Abstract
Male breast cancer is rare and has been the focus of limited research. Although the etiology is unclear, conditions increasing circulating prolactin (PRL), as well as estrogen, increase the risk of tumorigenesis. We modeled exposure to elevated PRL in transgenic mice, using the mammary-selective, estrogen-insensitive promoter neu-related lipocalin (NRL), to drive PRL expression. Male NRL-PRL mice did not develop mammary tumors. However, in cooperation with the well-characterized oncogene transforming growth factor-α (TGF-α), PRL induced mammary tumors in 100% of male bitransgenic mice. Similar to disease in human males, these tumors expressed variable levels of estrogen receptor-α (ER-α) and androgen receptors. However, carcinogenesis was not responsive to testicular steroids because castration did not alter latency to tumor development or tumor ER-α expression. Interestingly, both NRL-TGF-α/PRL and NRL-PRL males demonstrated increased ductal development, which occurred during puberty, similar to female mice. This outgrowth was diminished in NRL-PRL males treated with ICI 182,780, suggesting that PRL enhances ER-mediated growth. Treatment of MCF-7-derived cells with PRL increased phosphorylation of ER-α at residues implicated in unliganded ER-α activity. Together, these studies suggest that PRL expands the pool of cells susceptible to tumorigenesis, which is then facilitated by PRL and TGF-α cross talk. Activation of ER-α is one mechanism by which PRL may contribute to breast cancer and points to other therapeutic strategies for male patients.
An estimated 1690 new cases of male breast cancer will be diagnosed in the United States, and 460 men will die as a result of the disease this year.1 Because it is a rare disease, male breast cancer has been the focus of very limited research. Unlike breast cancer in women, the incidence in men is rising 1.1% annually, and men are more likely to have advanced disease and poorer survival compared to women.2 Conditions that elevate the ratio of circulating estrogens and androgens and increase the risk for this disease include Klinefelter’s syndrome, liver cirrhosis, estrogen therapy for prostate cancer, obesity, and testicular abnormalities.3,4 Similar to the postmenopausal disease in women, 85 to 91% of male breast cancers are estrogen receptor-α (ER-α)-positive and respond to tamoxifen therapy, although the survival advantage for ER-α-positive tumors is not as evident in males as females.5 Androgen receptor (AR) is also expressed in a significant number (39 to 80%) of tumors.6,7
Hyperprolactinemia is also a significant risk factor.3,8 At least one-third of a consecutive series of male breast cancer patients had elevated serum levels of prolactin (PRL), which also correlated with the size of the primary tumor.9 Drug treatments associated with PRL elevation and prolactinomas are also significantly linked to increased breast cancer risk.10 In a retrospective study, one in three men with breast cancer had detectable PRL receptor in their tumors.11 To study the effect of PRL on tumorigenesis, transgenic mice were generated that overexpress PRL under control of the mammary-selective, estrogen-insensitive promoter neu-related lipocalin (NRL).12 Although virgin female NRL-PRL mice developed mammary adenocarcinomas with a long latency, males did not demonstrate mammary tumor development, indicating that additional factors are necessary for tumorigenesis.
In breast tumors from women, expression of epidermal growth factor receptor (EGFR) family members has become an important indicator for both prognosis and treatment, and multiple therapies targeting these receptors have been developed.13 In male breast cancer, 20 to 76% of cases examined expressed EGFR; c-erbB2 has also been detected and may be a prognostic indicator, although it does not occur as frequently in the male disease.14,15 The well-characterized oncogene, transforming growth factor-α (TGF-α), binds to EGFR and activates either EGFR homodimers or heterodimers with its preferred partner, erbB2.16,17 Transgenic virgin female mice that overexpress TGF-α under control of the NRL promoter develop mammary tumors and preneoplastic lesions.18,19 Together, TGF-α and PRL potently cooperate; bitransgenic NRL-TGF-α/PRL females develop mammary tumors similar to those found in NRL-TGF-α females with a greatly reduced latency and 100% incidence.18
This hormone/growth factor interaction is also strongly oncogenic in the male. Bitransgenic NRL-TGF-α/PRL males developed mammary tumors with similar incidence and latency to that found in NRL-TGF-α/PRL females18; single transgenic males developed no lesions. Like male breast cancer in humans, the resulting tumors expressed variable levels of both ER-α and AR. Tumorigenesis in bitransgenic males proceeded independently of testicular steroids after castration after puberty. Transgenic PRL alone enhanced ductal elongation during puberty, which was inhibited by the ER-α antagonist ICI 182,780. Additionally, PRL, like TGF-α, induced phosphorylation of ER-α on serine residues associated with ligand-independent ER-α activity. Together these findings suggest that PRL alone enlarges the population of cells susceptible to transformation and that signaling cooperation between PRL and TGF-α is sufficient to drive oncogenic processes, resulting in hormonally insensitive mammary tumors. This novel model system offers important insight into PRL, ER-α, and TGF-α interactions in the pathogenesis of this disease in males and suggests therapeutic targets that may enhance survival in male breast cancer.
Materials and Methods
Materials
5-Bromo-2-deoxyurindine (BrdU) and 5-bromo-4-chloro-3-indolyl phosphate (BCIP) were obtained from Sigma Chemical Co. (St. Louis, MO). The following antibodies were used for immunohistochemistry and Western analyses: BrdU (MAS-250) from Accurate Scientific (Westbury, NY); ER-α (SC-542) and AR (SC-816) from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA); pS118-ER-α (16J4) and pER-K1/2 (Thr202/Tyr204; 9101) from Cell Signaling Technologies, Inc. (Danvers, MA); and pS167-ER-α (07-481) from Upstate-Cell Signaling Solutions (Lake Placid, NY).
