Abstract
The hallmark of IgA nephropathy (IgAN), the most common form of glomerulonephritis, is the presence of mesangial deposits containing IgA, specifically the IgA1 subclass, as the most prominent component. The deposited IgA is considered to be part of an immune complex. The family of enzymes known as bacterial IgA proteases exhibits substrate specificity that is essentially limited to the hinge region of IgA1. Here we demonstrate the ability of systemically administered IgA protease to remove glomerular IgA immune complexes, both the antigen and antibody components, in a passive mouse model of IgAN. Thus, IgA protease may have potential as a therapeutic agent for human IgAN.
IgA nephropathy (IgAN) is the most common form of glomerulonephritis throughout the world.1,2 The disease is defined, characterized, and diagnosed by virtue of mesangial deposits containing polyclonal, polymeric IgA as the principal component. Other isotypes of immunoglobulin and complement may be observed as well. Remarkably, although IgA in humans comprises two subclasses, IgA1 and IgA2, the mesangial deposits in IgAN contain IgA1 almost exclusively.
Clinically, IgAN is characterized by varying degrees of proteinuria, hematuria, and hypertension that may evolve into end-stage renal disease. Overt illness is typically episodic and often occurs concurrently with or soon after an upper respiratory or gastrointestinal syndrome. Although the pathogenesis of the disease is not clear, the fact that IgA is the main immunoglobulin of the mucosal immune system and the temporal association with upper respiratory or gastrointestinal upset suggest that IgAN may result from an exaggerated or poorly regulated IgA antibody response to a mucosal infection or to an inhaled or ingested inert antigen. As a result, immune complexes containing IgA reach the circulation and deposit in the renal mesangium. A specific antigen has not been identified, and the proposed pathogenesis allows for different infectious agents or other antigens over time to be involved in this usually intermittent disease. Because serum IgA may be elevated in such diseases as multiple myeloma and AIDS without producing renal deposits or causing nephritis, the IgA in IgAN is thought to have special features, such as abnormal glycosylation, that promote renal deposition.1,2,3,4,5
Regardless of the particular mechanism(s) of pathogenesis, there is consensus that the proximate cause of IgAN is the deposition of IgA-containing aggregates in the mesangium. Therefore, treatment aimed at removing such aggregates would seem logical. The approach taken in the present study was to develop a passive mouse model of IgAN, a variant of one described earlier,6 in which the deposited IgA is human IgA1. The mice were then injected systemically with bacterial IgA protease, an enzyme for which substrate specificity is virtually restricted to the hinge region of human IgA1,7,8,9 the isotype that defines clinical IgAN, in an effort to remove deposited IgA immune complexes from the kidneys.
Materials and Methods
IgA Immune Complexes
Human serum from outdated, pooled blood bank plasma was made 50% saturated at room temperature by addition of solid (NH4)2SO4. After 1 hour at 4°C, the suspension was centrifuged; the washed precipitate was dissolved in PBS, pH 7.2, dialyzed against this buffer, and passed through a calibrated Sephacryl 300 (GE Healthcare, Piscataway, NJ) column. Fractions containing dimeric (d) IgA were pooled. To separate the dIgA1 subclass from dIgA2, the IgA in PBS was applied to a column of immobilized jacalin (Pierce, Rockford, IL), a lectin that binds IgA1 but not IgA2. Bound dIgA1 was eluted with 0.25 mol/L d-galactose in PBS, dialyzed against PBS, concentrated, and stored at 4°C. Its purity was confirmed by SDS-polyacrylamide gel electrophoresis (PAGE) under reduced and unreduced conditions and by double immunodiffusion analysis with IgA subclass-specific antisera (The Binding Site, Birmingham, UK). For our experiments, dIgA1 was used to form immune complexes because the IgA1 deposits in human IgAN are predominantly oligomeric.2
To prepare IgA immune complexes, the quantitative precipitin reaction was used to determine the equivalence point with goat F(ab′)2 anti-human F(ab′)2 antibody (The Jackson Laboratory, Bar Harbor, ME). After preliminary experiments, it was decided to use soluble IgA immune complexes in twofold antigen excess (note that in our model system, IgA1 is the antigen) and to inject i.v. complexes containing 800 μg of dIgA1 and 400 μg of goat F(ab′)2 anti-human F(ab′)2 per mouse in 0.01 mol/L PBS, pH 7.2. Such complexes gave consistent deposition in the glomerular mesangium that was easily detectable by immunofluorescence for at least 24 hours. Control preparations contained dIgA1 plus F(ab′)2 fragments of normal goat IgG.
