Abstract
Considering the potential role of macrophage migration inhibitory factor (MIF) in the inflammation process in placenta when infected by pathogens, we investigated the production of this cytokine in chorionic villous explants obtained from human first-trimester placentas stimulated with soluble antigen from Toxoplasma gondii (STAg). Parallel cultures were performed with villous explants stimulated with STAg, interferon-γ (IFN-γ), or STAg plus IFN-γ. To assess the role of placental MIF on monocyte adhesiveness to human trophoblast, explants were co-cultured with human myelomonocytic THP-1 cells in the presence or absence of supernatant from cultures treated with STAg (SPN), SPN plus anti-MIF antibodies, or recombinant MIF. A significantly higher concentration of MIF was produced and secreted by villous explants treated with STAg or STAg plus IFN-γ after 24-hour culture. Addition of SPN or recombinant MIF was able to increase THP-1 adhesion, which was inhibited after treatment with anti-MIF antibodies. This phenomenon was associated with intercellular adhesion molecule expression by villous explants. Considering that the processes leading to vertical dissemination of T. gondii remain widely unknown, our results demonstrate that MIF production by human first-trimester placenta is up-regulated by parasite antigen and may play an essential role as an autocrine/paracrine mediator in placental infection by T. gondii.
Toxoplasma gondii is an obligate, intracellular coccidian and an important opportunistic pathogen for a wide range of hosts. In humans, toxoplasmosis is associated with severe congenital defects when the primary infection is acquired during the first trimester of pregnancy.1 Control for this infection is a result of complex and compartmented immunological mechanisms, in which cellular immunity is considered the key component of the host immune response because it is responsible for regulating T. gondii replication.2,3 Although B-cell-deficient mice have an impaired immune response toward this parasite,4,5 it is generally accepted that antibodies play a minor role.
Infection with T. gondii elicits a Th1-type immune response with prominent production of interferon- γ (IFN-γ), tumor necrosis factor-α (TNF-α), and interleukin-1β.4,5,6 The role of IFN-γ is intriguing. When IFN-γ is administered in vivo in a mouse model of toxoplasmosis, significant protection against T. gondii is observed.7,8 From these studies, it is suggested that IFN-γ is the major mediator of resistance against T. gondii. However, during gestation, a decreased placental and fetal infection was observed in a model of BALB/c mice during acute primary T. gondii infection after neutralization of IFN-γ.9 In vitro studies using the human choriocarcinoma cell line BeWo demonstrated that IFN-γ was unable to control replication of T. gondii.10
Macrophage migration inhibitory factor (MIF) is a cytokine capable of inhibiting the random migration of mononuclear cells in vitro and is produced by different cell types, including macrophages, lymphocytes, and fibroblasts, and by reproductive cells and tissues.11 MIF is expressed in human pregnancy by both fetal trophoblast and maternal uterus.12,13 MIF is a key regulator of immune and inflammatory responses. It is released on activation of macrophages by various pro-inflammatory stimuli, such as lipopolysaccharide, toxic shock syndrome toxin 1, malaria parasites, TNF-α, and IFN-γ.11,14,15 It is also involved in the activation of macrophages and killing of intracellular parasites such as Leishmania major.16 Previous studies have demonstrated that intervillous blood mononuclear cells in human placenta produced higher levels of MIF than peripheral blood mononuclear cells.17 The same authors demonstrated that levels of MIF in intervillous space in malaria-infected placenta were higher than in uninfected tissues.17 These data suggest a potential role of MIF to retain activated macrophages for killing intracellular parasites in human placenta. Herein, we investigated the effect of soluble T. gondii antigen in MIF expression by human first-trimester placenta and the potential role of MIF in regulating adhesion of monocytes to the villous trophoblast.
Materials and Methods
Cell Cultures
The human myelomonocytic THP-1 cell line (202-TIB; American Type Culture Collection, Manassas, VA) was cultured in RPMI 1640 supplemented with 10% fetal calf serum, 2 mmol/L glutamine, 100 U/ml penicillin, and 50 μg/ml streptomycin. Cells were incubated at 37°C in an atmosphere containing 5% CO2. The cell density ranged from 1 to 5 × 105 cells/ml in a total volume of 10 ml.
Human Chorionic Villous Explant Cultures
Placenta samples (n = 12) were obtained from patients undergoing elective termination of pregnancy (9–12 weeks of gestation) after informed consent in accordance with participating institutions’ Ethics Guidelines (Università degli Studi di Siena, Italy). Briefly, placental tissues were placed in ice-cold sterile PBS and processed within 2 hours of collection. The tissues were washed in PBS and aseptically dissected using a microscope to remove endometrial tissue and fetal membranes. Floating terminal villous with five to seven tips per explant was teased apart, as previously described.18 Explants were transferred to a 24-well plate and cultured in Dulbecco’s modified Eagle’s medium/F12 medium (pH 7.4) supplemented with 100 U/ml penicillin and 100 μg/ml streptomycin and incubated overnight at 37°C in a humidified 5% CO2 incubator.
Parasite and Antigen
T. gondii tachyzoites (RH strain) were maintained in Swiss mice by intraperitoneal serial passage at regular 48-hour intervals.19 Mouse peritoneal exudates were harvested and washed twice (720 × g, 10 minutes, 4°C) in PBS. To prepare soluble tachyzoite antigen (STAg), parasite suspensions were adjusted to 1 × 108 tachyzoites/ml, treated with protease inhibitors (10 μg/ml aprotinin, 50 μg/ml leupeptin, and 1.6 mmol/L phenylmethylsulfonyl fluoride), and then lysed by five freeze-thaw (liquid nitrogen and water bath at 37°C) cycles and further by ultrasound (six 60-Hz cycles for 1 minute each) on ice. After centrifugation (10,000 × g, 30 minutes at 4°C), supernatants were collected and filtered through a 0.2-μm-pore-size membrane (Corning Costar Corp., Cambridge, MA). The protein concentration was determined,20 and STAg aliquots were stored at −80°C.