Genotyping and Maintaining Mice
NRL-PRL mice [line 1647-13, TgN(Nrl-Prl)23EPS; line 1655-8, TgN(Nrl-Prl)24EPS]12 and NRL-TGF-α mice [line 1385-7, TgN(Nrl-Tgfa)25EPS]18,19 were generated as described. All lines were maintained in the FVB/N strain background. Tail biopsies were collected at weaning, and offspring were screened for the PRL12 and TGF-α transgenes18 as described previously. NRL-human placental alkaline phosphatase (hPAP) transgene was detected using the following polymerase chain reaction primers: forward, 5′-CTGATGAATGGGAGCAGTGGTGGAA-3′, and reverse, 5′-GCAGACACTCTATGCCTGTGTGGAG-3′. Serum levels of PRL were determined as described,12 and unless noted, results from NRL-PRL 1647-13 single transgenic and bitransgenic lines are shown for clarity. Animals were considered to be end stage when tumor diameter reached 1.5 cm. Mice were housed and handled in accordance with the Guide for Care and Use of Laboratory Animals in Association for Assessment and Accreditation of Laboratory Animal Care-accredited facilities. All procedures were approved by the University of Wisconsin–Madison Animal Care and Use Committee.
Castration
Male mice were castrated at 3 months of age for determining latency to tumor development. NRL-PRL line 1647-13 and the corresponding bitransgenic line were used for these studies. Glands from single transgenic and nontransgenic castrated and sham males were collected at 1 year of age to determine effect of castration on ductal proliferation and steroid receptor expression levels. To examine the effect of gonadal steroids on ductal elongation during puberty, 3-week-old NRL-PRL 1647-13 males were castrated and injected with 5 mg of Faslodex (ICI 182,780) subcutaneously once each week as described20 or treated with sham surgery, and mammary glands were collected for analysis at 3 months of age. This dose of Faslodex significantly reduced the uterine weight of intact females to that of ovariectomized mice.
Mammary Gland Whole Mount Analyses
Fourth inguinal mammary glands were pressed between two slides, fixed in 10% neutral buffered formalin overnight, and stored in 70% ethanol. Whole mounts were stained with carmine alum, dehydrated with graded ethanol, cleared of fat using xylenes, and stored in glycerol until analysis. Ductal elongation was measured by dividing the area of the mammary fat pad by the area of the ductal growth as described.18
hPAP Histochemistry
Fourth inguinal glands were pressed between two slides, fixed in 4% paraformalin at 4°C for 2 to 4 hours, and stored in 70% ethanol. For detection of transgene expression, whole mounts and tissue sections were placed in substrate buffer, heated to 65°C for 1 hour to block endogenous alkaline phosphatase activity, and incubated with BCIP as described previously.21 Tissue sections were counterstained with nuclear fast red (N 8002) from Sigma Chemical Co.
Histological Examination of Mammary Tissue
Mammary tissue was fixed in 10% neutral buffered formalin for 18 to 24 hours, embedded in paraffin, and cut into 6-μm sections. Morphological analyses were performed on hematoxylin and eosin-stained slides. Mice were injected with 200 mg/kg body weight of BrdU 1 hour before sacrifice to label cells undergoing DNA synthesis. Proliferating and epithelial cells expressing ER-α or pERK1/2 were detected using immunohistochemistry as described previously.12,18 To examine ER-α expression, deparaffinized slides were exposed to 0.5% H2O2 in methanol to block endogenous peroxidase activity, boiled for 15 minutes in 0.1 mol/L Tris, pH 9.0, for antigen retrieval, then blocked in 1:100 rabbit serum in TBST. Slides were incubated with primary antibody (ER-α, 1:1000; pERK1/2, 1:100), rinsed and incubated with secondary antibody, rinsed and incubated with peroxidase streptavidin and 3,3′ diaminobenzidine, and counterstained with hematoxylin. An irrelevant antibody was used as a negative control, and uterine tissue was used as a positive control for ER-α staining. To examine AR expression, the following changes were made to the immunohistochemistry protocol: slides underwent antigen retrieval in 0.1 mol/L citrate, pH 6.0, blocked in 5% milk in phosphate-buffered saline, and incubated overnight with the primary antibody (1:500). Sections of the testicles were used as positive controls and omission of the primary antibody as negative controls. BrdU, ER-α, and AR indices were determined by evaluating 500 epithelial cells from morphologically normal ducts in each gland and 1000 cells in macrocysts and adenosis lesions in glands from bitransgenic males, as previously described.12
Cell Culture and Western Analyses
MCF7-derived cells were grown in RPMI 1640 containing 10% horse serum and gangciclovir as reported previously.22 ER-α phosphorylation was evaluated using 106 cells/100-mm plates incubated in serum-free media for 24 hours before treatment with vehicle, 4 nmol/L PRL or 5.5 nmol/L TGF-α for 15 minutes. Cell lysates were harvested and analyzed by immunoblotting as described previously.22 Antibodies were diluted 1:1000 for ER-α and pS118 ER-α and 1:5000 for pS167 ER-α.
Statistical Analyses
Statistical analyses were performed as described using Prism version 4.03 (GraphPad Software, Inc., San Diego, CA).
