Abstract
Leukocyte recruitment into inflamed tissue proceeds in a cascade-like fashion. The first contact of neutrophils with the endothelium is mediated by selectins and their counterreceptors, followed by rolling of neutrophils along the endothelial wall of postcapillary venules and integrin-mediated arrest. While rolling, neutrophils collect different inflammatory signals that can activate several pathways. In addition to activation of neutrophils by ligation of G-protein-coupled receptors with chemokines and other chemoattractants, integrins and selectin ligands are also able to signal into the cell, where they initiate neutrophil extravasation, promote cytoskeletal rearrangement, and ultimately induce superoxide production and degranulation. These signaling pathways may be targeted by therapeutic interventions to inhibit specific functions of neutrophils without affecting others. This Review is focused on the signaling events during the interaction of neutrophils with the endothelium.
Neutrophils are produced in the bone marrow and circulate in the blood for a few hours before they are selectively recruited to tissues that have been injured by infection, trauma, or an autoimmune reaction. Neutrophils constitute the first line of defense against invading bacteria. A defect in neutrophil recruitment, such as seen in leukocyte adhesion deficiency, is associated with recurrent bacterial infections that can be lethal.1 On the other hand, inhibition of neutrophil recruitment has positive effects on ischemia-reperfusion injury,2 abacterial inflammation,3 and autoimmune disease.4 Understanding neutrophil activation, signaling, and recruitment can help identify therapeutic targets to selectively inhibit the specific functions of neutrophils without affecting others.
At sites of inflammation, neutrophils roll along the endothelium of postcapillary venules, collect inflammatory signals, arrest, and transmigrate. The first contact between neutrophils and endothelium of postcapillary venules is known as capture or tethering and is mediated by selectins and their counterreceptors.5 Selectin binding and the presentation of chemokines by endothelial cells induce activation of signaling pathways in neutrophils that cause changes in integrin conformation (inside-out signaling). Depending on the resulting integrin conformation, binding of activated integrins to their counterreceptors causes either slow rolling or arrest. Slow rolling of neutrophils along the wall of inflamed venules is mediated by the β2-integrins αLβ2 [lymphocyte function antigen (LFA)-1] and αMβ2 [macrophage antigen (Mac)-1].6 Partial activation of LFA-1 exposes the ligand binding site of the integrin leading to LFA-1-dependent rolling.7 Efficient conversion from rolling to firm adhesion is dependent on the time a leukocyte spends in close contact with the endothelium.8 Upon arrest, integrins bound to their ligands can signal into the neutrophil (outside-in signaling), stabilize the adhesion (postadhesion strengthening),9 activate different signaling pathways, and initiate transmigration. During leukocyte interaction with the endothelium, leukocytes receive signals from P-selectin glycoprotein ligand (PSGL)-1, L-selectin, G-protein-coupled receptors (GPCRs), and integrins, which can activate different pathways and subsequently lead to activation of the leukocyte with actin polymerization, crawling, transmigration through the endothelium, respiratory burst, and degranulation.
This Review focuses on the molecular mechanisms involved in neutrophil interactions with the endothelium, signaling events that are induced during this process, and functional consequences of their activation.
PSGL-1
PSGL-1 is a disulfide-bonded homodimer highly expressed on microvilli of leukocytes.10 Based on biochemical analysis, most of the PSGL-1 is thought to be located in lipid rafts.11 PSGL-1 binds L-selectin and P-selectin through a sialyl Lewis x (sLex)-containing O-glycan and a nearby tyrosine sulfate residue10 and E-selectin through the N-terminal region as well as other binding sites on PSGL-1. Binding of E-selectin to PSGL-1 requires sialylated and fucosylated O-glycans but not tyrosine sulfatation.10 Antibody blockade of the N-terminal region of PSGL-1 inhibits rolling of leukocytes on P-selectin and L-selectin in vivo.12,13 Elimination of PSGL-1 by gene targeting and homologous recombination reduces the number of neutrophils interacting with the endothelium,14,15 alters the rolling velocity on P-selectin and on E-selectin,14,16 and diminishes neutrophil recruitment into inflamed tissue.15
In addition to its adhesive function, PSGL-1 is also known to initiate intracellular signaling events upon ligand engagement. This downstream signaling leads to activation of neutrophils, including activation of β2-integrins,17 tyrosine phosphorylation,18 secretion of cytokines,18 transcriptional activation,19 and cytoskeletal rearrangement.20 The cytoplasmatic tail of human PSGL-1 consists of 63 amino acids and is highly conserved among species.
PSGL-1 can interact with the cytoskeleton. For this interaction, a juxtamembrane region of 18 amino acids of the PSGL-1 cytoplasmatic tail is necessary,19 which interacts with the proteins moesin and ezrin, which belong to the ezrin-moesin-radixin (ERM) family. ERM proteins function as linking proteins between the plasma membrane and the actin cytoskeleton and play an important role in the formation of protrusive plasma membrane structures.21 The C-terminal domain of the ERM proteins can bind F-actin, whereas the N-terminal domain binds to the cytoplasmatic tail of PSGL-1.19 Unless activated, the N- and C-terminal domains of ERM proteins are associated in a manner that prevents F-actin binding.22 The interaction of PSGL-1 with the cytoskeleton is required for rolling; the destruction of the cytoskeleton or the elimination of the cytoplasmatic tail of PSGL-1 is associated with an increase in rolling velocities on P-selectin, reduced number of adherent cells, and a reduced capability to stabilize rolling.23 Decoupling of PSGL-1 from the cytoskeletal network also reduces its ability to cluster on microvilli.24
ERM proteins contain an immunoreceptor tyrosine-based activation motifs-like motif in their N-terminal region,25 which can directly interact with spleen tyrosine kinase (Syk).19 PSGL-1 engagement induces tyrosine phosphorylation of Syk. Syk is involved in PSGL-1-dependent signaling and therefore in regulating rolling on P-selectin and E-selectin (Figure 1).11,16 Slow rolling on E-selectin and intercellular adhesion molecule-1 (ICAM-1) is abolished by pharmacological blockade of Syk and is absent in Syk-deficient bone marrow chimeric mice. Blocking of p38 mitogen-activated protein kinase also increases the rolling velocity and reduces the number of neutrophil-interacting cells with E-selectin and ICAM-1.16,17
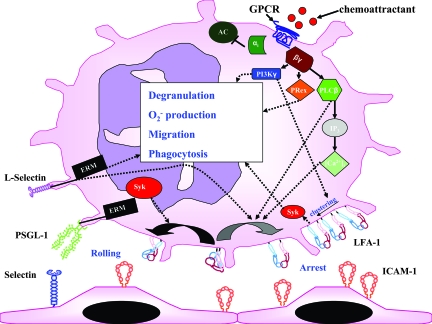
Figure 1.