Treatment of Explants
In each set of experiments, a single placenta was used, and for each treatment, explant cultures were set up in triplicate. After incubation overnight, the culture medium was removed, and the explant cultures were then incubated in Dulbecco’s modified Eagle’s medium/F12 supplemented as described above with the addition of medium alone, 30 μg/ml STAg, 100 IU/ml IFN-γ, 30 μg/ml STAg plus 100 IU/ml IFN-γ, or 0.5 to 10 μg/ml recombinant human MIF (rMIF), generously provided by Dr. R. Bucala (Yale University School of Medicine, New Haven, CT), and incubated for 24 or 48 hours. At the end of each incubation, cultures were stopped, supernatants were collected, and tissues were either fixed for 2 to 4 hours in 4% (v/v) formalin and embedded in paraffin for immunohistochemistry or frozen at −80°C for MIF concentration.
Cell Adhesion Assay
To assess the role of placental MIF on monocyte adhesiveness on villous explant cultures, explants from placenta were cultured into 24-well plates with supernatant collected and stored at −80°C from previous explant cultures treated for 24 hours with STAg (30 μg/ml) (SPN), SPN plus polyclonal goat anti-MIF antibodies (2.5 or 5 μg/ml) (R&D Systems, Abingdon, UK), SPN plus irrelevant antibodies (5 μg/ml rabbit IgG), rMIF (0.5 to 10 μg/ml), or fresh medium Dulbecco’s modified Eagle’s medium/F12. After 24 hours of treatment, villous explants were added to THP-1 cells (2 × 105 per well) and incubated for further 90 minutes. Unbound THP-1 cells were removed by gently washing three times with 200 μl of medium and then collected and counted in Burker chambers. Adherent THP-1 cells were estimated for each explant as the difference between the cells previously added and those unbound. Data represent the number of THP-1-adhered cells (×103) per volume of explant (mm3). The volume of villous explants was determined according to the Archimedes′ principle using a calibrated 1-ml volumetric glass pipette. A volume of 800 μl of medium was placed inside the pipette, followed by adding the villous, which became totally submerged in the medium. The amount of increased volume was assumed as the villous volume. Overall, the volume of villous explants was around 10 mm3.
Nitrite Determination
The nitrite concentration in the villous explant supernatants was determined by Griess test.21 Briefly, 100 μl of samples was added to each well of a 96-well plate, and 100 μl of a mixture (1:1) of 1% sulfanilamide dihydrochloride and 0.1% naphtylenediamide dihydrochloride in 2.5% H3PO4 was added to samples. The absorbance (A550) was determined in a microplate reader (Sclavo, Siena, Italy) with reference to a standard curve of sodium nitrite (Sigma Chemical Co., St. Louis, MO) with concentration ranging from 5 to 100 μmol/L. Each experiment was conducted in triplicate and repeated at least twice.
Western Blot
Frozen villous explants were thawed, minced with a razor blade, and homogenized on ice in RIPA buffer [50 mmol/L Tris-HCl, 150 mmol/L NaCl, 1% (v/v) Triton X-100, 1% (w/v) sodium deoxycholate, and 0.1% (w/v) SDS, pH 7.5] supplemented with complete protease inhibitor cocktail tablets (Roche Diagnostic, Mannheim, Germany). After centrifugation at 15,000 × g for 15 minutes at 4°C, 40 μg of total proteins was subjected to gel electrophoresis using 12% polyacrylamide gels under denaturant conditions (SDS-PAGE). Proteins were then electrotransferred to polyvinylidene difluoride membranes (Sigma Chemical Co.). Blotted membranes were incubated in blocking solution [3% not fat dry milk in PBS and 0.1% (v/v) Triton X-100] for 1 hour at room temperature and then incubated overnight with primary anti-human MIF monoclonal antibody (R&D Systems) at dilution of 1:500. The membranes were then exposed to horseradish peroxidase-conjugated rabbit anti-mouse secondary antibody. Detection by chemiluminescence reaction was performed using ECL kit (Perkin-Elmer Life Sciences, Boston, MA). Equal loading of the proteins was confirmed by staining the blots with a 10% Ponceau S solution (Sigma Chemical Co.). Densitometric analysis was performed by Quantity One Software (Bio-Rad, Milan, Italy). All measurements obtained in densitometry analysis of the Western blots were relative to Ponceau staining. This staining was performed according to Moore and Viselli,22 staining all of the proteins in the blot, including those from housekeeping genes such as actin or myosin, to show the amount of protein loading. Thus, this analysis was based on the ratio of the densitometric values obtained in MIF-specific chemiluminescence reaction and the major Ponceau-stained polyvinylidene difluoride membrane band.