Results
NRL Promoter Induces Expression in Male Mammary Epithelial Cells
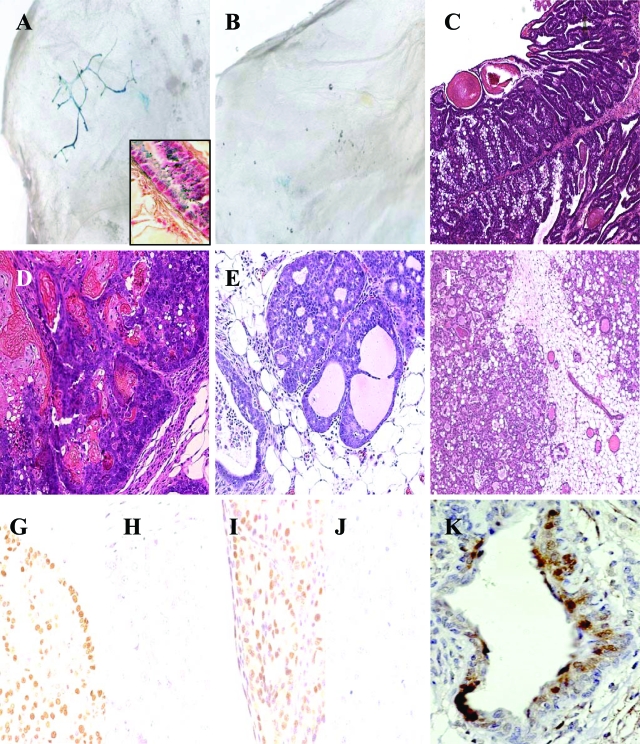
To study the distribution of NRL transgene expression in glands from male mice, transgenic mice bearing the marker gene hPAP were generated.19 Similar to the staining pattern in glands of female NRL-hPAP mice, hPAP was detectable in mammary epithelial cells throughout the ductal tree in males positive for the transgene (Figure 1A), unlike nontransgenic littermates (Figure 1B).
Figure 1.
Expression pattern of NRL promoter, mammary neoplasias, preneoplastic lesions, and steroid receptor expression. A: hPAP was uniformly expressed in mammary epithelial cells of transgenic male NRL-hPAP mice. Higher magnification of epithelial cells from gland of transgenic male NRL-hPAP exposed to BCIP substrate and counterstained with nuclear fast red. B: Glands from wild-type male mice did not stain positively for hPAP. C: Simple papillary epithelial lining of macrocyst from gland of NRL-TGF-α/PRL male. D: Squamous adenocarcinoma, with fibrous capsule and keratin deposition from gland of NRL-TGF-α/PRL male. E: Preneoplastic mammary intraepithelial neoplasia (MIN) from gland of NRL-TGF-α/PRL male. F: Adenosis surrounding region of normal ducts from gland of NRL-TGF-α/PRL male. Note hyperplastic glandular structures containing eosinophilic secretions. G: ER-α+ macrocyst. H: ER-α− macrocyst. I: AR+ macrocyst. J: AR− macrocyst. K: pERK1/2 expression in epithelial cells lining macrocyst. Original magnifications: ×25 (A, B); ×100 (C); ×200 (D–F); ×400 (G–K); ×600 (inset).
PRL and TGF-α Cooperatively Induce Tumors in Male Mice
Two lines of NRL-PRL males were crossed with NRL-TGF-α females to generate NRL-TGF-α/PRL bitransgenic males. Both resulting lines of bitransgenic males developed complex mammary macrocysts with 100% incidence and similar preneoplastic lesions, but males from the NRL-TGF-α/PRL 1655-8 line developed tumors with a significantly increased latency (9.1 ± 3.9 months, mean ± SD) compared to NRL-TGF-α/PRL 1647-13 males (5.7 ± 1.5 months). These end-stage lesions were similar to those found in glands of NRL-TGF-α and bitransgenic females (Table 1 and Figure 1C).18,19 Glands from NRL-TGF-α/PRL males also developed rare squamous adenocarcinomas (Figure 1D), as well as preneoplastic lesions including mammary intraepithelial neoplasias (MIN, Figure 1E), adenosis lesions (Figure 1F), and epithelial hyperplasias (not shown). Both bitransgenic lines demonstrated similar serum PRL levels compared to nontransgenic littermates. Neither line of NRL-PRL nor NRL-TGF-α males developed mammary tumors or preneoplastic lesions (evaluated at 1 year of age, Table 1; or a separate cohort at 19 to 24 months of age).
Table 1.
Mammary Morphology at End Stage
| Treatment | Mammary abnormalities
|
|
|---|---|---|
| Castrated | Sham surgery | |
| PRL × TGF-α | Macrocysts (10 of 12) | Macrocysts (9 of 9) |
| Complex/papillary (4 of 10) | Complex/papillary (6 of 9) | |
| Simple/papillary (6 of 10) | Simple/papillary (3 of 9) | |
| EH* (9 of 12) | EH* (7 of 9) | |
| Dilated ducts (8 of 12) | Dilated ducts (7 of 9) | |
| Adenosis (9 of 12) | Adenosis (3 of 9) | |
| MINs† (3 of 12) | MINs† (3 of 9) | |
| Squamous neoplasia (2 of 12) | ||
| TGF-α | No lesions present (8 of 8) | No lesions present (8 of 8) |
| PRL | No lesions present (9 of 9) | No lesions present (7 of 7) |
| Nontransgenic FVB/N | No lesions present (8 of 8) | No lesions present (8 of 8) |
End stage is defined as tumor size reaching 1.5 cm diameter or 1 year of age.
Epithelial hyperplasia.
Mammary intraepithelial neoplasias.
Given the high proportion of male breast cancers that are ER-α-positive, we examined steroid receptor expression in macrocysts of bitransgenic males. ER-α expression within cells lining macrocysts varied widely among individuals (Figure 1, G and H). Macrocysts from bitransgenic males also expressed variable levels of AR (Figure 1, I and J), similar to breast tumors from human males.