Activation of signaling pathways during the contact of neutrophils with the endothelium. E-selectin binding to PSGL-1 leads, through Syk, to partial LFA-1 activation (black curved arrow) that mediates rolling on ICAM-1 (shown as a dimer). L-selectin engagement may activate, through ERM proteins, the neutrophil and induce integrin activation. Activation of GPCRs leads to dissociation of the G protein in a Gα-subunit and a Gβγ-complex. The Gα-subunit inhibits some, but not all, adenylyl cyclase isoforms. The Gβγ-complex interacts directly with PI3Kγ, PLCβ, and P-Rex-1. The activation of these enzymes can induce degranulation, O2− production, migration, phagocytosis, integrin clustering, and full activation of the integrin (gray curved arrow). LFA-1 interaction with its counterreceptor triggers outside-in signaling and further activates the neutrophil. Arrows indicate signaling pathways, but interactions may be indirect. αiβγ, G-protein subunits; AC, adenylyl cyclase; [Ca2+]i, intracellular calcium; GPCR, G-protein-coupled receptor; IP3, inositol triphosphate; PRex, PtdIns(3,4,5)P3-dependent Rac exchanger.
L-Selectin
L-selectin is expressed by all blood neutrophils and is a type I transmembrane glycoprotein with an N-terminal C-type lectin domain, followed by an epidermal growth factor-like domain, two short consensus repeats, a transmembrane domain, and a cytoplasmatic tail. L-selectin interacts with sialylated ligands expressed by the endothelium and is involved in rolling and activation of leukocytes. The expressed levels, cytoskeletal anchoring, and the distribution of L-selectin determine the tethering efficiency and rolling velocity.26,27,28
Cross-linking of L-selectin on neutrophils can also initiate β2-integrin activation,29 but L-selectin-dependent rolling does not induce neutrophil arrest in flow chamber systems.30 L-selectin surface expression on neutrophils can be rapidly down-regulated by proteolytic cleavage of L-selectin near the cell surface by ADAM-17 (TACE) and at least one other “sheddase.”31 L-selectin-mediated neutrophil activation is of physiological importance, as shown by modulating L-selectin shedding. Blocking of L-selectin shedding in vivo increases signal input through L-selectin and results in an increase in neutrophil arrest, reduced neutrophil rolling velocities,32 and more stable rolling.33 It remains unclear whether these in vivo results are the consequences of increased L-selectin-mediated signaling or are due to increased exposure of neutrophils to the surface of activated endothelial cells when L- selectin shedding is inhibited.
Human and murine L-selectin cytoplasmatic tails are 100% conserved between residues Arg-356 and Ser-364 and have similar lengths (17 amino acids). The cytoplasmatic tail of L-selectin is constitutively associated with calmodulin34 and the actin-binding protein α-actinin27 and can also interact with proteins of the ERM family.35 The C terminus of the cytoplasmatic tail of L-selectin directly interacts with α-actinin and forms a complex with vinculin and talin.27 The presence of vinculin and talin increases the binding affinity of α-actinin for L-selectin.27 The binding site of L-selectin for α-actinin is located within the 53-kDa domain of α-actinin,27 suggesting that α-actinin functions as a direct linker between L-selectin and the cytoskeleton. Truncation of the α-actinin binding site, the 11 residues at the COOH-terminal end of the cytoplasmatic tail, or treatment with cytochalasin B disrupt the connection between L-selectin and the cytoskeleton and lead to reduced tethering and rolling in vivo and in vitro.26,28 Despite the reduced functionality of the truncated form of L-selectin lacking most of cytoplasmatic domain (11 of 17 amino acids), the mutant possesses an unchanged ability to recognize carbohydrates28 and is still localized on microvillar tips, suggesting that other cytoskeletal proteins are required to anchor L-selectin to microvilli.27
Calmodulin interacts with the cytoplasmatic tail of L-selectin in resting leukocytes.34 Activation of leukocytes leads to a release of calmodulin from the cytoplasmatic tail of L-selectin and causes the concomitant shedding of L-selectin. Abrogation of the interaction of calmodulin with the cytoplasmatic tail of L-selectin by mutagenesis or an antagonist leads to a higher turnover of cleaved L-selectin.34 These data show that calmodulin negatively regulates L-selectin shedding, likely by conformational change in the extracellular domain that renders the cleavage site inaccessible.
Two members of the ERM family, ezrin and moesin, can bind to the cytoplasmatic tail of L-selectin, but the binding properties are regulated differently. Moesin binds to the cytoplasmatic tail of L-selectin after stimulation, whereas ezrin is constitutively associated with the cytoplasmatic tail.35 Two residues (Arg-357 and Lys-362) in the ERM-binding domain of the human L-selectin tail contribute to the interaction with the N-terminal domain of moesin.36 Mutation of these two amino acids results in a reduction of induced shedding, lower levels of L-selectin in microvilli, and decreased tethering efficiency.36
L-selectin engagement of leukocytes by antibody or glyomimetics results in increased intracellular Ca2+-levels, cytoskeleton-independent, Src-kinase (p56lck)-dependent tyrosine phosphorylation of the cytoplasmatic tail of L- selectin and concomitant activation of downstream signaling pathways.37,38,39 Tyrosine phosphorylation of the cytoplasmatic tail of L-selectin leads to an association of L-selectin with the guanine nucleotide exchange factor SOS/Grb2.38 This signaling cascade also activates Ras, p38 mitogen-activated protein kinase, extracellular signal-regulated kinase 1/2 phosphorylation, and Rac and leads to increased O2− production38 (Figure 1).
Integrins
Integrins are type I transmembrane cell adhesion molecules consisting of two noncovalently associated subunits α and β that mediate cell-pathogen, cell-cell, and cell-extracellular matrix interactions.40 Neutrophils express the β2-integrins αLβ2 (LFA-1), αMβ2 (Mac-1), αxβ2, and low levels of α4β1. These integrins are involved in slow rolling,6 adhesion,40 postadhesion strengthening,9 neutrophil migration,41 respiratory burst,42 phagocytosis,43 and polarization and are a linkage between extracellular matrix and cytoskeleton.44 Binding of integrins to their ligands induces outside-in signaling with a concomitant activation of several intracellular signaling pathways. Mac-1 engagement is necessary for oxidative burst42 and phagocytosis.43 The extracellular domain of the α-chain of LFA-1 (αL) contains seven N-terminal homogeneous repeats organized into a β-propeller structure. The I domain is inserted in the third repeat and consists of two distinct sites: the metal ion-dependent adhesion site and the I-domain allosteric site. These sites require divalent cations for ligand binding and regulate the affinity of the integrin for their ligands.45 The membrane-proximal repeats contain a calcium-binding site that may be important for the orientation of the β-propeller as well as the association with the β-chain.46 The extracellular domain of the β-chain includes an I-like domain, which corresponds to the I domain of the α-chain, and both together represent the integrin binding site for the ligands. The legs of the α- and β-chain are formed by a series of globular domains that incorporate a knee or “genu.”47 In the “bent position”, the genu is folded, and the integrin assumes a compact structure in which the ligand-binding site is close to the membrane (<5 nm). In the “extended conformation state”, the integrin legs straighten, and the ligand-binding site is projected about 25 nm from the membrane.47 The cytoplasmatic tail of the α-chain of LFA-1 contains a GFFKR motif that is involved in the α/β association and in ligand recognition.48 The α/β association is also important in keeping the integrin in a low-affinity state. Mutational changes49 or complexes that interact with the cytoplasmatic domain break the α/β association, although details of this are not known for LFA-1. The cytoplasmatic tail of the β-subunit is important for the linkage with the cytoskeleton.
