Abstract
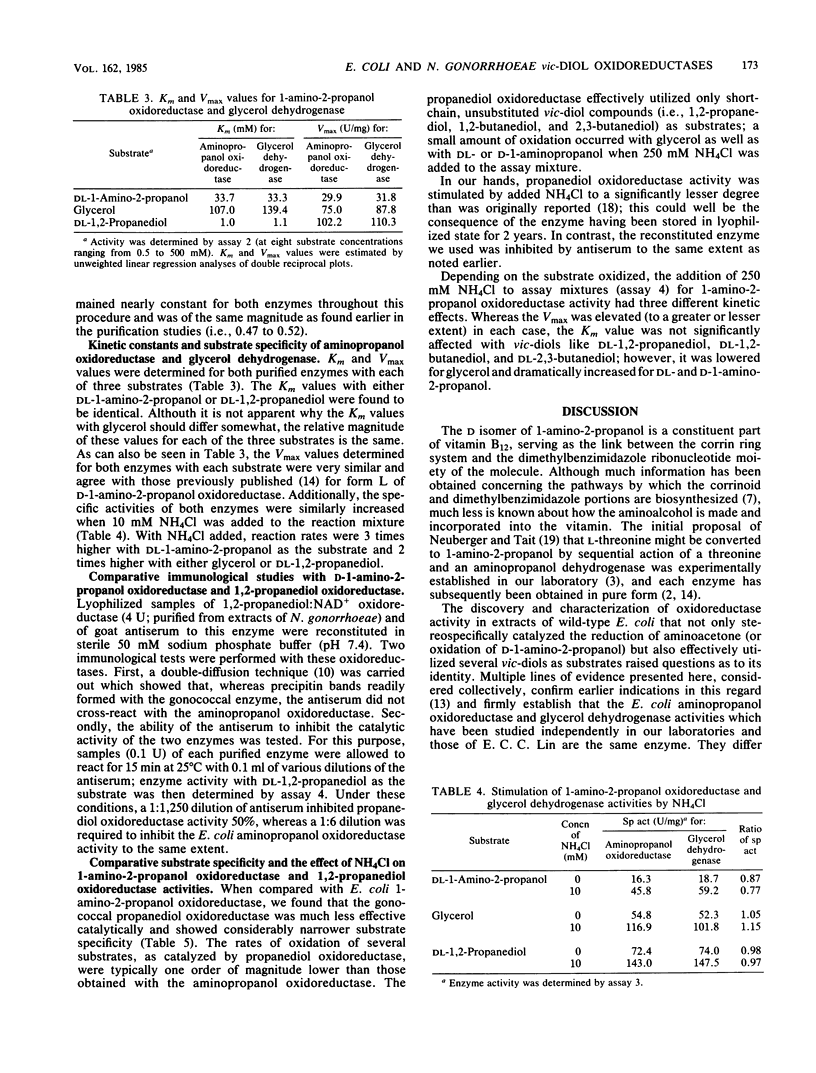
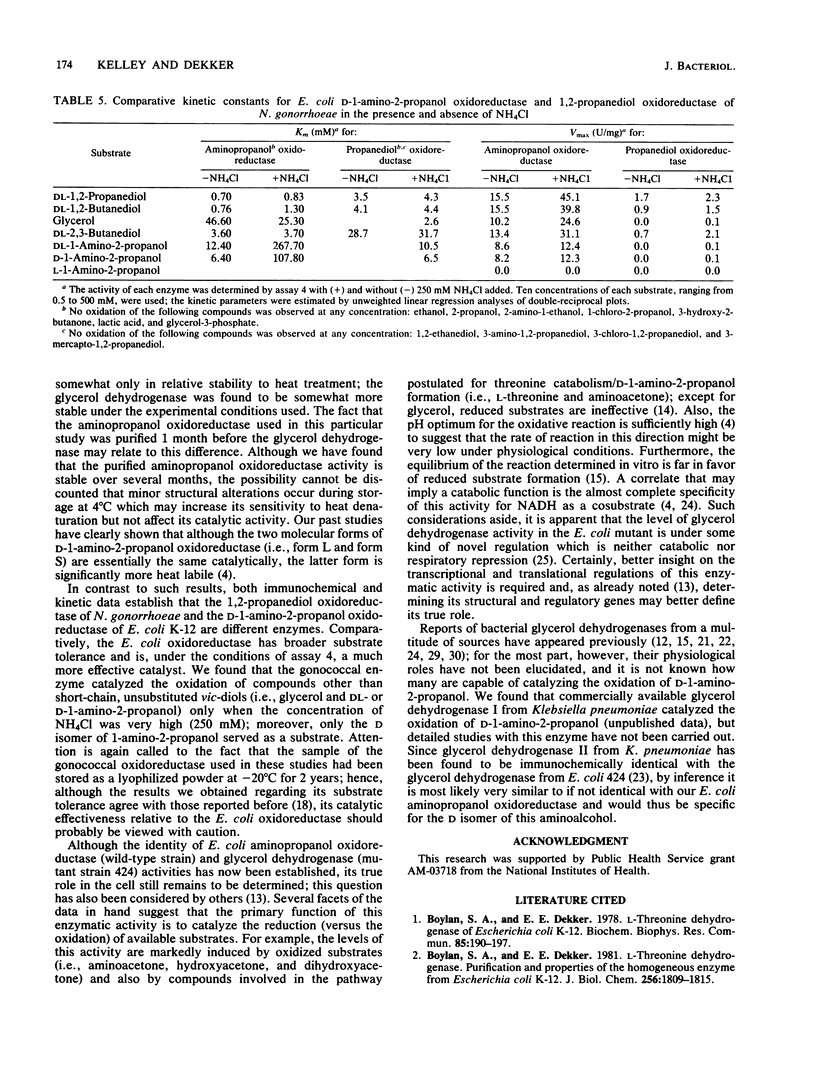
The properties of D-1-amino-2-propanol oxidoreductase from wild-type Escherichia coli have been compared with those of a glycerol dehydrogenase from mutant E. coli 424 and of a 1,2-propanediol oxidoreductase from Neisseria gonorrhoeae. Several independent lines of evidence indicate that the former two enzymes are identical. (i) Both enzymatic activities purified to virtual homogeneity in an identical manner, and the ratio of specific activities (glycerol/aminopropanol) remained constant at all stages. (ii) When electrophoresed, both purified enzymes showed a major as well as a minor band of protein coincident with activity, and these two bands from each enzyme had the same mobility. (iii) The subunit molecular weights and isoelectric points were identical for each enzyme, and (iv) kinetic constants (Km and Vmax values) determined with three different substrates were the same. The somewhat greater stability of the glycerol dehydrogenase to controlled heat denaturation at 74 degrees C was the only difference observed between these two enzymes. In contrast, D-1-amino-2-propanol oxidoreductase was found to be immunochemically and kinetically distinct from the 1,2-propanediol oxidoreductase from N. gonorrhoeae.
