/j.neurobiolaging.2004.09.019. [DOI] [PubMed] [Google Scholar]
- Goedert M, Jakes R. Expression of separate isoforms of human tau protein: correlation with the tau pattern in brain and effects on tubulin polymerization. EMBO J. 1990;9:4225–4230. doi: 10.1002/j.1460-2075.1990.tb07870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedert M, Jakes R, Spillantini MG, Hasegawa M, Smith MJ, Crowther RA. Assembly of microtubule-associated protein tau into Alzheimer-like filaments induced by sulphated glycosaminoglycans. Nature. 1996;383:550–553. doi: 10.1038/383550a0. [DOI] [PubMed] [Google Scholar]
- Delobel P, Lavenir I, Ghetti B, Holzer M, Goedert M. Cell-cycle markers in a transgenic mouse model of human tauopathy: increased levels of cyclin-dependent kinase inhibitors p21Cip1 and p27Kip1. Am J Pathol. 2006;168:878–887. doi: 10.2353/ajpath.2006.050540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirra SS, Murrell JR, Gearing M, Spillantini MG, Goedert M, Crowther RA, Levey AI, Jones R, Green J, Shoffner JM, Wainer BH, Schmidt ML, Trojanowski JQ, Ghetti B. Tau pathology in a family with dementia and a P301L mutation in tau. J Neuropathol Exp Neurol. 1999;58:335–345. doi: 10.1097/00005072-199904000-00004. [DOI] [PubMed] [Google Scholar]
- Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R, Metherate R, Mattson P, Akbari Y, LaFerla FM. Triple-transgenic model of Alzheimer’s disease with plaques and tangles: intracellular Abeta and synaptic dysfunction. Neuron. 2003;39:409–421. doi: 10.1016/s0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- Guillozet-Bongaarts AL, Cahill ME, Cryns VL, Reynolds MR, Berry RW, Binder LI. Pseudophosphorylation of tau at serine 422 inhibits caspase cleavage: in vitro evidence and implications for tangle formation in vivo. J Neurochem. 2006;97:1005–1014. doi: 10.1111/j.1471-4159.2006.03784.x. [DOI] [PubMed] [Google Scholar]
- Zehr C, Lewis J, McGowan E, Crook J, Lin WL, Godwin K, Knight J, Dickson DW, Hutton M. Apoptosis in oligodendrocytes is associated with axonal degeneration in P301L tau mice. Neurobiol Dis. 2004;15:553–562. doi: 10.1016/j.nbd.2003.12.011. [DOI] [PubMed] [Google Scholar]