IgA Protease
The Haemophilis influenzae IgA protease used in this study is designated IgA1-specific serine endopeptidase, family S6.9 IgA proteases of Gram-negative bacteria are secreted into the medium through the autotransporter pathway.10 Taking advantage of the intrinsic autoproteolytic processing, we designed and expressed a recombinant form of the H. influenzae enzyme that contains six contiguous histidine residues, a strategy that facilitates the purification of the mature enzyme via nickel-affinity chromatography. The His segment was introduced such that it would be located two residues from the carboxy terminus of the secreted, mature protease. The recombinant H. influenzae strain was designated Rd 6His; the modified enzyme iga gene in this strain is chromosomal. The autoprocessed, secreted protease has a molecular mass of 109 kDa and theoretical pI = 7.0. The six-histidine stretch did not adversely affect activity.
H. influenzae strain Rd 6His was grown in starter culture and then introduced to a 10-L fermenter containing brain-heart infusion broth supplemented with 10 μg/ml NAD and hemin (Sigma, St. Louis, MO). All purification steps after fermentation were performed at 4°C. The cell-free medium from a 10-L culture contained the protease activity and was concentrated to 400 ml, filtered, and dialyzed against 25 mmol/L Tris-HCl, pH 7.5, containing 0.025% sodium azide. The concentrate was applied to a DE-52 (Whatman, Maidstone, UK) anion exchange column and washed with 25 mmol/L Tris, and the flow-through containing the enzyme was collected. Enzyme activity was assayed on human myeloma IgA1 protein substrate. The enzyme was precipitated by addition of solid ammonium sulfate (390 g/L), redissolved in 50 mmol/L sodium phosphate, pH 7.5, and dialyzed against the same buffer, now containing 5 to 10 mmol/L imidazole. Concentrated protease was applied to a Ni-NTA-agarose column (Qiagen, Valencia, CA). The nickel-bound enzyme was washed with starting buffer containing 5 mmol/L imidazole, and then the protease was eluted with 100 mmol/L imidazole in 50 mmol/L sodium phosphate, pH 7.5, and filter concentrated. Overall recovery of enzyme activity relative to that in the original culture medium was ∼75%, and its purity was >95% as judged by SDS-PAGE.
Experiments In Vitro
Soluble IgA immune complexes in twofold antigen excess were treated with IgA protease for 1 hour at 37°C, subjected to SDS-PAGE under reducing conditions, and stained with Coomassie Blue.
Experiments In Vivo
Mice were injected with different quantities of soluble IgA1 immune complexes in two- to threefold antigen excess. By immunofluorescence the amounts of subsequent glomerular deposits did not vary significantly between 0.5 and 24 hours later. From the results, the following protocol was adopted. BALB/c female mice (Charles River Laboratories, Wilmington, MA), 8 to 10 weeks old, were injected i.v. with IgA1 immune complexes in twofold antigen excess [800 μg of human dIgA1 antigen plus 400 μg of goat F(ab′)2 anti-human F(ab′)2 antibody] followed, beginning 1 hour later, by varying amounts of IgA protease i.v. ± i.p., or saline (control). Mice were sacrificed 1 hour after injection of enzyme (or saline). Frozen sections (4 μm thick) of kidneys were fixed in acetone, stained with fluorescent antibodies, and examined in blinded fashion for the presence of human IgA (both Fc and Fab regions), goat F(ab′)2, and mouse C3. Fluorescent antibodies included fluoresceinated rabbit F(ab′)2 anti-human IgA (α chain specific), fluoresceinated rabbit F(ab′)2 anti-human F(ab′)2, rhodaminated rabbit F(ab′)2 anti-goat F(ab′)2 (all from The Jackson Laboratory), and fluoresceinated goat anti-mouse C3 (Cappel Laboratories, Cochranville, PA). The immunofluorescence intensity for each reactant on a scale of 0 to 4 was evaluated for at least 20 glomeruli per mouse with a Leica DMLB microscope (Leica, Wetzlar, Germany) equipped with epi-illumination.