Full text
PDF
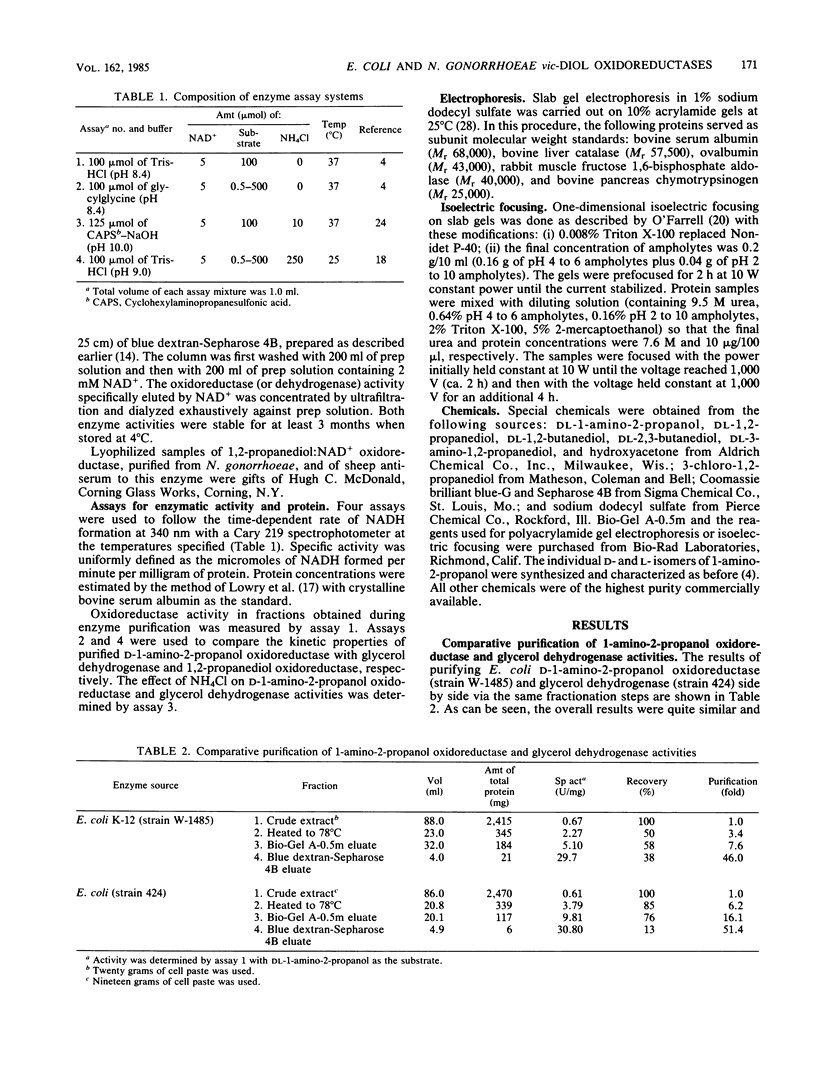
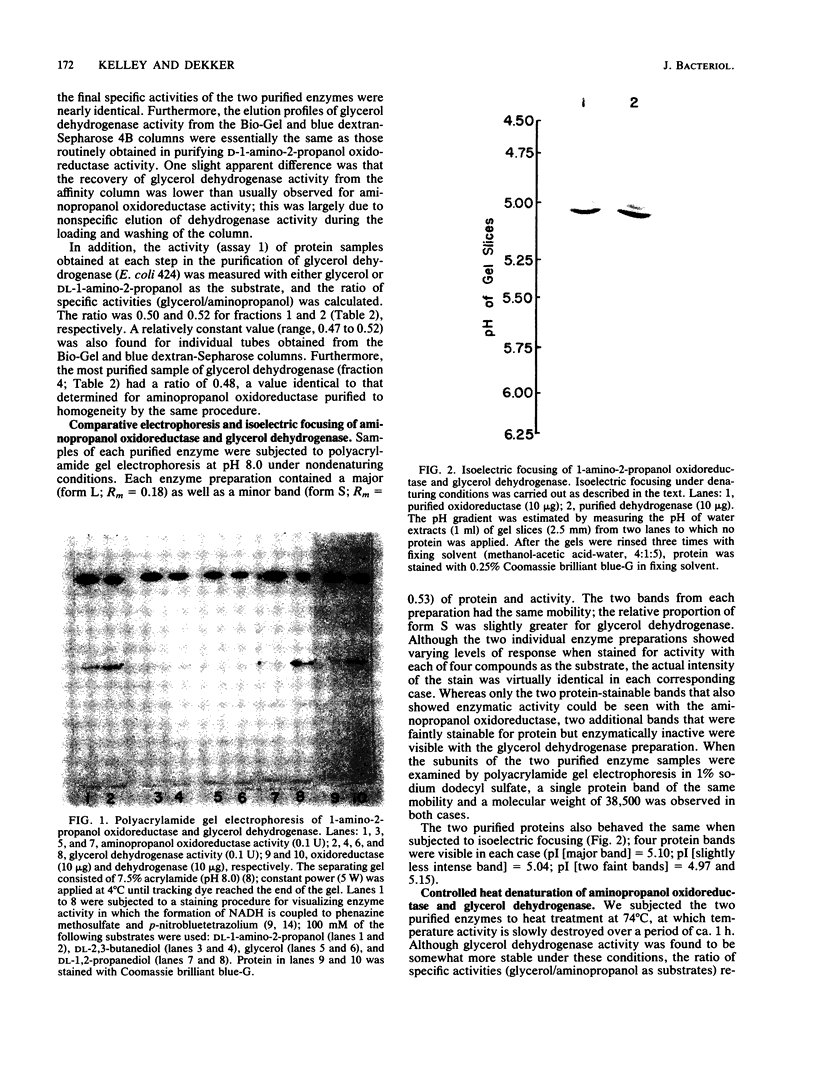

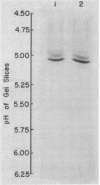
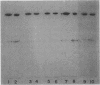
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boylan S. A., Dekker E. E. L-Threonine dehydrogenase of Escherichia coli K-12. Biochem Biophys Res Commun. 1978 Nov 14;85(1):190–197. doi: 10.1016/s0006-291x(78)80028-x. [DOI] [PubMed] [Google Scholar]
- Boylan S. A., Dekker E. E. L-threonine dehydrogenase. Purification and properties of the homogeneous enzyme from Escherichia coli K-12. J Biol Chem. 1981 Feb 25;256(4):1809–1815. [PubMed] [Google Scholar]
- Campbell R. L., Dekker E. E. Formation of D-1-amino-2-propanol from L-threonine by enzymes from Escherichia coli K-12. Biochem Biophys Res Commun. 1973 Jul 17;53(2):432–438. doi: 10.1016/0006-291x(73)90680-3. [DOI] [PubMed] [Google Scholar]
- Campbell R. L., Swain R. R., Dekker E. E. Purification, separation, and characterization of two molecular forms of D-1-amino-2-propanol:NAD+ oxidoreductase activity from extracts of Escherichia coli K-12. J Biol Chem. 1978 Oct 25;253(20):7282–7288. [PubMed] [Google Scholar]
- Dekker E. E., Swain R. R. Formation of Dg-1-amino-2-propanol by a highly purified enzyme from Escherichia coli. Biochim Biophys Acta. 1968 May;158(2):306–307. doi: 10.1016/0304-4165(68)90150-5. [DOI] [PubMed] [Google Scholar]
- FRASER D., JERREL E. A. The amino acid composition of T3 bacteriophage. J Biol Chem. 1953 Nov;205(1):291–295. [PubMed] [Google Scholar]
- Goldstein I. J., So L. L. Protein-carbonhydrate interaction. 3. Agar gel-diffusion studies on the interaction of Concanavalin A, a lectin isolated from jack bean, with polysaccharides. Arch Biochem Biophys. 1965 Aug;111(2):407–414. doi: 10.1016/0003-9861(65)90203-1. [DOI] [PubMed] [Google Scholar]