Tumorigenesis in NRL-TGF-α/PRL Males Is Insensitive to Testicular Steroids
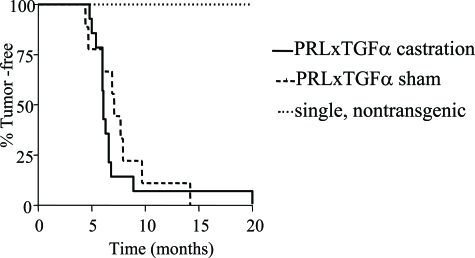
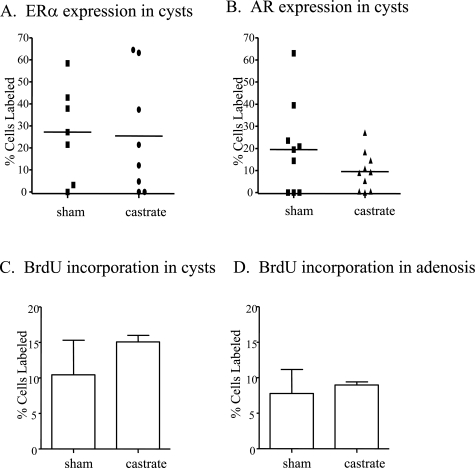
To examine the effect of testicular steroids on tumorigenesis, males of all genotypes underwent castration or sham surgery shortly after puberty at 3 months of age. As expected, no single transgenic or nontransgenic males developed tumors after 1 year. Both castrated and sham surgery-treated bitransgenic males developed mammary macrocysts with 100% incidence and similar latency (Figure 2). These tumors displayed a wide variation in ER-α and AR expression, which was not affected by castration (Figure 3, A and B). Gonadal removal also did not significantly alter either the tumor histotype or the complement of preneoplastic lesions (Table 1). The proliferation rates of epithelial cells lining the macrocysts and preneoplastic adenosis lesions were not significantly altered in castrated compared to sham-treated males (Figure 3, C and D), indicating that cellular turnover in these lesions is not dependent on testicular steroids.
Figure 2.
Castration did not affect tumor latency in NRL-TGF-α/PRL male mice. Bitransgenic, single transgenic, and nontransgenic male mice underwent castration or sham surgery at 3 months of age and were monitored for tumor development. End stage was defined when tumors reached 1.5 cm in diameter. The latencies were compared by Kaplan-Meier analysis, and differences were detected using the Mantel-Haenszel test. All bitransgenic male mice developed mammary tumors with no significant difference in latency (P = 0.14). No castrated and sham-treated single transgenic and nontransgenic littermates developed mammary lesions.
Figure 3.
Effect of castration on ER-α and AR expression in macrocysts and proliferation in mammary lesions of NRL-TGF-α/PRL male mice. Percentage of ER-α+ (A) and AR+ (B) cells present in mammary macrocysts. Each symbol represents a single macrocyst. Proliferation in macrocysts (C) and adenosis lesions (D) was determined as described in Materials and Methods and expressed as mean ± SD.
Morphologically normal ducts in the bitransgenic males were also insensitive to the removal of testicular steroids. Castration did not affect proliferation (percent BrdU-labeled cells: sham, 4.9 ± 1.3; castrated, 5.9 ± 2.9; mean ± SD) or ER-α expression (percent ER-α-labeled cells: sham, 25.3 ± 4.0; castrated, 20.7 ± 8.2; mean ± SD). However, it did significantly decrease AR levels (percent AR-labeled cells: sham, 30.2 ± 14.0; castrated, 9.1 ± 3.6, mean ± SD; P = 0.0012), consistent with ligand-induced protein stabilization.23 This lack of mitogenic dependence on gonadal steroids is similar to the independence from ovarian hormones observed in ovariectomized bitransgenic females (L.M. Arendt et al, manuscript submitted).
PRL Enhances Ductal Elongation in NRL-PRL Males
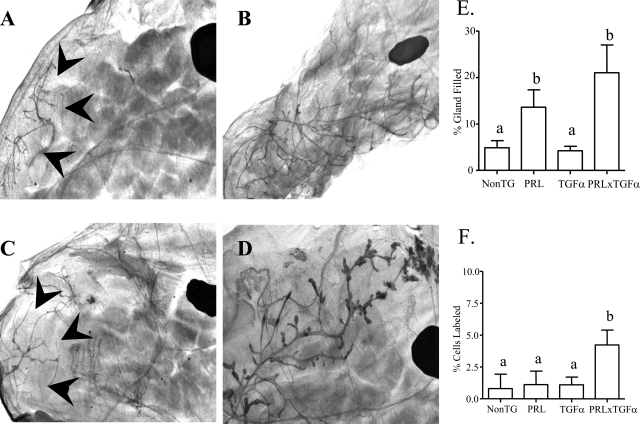
Mammary sexual dimorphism begins between embryonic days 13 and 14, when the testes begin production of androgens, inciting the fibroblasts surrounding the epithelial bud to condense, which induces the regression of the mammary tree.24,25 As a result, male mice and rats lack nipples and their mammary glands are not connected to the skin,26 although a residual ductal tree may be present depending on the strain of mouse examined.27 In the FVB/N males examined here, no remnant was identifiable in 0 to 10% of the males of each of the four genotypes, and there was no significant difference in this incidence among genotypes. Mature nontransgenic and NRL-TGF-α males demonstrated very little ductal elongation into the mammary fat pad (Figure 4, A and C). In contrast, the ductal system was significantly enhanced in glands of both NRL-PRL and bitransgenic males (Figure 4, B, D, and E). The extent of branching exhibited considerable variation within a genotype. However, combined analyses of multiple animals revealed the same pattern of differences as the extent of gland filling (data not shown), suggesting that these processes are linked in this model.
Figure 4.