The β2-integrins can dynamically regulate their adhesiveness by altering their affinity and avidity. “Affinity” is defined as a measure of the equilibrium interaction of monovalent receptors and ligand in solution or on a surface. “Avidity” describes the interaction of two surfaces in which receptors and ligands come together to form many molecular interactions. Stimuli received by cell surface receptors for cytokines, adhesion molecule receptors, and chemokines initiate intracellular signals that lead to a transient change of the integrin conformation. In addition, activation by phorbol esters may enhance lateral mobility in the plasma membrane and change integrin anchorage with the cytoskeleton.50 The affinity states of LFA-1 are regulated by the conformation of the I domain. The position of the seventh α-helix controls the conformation of the magnesium-containing ligand binding site in the I domain.51 The different positions are associated with the three distinct affinity states (low-, intermediate-, and high-affinity state),51 but additional states may exist, and the equilibrium is dynamic.
The main component of integrin avidity regulation is based on the postligand binding interaction of the integrin with the cytoskeleton. Lateral mobility leads to an accumulation of integrins in areas where ligands are available, a process called “clustering,” which regulates cell avidity for surface-presented integrin ligands. These features assure specific binding of leukocytes at sites of inflammation.
LFA-1 can control the rolling velocities of leukocytes in vivo,6,16 as well as their adhesion52 and migration52,53 (Figure 1). During neutrophil migration through the endothelium, LFA-1 forms ring-like clusters at the neutrophil-endothelial junction.53 These clusters move from the leading edge to the neutrophil uropod, always in contact with their major ligand ICAM-1.53
Mac-1 is stored in neutrophil secretory, secondary, and tertiary granules, ready to be released after cell activation.54 In addition to ICAM-1, Mac-1 has several other ligands, including different bacterial and fungal glycoproteins, heparin, coagulation factor Xa, fibrinogen, and complement C3bi.55 Like LFA-1, Mac-1 can also regulate the rolling velocity of leukocytes in vivo6 and, depending on the model, participates in neutrophil migration. Mac-1 is also involved in neutrophil-platelet interactions56 and intravascular crawling of monocytes57 and neutrophils58 and is critical for certain forms of phagocytosis.43
G Protein-Coupled Receptors
Neutrophils can be activated by soluble stimuli including N-formyl-l-methionyl-l-leucyl-l-phenylalanine, complement C5a, platelet-activating factor, leukotriene B4, and the chemokines CXCL1, 2, 3, 4, 5, 6, 7, and 8.59 Chemoattractants can induce arrest from rolling, cytoskeleton rearrangement, cell polarization, migration along a concentration gradient, degranulation, and respiratory burst.60 Chemoattractants bind to GPCRs, which undergo a conformational change after ligand binding.60
G proteins consist of an α-subunit and a βγ-complex.61 G proteins are classified according their α-subunit, where neutrophils express Gs, Gi, and Gq family members. Gi proteins are the most abundant and important G proteins in neutrophils, because they mediate almost all pro-inflammatory effects of chemoattractants. The Gαi family consists of the subunits Gαz, Gαo, Gαi1, Gαi2, and Gαi362 and can be blocked by pertussis toxin with the exception of Gαz. Gαi2 and Gαi3 are expressed abundantly in leukocytes.63 Five different β-subunits and 12 γ-subunits exist, forming a number of Gβγ-complexes.61
The binding of a chemoattractant to its receptor results in the activation of the associated G protein, which dissociates into the GTP-bound Gα-subunit and the Gβγ-complex60 (Figure 1). The majority of neutrophil responses induced by chemoattractants except platelet activating factor can be inhibited by pertussis toxin, which shows that the Gαi class of G protein is associated with relevant chemoattractant receptors in neutrophils. The Gβγ-subunit is able to activate phosphatidylinositol 3-kinase (PI3K) γ and phospholipase C (PLC) β2 and, to a lesser extent, PLC β3.64,65 PLC β hydrolyzes phosphatidylinositol 4,5-biphosphate to produce inositol triphosphate and diacylglycerol. Inositol triphosphate mobilizes Ca2+ from nonmitochondrial stores, and diacylglycerol activates Ca2+-dependent and Ca2+-independent protein kinase C isoenzymes. Protein kinase C isoenzymes are important for activating cytotoxic effector function of neutrophils such as O2− formation.66 Neutrophils from mice lacking PLC β2 show deficient chemoattractant-induced Ca2+ release and Mac-1 up-regulation but enhanced chemotaxis and increased leukocyte recruitment in response to fMet-Leu-Phe.67 PLC β2- and PLC β3-deficient mice show a complete deficiency of chemokine-stimulated O2− formation.68 A recent study showed that PLC is involved in chemokine-induced α4β1-integrin affinity up-regulation and arrest of monocytes.69
Certain Gβγ-subunits can also activate PI3Kγ,70 which catalyzes the phosphorylation of phospatidylinositol-3,4-biphosphate to phospatidylinositol-3,4,5-triphosphate. Phospatidylinositol-3,4,5-triphosphate binds to proteins containing pleckstrin homology domains, resulting in downstream signaling. For example, protein kinase B (also known as Akt) is activated by PI3Kγ after exposure to chemoattractants. Protein kinase B translocates to the leading edge of migrating neutrophils.71 PI3Kγ also regulates other effectors including low-molecular mass GTP binding proteins of the Rho family.71 Elimination of PI3Kγ leads to reduced O2− formation,65 failure of postadhesion strengthening,72 and reduced chemotaxis73 but is not involved in arrest.72
