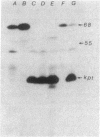
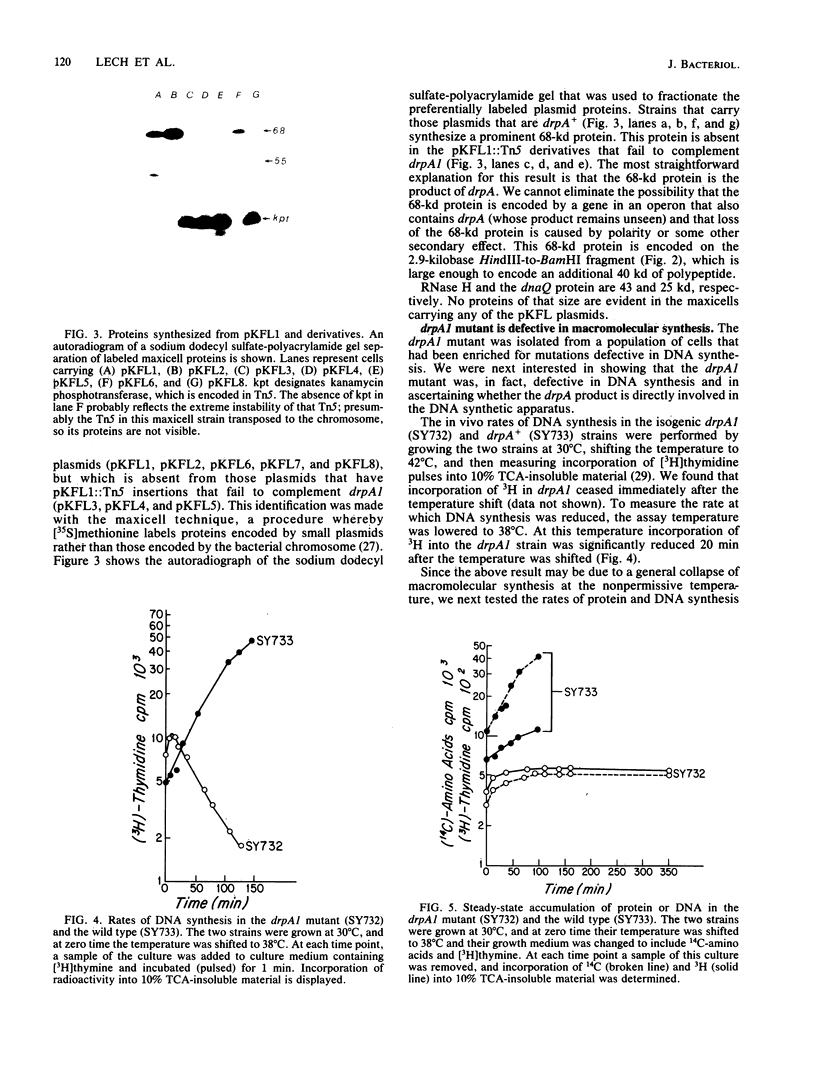
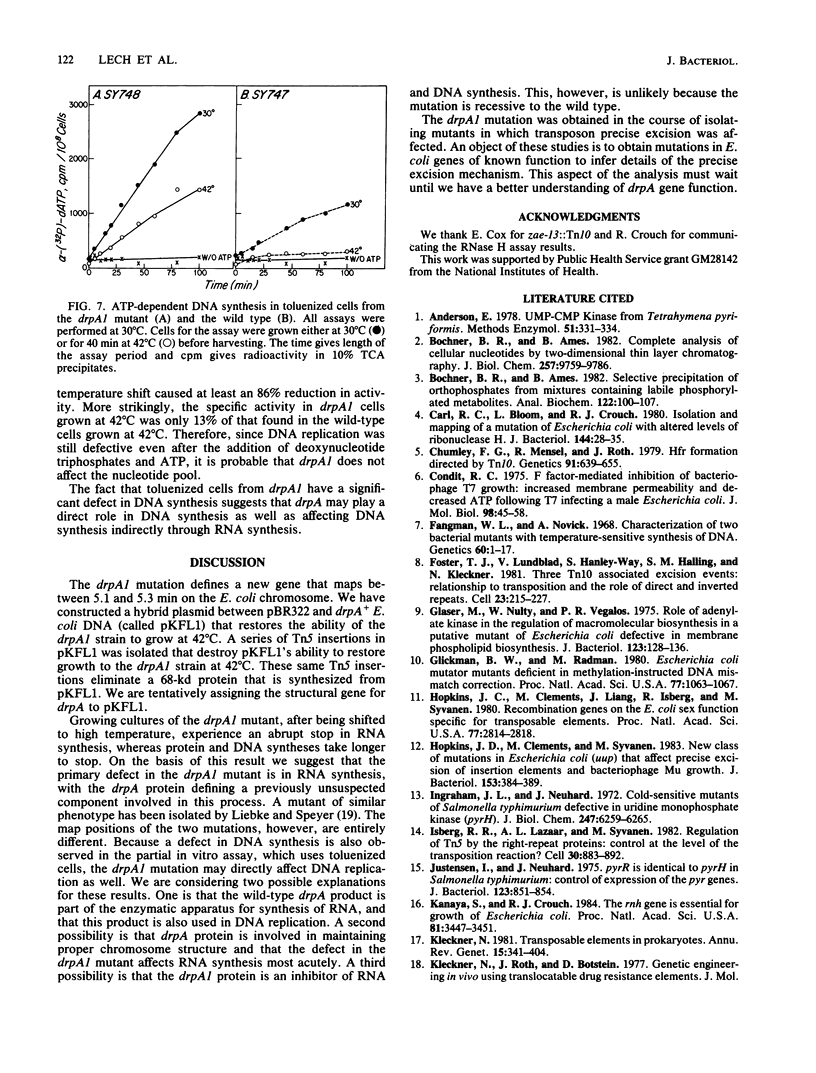
Abstract
The mutation drpA1 defines a new gene in Escherichia coli K-12 that maps at about 5.2 min. This mutation was obtained after enriching a population of cells for temperature sensitive dna mutations with the [3H]thymidine "suicide" technique followed by screening for mutants defective in transposon Tn5 precise excision. When growing cells carrying the drpA1 allele were shifted to the nonpermissive temperature, we showed that DNA, RNA, and protein syntheses shut off quickly, with the cessation of RNA synthesis occurring first. A recombinant plasmid between pBR322 and an HindIII fragment from wild-type E. coli restores the growth defect in drpA1 mutants. Using transposon Tn5 mutagenesis of this plasmid, we have been able to correlate the presence of a 68-kilodalton protein, as observed with the maxicell technique, with the ability of this plasmid to restore growth to drpA1 mutants.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson E. P. UMP-CMP kinase from Tetrahymena pyriformis. Methods Enzymol. 1978;51:331–337. doi: 10.1016/s0076-6879(78)51044-6. [DOI] [PubMed] [Google Scholar]
- Bochner B. R., Ames B. N. Complete analysis of cellular nucleotides by two-dimensional thin layer chromatography. J Biol Chem. 1982 Aug 25;257(16):9759–9769. [PubMed] [Google Scholar]
- Bochner B. R., Ames B. N. Selective precipitation orthophosphate from mixtures containing labile phosphorylated metabolites. Anal Biochem. 1982 May 1;122(1):100–107. doi: 10.1016/0003-2697(82)90257-3. [DOI] [PubMed] [Google Scholar]
- Carl P. L., Bloom L., Crouch R. J. Isolation and mapping of a mutation in Escherichia coli with altered levels of ribonuclease H. J Bacteriol. 1980 Oct;144(1):28–35. doi: 10.1128/jb.144.1.28-35.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chumley F. G., Menzel R., Roth J. R. Hfr formation directed by tn10. Genetics. 1979 Apr;91(4):639–655. doi: 10.1093/genetics/91.4.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condit R. C. F factor-mediated inhibition of bacteriophage T7 growth: increased membrane permeability and decreased ATP levels following T7 infection of male Escherichia coli. J Mol Biol. 1975 Oct 15;98(1):45–59. doi: 10.1016/s0022-2836(75)80100-8. [DOI] [PubMed] [Google Scholar]