Representative whole mounts from inguinal glands of males of the different genotypes. A: Nontransgenic. B: NRL-PRL. C: NRL-TGF-α. D: NRL-TGF-α/PRL. The large dark oval in each mammary gland is a lymph node, and arrowheads mark boundaries of ductal elongation. E: Quantitation of ductal elongation into mammary fat pad of male mice. F: Proliferation rates as determined by BrdU labeling in glands of male mice. Glands were collected from single and nontransgenic males at 1 year of age or after macrocyst development in bitransgenic males, and prepared and evaluated as described in Materials and Methods. Data are expressed as mean ± SD. Different lowercase letters denote statistical differences among the different lines as determined by the Kruskal-Wallis test followed by Mann-Whitney post test (P < 0.05). Original magnifications, ×10.
Relatively little is known about the hormonal regulation of growth of this rudimentary mammary tree in glands from either male humans or mice. Within each genotype, castration at 3 months of age did not alter the extent of ductal elongation (data not shown), indicating that testicular steroids did not enhance this ductal development once adulthood was reached. Although ductal length in mature males was not significantly different between NRL-PRL and bitransgenic males, only bitransgenic adult males demonstrated elevated epithelial cell proliferation at 1 year of age (Figure 4F), suggesting that ductal growth is transient in the single transgenic NRL-PRL males. The continued elevated rate of proliferation observed in the bitransgenic glands, in stark contrast to those of single transgenic males (Figure 4F), also indicates that PRL and TGF-α interactions result in dysregulated proliferation, potentially an important contributor to the mammary pathology observed in this genotype.
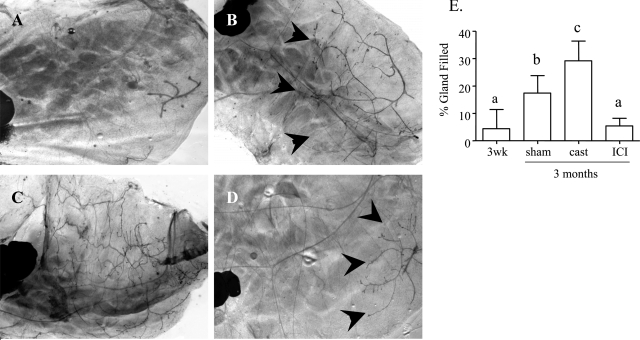
To examine the timing of ductal elongation in NRL-PRL males, glands were collected from males at 3 weeks of age, before puberty, and at 3 months of age. Glands from 3-week-old NRL-PRL males demonstrated only rudimentary growth (Figure 5, A and E), in contrast to the significant ductal development at 3 months (Figure 5, B and E), which was similar to that observed in 1-year-old NRL-PRL males (Figure 4A). This indicated that ductal elongation in males, like females, is a transient pubertal event. In females, this growth is initiated by elevated serum estrogen, acting through ER-α.28 To explore the role of ER-α in this event in males, NRL-PRL males were castrated or treated with ICI 182,780 weekly starting at 3 weeks of age, and ductal elongation was measured at 3 months of age. Castration significantly enhanced ductal outgrowth compared to sham-treated males (Figure 5, C–E). In contrast, ICI 182,780 treatment blocked this event, limiting growth to that found in nontransgenic or NRL-TGF-α males (compare Figure 5E to Figure 4E), suggesting that PRL-induced ductal elongation in males also is mediated through ER-α. There was no significant difference in body weight among castrated, sham-treated, or ICI 182,780-treated males (data not shown).
Figure 5.
Ductal elongation is significantly inhibited in NRL-PRL males treated with ICI 182,780. A: Rudimentary ductal growth in glands of 3-week-old NRL-PRL male. B: Glands from 3-month-old NRL-PRL males demonstrated ductal elongation during puberty. C: Ductal elongation was significantly enhanced in glands of 3-month-old NRL-PRL males castrated at 3 weeks of age. D: Ductal elongation was significantly inhibited in glands of 3-month-old NRL-PRL males after weekly injections of ICI 182,780 starting at 3 weeks of age. E: Quantitation of ductal elongation into mammary fat pad as described in Materials and Methods. Data are expressed as mean ± SD. Different lowercase letters denote statistical differences among the different lines as determined by the Kruskal-Wallis test followed by Mann-Whitney post test (P < 0.05). Original magnifications: ×20 (A); ×10 (B–D).
PRL and TGF-α Increase ER-α Phosphorylation
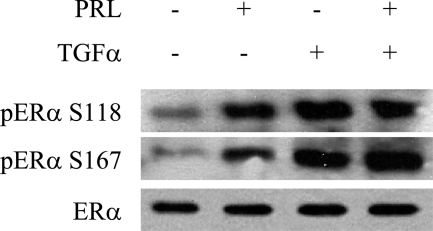
Ductal elongation in NRL-PRL males was significantly diminished in response to treatment with ICI 182,780, indicating an interaction of PRL with ER-α. We have previously shown both in vivo and in vitro that PRL and TGF-α cooperatively induce prolonged ERK1/218 and Akt phosphorylation (L.M. Arendt et al, manuscript submitted), and many tumors in the current study exhibited high levels of nuclear pERK1/2 (Figure 1K). Both ERK1/2 and Akt have been implicated in phosphorylation of ER-α and unliganded ER-α activity.29,30,31 To determine whether PRL initiation of these pathways resulted in ER-α phosphorylation, MCF-7-derived cells were treated with vehicle, PRL, and/or TGF-α for 15 minutes. In response to treatment, PRL, like TGF-α, induced phosphorylation of ER-α at S118 and S167 (Figure 6).
Figure 6.
