Abstract
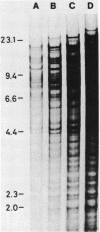
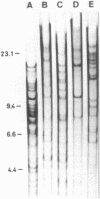
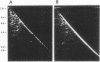
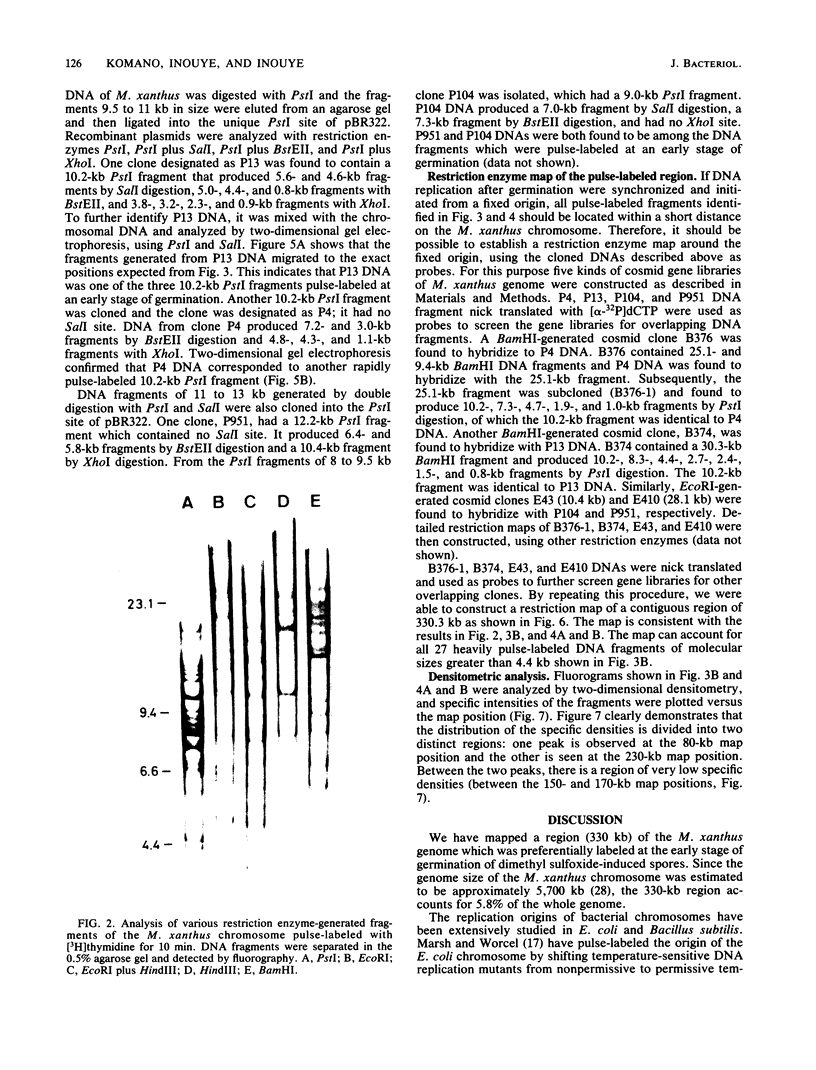
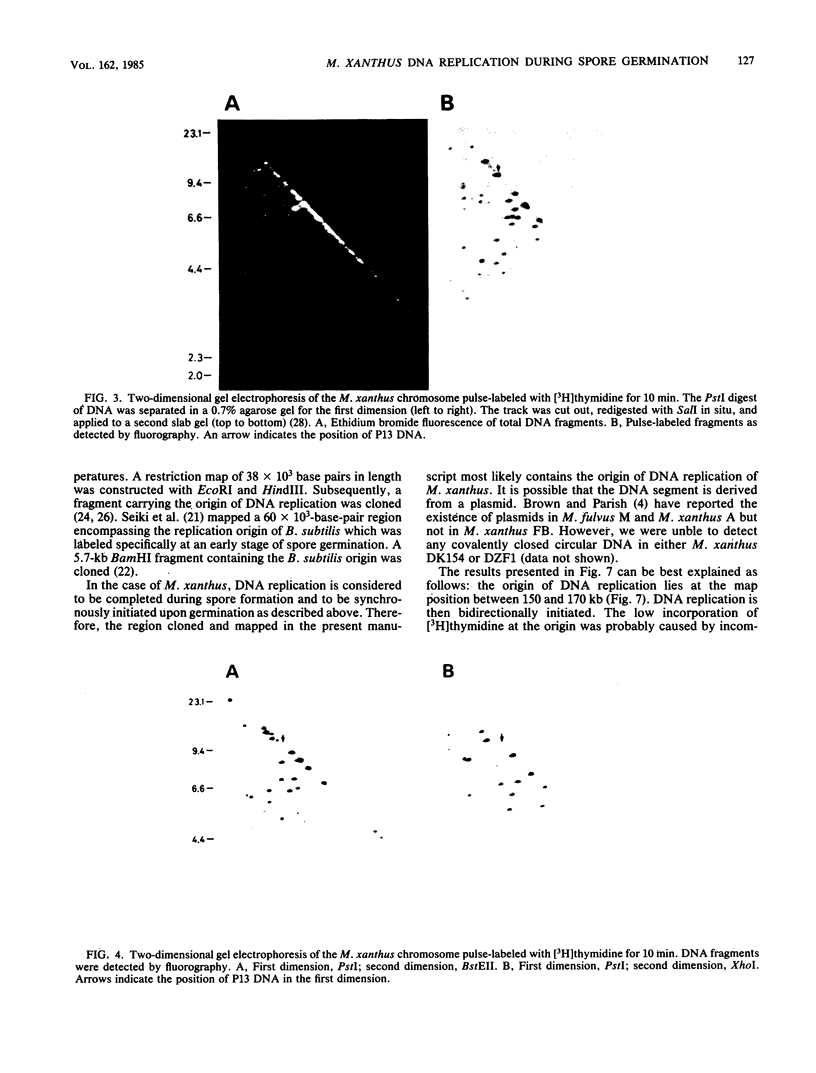
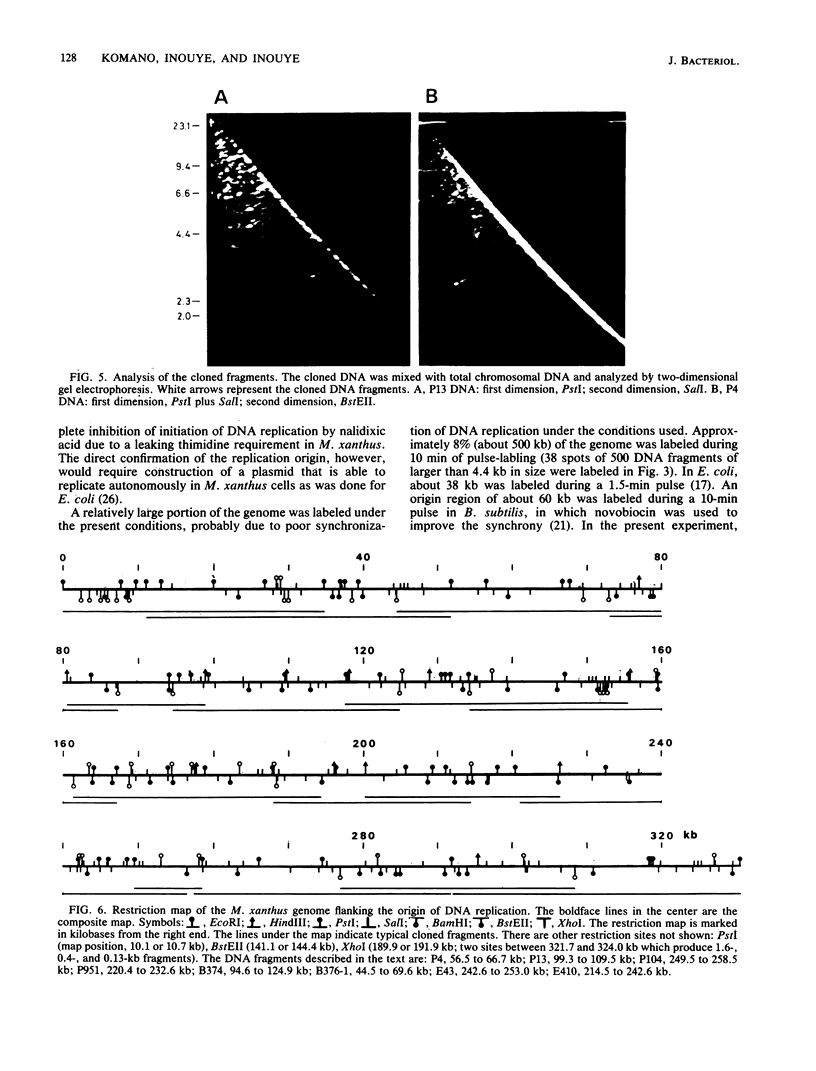
Myxococcus xanthus was pulse-labeled with [3H]thymidine immediately after germination of dimethyl sulfoxide-induced spores. The restriction enzyme digests of the total chromosomal DNA from the pulse-labeled cells were analyzed by one-dimensional as well as two-dimensional agarose gel electrophoresis. Four PstI fragments preferentially labeled at a very early stage of germination were cloned into the unique PstI site of pBR322. By using these clones as probes, a restriction enzyme map was established covering approximately 6% of the total M. xanthus genome (330 X 10(3) base pairs). The distribution of the specific activities of the restriction fragments pulse-labeled after germination suggests a bidirectional mode of DNA replication from a fixed origin.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beckmann J. S., Johnson P. F., Abelson J. Cloning of yeast transfer RNA genes in Escherichia coli. Science. 1977 Apr 8;196(4286):205–208. doi: 10.1126/science.322282. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Brown N. L., Parish J. H. Extrachromosomal DNA in chloramphenicol resistant myxococcus strains. J Gen Microbiol. 1976 Mar;93(1):63–68. doi: 10.1099/00221287-93-1-63. [DOI] [PubMed] [Google Scholar]
- Campos J. M., Geisselsoder J., Zusman D. R. Isolation of bacteriophage MX4, a generalized transducing phage for Myxococcus xanthus. J Mol Biol. 1978 Feb 25;119(2):167–178. doi: 10.1016/0022-2836(78)90431-x. [DOI] [PubMed] [Google Scholar]
- Clarke L., Carbon J. Functional expression of cloned yeast DNA in Escherichia coli: specific complementation of argininosuccinate lyase (argH) mutations. J Mol Biol. 1978 Apr 25;120(4):517–532. doi: 10.1016/0022-2836(78)90351-0. [DOI] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Hsu L. Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2110–2114. doi: 10.1073/pnas.69.8.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins J., Brüning H. J. Plasmids useable as gene-cloning vectors in an in vitro packaging by coliphage lambda: "cosmids". Gene. 1978 Oct;4(2):85–107. doi: 10.1016/0378-1119(78)90023-9. [DOI] [PubMed] [Google Scholar]