- Hou C. T., Patel R. N., Laskin A. I., Barnabe N., Barist I. Purification and properties of a NAD-linked 1,2-propanediol dehydrogenase from propane-grown Pseudomonas fluorescens NRRL B-1244. Arch Biochem Biophys. 1983 May;223(1):297–308. doi: 10.1016/0003-9861(83)90595-7. [DOI] [PubMed] [Google Scholar]
- Jin R. Z., Tang J. C., Lin E. C. Experimental evolution of a novel pathway for glycerol dissimilation in Escherichia coli. J Mol Evol. 1983;19(6):429–436. doi: 10.1007/BF02102318. [DOI] [PubMed] [Google Scholar]
- Kelley J. J., Dekker E. E. D-1-amino-2-propanol:NAD+ oxidoreductase. Purification and general properties of the large molecular form of the enzyme from Escherichia coli K12. J Biol Chem. 1984 Feb 25;259(4):2124–2129. [PubMed] [Google Scholar]
- LIN E. C., MAGASANIK B. The activation of glycerol dehydrogenase from Aerobacter aerogenes by monovalent cations. J Biol Chem. 1960 Jun;235:1820–1823. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lowe D. A., Turner J. M. Microbial metabolism of amino ketones: D-1-aminopropan-2-ol and aminoacetone metabolism in Escherichia coli. J Gen Microbiol. 1970 Sep;63(1):49–61. doi: 10.1099/00221287-63-1-49. [DOI] [PubMed] [Google Scholar]
- McDonald H. C., Takeguchi M. M., Detar C. C., Simon P. A., Livsey K. A., Odstrchel G., Kaplan N. O., Weetall H. H. The isolation and characterization of a 1,2-propanediol oxidoreductase from Neisseria gonorrhoeae. J Gen Microbiol. 1980 Aug;119(2):451–458. doi: 10.1099/00221287-119-2-451. [DOI] [PubMed] [Google Scholar]
- NEUBERGER A., TAIT G. H. The enzymic conversion of threonine to aminoacetone. Biochim Biophys Acta. 1960 Jun 17;41:164–165. doi: 10.1016/0006-3002(60)90388-7. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Sasaki I., Itoh N., Goto H., Yamamoto R., Tanaka H., Yamashita K., Yamashita J., Horio T. Specific affinity of glycerol dehydrogenase from Geotrichum candidium for 10-carboxydecyl-sepharose: its application to chromatography for purification of the enzyme. J Biochem. 1982 Jan;91(1):211–217. doi: 10.1093/oxfordjournals.jbchem.a133678. [DOI] [PubMed] [Google Scholar]