PRL and TGF-α induce phosphorylation of ER-α. Representative immunoblot of MCF-7-derived cells treated with either 4 nmol/L PRL or 5.5 nmol/L TGF-α, which induced increased levels of S118 and S167 phosphorylated ER-α compared to vehicle.
Discussion
The factors that contribute to the development of breast cancer in males are not well understood. Here we show that the combination of two endogenous factors important in normal breast development in females, PRL and TGF-α, cooperated to cause tumors with 100% incidence in male mice, in striking contrast to the lack of pathology with either transgene alone. Like breast tumors in men, these lesions expressed both ER-α and AR. However, the latency of these lesions as well as cellular turnover in the context of these dysregulated factors was independent of testicular steroids.
Although single transgenic NRL-PRL males developed no evident structural abnormalities, they displayed greatly increased ductal elongation, coincident with a transient increase in proliferation at puberty. In contrast to events in the adult bitransgenic males, this was sensitive to manipulation of steroid signals, enhanced by castration, and inhibited by the ER-α-selective inhibitor ICI 182,780. In female mice, ductal elongation occurs during puberty in the context of increased circulating estrogen acting through ER-α.32 Genetically male mammary epithelial cells respond to hormonal context like those of females, after castration and supplemental steroids,33,34 or transfer to a female mammary fat pad.35,36 The ability of ICI 182,780 to abrogate PRL-induced ductal elongation indicates that ER mediates the growth observed in the current study, overcoming inhibitory signals from testicular androgens.26,37,38 The ability of PRL to phosphorylate ER-α residues that are associated with unliganded activity29,30,31 suggests that PRL-induced activation of ER-α itself may be responsible. This action of PRL may be one mechanism underlying the association of circulating PRL with male, as well as female, breast cancer.
Interestingly, glands from NRL-PRL and NRL-TGF-α males do not demonstrate any evidence of preneoplastic lesion formation. This is particularly surprising in NRL-PRL males, in light of the expansion of the ductal tree. However, in females, PRL-induced tumors display a different histotype than TGF-α and bitransgenic animals with a much longer latency. Androgenic activity in males may oppose other processes critical for PRL-mediated tumorigenesis. Evidence from other models also suggests that although activation of ER-α can drive ductal elongation in male glands, it is not sufficient for lesion formation. Overexpression of P450 aromatase under control of the ubiquitin C promoter, which elevates circulating estrogen and reduces testosterone, significantly enhances ductal elongation and alveolar development in males.39 MMTV-directed expression of metastasis associated protein-1, a possible co-repressor of ER-α, up-regulates a downstream target of ER-α, cyclin D1, inducing ductal elongation in males.40 Overexpression of ER-α alone is not sufficient to induce lesion formation in female mice41; we would predict that this also would be the case in males. In contrast to NRL-PRL males, NRL-TGF-α males did not exhibit ductal development, despite the ability of TGF-α, like PRL, to phosphorylate ER-α in vitro. This may result from TGF-α-induced down-regulation of ER-α. Glands of NRL-TGF-α females exhibited significantly reduced ER-α expression in morphologically normal structures compared to nontransgenic or NRL-PRL females.18 However, PRL was able to reverse this decline in bitransgenic females, consistent with our current studies.
PRL and TGF-α potently cooperate at downstream pathways, which may contribute to their dramatic increase in oncogenicity in combination. In vitro, PRL and TGF-α together prolong ERK1/2 and Akt activity18 (L.M. Arendt et al, manuscript submitted), two pathways which have been implicated in proliferation, survival, and motility,42,43,44 as well as unliganded ER-α activation.29,30,31 Synergistic activation of these processes critical for neoplastic progression in an expanded population of epithelial cells susceptible to tumorigenesis is likely to underlie our observations.
Breast cancer in men is relatively rare; however, unlike the disease in women, the incidence is rising. Male breast tumors demonstrate high levels of ER-α expression, similar to those in postmenopausal women. Moreover, like women, male patients demonstrate resistance to antiestrogens, such as tamoxifen, or develop resistance after treatment. In vivo, PRL in combination with TGF-α induces ER-α-positive, but estrogen-insensitive, disease. By understanding cross talk among PRL, TGF-α, and ER-α, it may be possible to increase the number of patients initially responsive to endocrine therapies as well as increase survival.
Acknowledgments
We thank Tara Grafwallner-Huseth and Debra Rugowski for assistance with data collection and Drs. Eric Sandgren and Terri Rose-Hellekant for insightful discussions.
Footnotes
Address reprint requests to Linda A. Schuler, Department of Comparative Biosciences, University of Wisconsin, 2015 Linden Dr., Madison, WI 53706. E-mail: schulerl@svm.vetmed.wisc.edu.
Supported by the National Center for Research Resources (a component of the National Institutes of Health, grant number K01-RR021858 to L.M.A.), the National Institutes of Health (grants R01-CA 78312 and DK-62783 to L.A.S. and T32-AG00265), and the University of Wisconsin Center for Women’s Health and Women’s Health Research.
The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the National Center for Research Resources or National Institutes of Health.