- Fangman W. L., Novick A. Characterization of two bacterial mutants with temperature-sensitive synthesis of DNA. Genetics. 1968 Sep;60(1):1–17. doi: 10.1093/genetics/60.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster T. J., Lundblad V., Hanley-Way S., Halling S. M., Kleckner N. Three Tn10-associated excision events: relationship to transposition and role of direct and inverted repeats. Cell. 1981 Jan;23(1):215–227. doi: 10.1016/0092-8674(81)90286-5. [DOI] [PubMed] [Google Scholar]
- Glaser M., Nulty W., Vagelos P. R. Role of adenylate kinase in the regulation of macromolecular biosynthesis in a putative mutant of Escherichia coli defective in membrane phospholipid biosynthesis. J Bacteriol. 1975 Jul;123(1):128–136. doi: 10.1128/jb.123.1.128-136.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glickman B. W., Radman M. Escherichia coli mutator mutants deficient in methylation-instructed DNA mismatch correction. Proc Natl Acad Sci U S A. 1980 Feb;77(2):1063–1067. doi: 10.1073/pnas.77.2.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins J. D., Clements M. B., Liang T. Y., Isberg R. R., Syvanen M. Recombination genes on the Escherichia coli sex factor specific for transposable elements. Proc Natl Acad Sci U S A. 1980 May;77(5):2814–2818. doi: 10.1073/pnas.77.5.2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins J. D., Clements M., Syvanen M. New class of mutations in Escherichia coli (uup) that affect precise excision of insertion elements and bacteriophage Mu growth. J Bacteriol. 1983 Jan;153(1):384–389. doi: 10.1128/jb.153.1.384-389.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingraham J. L., Neuhard J. Cold-sensitive mutants of Salmonella typhimurium defective in uridine monophosphate kinase (pyrH). J Biol Chem. 1972 Oct 10;247(19):6259–6265. [PubMed] [Google Scholar]
- Isberg R. R., Lazaar A. L., Syvanen M. Regulation of Tn5 by the right-repeat proteins: control at the level of the transposition reaction? Cell. 1982 Oct;30(3):883–892. doi: 10.1016/0092-8674(82)90293-8. [DOI] [PubMed] [Google Scholar]
- Justesen J., Neuhard J. pyrR identical to pyrH in Salmonella typhimurium: control of expression of the pyr genes. J Bacteriol. 1975 Sep;123(3):851–854. doi: 10.1128/jb.123.3.851-854.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanaya S., Crouch R. J. The rnh gene is essential for growth of Escherichia coli. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3447–3451. doi: 10.1073/pnas.81.11.3447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleckner N. Transposable elements in prokaryotes. Annu Rev Genet. 1981;15:341–404. doi: 10.1146/annurev.ge.15.120181.002013. [DOI] [PubMed] [Google Scholar]