Rac is one of the GTPases of the Rho family that is activated after GPCR activation. Rac1 and Rac2 are both involved in regulation of phagocyte migration, whereas Rac2 also regulates activation of neutrophil respiratory burst.74,75 Guanine nucleotide exchange factors activate Rac by exchanging GDP for GTP. Two other guanine nucleotide exchange factors, Vav1 and P-Rex-1, are involved in Rac2 activation.76,77 Vav protein activity is regulated by tyrosine kinases, including Src family kinases and Syk,77 whereas P-Rex1 is activated in a synergistic fashion by phospatidylinositol-3,4,5-triphosphate and the Gβγ-complex.78 Vav guanine nucleotide exchange factors are also involved in adhesion strengthening.79 Full activation of Rac is required for NADPH oxidase activation, whereas partial activation of Rac is sufficient for neutrophil chemotaxis. Rac1 and Rac2 can also be regulated by dedicator of cytokinesis 2.80 Dedicator of cytokinesis 2 deficiency leads to an inhibition of cell polarity and translocation speed.80 p21-activated kinase is downstream from Rac, and after activation, p21-activated kinase can induce phosphorylation of extracellular signal-regulated kinase 1/2, p38 mitogen-activated protein kinase, and c-Jun N-terminal kinase.81,82
After activation of the GPCR and the release of the Gβγ-complex, the Gαi-subunit inhibits some, but not all, adenylyl cyclase isoforms (Figure 1).83 This results in reduced cAMP levels and cAMP-dependent protein kinase activity. Small GTPases are also effectors of the Gαi-subunit after activation.60 Ras, one of the GTPases downstream of the Gαi-subunit, can activate PI3K by binding directly to the 110-kDa catalytic subunit.84 A recent study using Gαi2-deficient mice demonstrated that Gαi2 in nonhematopoietic cells is involved in migration of leukocytes into the lung in a model of allergy and after lipopolysaccharide stimulation, whereas Gαi2 in leukocytes regulates chemotaxis in response to CXCL2/3.85 Gαi2 in neutrophils is also required for chemokine-induced arrest.86
Functional Consequences
In addition to the described signaling inputs, the adhesion of neutrophils to biological surfaces by integrins is another powerful activator (outside-in signaling).87 Depending on the magnitude of signaling inputs, neutrophils may become fully activated and manifest a number of functional responses like spreading, transmigration, phagocytosis, superoxide production, and degranulation. Among the most important functional response of activated neutrophils is the ability to rearrange their actin cytoskeleton and chemotax toward inflammatory stimuli.88 Activated neutrophils have the ability to arrange the subunits of the NADPH oxidase at the phagosome membrane and undergo respiratory burst, resulting in release of oxygen radical intermediates.42 Neutrophils spreading over biological surfaces or transmigrating into inflamed tissue release their granule components, including metal-binding proteins, peroxidases, and hydrolytic enzymes.89 During degranulation, granule membranes fuse with the cytoplasmic membrane, and this process provides a rich source of new cell surface receptors that can interact with the environment.90 In addition to these functions, adhesion of neutrophils augments the ability to phagocytose foreign particles and pathogens,91,92 and also induces signaling events that regulate neutrophil apoptosis.93 Stimulation of neutrophils in suspension with inflammatory mediators does not induce significant granule secretion or sustained respiratory burst. To initiate functional responses of cell spreading, migration, respiratory burst, and granule secretion, neutrophils must be co-stimulated with both an inflammatory mediator and an adhesive surface44 or pharmacological intervention.
Conclusions
Selectin- and selectin ligand-induced signaling, G-protein-coupled receptor signaling, and outside-in signaling of integrins control activation of neutrophils and their functional responses (spreading, migration, respiratory burst, and degranulation), but the precise signaling pathways and the points of convergence are poorly understood. Many different clinical trials tried to modulate leukocyte trafficking by blocking molecules that are involved in distinct steps of the multistep trafficking cascade. Clinical trials blocking β2-integrins to block neutrophil trafficking in the context of ischemia-reperfusion injury have not shown any benefits. Antibody blockade of ICAM-1 had negative effects on strokes in a phase II trial.94 However, the treatment with Natalizumab, a monoclonal antibody against α4β1, is very efficacious in patients with Crohn’s disease95 and multiple sclerosis.96 Inhibition of the αL-integrin has beneficial effects in treating graft-versus-host disease, transplant rejection, and psoriasis. Different antagonists of chemokine receptors are now being tested in preclinical and early clinical settings. Blocking of adhesion molecules or GPCRs is a “double-edged-sword” approach, because this treatment can lead to impaired host response. Uncovering the proximal and distal signaling pathways and their interconnections may help to develop better therapeutics that modulate neutrophil function in inflammatory disease.
Acknowledgments
We thank illustrator Ben Weston for assistance with Figure 1.
Footnotes
Address reprint requests to Klaus Ley, M.D., La Jolla Institute for Allergy and Immunology, Division of Inflammation Biology, 9420 Athena Circle Drive, La Jolla, CA 92037. E-mail: klaus@ljai.org.
Supported by a grant of the Deutsche Forschungsgemeinschaft (DFG AZ 428/2-1 to A.Z.) and by grants from the National Institutes of Health (HL58108, 55798, and 73361 to K.L.).
References
- Anderson DC, Springer TA. Leukocyte adhesion deficiency: an inherited defect in the Mac-1, LFA-1, and p150,95 glycoproteins. Annu Rev Med. 1987;38:175–194. doi: 10.1146/annurev.me.38.020187.001135. [DOI] [PubMed] [Google Scholar]
- Singbartl K, Green SA, Ley K. Blocking P-selectin protects from ischemia/reperfusion-induced acute renal failure. FASEB J. 2000;14:48–54. doi: 10.1096/fasebj.14.1.48. [DOI] [PubMed] [Google Scholar]