Immunocytochemistry
Paraffin-embedded explants were cut into 4-μm sections and incubated for 10 minutes at room temperature with 5% acetic acid to block endogenous alkaline phosphatase. Sections were treated with 2% normal rabbit serum diluted in 0.05 mol/L Tris-buffered saline (pH 7.4) for 30 minutes at 37°C to block nonspecific binding sites. The preparations were incubated for 12 hours at 4°C with mouse monoclonal antibody anti-MIF or goat polyclonal anti-intercellular adhesion molecule (ICAM)-1 (R&D Systems) at dilution of 1:200 and 1:300, respectively. Negative controls were performed by replacement of the primary antibody with normal mouse serum or normal goat, respectively, for MIF and ICAM-1. The preparations were then rinsed in Tris-buffered saline and incubated with biotinylated rabbit anti-mouse IgG (Sigma Chemical Co.) or biotinylated rabbit anti-goat IgG (DAKO, Glostrup, Denmark) for 30 minutes at 37°C. The reaction signal was amplified using the ABC system (Biomeda, Foster City, CA), developed with fast red-naphthol (Sigma Chemical Co.), and counterstained with Mayer’s hematoxylin.
Enzyme-Linked Immunosorbent Assay
MIF release in supernatant of villous explant cultures was measured by a colorimetric sandwich enzyme-linked immunosorbent assay. Briefly, 96-well plates were coated overnight with monoclonal antibody anti-human MIF (2.0 μg/ml) (R&D Systems), washed and blocked with blocking solution (1% bovine serum albumin and 5% sucrose in PBS), and incubated at room temperature for 1.5 hours. After washing, 100 μl of samples was added in duplicate and incubated for 2 hours at room temperature. The plates were then washed three times, and 200 ng/ml biotinylated goat anti-human MIF (R&D Systems) was added and incubated for 2 hours at room temperature. The plates were washed again and streptavidin-horseradish peroxidase (Zymed, San Francisco, CA) was added to each well and incubated for 20 minutes at room temperature. After addition of 3,3′,5,5′-tetramethylbenzidine (Zymed Laboratories), immunocomplexes were quantified using an enzyme-linked immunosorbent assay microplate reader (SR 400; Sclavo, Siena, Italy). MIF concentration was calculated by extrapolation from a standard curve (range, 25 to 2000 pg/ml) using bacterially expressed rMIF (R&D Systems). The sensitivity limit was 18 pg/ml. Intra- and interassay coefficients of variation were 3.86 ± 0.95 and 9.14 ± 0.47%, respectively. To normalize explants from different sizes, a ratio between MIF production (pg/ml) and their correspondent protein contents (mg/ml) was calculated, and the data were expressed in picograms/milligram.
Statistical Analysis
All data were expressed as the means ± SD of three to four separate experiments performed in triplicate. Differences between the means were analyzed using the Student’s unpaired t-test or Mann-Whitney test when appropriate and were considered statistically significant when P < 0.05.
Results
MIF Is Induced by Chorionic Villous Explants Exposed to STAg, IFN-γ, or STAg plus IFN-γ
We first tested whether MIF was regulated by chorionic villous explants exposed to STAg, IFN-γ, or STAg plus IFN-γ with respect to unstimulated cultures (controls). Explants were incubated for 24 or 48 hours, and culture supernatants and tissue extracts were assayed for MIF concentration. Tissues were also analyzed by immunocytochemistry using anti-MIF antibodies.
Release of MIF into Culture Medium
As shown in Figure 1, increased levels of MIF were observed at 24 hours in culture supernatants from tissues exposed to any treatment when compared with controls. However, this increase was significantly higher only in the presence of STAg, either alone or in combination with IFN-γ. In cultures maintained for 48 hours, release of MIF by unstimulated cultures was higher than at 24 hours, but no significant effect was observed after treatment with STAg, IFN-γ, or STAg plus IFN-γ (Figure 1).
Figure 1.
MIF secretion by chorionic villous explants treated with soluble T. gondii antigen (30 μg/ml STAg), 100 U/ml IFN-γ, STAg plus IFN-γ, or medium alone (controls). The supernatants were collected after 24 and 48 hours and assayed for MIF concentrations by sandwich enzyme-linked immunosorbent assay. Statistically significant differences were determined using two-tailed Student’s t-test. *P < 0.05 in comparison with controls at 24 hours. Data are representative of 12 different placenta explants.
Intracellular MIF
Western blot analysis showed a specific band of 12.5 kDa corresponding to predicted molecular mass of MIF in all examined samples (Figure 2A). Densitometric analysis revealed that intracellular MIF was significantly induced by each treatment, including STAg, IFN-γ, or STAg plus IFN-γ at 24 hours (Figure 2B). At 48 hours, intracellular MIF was dramatically increased in untreated cultures, whereas it was significantly reduced in cultures treated with STAg or IFN-γ (Figure 2B).
Figure 2.
Intracellular MIF in chorionic villous explants with soluble T. gondii antigen (30 μg/ml STAg), 100 U/ml IFN-γ, STAg plus IFN-γ, or controls. A: Top, representative Western blot analysis of explant lysates analyzed by SDS/PAGE, followed by detection with anti-MIF antibodies. Bottom, membranes stained with Ponceau S to access the total protein content loaded in each lane. B: The histogram represents densitometric measurement of Western blot bands. Statistically significant differences were determined using two-tailed Student’s t-test. *P < 0.05 in comparison with controls at 24 hours; #P < 0.05 in comparison with controls at 48 hours. Data are representative of 12 different placenta explants.