Some mice that were injected with immune complexes as above, but not injected further, were sacrificed 2 hours later, and their kidneys were removed and frozen. Kidney sections, 4 μm thick, were cut in a cryostat, picked up on saline-coated glass slides, air-dried unfixed, and stored at −20°C. As needed, sections were rehydrated in PBS and overlaid with IgA protease, at 10, 100, or 1000 μg/ml in PBS containing 1% bovine serum albumin. Sections were incubated for 1 hour at 37°C, rinsed in PBS, stained with fluoresceinated rabbit F(ab′)2 anti-human IgA (α chain specific), and examined as above.
For electron microscopy, pieces of renal cortex were fixed in triple aldehyde/dimethyl sulfoxide, postfixed in potassium ferrocyanide/aqueous osmium tetroxide, and embedded in Epon epoxy.11,12 Ultra-thin sections (80 nm) were stained with uranyl acetate/lead citrate.13 Glomeruli were visualized and photographed in a Zeiss CEM 902 microscope (Zeiss, Oberkochen, Germany). Deposits were evaluated in blinded fashion. The number, size, and density of deposits were assessed from digital images with NIH Image software (http://rsb.info.nih.gov/nih-image/) running on a Power Mac G4 processor (Apple Computer, Cupertino, CA). The localization within the glomerulus and the character of deposits (eg, electron-dense, -lucent, partially lucent, or tactoid) were also recorded.
Statistical Analysis
Four independent experiments were performed on mice treated with varying doses of IgA protease (i.v. ± i.p.), together with controls given saline instead of enzyme. Fluorescence intensity was evaluated by two-way analysis of variance, stratifying the enzyme dose (0 for the saline-treated groups) and the individual experiment. There was statistically significant experiment-wise variation and significant interaction between dose and experiment; therefore, one-way analysis of variance was performed within each experiment to evaluate enzyme dose effect. There was no dose effect within any single experiment at enzyme doses above 5 μg/mouse. In all four experiments, IgA protease significantly reduced glomerular immune deposits compared with saline-treated controls. Immunofluorescence scores normalized to the mean value of saline-treated mice within each experiment were normally distributed, and interexperimental variation (and interaction) was not significant by two-way analysis of variance. Accordingly, normalized data from the four independent experiments were pooled in a one-way analysis of variance design. Another experiment examined the capacity of IgA protease to digest glomerular immune deposits in tissue sections. Fluorescence intensity scores from this experiment were analyzed by one-way analysis of variance, without normalization. All post hoc comparisons used Bonferroni’s corrected t-tests. The same statistical methods were used for evaluating electron microscopy results, but without normalization applied.