- DWORKIN M., GIBSON S. M. A SYSTEM FOR STUDYING MICROBIAL MORPHOGENESIS: RAPID FORMATION OF MICROCYSTS IN MYXOCOCCUS XANTHUS. Science. 1964 Oct 9;146(3641):243–244. doi: 10.1126/science.146.3641.243. [DOI] [PubMed] [Google Scholar]
- Hohn B., Collins J. A small cosmid for efficient cloning of large DNA fragments. Gene. 1980 Nov;11(3-4):291–298. doi: 10.1016/0378-1119(80)90069-4. [DOI] [PubMed] [Google Scholar]
- Inouye S., Ike Y., Inouye M. Tandem repeat of the genes for protein S, a development-specific protein of Myxococcus xanthus. J Biol Chem. 1983 Jan 10;258(1):38–40. [PubMed] [Google Scholar]
- Kimchi A., Rosenberg E. Linkages between deoxyribonucleic acid synthesis and cell division in Myxococcus xanthus. J Bacteriol. 1976 Oct;128(1):69–79. doi: 10.1128/jb.128.1.69-79.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komano T., Inouye S., Inouye M. Patterns of protein production in Myxococcus xanthus during spore formation induced by glycerol, dimethyl sulfoxide, and phenethyl alcohol. J Bacteriol. 1980 Dec;144(3):1076–1082. doi: 10.1128/jb.144.3.1076-1082.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- Marsh R. C., Worcel A. A DNA fragment containing the origin of replication of the Escherichia coli chromosome. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2720–2724. doi: 10.1073/pnas.74.7.2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K., Inouye M. Inactivation of the Serratia marcescens gene for the lipoprotein in Escherichia coli by insertion sequences, IS1 and IS5; sequence analysis of junction points. Mol Gen Genet. 1981;183(1):107–114. doi: 10.1007/BF00270147. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rosenberg E., Katarski M., Gottlieb P. Deoxyribonucleic acid synthesis during exponential growth and microcyst formation in Myxococcus xanthus. J Bacteriol. 