References
- American Cancer Society Atlanta: American Cancer Society; Cancer Facts and Figures, 2006–2007. 2007 [Google Scholar]
- Nahleh ZA. Hormonal therapy for male breast cancer: a different approach for a different disease. Cancer Treat Rev. 2006;32:101–105. doi: 10.1016/j.ctrv.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Krause W. Male breast cancer—an andrological disease: risk factors and diagnosis. Andrologia. 2004;36:346–354. doi: 10.1111/j.1439-0272.2004.00639.x. [DOI] [PubMed] [Google Scholar]
- Giordano SH. A review of the diagnosis and management of male breast cancer. Oncologist. 2005;10:471–479. doi: 10.1634/theoncologist.10-7-471. [DOI] [PubMed] [Google Scholar]
- O’Malley C, Shema S, White E, Glaser S. Incidence of male breast cancer in California, 1988–2000: racial/ethnic variation in 1759 men. Breast Cancer Res Treat. 2005;93:145–150. doi: 10.1007/s10549-005-4517-z. [DOI] [PubMed] [Google Scholar]
- Freemark M, Handwerger S. The glycogenic effects of placental lactogen and growth hormone in ovine fetal liver are mediated through binding to specific fetal ovine placental lactogen receptors. Endocrinology. 1986;118:613–618. doi: 10.1210/endo-118-2-613. [DOI] [PubMed] [Google Scholar]
- Kidwai N, Gong Y, Sun X, Deshpande CG, Yeldandi AV, Rao MS, Badve S. Expression of androgen receptor and prostate-specific antigen in male breast carcinoma. Breast Cancer Res. 2004;6:R18–R23. doi: 10.1186/bcr733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fentiman IS, Fourquet A, Hortobagyi GN. Male breast cancer. Lancet. 2006;367:595–604. doi: 10.1016/S0140-6736(06)68226-3. [DOI] [PubMed] [Google Scholar]
- Olsson H, Alm P, Aspegren K, Gullberg B, Jonsson PE, Ranstam J. Increased plasma prolactin levels in a group of men with breast cancer—a preliminary study. Anticancer Res. 1990;10:59–62. [PubMed] [Google Scholar]
- Olsson H, Ranstam J. Head trauma and exposure to prolactin-elevating drugs as risk factors for male breast cancer. J Natl Cancer Inst. 1988;80:679–683. doi: 10.1093/jnci/80.9.679. [DOI] [PubMed] [Google Scholar]
- Gill S, Peston D, Vonderhaar BK, Shousha S. Expression of prolactin receptors in normal, benign, and malignant breast tissue: an immunohistological study. J Clin Pathol. 2001;54:956–960. doi: 10.1136/jcp.54.12.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose-Hellekant TA, Arendt LM, Schroeder MD, Gilchrist K, Sandgren EP, Schuler LA. Prolactin induces ER-a-positive and ER-a-negative mammary cancer in transgenic mice. Oncogene. 2003;22:4664–4674. doi: 10.1038/sj.onc.1206619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan SK, Hill ME, Gullick WJ. The role of the epidermal growth factor receptor in breast cancer. J Mammary Gland Biol Neoplasia. 2006;11:3–11. doi: 10.1007/s10911-006-9008-2. [DOI] [PubMed] [Google Scholar]
- Giordano SH, Buzdar AU, Hortobagyi GN. Breast cancer in men. Ann Intern Med. 2002;137:678–687. doi: 10.7326/0003-4819-137-8-200210150-00013. [DOI] [PubMed] [Google Scholar]
- Idelevich E, Mozes M, Ben Baruch N, Huszar M, Kruglikova A, Katsnelson R, Shani A. Oncogenes in male breast cancer. Am J Clin Oncol. 2003;26:259–261. doi: 10.1097/01.COC.0000020582.25017.5D. [DOI] [PubMed] [Google Scholar]
- Yarden Y. The EGFR family and its ligands in human cancer: signalling mechanisms and therapeutic opportunities. Eur J Cancer. 2001;37:S3–S8. doi: 10.1016/s0959-8049(01)00230-1. [DOI] [PubMed] [Google Scholar]
- Holbro T, Civenni G, Hynes NE. The ErbB receptors and their role in cancer progression. Exp Cell Res. 2003;284:99–110. doi: 10.1016/s0014-4827(02)00099-x. [DOI] [PubMed] [Google Scholar]
- Arendt LM, Rose-Hellekant TA, Sandgren EP, Schuler LA. Prolactin potentiates TGF-α induction of mammary neoplasia in transgenic mice. Am J Pathol. 2006;168:1365–1374. doi: 10.2353/ajpath.2006.050861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose-Hellekant TA, Schroeder MD, Brockman JL, Zhdankin O, Bolstad R, Chen KS, Gould MN, Schuler LA, Sandgren EP. Estrogen receptor positive mammary tumorigenesis in TGFα transgenic mice progresses with progesterone receptor loss. Oncogene. 2007;26:5238–5246. doi: 10.1038/sj.onc.1210340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho HW, Nie R, Carnes K, Zhou Q, Sharief NA, Hess RA. The antiestrogen ICI 182,780 induces early effects on the adult male mouse reproductive tract and long-term decreased fertility without testicular atrophy. Reprod Biol Endocrinol. 2003;1:57. doi: 10.1186/1477-7827-1-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisseberth WC, Brettingen NT, Lohse JK, Sandgren EP. Ubiquitous expression of marker transgenes in mice and rats. Dev Biol. 1999;214:128–138. doi: 10.1006/dbio.1999.9417. [DOI] [PubMed] [Google Scholar]
- Schroeder MD, Symowicz J, Schuler LA. Prolactin modulates cell cycle regulators in mammary tumor epithelial cells. Mol Endocrinol. 2002;16:45–57. doi: 10.1210/mend.16.1.0762. [DOI] [PubMed] [Google Scholar]