Immunocytochemistry
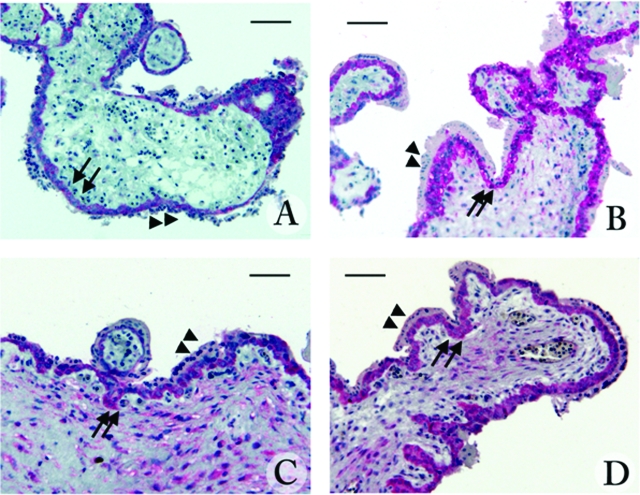
Immunocytochemistry performed using anti-MIF antibodies on explant cultures at 24 hours of incubation after treatment with STAg, IFN-γ, or STAg plus IFN-γ showed immunoreactivity for MIF in all examined sections (Figure 3). MIF immunostaining was generally more intense in treated (Figure 3, B–D) than in untreated (Figure 3A) cultures, and it was mainly localized in the villous cytotrophoblast, the inner layer of villi, whereas the outer layer (syncytiotrophoblast) was less stained. Immunostaining in treated cultures was also highly present in the villous stroma (Figure 3, B–D).
Figure 3.
Localization of MIF in 9-week chorionic explant cultures untreated (A) or treated with soluble T. gondii antigen (30 μg/ml STAg) (B), 100 U/ml IFN-γ (C), or STAg plus IFN-γ (D) for 24 hours. The expression of MIF was identified by immunophosphatase staining with anti-human MIF monoclonal antibody and counterstained with Mayer’s hematoxylin. Arrows indicate staining in the cytotrophoblast layers, and arrowheads indicate syncytiotrophoblast. Scale bar = 50 μm.
MIF Induces ICAM-1 Expression and Adhesion of THP-1 Cells to Villous Explants
The second set of experiments was conducted to determine whether MIF is able to sustain adhesion of THP-1 on villous explants. We first analyzed expression of ICAM-1, a molecule known to mediate leukocyte trafficking across endothelial and epithelial barriers, by explant cultures and human first-trimester placenta tissues. Second, we investigated the role of MIF and particularly trophoblast MIF in the adhesion of THP-1 on villous explants.
ICAM-1 Expression
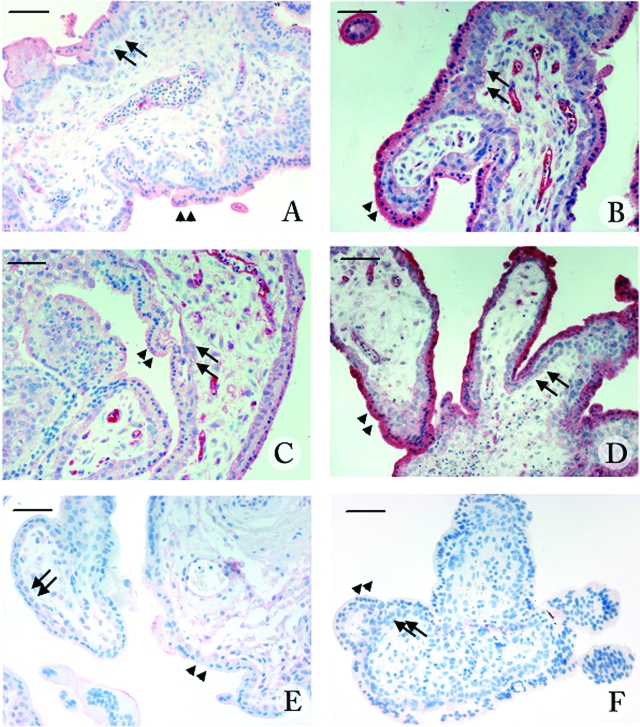
Immunocytochemistry for ICAM-1 expression was performed in chorionic villous explants incubated for 24 hours with fresh medium (controls) or supernatants collected from previous explant cultures exposed to STAg (30 μg/ml) (SPN), SPN plus anti-MIF antibodies (2.5 or 5 μg/ml), or rMIF (10 μg/ml). To test whether culture conditions affected ICAM-1 expression, immunohistochemistry was also performed in placental tissues fixed immediately after collection. Immunocytochemical analysis showed that ICAM-1 expression was scarcely detectable in first trimester tissues and in untreated cultures (Figure 4, A and E), whereas it was visibly up-regulated in cultures exposed to STAg-induced trophoblast MIF (SPN) (Figure 4B) or rMIF (Figure 4D). ICAM-1 expression was mainly localized in the syncytiotrophoblast, in some elements of the villous stroma, and, limited to cultures exposed to SPN or rMIF, in the fetal endothelial cells. Treatment with SPN plus anti-MIF antibodies reduced ICAM-1 expression mainly in the syncytiotrophoblast in a dose-dependent manner, whereas the intense staining in the endothelial cells and villous stroma remained unaffected (Figure 4C). No immunoreactivity was detected in controls performed by substituting the primary antibody with normal goat serum (Figure 4F).
Figure 4.
Localization of ICAM-1 in 9-week placental villous explants untreated (A) or treated with supernatant from villous explant cultures treated with STAg for 24 hours (SPN) (B), SPN plus anti-human MIF polyclonal antibody (5 μg/ml) (C), rMIF (10 μg/ml) (D). First-trimester human placenta (E) and negative control of first-trimester placenta obtained by omission of the primary antibody (F). The expression of ICAM-1 was identified by immunophophatase staining with anti-human ICAM-1 antibody and counterstained with Mayer’s hematoxylin. Arrows indicate the cytotrophoblast layers, and arrowheads indicate the syncytiotrophoblast layers. Scale bar = 50 μm.