Results
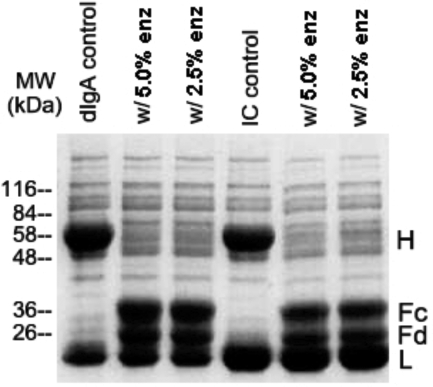
The goal of this research is to use highly specific IgA protease to cleave immune complexes containing polymeric IgA1 that have deposited in the glomerulus. Before investigating the effects of IgA protease in vivo, we wanted to know that the enzyme is capable of digesting immune-complexed IgA1 in vitro. Accordingly, soluble IgA immune complexes in twofold antigen excess, the same complexes envisioned for the studies in vivo, were exposed to IgA protease for 1 hour at 37°C and then subjected to SDS-PAGE under reducing conditions. From the results in Figure 1 it is evident that at two different weight ratios of enzyme to IgA substrate (5% and 2.5%), the H chains of both free and complexed IgA were equally and essentially completely cleaved into Fc and Fd fragments (Fd is the part of the H chain contained in the Fab fragment). Similar results were obtained when the digestion was done in the presence of an additional 4 mg/ml IgA1, twice its normal serum concentration (results not shown).
Figure 1.
Cleavage of free and immune-complexed IgA by IgA protease in vitro. Human dIgA1 and IgA immune complexes (IC), the latter containing IgA1 antigen and goat F(ab′)2 anti-human F(ab′)2 antibody, were digested with two strengths of H. influenzae IgA protease (either 5% or 2.5% enzyme by weight of IgA), subjected under reducing conditions to SDS-PAGE, and stained with Coomassie Blue. H and L indicate intact H(α) and L polypeptide chains, and Fc and Fd indicate the H chain fragments after IgA protease cleavage at the IgA hinge. After 1 hour at 37°C at both enzyme/substrate weight ratios, the enzyme cleaved the IgA, whether free or complexed.
The next experiment was designed to test whether i.v. IgA protease is also capable of digesting IgA immune complexes that had deposited in the kidneys. In pilot experiments, i.v. injection of 800 μg of dimeric human IgA1 complexed with 400 μg of F(ab′)2 fragments of goat anti-human F(ab′)2 (twofold antigen excess) gave rise to prominent mesangial immunofluorescence for both reactants throughout the glomerulus in virtually all glomeruli. Staining for mouse C3 was negative. The kidneys of mice given a non-immune mixture of 800 μg of IgA1 and 400 μg of irrelevant goat F(ab′)2 fragments exhibited only weak and irregular background immunofluorescence. Accordingly, we used a protocol in which mice were given i.v. injections of the immune complexes just described, followed by varying doses of IgA protease beginning 1 hour later.
Mice given IgA immune complexes followed by a single i.v. dose of 5 μg of enzyme had significantly greater glomerular immune deposits than did those not given immune complexes (negative control mice) (data not shown). The 5-μg dose of IgA protease had only a slight, but significant (P < 0.05), effect on glomerular immunofluorescence (data not shown). After treatment with ≥10 μg of IgA protease, glomeruli had significantly (see below) less human Fcα, human F(ab′)2, and goat F(ab′)2 deposition than did glomeruli from mice given saline rather than enzyme. Increasing the dose of i.v. enzyme beyond 10 μg/mouse and including i.p. enzyme as well did not enhance the therapeutic effect. Accordingly, the groups of mice given greater than 5 μg of enzyme were pooled for statistical analysis.