- Zarbock A, Schmolke M, Spieker T, Jurk K, Van Aken H, Singbartl K. Acute uremia but not renal inflammation attenuates aseptic acute lung injury: a critical role for uremic neutrophils. J Am Soc Nephrol. 2006;17:3124–3131. doi: 10.1681/ASN.2006040358. [DOI] [PubMed] [Google Scholar]
- Chiriac M, Roesler J, Sindrilaru A, Scharffetter-Kochanek K, Zillikens D, Sitaru C. NADPH oxidase is required for neutrophil-dependent autoantibody-induced tissue damage. J Pathol. 2007;212:56–65. doi: 10.1002/path.2157. [DOI] [PubMed] [Google Scholar]
- Kansas GS. Selectins and their ligands: current concepts and controversies. Blood. 1996;88:3259–3287. [PubMed] [Google Scholar]
- Dunne JL, Ballantyne CM, Beaudet AL, Ley K. Control of leukocyte rolling velocity in TNF-alpha-induced inflammation by LFA-1 and Mac-1. Blood. 2002;99:336–341. doi: 10.1182/blood.v99.1.336. [DOI] [PubMed] [Google Scholar]
- Salas A, Shimaoka M, Kogan AN, Harwood C, von Andrian UH, Springer TA. Rolling adhesion through an extended conformation of integrin alphaLbeta2 and relation to alpha I and beta I-like domain interaction. Immunity. 2004;20:393–406. doi: 10.1016/s1074-7613(04)00082-2. [DOI] [PubMed] [Google Scholar]
- Jung U, Norman KE, Scharffetter-Kochanek K, Beaudet AL, Ley K. Transit time of leukocytes rolling through venules controls cytokine-induced inflammatory cell recruitment in vivo. J Clin Invest. 1998;102:1526–1533. doi: 10.1172/JCI119893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley K, Zarbock A. Hold on to your endothelium: postarrest steps of the leukocyte adhesion cascade. Immunity. 2006;25:185–187. doi: 10.1016/j.immuni.2006.08.001. [DOI] [PubMed] [Google Scholar]
- McEver RP, Cummings RD. Role of PSGL-1 binding to selectins in leukocyte recruitment. J Clin Invest. 1997;100:S97–S103. [PubMed] [Google Scholar]
- Abbal C, Lambelet M, Bertaggia D, Gerbex C, Martinez M, Arcaro A, Schapira M, Spertini O. Lipid raft adhesion receptors and Syk regulate selectin-dependent rolling under flow conditions. Blood. 2006;108:3352–3359. doi: 10.1182/blood-2006-04-013912. [DOI] [PubMed] [Google Scholar]
- Norman KE, Moore KL, McEver RP, Ley K. Leukocyte rolling in vivo is mediated by P-selectin glycoprotein ligand-1. Blood. 1995;86:4417–4421. [PubMed] [Google Scholar]
- Sperandio M, Smith ML, Forlow SB, Olson TS, Xia L, McEver RP, Ley K. P-selectin glycoprotein ligand-1 mediates L-selectin-dependent leukocyte rolling in venules. J Exp Med. 2003;197:1355–1363. doi: 10.1084/jem.20021854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia L, Sperandio M, Yago T, McDaniel JM, Cummings RD, Pearson-White S, Ley K, McEver RP. P-selectin glycoprotein ligand-1-deficient mice have impaired leukocyte tethering to E-selectin under flow. J Clin Invest. 2002;109:939–950. doi: 10.1172/JCI14151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Hirata T, Croce K, Merrill-Skoloff G, Tchernychev B, Williams E, Flaumenhaft R, Furie BC, Furie B. Targeted gene disruption demonstrates that P-selectin glycoprotein ligand 1 (PSGL-1) is required for P-selectin-mediated but not E-selectin-mediated neutrophil rolling and migration. J Exp Med. 1999;190:1769–1782. doi: 10.1084/jem.190.12.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarbock A, Lowell C, Ley K. Spleen tyrosine kinase Syk is necessary for E-selectin-induced aLβ2 integrin (LFA-1)-mediated rolling on intercellular adhesion molecule-1 (ICAM-1). Immunity. 2007;26:773–783. doi: 10.1016/j.immuni.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon SI, Hu Y, Vestweber D, Smith CW. Neutrophil tethering on E-selectin activates beta 2 integrin binding to ICAM-1 through a mitogen-activated protein kinase signal transduction pathway. J Immunol. 2000;164:4348–4358. doi: 10.4049/jimmunol.164.8.4348. [DOI] [PubMed] [Google Scholar]
- Hidari KI, Weyrich AS, Zimmerman GA, McEver RP. Engagement of P-selectin glycoprotein ligand-1 enhances tyrosine phosphorylation and activates mitogen-activated protein kinases in human neutrophils. J Biol Chem. 1997;272:28750–28756. doi: 10.1074/jbc.272.45.28750. [DOI] [PubMed] [Google Scholar]
- Urzainqui A, Serrador JM, Viedma F, Yanez-Mo M, Rodriguez A, Corbi AL, Alonso-Lebrero JL, Luque A, Deckert M, Vazquez J, Sanchez-Madrid F. ITAM-based interaction of ERM proteins with Syk mediates signaling by the leukocyte adhesion receptor PSGL-1. Immunity. 2002;17:401–412. doi: 10.1016/s1074-7613(02)00420-x. [DOI] [PubMed] [Google Scholar]
- Ba X, Chen C, Gao Y, Zeng X. Signaling function of PSGL-1 in neutrophil: tyrosine-phosphorylation-dependent and c-Abl-involved alteration in the F-actin-based cytoskeleton. J Cell Biochem. 2005;94:365–373. doi: 10.1002/jcb.20213. [DOI] [PubMed] [Google Scholar]
- Bretscher A, Chambers D, Nguyen R, Reczek D. ERM-Merlin and EBP50 protein families in plasma membrane organization and function. Annu Rev Cell Dev Biol. 2000;16:113–143. doi: 10.1146/annurev.cellbio.16.1.113. [DOI] [PubMed] [Google Scholar]
- Huang L, Wong TY, Lin RC, Furthmayr H. Replacement of threonine 558, a critical site of phosphorylation of moesin in vivo, with aspartate activates F-actin binding of moesin: regulation by conformational change. J Biol Chem. 1999;274:12803–12810. doi: 10.1074/jbc.274.18.12803. [DOI] [PubMed] [Google Scholar]
- Snapp KR, Heitzig CE, Kansas GS. Attachment of the PSGL-1 cytoplasmic domain to the actin cytoskeleton is essential for leukocyte rolling on P-selectin. Blood. 2002;99:4494–4502. doi: 10.1182/blood.v99.12.4494. [DOI] [PubMed] [Google Scholar]
- Bruehl RE, Moore KL, Lorant DE, Borregaard N, Zimmerman GA, McEver RP, Bainton DF. Leukocyte activation induces surface redistribution of P-selectin glycoprotein ligand-1. J Leukoc Biol. 1997;61:489–499. doi: 10.1002/jlb.61.4.489. [DOI] [PubMed] [Google Scholar]
- Rozsnyay Z, Sarmay G, Zoller M, Gergely J. Membrane-bound ezrin is involved in B-cell receptor-mediated signaling: potential role of an ITAM-like ezrin motif. Immunol Lett. 1996;54:163–169. doi: 10.1016/s0165-2478(96)02667-3. [DOI] [PubMed] [Google Scholar]