THP-1 Adhesion
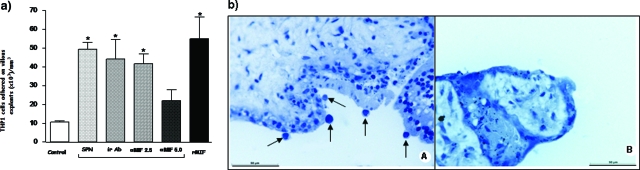
To analyze monocyte adhesion to explant cultures, we performed co-culture studies by adding suspensions of THP-1 cells (2 × 105/ml) to explant cultures exposed for 24 hours to SPN, SPN plus anti-MIF antibodies (2.5 and 5 μg/ml), SPN plus irrelevant antibodies (5 μg/ml), or rMIF (10 μg/ml). Results showed that treatment of explants with SPN or SPN plus irrelevant antibodies increased significantly monocyte adhesion to trophoblast as much as did rMIF treatment. Interestingly, monocyte adhesion was significantly reduced when SPN was treated with 5 μg/ml anti-MIF antibody, and this phenomenon was dose dependent because 2.5 μg/ml anti-MIF antibody was unable to impair monocyte adhesion (Figure 5, a and b).
Figure 5.
a: Number of THP1 cells adhered on villous explant surface untreated (Control) or treated with supernatant (SPN) of villous explants containing MIF (0.65 μg/ml) or with SPN plus irrelevant antibody (5 μg/ml ir Ab) or SPN plus anti-human MIF (2.5 and 5 μg/ml) or medium containing rMIF (10 μg/ml) for 24 hours. Statistically significant differences were determined using the Mann-Whitney test. *P < 0.05 in comparison with medium alone. b: Villous explant treated with rMIF (10 μg/ml) for 24 hours showing THP1 adhered on its surface (arrows) (A) and untreated villous explant (Control) (B) stained with Mayer’s hematoxylin. Scale bar = 50 μm.
Production of Nitrite
No production of nitrite was detected in supernatants from villous explants either untreated or treated with STAg, IFN-γ, or STAg plus IFN-γ (data not shown), showing that in this experimental design, the increased expression of MIF and, consequently, ICAM-1 did not interfere with the production of nitrogen monoxide.
Discussion
MIF has been demonstrated in the human endometrium and decidua of first-trimester placentas, placental villi, cytotrophoblast and in the trophoblast cell islands.12,23 Therefore, MIF has been implicated in embryo implantation and other reproductive functions.23 In addition, the steroid hormones, such as estrogen, progesterone, and cortisol, all of which may be immunomodulatory, are elevated during pregnancy. Particularly, cortisol has been reported to suppress macrophage function24 and to decrease immunity to malaria during pregnancy.25 It has also been reported that MIF is able to activate macrophages and to overcome the immunosupressive effect of glucocorticoid hormones, such as cortisol. Thus, MIF may play an important and critical role, along with IFN-γ and/or TNF-α, in activating macrophages to clear malaria parasites.17 MIF has been shown to be effective in activating macrophages to kill intracellular parasites, such as L. major.16
The present study demonstrates for the first time that MIF production and secretion of this molecule by villous explants are strongly associated with stimulation by STAg or STAg plus IFN-γ. Interestingly, some major discrepancies were observed between MIF secretion and intracellular MIF, probably demonstrating important differences concerning the biology of MIF secretion versus MIF intracellular production. STAg plus IFN-γ stimulation for 24 hours increased both MIF secretion and MIF intracellular production, as was also seen for STAg alone. After 48 hours, however, STAg or IFN-γ stimulation alone impaired intracellular MIF production. In addition, this impairment disappeared when both stimuli were used simultaneously for 48 hours. These findings suggest that STAg alone or in combination with IFN-γ may play a critical role in MIF production and secretion. Additionally, we observed that stimulation of villous explants by STAg and IFN-γ failed to induce NO production. A previous study has demonstrated that MIF is unable to induce TNF-α and NO production, as well to increase the inducible nitric oxide synthase mRNA expression by macrophages, because TNF-α production is required for autocrine stimulation of primed macrophages for NO generation.26,27 In the present study, the lack of production of NO by villous trophoblast stimulated with STAg and/or IFN-γ could be dependent on mechanisms similar to those observed in macrophages.