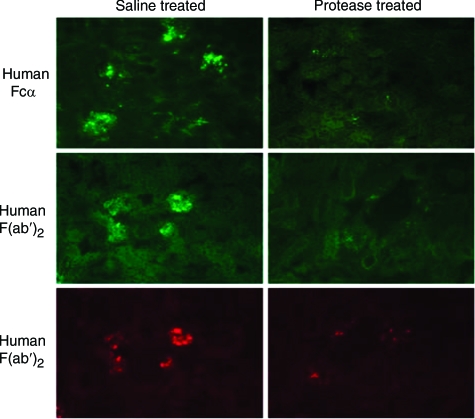
As indicated in Figure 2 and Table 1, IgA protease reduced the immunofluorescence score for Fcα by 60% (F = 45.7, P < 0.001 versus saline control). However, staining only for Fcα could not exclude the possibility that the IgA protease had cleaved the Fcα but that immune complexes containing human Fabα still remained in the glomeruli. This possibility was excluded by the results of also staining with fluorescent anti-human F(ab′)2. When stained for human F(ab′)2, the immunofluorescence score was reduced by 62% (F = 11.3, P < 0.001 vs saline control), to a level not significantly different from background. Thus, both portions of the IgA1, Fc and Fab, were removed from the glomeruli by the IgA protease. These results suggest that IgA protease is indeed capable of diminishing the quantity of glomerular IgA immune complexes. In keeping with this interpretation, the results also show that the goat F(ab′)2 antibody was removed as well by the IgA protease treatment [relative immunofluorescence intensity reduced by 41% (F = 15.2, P < 0.001 versus saline]. From the combined data in Figure 2 and Table 1, it can be concluded that both the antigen and antibody components of the injected immune complexes, ie, the human IgA1 antigen and the goat F(ab′)2 anti-human F(ab′)2 antibody, were removed by IgA protease treatment. The enzyme was also effective when given 24 hours (rather than 1 hour) after the injection of immune complexes (Table 2). In these experiments there was no apparent toxicity from the injected enzyme, as assessed by clinical and morphological observation and evaluation.
Figure 2.
Immunofluorescence photomicrographs of kidney sections from mice injected i.v. 2 hours before sacrifice with immune complexes composed of human IgA1 and goat F(ab′)2 anti-human F(ab′)2 and 1 hour before sacrifice with IgA1 protease (right column) or saline (left column). The top and middle rows (fluorescein fluorescence) show the human IgA component of the complexes. The bottom row (rhodamine fluorescence) shows the goat immunoglobulin component. The IgA is detected with anti-human Fcα and anti-human F(ab′)2 in the top and middle rows, respectively. Note that the enzyme removed most of the deposited immune complexes, both the human IgA antigen and the goat antibody.
Table 1.
Immunofluorescence Analysis of Kidney Sections from Mice Injected with IgA Immune Complexes and Then with IgA Protease
| Immune complexes | Treatment | Number of mice | Relative immunofluorescence intensity*
|
||
|---|---|---|---|---|---|
| Human Fcα | Human Fab′ | Goat Fab′ | |||
| Yes | IgA protease (≥10 μg) | 26 | 40.2 ± 3.8† | 37.6 ± 7.5† | 59.3 ± 5.8† |
| Yes | Saline | 17 | 100 ± 6.1‡ | 100 ± 6.5‡ | 100 ± 5.9‡ |
| No | None | 4 | 0 ± 0 | 19.9 ± 7.4 | 9.1 ± 5.3 |
Values are mean (± SE) normalized fluorescence intensity (saline control = 100) pooled from four independent experiments.
F ≥ 11.3, P < 0.001 versus saline treatment.
F ≥ 11.3, P < 0.001 versus no immune complexes.
Table 2.
Immunofluorescence Scores of Kidney Sections from Mice Injected with IgA Immune Complexes and 24 Hours Later with Enzyme (4 Mice) or Saline (4 Mice)
| Human IgA
|
Goat F(ab′)2
|
||
|---|---|---|---|
| Enzyme | Saline | Enzyme | Saline |
| 0 | + | 0 | ++ |
| 0 | + + | + | +++ |
| 0 | + | + | ++ |
| 0 | ++ | 0 | +++ |
Immunofluorescence intensity is indicated on a scale of 0 to 4+.