- Scharschmidt M., Pfleiderer G., Metz H., Brümmer W. Isolierung und Charakterisierung von Glycerin-Dehydrogenase aus Bacillus megaterium. Hoppe Seylers Z Physiol Chem. 1983 Jul;364(7):911–921. [PubMed] [Google Scholar]
- TAYLOR M. B., JUNI E. Stereoisomeric specificities of 2,3-butanediol dehydrogenases. Biochim Biophys Acta. 1960 Apr 22;39:448–457. doi: 10.1016/0006-3002(60)90197-9. [DOI] [PubMed] [Google Scholar]
- Tang C. T., Ruch F. E., Jr, Lin C. C. Purification and properties of a nicotinamide adenine dinucleotide-linked dehydrogenase that serves an Escherichia coli mutant for glycerol catabolism. J Bacteriol. 1979 Oct;140(1):182–187. doi: 10.1128/jb.140.1.182-187.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J. C., Forage R. G., Lin E. C. Immunochemical properties of NAD+-linked glycerol dehydrogenases from Escherichia coli and Klebsiella pneumoniae. J Bacteriol. 1982 Dec;152(3):1169–1174. doi: 10.1128/jb.152.3.1169-1174.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J. C., St Martin E. J., Lin E. C. Derepression of an NAD-linked dehydrogenase that serves an Escherichia coli mutant for growth on glycerol. J Bacteriol. 1982 Dec;152(3):1001–1007. doi: 10.1128/jb.152.3.1001-1007.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuner J. M. Microbial metabolism of amino ketones. Aminoacetone formation from 1-aminopropan-2-ol by a dehydrgenase in Escerichia coli. Biochem J. 1966 May;99(2):427–433. doi: 10.1042/bj0990427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Pringle J. R., Osborn M. Measurement of molecular weights by electrophoresis on SDS-acrylamide gel. Methods Enzymol. 1972;26:3–27. doi: 10.1016/s0076-6879(72)26003-7. [DOI] [PubMed] [Google Scholar]