1967 Apr;93(4):1402–1408. doi: 10.1128/jb.93.4.1402-1408.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiki M., Ogasawara N., Yoshikawa H. Structure and function of the region of the replication origin of the Bacillus subtilis chromosome. I. Isolation and characterization of plasmids containing the origin region. Mol Gen Genet. 1981;183(2):220–226. doi: 10.1007/BF00270621. [DOI] [PubMed] [Google Scholar]
- Shimkets L. J., Gill R. E., Kaiser D. Developmental cell interactions in Myxococcus xanthus and the spoC locus. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1406–1410. doi: 10.1073/pnas.80.5.1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkin S. S., Rosenberg E. Induction of morphogenesis by methionine starvation in Myxococcus xanthus: polyamine control. J Bacteriol. 1970 Sep;103(3):641–649. doi: 10.1128/jb.103.3.641-649.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda S., Hirota Y. Cloning and mapping of the replication origin of Escherichia coli. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5458–5462. doi: 10.1073/pnas.74.12.5458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee T., Inouye M. Reexamination of the genome size of myxobacteria, including the use of a new method for genome size analysis. J Bacteriol. 1981 Mar;145(3):1257–1265. doi: 10.1128/jb.145.3.1257-1265.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee T., Inouye M. Two-dimensional DNA electrophoresis applied to the study of DNA methylation and the analysis of genome size in Myxococcus xanthus. J Mol Biol. 1982 Jan 15;154(2):181–196. doi: 10.1016/0022-2836(82)90059-6. [DOI] [PubMed] [Google Scholar]
- Zusman D., Rosenberg E. Deoxyribonucleic acid synthesis during microcyst germination in Myxococcus xanthus. J Bacteriol. 1968 Oct;96(4):981–986. doi: 10.1128/jb.96.4.981-986.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Meyenburg K., Hansen F. G., Nielsin L. D., Riise E. Origin of replication, oriC, or the Escherichia coli chromosome on specialized transducing phages lambda asn. Mol Gen Genet. 1978 Apr 17;160(3):287–295. doi: 10.1007/BF00332972. [DOI] [PubMed] [Google Scholar]