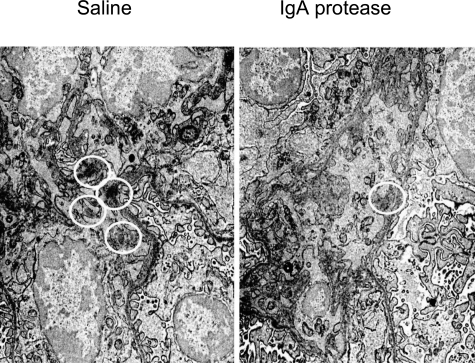
By electron microscopy, small (250- to 400-nm), discrete, amorphous electron-dense deposits were detected in paramesangial sites and in the mesangial matrix in all mice, but no deposits were present in the glomerular capillary walls. Mice treated with IgA protease had 1.47 ± 0.22 deposits per 100 μm2 of mesangial area, far fewer (P < 0.002) than the 3.89 ± 0.60 deposits per 100 μm2 detected in control mice treated with saline (Figure 3). The mesangial electron-dense deposits did not differ statistically in size or density between the two treatment groups, and no lucent, partially lucent, or fibrillar deposits were present. Although 92% of the mesangial zones from mice given saline had at least one deposit, only 60% of mesangial areas from mice treated with enzyme had any deposits (P < 0.05 by the difference between percentages; p = NS by Fisher’s exact test).
Figure 3.
Electron micrographs of representative mesangial regions from mice injected i.v. with immune complexes (as detailed for Figure 2 and in Materials and Methods), followed by saline (left panel) or IgA1 protease (right panel). Amorphous electron-dense deposits (circled) are significantly less plentiful in mice given enzyme.
To provide additional evidence that IgA protease is capable of digesting IgA immune complexes in situ if it can access the glomerulus, kidney sections from mice that had been injected i.v. with IgA immune complexes were exposed to solutions of IgA protease. The results in Table 3 show that the enzyme at concentrations ≥100 μg/ml lowered the IgA immunofluorescence score from 1.5 to 0.7 (P < 0.01). In contrast, enzyme at 10 μg/ml was without effect.
Table 3.
Immunofluorescence Analysis of Human Fcα in IgA Protease-Exposed Kidney Sections from Mice Injected with IgA Immune Complexes
| Exposed to IgA protease (≥100 μg/ml) | Controls (no enzyme) |
|---|---|
| 0.67 ± 0.17* | 1.50 ± 0.14 |
Immunofluorescence was scored on a scale of 0 to 4 for sections exposed to 10, 100, or 1000 μg/ml IgA protease or to bovine serum albumin (control). Values are mean scores (± SE).
F = 5.8, P < 0.01 versus no enzyme controls.
Discussion
IgAN is a major form of glomerular disease throughout the world in terms of both incidence and severity. Unfortunately, effective therapy is lacking. In the present work, we investigated the potential of specific enzyme therapy for two reasons. First, we and others had previously demonstrated in mice, rats, and rabbits that nonspecific proteolytic enzymes are capable of removing glomerular immune complexes in passive serum sickness and membranous nephropathy models without causing demonstrable toxicity.14,15,16,17 Similarly, dextranase, as well as nonspecific proteases, was capable of digesting dextran-containing immune complexes in active and passive models of IgAN.18 Second, for unknown reasons, in human IgAN the involved IgA is limited to the IgA1 subclass, the isotype for which microbial IgA proteases have a high degree of substrate specificity. IgA proteases are a family of bacterial enzymes that attack the hinge region of human IgA1; they do not attack the H chain of human IgA2 proteins. We thought it reasonable to exploit the IgA proteases to digest glomerular IgA1-containing immune complexes in vivo. Moreover, the resistance of other proteins to cleavage by these enzymes reduces the risk of systemic toxicity.