- Dwir O, Kansas GS, Alon R. Cytoplasmic anchorage of L-selectin controls leukocyte capture and rolling by increasing the mechanical stability of the selectin tether. J Cell Biol. 2001;155:145–156. doi: 10.1083/jcb.200103042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavalko FM, Walker DM, Graham L, Goheen M, Doerschuk CM, Kansas GS. The cytoplasmic domain of L-selectin interacts with cytoskeletal proteins via alpha-actinin: receptor positioning in microvilli does not require interaction with alpha-actinin. J Cell Biol. 1995;129:1155–1164. doi: 10.1083/jcb.129.4.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kansas GS, Ley K, Munro JM, Tedder TF. Regulation of leukocyte rolling and adhesion to high endothelial venules through the cytoplasmic domain of L-selectin. J Exp Med. 1993;177:833–838. doi: 10.1084/jem.177.3.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon SI, Burns AR, Taylor AD, Gopalan PK, Lynam EB, Sklar LA, Smith CW. L-selectin (CD62L) cross-linking signals neutrophil adhesive functions via the Mac-1 (CD11b/CD18) beta 2-integrin. J Immunol. 1995;155:1502–1514. [PubMed] [Google Scholar]
- Lawrence MB, Berg EL, Butcher EC, Springer TA. Rolling of lymphocytes and neutrophils on peripheral node addressin and subsequent arrest on ICAM-1 in shear flow. Eur J Immunol. 1995;25:1025–1031. doi: 10.1002/eji.1830250425. [DOI] [PubMed] [Google Scholar]
- Smalley DM, Ley K. L-selectin: mechanisms and physiological significance of ectodomain cleavage. J Cell Mol Med. 2005;9:255–266. doi: 10.1111/j.1582-4934.2005.tb00354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafezi-Moghadam A, Ley K. Relevance of L-selectin shedding for leukocyte rolling in vivo. J Exp Med. 1999;189:939–948. doi: 10.1084/jem.189.6.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafezi-Moghadam A, Thomas KL, Prorock AJ, Huo Y, Ley K. L-selectin shedding regulates leukocyte recruitment. J Exp Med. 2001;193:863–872. doi: 10.1084/jem.193.7.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn J, Walcheck B, Migaki GI, Jutila MA, Kishimoto TK. Calmodulin regulates L-selectin adhesion molecule expression and function through a protease-dependent mechanism. Cell. 1998;92:809–818. doi: 10.1016/s0092-8674(00)81408-7. [DOI] [PubMed] [Google Scholar]
- Ivetic A, Deka J, Ridley A, Ager A. The cytoplasmic tail of L-selectin interacts with members of the Ezrin-Radixin-Moesin (ERM) family of proteins: cell activation-dependent binding of Moesin but not Ezrin. J Biol Chem. 2002;277:2321–2329. doi: 10.1074/jbc.M109460200. [DOI] [PubMed] [Google Scholar]
- Ivetic A, Florey O, Deka J, Haskard DO, Ager A, Ridley AJ. Mutagenesis of the ezrin-radixin-moesin binding domain of L-selectin tail affects shedding, microvillar positioning, and leukocyte tethering. J Biol Chem. 2004;279:33263–33272. doi: 10.1074/jbc.M312212200. [DOI] [PubMed] [Google Scholar]
- Laudanna C, Constantin G, Baron P, Scarpini E, Scarlato G, Cabrini G, Dechecchi C, Rossi F, Cassatella MA, Berton G. Sulfatides trigger increase of cytosolic free calcium and enhanced expression of tumor necrosis factor-alpha and interleukin-8 mRNA in human neutrophils: evidence for a role of L-selectin as a signaling molecule. J Biol Chem. 1994;269:4021–4026. [PubMed] [Google Scholar]
- Brenner B, Gulbins E, Schlottmann K, Koppenhoefer U, Busch GL, Walzog B, Steinhausen M, Coggeshall KM, Linderkamp O, Lang F. L-selectin activates the Ras pathway via the tyrosine kinase p56lck. Proc Natl Acad Sci USA. 1996;93:15376–15381. doi: 10.1073/pnas.93.26.15376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddell TK, Fialkow L, Chan CK, Kishimoto TK, Downey GP. Signaling functions of L-selectin: enhancement of tyrosine phosphorylation and activation of MAP kinase. J Biol Chem. 1995;270:15403–15411. doi: 10.1074/jbc.270.25.15403. [DOI] [PubMed] [Google Scholar]
- Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- Lindbom L, Werr J. Integrin-dependent neutrophil migration in extravascular tissue. Semin Immunol. 2002;14:115–121. doi: 10.1006/smim.2001.0348. [DOI] [PubMed] [Google Scholar]
- Nathan C, Srimal S, Farber C, Sanchez E, Kabbash L, Asch A, Gailit J, Wright SD. Cytokine-induced respiratory burst of human neutrophils: dependence on extracellular matrix proteins and CD11/CD18 integrins. J Cell Biol. 1989;109:1341–1349. doi: 10.1083/jcb.109.3.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayadas TN, Cullere X. Neutrophil beta2 integrins: moderators of life or death decisions. Trends Immunol. 2005;26:388–395. doi: 10.1016/j.it.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Berton G, Lowell CA. Integrin signalling in neutrophils and macrophages. Cell Signal. 1999;11:621–635. doi: 10.1016/s0898-6568(99)00003-0. [DOI] [PubMed] [Google Scholar]
- Luo BH, Carman CV, Springer TA. Structural basis of integrin regulation and signaling. Annu Rev Immunol. 2007;25:619–647. doi: 10.1146/annurev.immunol.25.022106.141618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer TA. Folding of the N-terminal, ligand-binding region of integrin alpha-subunits into a beta-propeller domain. Proc Natl Acad Sci USA. 1997;94:65–72. doi: 10.1073/pnas.94.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi J, Petre BM, Walz T, Springer TA. Global conformational rearrangements in integrin extracellular domains in outside-in and inside-out signaling. Cell. 2002;110:599–511. doi: 10.1016/s0092-8674(02)00935-2. [DOI] [PubMed] [Google Scholar]
- Lu CF, Springer TA. The alpha subunit cytoplasmic domain regulates the assembly and adhesiveness of integrin lymphocyte function-associated antigen-1. J Immunol. 1997;159:268–278. [PubMed] [Google Scholar]
- Wegener KL, Partridge AW, Han J, Pickford AR, Liddington RC, Ginsberg MH, Campbell ID. Structural basis of integrin activation by talin. Cell. 2007;128:171–182. doi: 10.1016/j.cell.2006.10.048. [DOI] [PubMed] [Google Scholar]