- Brys M. Androgens and androgen receptor: do they play a role in breast cancer? Med Sci Monit. 2000;6:433–438. [PubMed] [Google Scholar]
- Kratochwil K, Schwartz P. Tissue interaction in androgen response of embryonic mammary rudiment of mouse: identification of target tissue for testosterone. Proc Natl Acad Sci USA. 1976;73:4041–4044. doi: 10.1073/pnas.73.11.4041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuberger B, Fitzka I, Wasner G, Kratochwil K. Induction of androgen receptor formation by epithelium-mesenchyme interaction in embryonic mouse mammary gland. Proc Natl Acad Sci USA. 1982;79:2957–2961. doi: 10.1073/pnas.79.9.2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao DJ, Dickson RB. Roles of androgens in the development, growth, and carcinogenesis of the mammary gland. J Steroid Biochem Mol Biol. 2002;80:175–189. doi: 10.1016/s0960-0760(01)00185-6. [DOI] [PubMed] [Google Scholar]
- Hovey RC, Trott JF, Vonderhaar BK. Establishing a framework for the functional mammary gland: from endocrinology to morphology. J Mammary Gland Biol Neoplasia. 2002;7:17–38. doi: 10.1023/a:1015766322258. [DOI] [PubMed] [Google Scholar]
- Bocchinfuso WP, Korach KS. Mammary gland development and tumorigenesis in estrogen receptor knockout mice. J Mammary Gland Biol Neoplasia. 1997;2:323–334. doi: 10.1023/a:1026339111278. [DOI] [PubMed] [Google Scholar]
- Lannigan DA. Estrogen receptor phosphorylation. Steroids. 2003;68:1–9. doi: 10.1016/s0039-128x(02)00110-1. [DOI] [PubMed] [Google Scholar]
- Shupnik MA. Crosstalk between steroid receptors and the c-Src-receptor tyrosine kinase pathways: implications for cell proliferation. Oncogene. 2004;23:7979–7989. doi: 10.1038/sj.onc.1208076. [DOI] [PubMed] [Google Scholar]
- Coleman KM, Smith CL. Intracellular signaling pathways: nongenomic actions of estrogens and ligand-independent activation of estrogen receptors. Front Biosci. 2001;6:D1379–D1391. doi: 10.2741/coleman. [DOI] [PubMed] [Google Scholar]
- Hewitt SC, Harrell JC, Korach KS. Lessons in estrogen biology from knockout and transgenic animals. Annu Rev Physiol. 2005;67:285–308. doi: 10.1146/annurev.physiol.67.040403.115914. [DOI] [PubMed] [Google Scholar]
- Flux DS. Mammary gland growth in male mice of the CHI strain after hypophysectomy and castration. J Endocrinol. 1958;17:300–306. doi: 10.1677/joe.0.0170300. [DOI] [PubMed] [Google Scholar]
- Daane TA, Lyons WR. Effect of estrone, progesterone and pituitary mammotropin on the mammary glands of castrated C3H male mice. Endocrinology. 1954;55:191–199. doi: 10.1210/endo-55-2-191. [DOI] [PubMed] [Google Scholar]
- Hoshino K. Morphogenesis and growth potentiality of mammary glands in mice. II. Quantitative transplantation of mammary glands of normal male mice. J Natl Cancer Inst. 1963;30:585–591. [PubMed] [Google Scholar]
- Blair PB, Moretti RL. Transplantation of isologous male mammary gland tissue into the mammary fat-pads of female mice. Transplantation. 1970;10:44–52. doi: 10.1097/00007890-197007000-00004. [DOI] [PubMed] [Google Scholar]
- Dobs A, Darkes MJ. Incidence and management of gynecomastia in men treated for prostate cancer. J Urol. 2005;174:1737–1742. doi: 10.1097/01.ju.0000176461.75794.f8. [DOI] [PubMed] [Google Scholar]
- Autorino R, Perdona S, D’Armiento M, De Sio M, Damiano R, Cosentino L, Di Lorenzo G. Gynecomastia in patients with prostate cancer: update on treatment options. Prostate Cancer Prostatic Dis. 2006;9:109–114. doi: 10.1038/sj.pcan.4500859. [DOI] [PubMed] [Google Scholar]
- Li X, Warri A, Makela S, Ahonen T, Streng T, Santti R, Poutanen M. Mammary gland development in transgenic male mice expressing human P450 aromatase. Endocrinology. 2002;143:4074–4083. doi: 10.1210/en.2002-220181. [DOI] [PubMed] [Google Scholar]
- Bagheri-Yarmand R, Talukder AH, Wang RA, Vadlamudi RK, Kumar R. Metastasis-associated protein 1 deregulation causes inappropriate mammary gland development and tumorigenesis. Development. 2004;131:3469–3479. doi: 10.1242/dev.01213. [DOI] [PubMed] [Google Scholar]
- Frech MS, Halama ED, Tilli MT, Singh B, Gunther EJ, Chodosh LA, Flaws JA, Furth PA. Deregulated estrogen receptor α expression in mammary epithelial cells of transgenic mice results in the development of ductal carcinoma in situ. Cancer Res. 2005;65:681–685. [PMC free article] [PubMed] [Google Scholar]
- Santen RJ, Song RX, McPherson R, Kumar R, Adam L, Jeng MH, Yue W. The role of mitogen-activated protein (MAP) kinase in breast cancer. J Steroid Biochem Mol Biol. 2002;80:239–256. doi: 10.1016/s0960-0760(01)00189-3. [DOI] [PubMed] [Google Scholar]
- Altomare DA, Testa JR. Perturbations of the AKT signaling pathway in human cancer. Oncogene. 2005;24:7455–7464. doi: 10.1038/sj.onc.1209085. [DOI] [PubMed] [Google Scholar]
- Kim D, Dan HC, Park S, Yang L, Liu Q, Kaneko S, Ning J, He L, Yang H, Sun M, Nicosia SV, Cheng JQ. AKT/PKB signaling mechanisms in cancer and chemoresistance. Front Biosci. 2005;10:975–987. doi: 10.2741/1592. [DOI] [PubMed] [Google Scholar]