- Liebke H. H., Speyer J. F. A new gene in E. coli RNA synthesis. Mol Gen Genet. 1983;189(2):314–320. doi: 10.1007/BF00337823. [DOI] [PubMed] [Google Scholar]
- Maki H., Horiuchi T., Sekiguchi M. Structure and expression of the dnaQ mutator and the RNase H genes of Escherichia coli: overlap of the promoter regions. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7137–7141. doi: 10.1073/pnas.80.23.7137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moses R. E., Richardson C. C. Replication and repair of DNA in cells of Escherichia coli treated with toluene. Proc Natl Acad Sci U S A. 1970 Oct;67(2):674–681. doi: 10.1073/pnas.67.2.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neidhardt F. C., Bloch P. L., Smith D. F. Culture medium for enterobacteria. J Bacteriol. 1974 Sep;119(3):736–747. doi: 10.1128/jb.119.3.736-747.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RANDERATH E., RANDERATH K. RESOLUTION OF COMPLEX NUCLEOTIDE MIXTURES BY TWO-DIMENSIONAL ANION-EXCHANGE THIN-LAYER CHROMATOGRAPHY. J Chromatogr. 1964 Oct;16:126–129. doi: 10.1016/s0021-9673(01)82446-8. [DOI] [PubMed] [Google Scholar]
- RANDERATH K., RANDERATH E. ION-EXCHANGE CHROMATOGRAPHY OF NUCLEOTIDES ON POLY-(ETHYLENEIMINE)-CELLULOSE THIN LAYERS. J Chromatogr. 1964 Oct;16:111–125. doi: 10.1016/s0021-9673(01)82445-6. [DOI] [PubMed] [Google Scholar]
- Sancar A., Hack A. M., Rupp W. D. Simple method for identification of plasmid-coded proteins. J Bacteriol. 1979 Jan;137(1):692–693. doi: 10.1128/jb.137.1.692-693.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheuermann R., Tam S., Burgers P. M., Lu C., Echols H. Identification of the epsilon-subunit of Escherichia coli DNA polymerase III holoenzyme as the dnaQ gene product: a fidelity subunit for DNA replication. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7085–7089. doi: 10.1073/pnas.80.23.7085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler J. A., Gross J. D. Escherichia coli mutants temperature-sensitive for DNA synthesis. Mol Gen Genet. 1971;113(3):273–284. doi: 10.1007/BF00339547. [DOI] [PubMed] [Google Scholar]