It has been demonstrated that MIF is able to up-regulate the expression of adhesion molecules (ICAM-1 and vascular cell adhesion molecule-1).28 Accordingly, in the present study, our findings evidenced that MIF is associated with increased ICAM-1 expression in syncytiotrophoblast and that the augmentation of adhesion of THP-1 to villous explants is mainly due to a substantial up-regulation of ICAM-1 expression on MIF-treated villous. Hence, these data support a central role for MIF to increase ICAM-1 expression and the cell adhesion process. Because MIF is secreted in response to STAg stimulation, it can be hypothesized that T. gondii is able to subvert the regulation of host cell adhesion and likely exploits the natural pathways of cellular migration for parasite dissemination of the host.29
Endothelial cell surface ICAM-1 appears to contribute to the adhesion and transmigration of leukocytes that express its ligand molecule leukocyte function-associated antigen-1, such as neutrophils, monocytes, lymphocytes and natural killer cells.30,31 The syncytiotrophoblast, which delimits the villous stroma, has an endothelial function that enables regulation of maternal-fetal exchanges.32 ICAM-1 overexpression has been described in cultured syncytiotrophoblast pretreated with inflammatory cytokines, which have also the ability to increase the adhesion of monocytes to syncytiotrophoblast.33 Pretreatment of syncytiotrophoblast with the inflammatory cytokines IFN-γ, TNF-α, and interleukin-1α greatly increases the number of monocytes bound on the surface by up-regulation of apical ICAM-1 expression.33 Additionally, the adhesion of monocytes to syncytiotrophoblast was inhibited >80% when using anti-ICAM-1 antibodies. These results suggest that villous trophoblast ICAM-1 overexpression occurs only during an immune inflammatory reaction and that increased expression of this molecule may represent an important pathological feature in immunoinflammatory disorders of the placenta characterized by an excessive accumulation of leukocytes in the intervillous space as observed during infections and villitis.33 Accordingly, two recent trophoblast culture studies involving HIV-1 and human cytomegalovirus placental infection have shown trophoblastic ICAM-1 overexpression,34,35 suggesting that this molecule could be an interacting agent that facilitates pathogen transfer across the placental barrier.34
A number of parasitic, bacterial and viral pathogens have been shown to cross biological barriers using trafficking within leukocytes (ie, Torjan-horse) by transcytosis and paracellular migration.36,37,38 In contrast, the passage of T. gondii across the epithelium barrier in vitro occurs by direct penetration, but it does not result in damage to the monolayer or individual cells.39
In a recent study, Pfaff et al40 demonstrated that BeWo cell monolayers stimulated with the supernatant of T. gondii-infected peripheral blood mononuclear cells showed a large increase in THP-1 cell adhesion and ICAM-1 up-regulation apparently dependent on IFN-γ, but the treatment of BeWo cells with IFN-γ failed to induce adhesion up-regulation. Based on the findings obtained by these authors, it is understood that another molecule is mediating this effect, and we hypothesize that MIF may be this mediator, because our data clearly demonstrated that STAg up-regulates MIF, which modulates the expression of ICAM-1 and adhesion of THP-1 cells on the surface of villous explants.
ICAM-1 is present on the apical surface of a number of biological barriers, and its expression is up-regulated during infection of human cells with T. gondii.41 This parasite has an evolutionarily conserved family of transmembrane adhesins called MIC2,42 which binds to ICAM-1 on host cells, and this interaction probably facilitates transmigration of T. gondii.42 Thus, we hypothesized that the enhanced expression of ICAM-1 induced by MIF could favor the adhesion of monocytes potentially infected with T. gondii, and, in turn, the parasite on the villous surface could facilitate the dissemination of the infection into the deep placental tissues. To our knowledge, the present study demonstrates for the first time that MIF production and secretion of this molecule by villous explants are strongly associated with stimulation by STAg or STAg plus IFN-γ. It has already been described that MIF is able to up-regulate the expression of ICAM-128 and that this adhesion molecule is present on the apical surface of a number of biological barriers, with its expression up-regulated during infection of human cells with T. gondii.41 Our findings, however, allow us to put the following sequence of events together, First, STAg binds to the syncitiotrophoblast and is taken up and transported to the underlying cytotrophoblast, which then up-regulates MIF. Then MIF acts on the basal surface of the syncitiotrophoblast to up-regulate apical surface ICAM-1 expression, which in turn binds either STAg or monocytes activated by STAg.
In conclusion, the present study provides the first evidence that T. gondii antigen is able to induce production and secretion of MIF by villous explants. In addition, the expression of ICAM-1 by trophoblast cells is up-regulated by MIF and consequently increases the adhesion of THP-1 on villous explants. Based on these findings, we suggest that MIF may play an important role in immune response to T. gondii in the placental microenvironment.
Footnotes
Address reprint requests to Eloisa Amália Vieira Ferro, Instituto de Ciências Biomédicas, Universidade Federal de Uberlândia, Av. Pará, 1720, Uberlândia, Minas Gerais, Brasil 38405320. E-mail: eloisa@umuarama.ufu.br.
Supported by Brazilian Research Agencies (Conselho Nacional de Desenvolvimento Científico e Technológico, Coordenadoria de Aperfeiçoamento de Pessoal do Ensino Superior, and Fundação de Ampara a Pesquisa do Estado de Minas Gerais) and by a research grant (Piano di Ateneo per la Ricerca) from the University of Siena, Siena, Italy.