Because animal IgAs are not substrates for IgA protease, we had to develop a passive model in which the IgA is human IgA1. It would have been convenient to use IgA1 from cases of multiple myeloma, which are ready sources of large amounts of suitable substrate. We decided against this approach because of the possibility that a given myeloma protein, being the product of a neoplastic clone of plasma cells, might have distinctive properties not typical of IgA1 in general. Instead we decided to purify IgA1 from pooled normal human plasma in which no single clonal IgA would predominate. Because we wanted to use human IgA1 in our model system, the IgA had to be the antigen rather than the antibody. For our purposes, this distinction is irrelevant. Moreover, there is evidence in human IgAN that the IgA can, at least in some cases, also be an antigen.19 For the antibody in our passive model, we used commercially available F(ab′)2 fragments of goat IgG antibody to human F(ab′)2 fragments to form complexes of human IgA1 cross-linked at their antigen-binding F(ab) domains. We chose goat F(ab′)2 fragments because they lack the Fc portion of IgG that could cause binding in vivo to cellular Fcγ receptors; in addition, the Fcγ domain could potentially activate proinflammatory mediator systems. The essence of the model we designed was to bring about the accumulation of immune complexes containing human IgA1 in the glomeruli of a living mouse.
After determining that the model was satisfactory for our purposes, we tested the ability of IgA protease to decrease the amount of injected IgA immune complexes deposited in the kidneys in vivo. We showed that the quantity of deposited complexes was in fact reduced by an enzyme that is only capable of digesting IgA1 at a single site, the peptide bond between amino acid residues 231–232 in each heavy (α) chain. Cleavage there depleted both components of the immune complexes, the IgA antigen and the goat F(ab′)2 antibody. To explain the enzyme effect, two mechanisms can be envisioned: digestion of circulating immune complexes and digestion of deposited complexes. These mechanisms are not mutually exclusive and indeed would act synergistically in furthering a therapeutic effect. The results of the experiments in Figure 1 (digestion of soluble complexes) and Table 3 (digestion of deposited complexes in situ) demonstrate that both mechanisms are feasible. Furthermore, there was no evidence of any untoward effects from the injected IgA protease, which is in keeping with its highly limited substrate specificity.
In conclusion, we have demonstrated as a “proof of principle” the ability of exogenous IgA protease to reduce the quantity of glomerular IgA immune complexes in a passive mouse model. The results suggest that further investigation of the enzyme’s therapeutic potential for human IgAN is warranted.
Acknowledgments
We acknowledge the contributions of Drs. Anne Kane and Andrew Wright and of Marc Buren.
Footnotes
Address reprint requests to Michael E. Lamm, Case Western Reserve University, 10900 Euclid Avenue, Wolstein Research Building, Room 5531, Cleveland, OH 44106. E-mail: mel6@case.edu.
Supported by National Institutes of Health grants DK071675, AI36359, DK59334, DE15844, and DK34928, and the core facilities of the Tufts GRASP Digestive Disease Research Center, P30DK034928.
References
- Emancipator SN. IgA nephropathy and Henoch-Schonlein syndrome. Jennette JC, Olson JL, Schwartz MM, Silva FG, editors. Philadelphia: Lippincott-Raven; Heptinstall’s Pathology of the Kidney. 1998:pp 479–539. [Google Scholar]
- Emancipator SN, Mestecky J, Lamm ME. IgA nephropathy and related diseases, in Mucosal Immunology. Mestecky J, Bienenstock J, Lamm ME, Mayer L, McGhee JR, Strober W, editors. San Diego: Elsevier; 2005:pp 1580–1600. [Google Scholar]