- Kucik DF, Dustin ML, Miller JM, Brown EJ. Adhesion-activating phorbol ester increases the mobility of leukocyte integrin LFA-1 in cultured lymphocytes. J Clin Invest. 1996;97:2139–2144. doi: 10.1172/JCI118651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimaoka M, Xiao T, Liu JH, Yang Y, Dong Y, Jun CD, McCormack A, Zhang R, Joachimiak A, Takagi J, Wang JH, Springer TA. Structures of the alpha L I domain and its complex with ICAM-1 reveal a shape-shifting pathway for integrin regulation. Cell. 2003;112:99–111. doi: 10.1016/s0092-8674(02)01257-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding ZM, Babensee JE, Simon SI, Lu H, Perrard JL, Bullard DC, Dai XY, Bromley SK, Dustin ML, Entman ML, Smith CW, Ballantyne CM. Relative contribution of LFA-1 and Mac-1 to neutrophil adhesion and migration. J Immunol. 1999;163:5029–5038. [PubMed] [Google Scholar]
- Shaw SK, Ma S, Kim MB, Rao RM, Hartman CU, Froio RM, Yang L, Jones T, Liu Y, Nusrat A, Parkos CA, Luscinskas FW. Coordinated redistribution of leukocyte LFA-1 and endothelial cell ICAM-1 accompany neutrophil transmigration. J Exp Med. 2004;200:1571–1580. doi: 10.1084/jem.20040965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borregaard N, Kjeldsen L, Sengelov H, Diamond MS, Springer TA, Anderson HC, Kishimoto TK, Bainton DF. Changes in subcellular localization and surface expression of L-selectin, alkaline phosphatase, and Mac-1 in human neutrophils during stimulation with inflammatory mediators. J Leukoc Biol. 1994;56:80–87. doi: 10.1002/jlb.56.1.80. [DOI] [PubMed] [Google Scholar]
- Ross GD. Regulation of the adhesion versus cytotoxic functions of the Mac-1/CR3/alphaMbeta2-integrin glycoprotein. Crit Rev Immunol. 2000;20:197–222. [PubMed] [Google Scholar]
- Zarbock A, Polanowska-Grabowska RK, Ley K. Platelet-neutrophil-interactions: linking hemostasis and inflammation. Blood Rev. 2007;21:99–111. doi: 10.1016/j.blre.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Schenkel AR, Mamdouh Z, Muller WA. Locomotion of monocytes on endothelium is a critical step during extravasation. Nat Immunol. 2004;5:393–400. doi: 10.1038/ni1051. [DOI] [PubMed] [Google Scholar]
- Phillipson M, Heit B, Colarusso P, Liu L, Ballantyne CM, Kubes P. Intraluminal crawling of neutrophils to emigration sites: a molecularly distinct process from adhesion in the recruitment cascade. J Exp Med. 2006;203:2569–2575. doi: 10.1084/jem.20060925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson TS, Ley K. Chemokines and chemokine receptors in leukocyte trafficking. Am J Physiol Regul Integr Comp Physiol. 2002;283:R7–R28. doi: 10.1152/ajpregu.00738.2001. [DOI] [PubMed] [Google Scholar]
- Bokoch GM. Chemoattractant signaling and leukocyte activation. Blood. 1995;86:1649–1660. [PubMed] [Google Scholar]
- Wettschureck N, Offermanns S. Mammalian G proteins and their cell type specific functions. Physiol Rev. 2005;85:1159–1204. doi: 10.1152/physrev.00003.2005. [DOI] [PubMed] [Google Scholar]
- Wilkie TM, Gilbert DJ, Olsen AS, Chen XN, Amatruda TT, Korenberg JR, Trask BJ, de Jong P, Reed RR, Simon MI, Jenkins NA, Copeland NG. Evolution of the mammalian G protein alpha subunit multigene family. Nat Genet. 1992;1:85–91. doi: 10.1038/ng0592-85. [DOI] [PubMed] [Google Scholar]
- Jiang M, Spicher K, Boulay G, Martin-Requero A, Dye CA, Rudolph U, Birnbaumer L. Mouse gene knockout and knockin strategies in application to alpha subunits of Gi/Go family of G proteins. Methods Enzymol. 2002;344:277–298. doi: 10.1016/s0076-6879(02)44721-0. [DOI] [PubMed] [Google Scholar]
- Camps M, Carozzi A, Schnabel P, Scheer A, Parker PJ, Gierschik P. Isozyme-selective stimulation of phospholipase C-beta 2 by G protein beta gamma-subunits. Nature. 1992;360:684–686. doi: 10.1038/360684a0. [DOI] [PubMed] [Google Scholar]
- Hirsch E, Katanaev VL, Garlanda C, Azzolino O, Pirola L, Silengo L, Sozzani S, Mantovani A, Altruda F, Wymann MP. Central role for G protein-coupled phosphoinositide 3-kinase gamma in inflammation. Science. 2000;287:1049–1053. doi: 10.1126/science.287.5455.1049. [DOI] [PubMed] [Google Scholar]
- Mayer AM, Brenic S, Glaser KB. Pharmacological targeting of signaling pathways in protein kinase C-stimulated superoxide generation in neutrophil-like HL-60 cells: effect of phorbol ester, arachidonic acid and inhibitors of kinase(s), phosphatase(s) and phospholipase A2. J Pharmacol Exp Ther. 1996;279:633–644. [PubMed] [Google Scholar]
- Jiang H, Kuang Y, Wu Y, Xie W, Simon MI, Wu D. Roles of phospholipase C beta2 in chemoattractant-elicited responses. Proc Natl Acad Sci USA. 1997;94:7971–7975. doi: 10.1073/pnas.94.15.7971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Jiang H, Xie W, Zhang Z, Smrcka AV, Wu D. Roles of PLC-beta2 and -beta3 and PI3Kgamma in chemoattractant-mediated signal transduction. Science. 2000;287:1046–1049. doi: 10.1126/science.287.5455.1046. [DOI] [PubMed] [Google Scholar]
- Hyduk SJ, Chan JR, Duffy ST, Chen M, Peterson MD, Waddell TK, Digby GC, Szaszi K, Kapus A, Cybulsky MI. Phospholipase C, calcium, and calmodulin are critical for alpha4beta1 integrin affinity up-regulation and monocyte arrest triggered by chemoattractants. Blood. 2007;109:176–184. doi: 10.1182/blood-2006-01-029199. [DOI] [PubMed] [Google Scholar]
- Dekker LV, Segal AW. Perspectives: signal transduction: signals to move cells. Science. 2000;287:982–983, 985. doi: 10.1126/science.287.5455.982. [DOI] [PubMed] [Google Scholar]
- Servant G, Weiner OD, Herzmark P, Balla T, Sedat JW, Bourne HR. Polarization of chemoattractant receptor signaling during neutrophil chemotaxis. Science. 2000;287:1037–1040. doi: 10.1126/science.287.5455.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DF, Deem TL, Bruce AC, Reutershan J, Wu D, Ley K. Leukocyte phosphoinositide-3 kinase {gamma} is required for chemokine-induced, sustained adhesion under flow in vivo. J Leukoc Biol. 2006;80:1491–1499. doi: 10.1189/jlb.0306227. [DOI] [PubMed] [Google Scholar]