References
- Remington JS, McLeod R, Thuliez P, Desmonsts G. Toxoplasmosis. JS Remington, Klein OJ., editors. Philadelphia, PA: W.B. Saunders; Infectious Disease of the Fetus and Newborn Infant. (ed 5) 2001:pp 205–356. [Google Scholar]
- Lindberg RE, Frenkel JK. Cellular immunity to Toxoplasma and Besnoitia in hamsters: specificity and the effects of cortisol. Infect Immun. 1977;15:855–862. doi: 10.1128/iai.15.3.855-862.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlüter D, Lohler J, Deckert M, Hof H, Schwendemann G. Toxoplasma encephalitis of immunocompetent and nude mice: immunohistochemical characterisation of Toxoplasma antigen, infiltrates and major histocompatibility complex gene products. J Neuroimmunol. 1991;31:185–198. doi: 10.1016/0165-5728(91)90040-e. [DOI] [PubMed] [Google Scholar]
- Denkers EY, Gazzinelli RT. Regulation and function of T-cell-mediated immunity during Toxoplasma gondii infection. Clin Microbiol Rev. 1998;11:569–588. doi: 10.1128/cmr.11.4.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang H, Remington JS, Suzuki YR. Decreased resistance of B cell-deficient mice to infection with Toxoplasma gondii despite unimpaired expression of IFN-gamma, TNF-alpha, and inducible nitric oxide synthase. J Immunol. 2000;164:2629–2634. doi: 10.4049/jimmunol.164.5.2629. [DOI] [PubMed] [Google Scholar]
- Chang HR, Grau GE, Pechere JC. Role of TNF and IL-1 in infections with Toxoplasma gondii. Immunology. 1990;69:33–37. [PMC free article] [PubMed] [Google Scholar]
- McCabe RE, Luft BJ, Remington JS. Effect of murine interferon gamma on murine toxoplasmosis. J Infect Dis. 1984;150:961–962. doi: 10.1093/infdis/150.6.961. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Orellana MA, Robbert DS, Jack SR. Interferon-γ: the major mediator of resistance against Toxoplasma gondii. Science. 1988;240:516–518. doi: 10.1126/science.3128869. [DOI] [PubMed] [Google Scholar]
- Abou-Bacar A, Pfaff AW, Georges S, Letscher-Bru V, Filisetti D, Villard O, Antoni E, Klein JP, Candolfi E. Role of NK cells and gamma interferon in transplacental passage of Toxoplasma gondii in a mouse model of primary infection. Infect Immun. 2004;72:1397–1401. doi: 10.1128/IAI.72.3.1397-1401.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira JG, Silva NM, Santos AA, Souza MA, Ferreira GL, Mineo JR, Ferro EA. BeWo trophoblasts are unable to control replication of Toxoplasma gondii, even in the presence of exogenous IFN-gamma. Placenta. 2006;27:691–698. doi: 10.1016/j.placenta.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Bernhagen J, Calandra T, Bucala R. Regulation of the immune response by macrophage migration inhibitory factor: biological and structural features. J Mol Med. 1998;76:151–161. doi: 10.1007/s001090050204. [DOI] [PubMed] [Google Scholar]
- Ietta F, Wu Y, Romagnoli R, Soleymanlou N, Orsini B, Zamudio S, Paulesu L, Caniggia I. Oxygen regulation of macrophage migration inhibitory factor in human placenta. Am J Physiol Endocrinol Metab. 2007;292:E272–E280. doi: 10.1152/ajpendo.00086.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arcuri F, Ricci C, Ietta F, Cintorino M, Tripodi SA, Cetin I, Garzia E, Schatz F, Lemi P, Santopietro R, Paulesu L. Macrophage migration inhibitory factor in human endometrium: expression and localization during the menstrual cycle and early pregnancy. Biol Reprod. 2001;64:1200–1205. doi: 10.1095/biolreprod64.4.1200. [DOI] [PubMed] [Google Scholar]
- Chaisavaneeyakorn S, Lucchi N, Abramowsky C, Othoro C, Chaiyaroj SC, Shi YP, Nahlen BL, Peterson DS, Moore JM, Udhayakumar V. Immunohistological characterization of macrophage migration inhibitory factor expression in Plasmodium falciparum-infected placentas. Infect Immun. 2005;73:3287–3293. doi: 10.1128/IAI.73.6.3287-3293.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martiney JA, Sherry B, Metz CN, Espinoza M, Ferrer AS, Calandra T, Broxmeyer HE, Bucala R. Macrophage migration inhibitory factor release by macrophages after ingestion of Plasmodium chabaudi-infected erythrocytes: possible role in the pathogenesis of malarial anemia. Infect Immun. 2000;68:2259–2267. doi: 10.1128/iai.68.4.2259-2267.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jüttner S, Bernhagen J, Metz CN, Rollinghoff M, Bucala R, Gessner A. Migration inhibitory factor induces killing of Leishmania major by macrophages: dependence on reactive nitrogen intermediates and endogenous TNF-alpha. J Immunol. 1998;161:2383–2390. [PubMed] [Google Scholar]
- Chaisavaneeyakorn S, Moore JM, Othoro C, Otieno J, Chaiyaroj SC, Shi YP, Nahlen BL, Lal AA, Udhayakumar V. Immunity to placental malaria: IV. Placental malaria is associated with up-regulation of macrophage migration inhibitory factor in intervillous blood. J Infect Dis. 2002;186:1371–1375. doi: 10.1086/344322. [DOI] [PubMed] [Google Scholar]
- Caniggia I, Taylor CV, Ritchie JW, Lye SJ, Letarte M. Endoglin regulates trophoblast differentiation along the invasive pathway in human placental villous explants. Endocrinology. 1997;138:4977–4988. doi: 10.1210/endo.138.11.5475. [DOI] [PubMed] [Google Scholar]