- Mestecky J, Tomana N. Structural heterogeneity of glycans in immunoglobulin molecules: implications in IgA nephropathy. Nephrology. 1997;3:S653–S657. [Google Scholar]
- Zickerman AM, Allen AC, Talwar V, Olczak SA, Brownlee A, Holland M, Furness PN, Brunskill NJ, Feehally J. IgA myeloma presenting as Henoch-Schonlein purpura with nephritis. Am J Kidney Dis. 2000;36:E19. doi: 10.1053/ajkd.2000.16221. [DOI] [PubMed] [Google Scholar]
- Mestecky J, Novak J, Julian BA, Tomana M. Pathogenic potential of galactose-deficient IgA1 in IgA nephropathy. Nephrology. 2002;7:S92–S99. [Google Scholar]
- Rifai A, Small PA, Teague PO, Ayoub EM. Experimental IgA nephropathy. J Exp Med. 1979;150:1161–1173. doi: 10.1084/jem.150.5.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaut AG, Gilbert JV, Artenstein MS, Capra JD. Neisseria gonorrhoeae and Neisseria meningitidis: extracellular enzyme cleaves human immunoglobulin A. Science. 1975;190:1103–1105. doi: 10.1126/science.810892. [DOI] [PubMed] [Google Scholar]
- Plaut AG. IgA-specific metalloendopeptidase. Barrett AJ, Rawlings ND, Woessner JF, editors. London: Elsevier; Handbook of Proteolytic Enzymes. 2004:pp 772–777. [Google Scholar]
- Poulsen K, Kilian M. IgA-specific serine endopeptidase. Barrett AJ, Rawlings ND, Woessner JF, editors. London: Elsevier; Handbook of Proteolytic Enzymes. 2004:pp 1758–1761. [Google Scholar]
- Henderson IR, Navarro-Garcia F, Desvaux M, Fernandez RC, Ala’Aldeen D. Type V protein secretion pathway: the autotransporter story. Microb Mol Biol Rev. 2004;68:692–744. doi: 10.1128/MMBR.68.4.692-744.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalt MR, Tandler B. A study of fixation of early amphibian embryos for electron microscopy. J Ultrastruct Res. 1971;36:633–645. doi: 10.1016/s0022-5320(71)90020-7. [DOI] [PubMed] [Google Scholar]
- Karnovsky MJ. Use of ferrocyanide-reduced osmium tetroxide in electron microscopy. Proceedings of the 11th Annual Meeting, American Society of Cell Biology. 1971:p 146. [Google Scholar]
- Cash AD, Aliev G, Siedlak SL, Nunomura A, Fujioka H, Zhu X, Raina AK, Vinters HV, Tabaton M, Johnson AB, Paula-Barbosa M, Avila J, Jones PK, Castellani RJ, Smith MA, Perry G. Microtubule reduction in Alzheimer’s disease and aging is independent of tau filament formation. Am J Pathol. 2003;162:1623–1627. doi: 10.1016/s0002-9440(10)64296-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa M, Emancipator SN, Lamm ME. Removal of glomerular immune complexes in passive serum sickness nephritis by treatment in vivo with proteolytic enzymes. Lab Invest. 1986;55:551–556. [PubMed] [Google Scholar]
- Nakazawa M, Emancipator SN, Lamm ME. Proteolytic enzyme treatment reduces glomerular immune deposits and proteinuria in passive Heymann nephritis. J Exp Med. 1986;164:1973–1987. doi: 10.1084/jem.164.6.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffen C, Menzel J. In vivo-abbau von immunkomplexen in der niere durch oral applizierte enzyme. Wien Klin Wochenschr. 1987;99:525–531. [PubMed] [Google Scholar]
- White RB, Lowrie L, Stork JE, Iskandar SS, Lamm ME, Emancipator SN. Targeted enzyme therapy of experimental glomerulonephritis in rats. J Clin Invest. 1991;87:1819–1827. doi: 10.1172/JCI115203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gesualdo L, Ricanati S, Hassan MO, Emancipator SN, Lamm ME. Enzymolysis of glomerular immune deposits in vivo with dextranase/protease ameliorates proteinuria, hematuria, and mesangial proliferation in murine experimental IgA nephropathy. J Clin Invest. 1990;86:715–722. doi: 10.1172/JCI114767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomana M, Matousovic K, Julian BA, Radl J, Konecny K, Mestecky J. Galactose-deficient IgA1 in sera of IgA nephropathy patients is present in complexes with IgG. Kidney Int. 1997;52:509–516. doi: 10.1038/ki.1997.361. [DOI] [PubMed] [Google Scholar]