- Hannigan M, Zhan L, Li Z, Ai Y, Wu D, Huang CK. Neutrophils lacking phosphoinositide 3-kinase gamma show loss of directionality during N-formyl-Met-Leu-Phe-induced chemotaxis. Proc Natl Acad Sci USA. 2002;99:3603–3608. doi: 10.1073/pnas.052010699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y, Filippi MD, Cancelas JA, Siefring JE, Williams EP, Jasti AC, Harris CE, Lee AW, Prabhakar R, Atkinson SJ, Kwiatkowski DJ, Williams DA. Hematopoietic cell regulation by Rac1 and Rac2 guanosine triphosphatases. Science. 2003;302:445–449. doi: 10.1126/science.1088485. [DOI] [PubMed] [Google Scholar]
- Glogauer M, Marchal CC, Zhu F, Worku A, Clausen BE, Foerster I, Marks P, Downey GP, Dinauer M, Kwiatkowski DJ. Rac1 deletion in mouse neutrophils has selective effects on neutrophil functions. J Immunol. 2003;170:5652–5657. doi: 10.4049/jimmunol.170.11.5652. [DOI] [PubMed] [Google Scholar]
- Dong X, Mo Z, Bokoch G, Guo C, Li Z, Wu D. P-Rex1 is a primary Rac2 guanine nucleotide exchange factor in mouse neutrophils. Curr Biol. 2005;15:1874–1879. doi: 10.1016/j.cub.2005.09.014. [DOI] [PubMed] [Google Scholar]
- Kim C, Marchal CC, Penninger J, Dinauer MC. The hemopoietic Rho/Rac guanine nucleotide exchange factor Vav1 regulates N-formyl-methionyl-leucyl-phenylalanine-activated neutrophil functions. J Immunol. 2003;171:4425–4430. doi: 10.4049/jimmunol.171.8.4425. [DOI] [PubMed] [Google Scholar]
- Welch HC, Coadwell WJ, Ellson CD, Ferguson GJ, Andrews SR, Erdjument-Bromage H, Tempst P, Hawkins PT, Stephens LR. P-Rex1, a PtdIns(3,4,5)P3- and Gbetagamma-regulated guanine-nucleotide exchange factor for Rac. Cell. 2002;108:809–821. doi: 10.1016/s0092-8674(02)00663-3. [DOI] [PubMed] [Google Scholar]
- Gakidis MA, Cullere X, Olson T, Wilsbacher JL, Zhang B, Moores SL, Ley K, Swat W, Mayadas T, Brugge JS. Vav GEFs are required for beta2 integrin-dependent functions of neutrophils. J Cell Biol. 2004;166:273–282. doi: 10.1083/jcb.200404166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunisaki Y, Nishikimi A, Tanaka Y, Takii R, Noda M, Inayoshi A, Watanabe K, Sanematsu F, Sasazuki T, Sasaki T, Fukui Y. DOCK2 is a Rac activator that regulates motility and polarity during neutrophil chemotaxis. J Cell Biol. 2006;174:647–652. doi: 10.1083/jcb.200602142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fumagalli L, Zhang H, Baruzzi A, Lowell CA, Berton G. The SRC family kinases hck and fgr regulate neutrophil responses to N-formyl-methionyl-leucyl-phenylalanine. J Immunol. 2007;178:3874–3885. doi: 10.4049/jimmunol.178.6.3874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C, Dinauer MC. Rac2 is an essential regulator of neutrophil nicotinamide adenine dinucleotide phosphate oxidase activation in response to specific signaling pathways. J Immunol. 2001;166:1223–1232. doi: 10.4049/jimmunol.166.2.1223. [DOI] [PubMed] [Google Scholar]
- Sunahara RK, Dessauer CW, Gilman AG. Complexity and diversity of mammalian adenylyl cyclases. Annu Rev Pharmacol Toxicol. 1996;36:461–480. doi: 10.1146/annurev.pa.36.040196.002333. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Viciana P, Warne PH, Dhand R, Vanhaesebroeck B, Gout I, Fry MJ, Waterfield MD, Downward J. Phosphatidylinositol-3-OH kinase as a direct target of Ras. Nature. 1994;370:527–532. doi: 10.1038/370527a0. [DOI] [PubMed] [Google Scholar]
- Pero RS, Borchers MT, Spicher K, Ochkur SI, Sikora L, Rao SP, Abdala-Valencia H, O’Neill KR, Shen H, McGarry MP, Lee NA, Cook-Mills JM, Sriramarao P, Simon MI, Birnbaumer L, Lee JJ. G{alpha}i2-mediated signaling events in the endothelium are involved in controlling leukocyte extravasation. Proc Natl Acad Sci USA. 2007;104:4371–4376. doi: 10.1073/pnas.0700185104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarbock A, Deem TL, Burcin TL, Ley K. Gαi2 is required for chemokine-induced neutrophil arrest. Blood. 2007;110:3773–3779. doi: 10.1182/blood-2007-06-094565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berton G, Yan SR, Fumagalli L, Lowell CA. Neutrophil activation by adhesion: mechanisms and pathophysiological implications. Int J Clin Lab Res. 1996;26:160–177. doi: 10.1007/BF02592978. [DOI] [PubMed] [Google Scholar]
- Liu Y, Shaw SK, Ma S, Yang L, Luscinskas FW, Parkos CA. Regulation of leukocyte transmigration: cell surface interactions and signaling events. J Immunol. 2004;172:7–13. doi: 10.4049/jimmunol.172.1.7. [DOI] [PubMed] [Google Scholar]
- Richter J, Ng-Sikorski J, Olsson I, Andersson T. Tumor necrosis factor-induced degranulation in adherent human neutrophils is dependent on CD11b/CD18-integrin-triggered oscillations of cytosolic free Ca2+. Proc Natl Acad Sci USA. 1990;87:9472–9476. doi: 10.1073/pnas.87.23.9472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borregaard N, Cowland JB. Granules of the human neutrophilic polymorphonuclear leukocyte. Blood. 1997;89:3503–3521. [PubMed] [Google Scholar]
- Zhou MJ, Brown EJ. CR3 (Mac-1, alpha M beta 2. CD11b/CD18) and Fc gamma RIII cooperate in generation of a neutrophil respiratory burst: requirement for Fc gamma RIII and tyrosine phosphorylation. J Cell Biol. 1994;125:1407–1416. doi: 10.1083/jcb.125.6.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown EJ. Complement receptors, adhesion, and phagocytosis. Infect Agents Dis. 1992;1:63–70. [PubMed] [Google Scholar]
- Coxon A, Rieu P, Barkalow FJ, Askari S, Sharpe AH, von Andrian UH, Arnaout MA, Mayadas TN. A novel role for the beta 2 integrin CD11b/CD18 in neutrophil apoptosis: a homeostatic mechanism in inflammation. Immunity. 1996;5:653–666. doi: 10.1016/s1074-7613(00)80278-2. [DOI] [PubMed] [Google Scholar]
- Yonekawa K, Harlan JM. Targeting leukocyte integrins in human diseases. J Leukoc Biol. 2005;77:129–140. doi: 10.1189/jlb.0804460. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Goldin E, Gordon FH, Malchow HA, Rask-Madsen J, Rutgeerts P, Vyhnalek P, Zadorova Z, Palmer T, Donoghue S. Natalizumab for active Crohn’s disease. N Engl J Med. 2003;348:24–32. doi: 10.1056/NEJMoa020732. [DOI] [PubMed] [Google Scholar]
- Miller DH, Khan OA, Sheremata WA, Blumhardt LD, Rice GP, Libonati MA, Willmer-Hulme AJ, Dalton CM, Miszkiel KA, O’Connor PW. A controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med. 2003;348:15–23. doi: 10.1056/NEJMoa020696. [DOI] [PubMed] [Google Scholar]