- Mineo JR, Camargo ME, Ferreira AW. Enzyme-linked immunosorbent assay for antibodies to Toxoplasma gondii polysaccharides in human toxoplasmosis. Infect Immun. 1980;27:283–287. doi: 10.1128/iai.27.2.283-287.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Green LC, Wagner DA, Glogwski J, Skipper PL, Wishnok JS, Tannenbaum SR. Analysis of nitrate, nitrite, and [15N] nitrate in biological fluids. Anal Biochem. 1982;126:131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- Moore MK, Viselli SM. Staining and quantification of proteins transferred to polyvinylidene fluoride membranes. Anal Biochem. 2000;279:241–242. doi: 10.1006/abio.2000.4482. [DOI] [PubMed] [Google Scholar]
- Arcuri F, Cintorino M, Vatti R, Carducci A, Liberatori S, Paulesu L. Expression of macrophage migration inhibitory factor transcript and protein by first-trimester human trophoblasts. Biol Reprod. 1999;60:1299–1303. doi: 10.1095/biolreprod60.6.1299. [DOI] [PubMed] [Google Scholar]
- Weinberg ED. Pregnancy-associated depression of cell-mediated immunity. Rev Infect Dis. 1984;6:814–831. doi: 10.1093/clinids/6.6.814. [DOI] [PubMed] [Google Scholar]
- Vleugels MP, Eling WM, Rolland R, Graaf R. Cortisol and loss of malaria immunity in human pregnancy. Br J Obstet Gynaecol. 1987;94:758–764. doi: 10.1111/j.1471-0528.1987.tb03722.x. [DOI] [PubMed] [Google Scholar]
- Herriott MJ, Jiang H, Stewart CA, Fast DJ, Leu RW. Mechanistic differences between migration inhibitory factor (MIF) and IFN-gamma for macrophage activation. J Immunol. 1993;150:4524–4531. [PubMed] [Google Scholar]
- Drapier JC, Wietzerbin J, Hibbs JB., Jr Interferon-gamma and tumor necrosis factor induce the L-arginine-dependent cytotoxic effector mechanism in murine macrophages. Eur J Immunol. 1988;18:1587–1592. doi: 10.1002/eji.1830181018. [DOI] [PubMed] [Google Scholar]
- Lan HY, Bacher M, Yang N, Mu W, Nikolic-Paterson DJ, Metz C, Meinhardt A, Bucala R, Atkins RC. The pathogenic role of macrophage migration inhibitory factor in immunologically induced kidney disease in the rat. J Exp Med. 1997;185:1455–1465. doi: 10.1084/jem.185.8.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert H, Hitziger N, Dellacasa I, Svensson M, Barragan A. Induction of dendritic cell migration upon Toxoplasma gondii infection potentiates parasite dissemination. Cell Microbiol. 2006;8:1611–1623. doi: 10.1111/j.1462-5822.2006.00735.x. [DOI] [PubMed] [Google Scholar]
- Springer TA. Adhesion receptors of the immune system. Nature. 1990;346:425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- Schenkel AR, Mamdouh Z, Muller WA. Locomotion of monocytes on endothelium is a critical step during extravasation. Nat Immunol. 2004;5:393–400. doi: 10.1038/ni1051. [DOI] [PubMed] [Google Scholar]
- Fox H. Infections and inflammatory lesions of the placenta. Fox H, editor. London: Saunders,; Pathology of the Placenta. (ed 2) 1997:pp 294–343. [Google Scholar]
- Xiao J, Garcia-Lloret M, Winkler-Lowen B, Miller R, Simpson K, Guilbert LJ. ICAM-1-mediated adhesion of peripheral blood monocytes to the maternal surface of placental syncytiotrophoblasts: implications for placental villitis. Am J Pathol. 1997;150:1845–1860. [PMC free article] [PubMed] [Google Scholar]
- Arias RA, Munoz LD, Munoz-Fernandez MA. Transmission of HIV-1 infection between trophoblast placental cells and T-cells take place via an LFA-1-mediated cell to cell contact. Virology. 2003;307:266–277. doi: 10.1016/s0042-6822(02)00040-5. [DOI] [PubMed] [Google Scholar]
- Chan G, Stinski MF, Guilbert LJ. Human cytomegalovirus-induced upregulation of intercellular cell adhesion molecule-1 on villous syncytiotrophoblasts. Biol Reprod. 2004;71:797–803. doi: 10.1095/biolreprod.104.028118. [DOI] [PubMed] [Google Scholar]
- Kerr JR. Cell adhesion molecules in the pathogenesis of and host defence against microbial infection. Mol Pathol. 1999;52:220–230. doi: 10.1136/mp.52.4.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang SH, Jong AY. Cellular mechanisms of microbial proteins contributing to invasion of the blood-brain barrier. Cell Microbiol. 2001;3:277–287. doi: 10.1046/j.1462-5822.2001.00116.x. [DOI] [PubMed] [Google Scholar]
- Juliano PB, Blotta MH, Altemani AM. ICAM-1 is overexpressed by villous trophoblasts in placentitis. Placenta. 2006;27:750–757. doi: 10.1016/j.placenta.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Barragan A, Brossier F, Sibley LD. Transepithelial migration of Toxoplasma gondii involves an interaction of intercellular adhesion molecule 1 (ICAM-1) with the parasite adhesin MIC2. Cell Microbiol. 2005;7:561–568. doi: 10.1111/j.1462-5822.2005.00486.x. [DOI] [PubMed] [Google Scholar]
- Pfaff AW, Georges S, Abou-Bacar A, Letscher-Bru V, Klein JP, Mousli M, Candolfi E. Toxoplasma gondii regulates ICAM-1 mediated monocyte adhesion to trophoblasts. Immunol Cell Biol. 2005;83:483–489. doi: 10.1111/j.1440-1711.2005.01356.x. [DOI] [PubMed] [Google Scholar]
- Nagineni CN, Detrick B, Hooks JJ. Toxoplasma gondii infection induces gene expression and secretion of interleukin 1 (IL-1), IL-6, granulocyte-macrophage colony-stimulating factor, and intercellular adhesion molecule 1 by human retinal pigment epithelial cells. Infect Immun. 2000;68:407–410. doi: 10.1128/iai.68.1.407-410.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan KL, Carruthers VB, Sibley LD, Ajioka JW. Molecular characterisation of an expressed sequence tag locus of Toxoplasma gondii encoding the micronemal protein MIC2. Mol Biochem Parasitol. 1997;84:203–214. doi: 10.1016/s0166-6851(96)02796-x. [DOI] [PubMed] [Google Scholar]