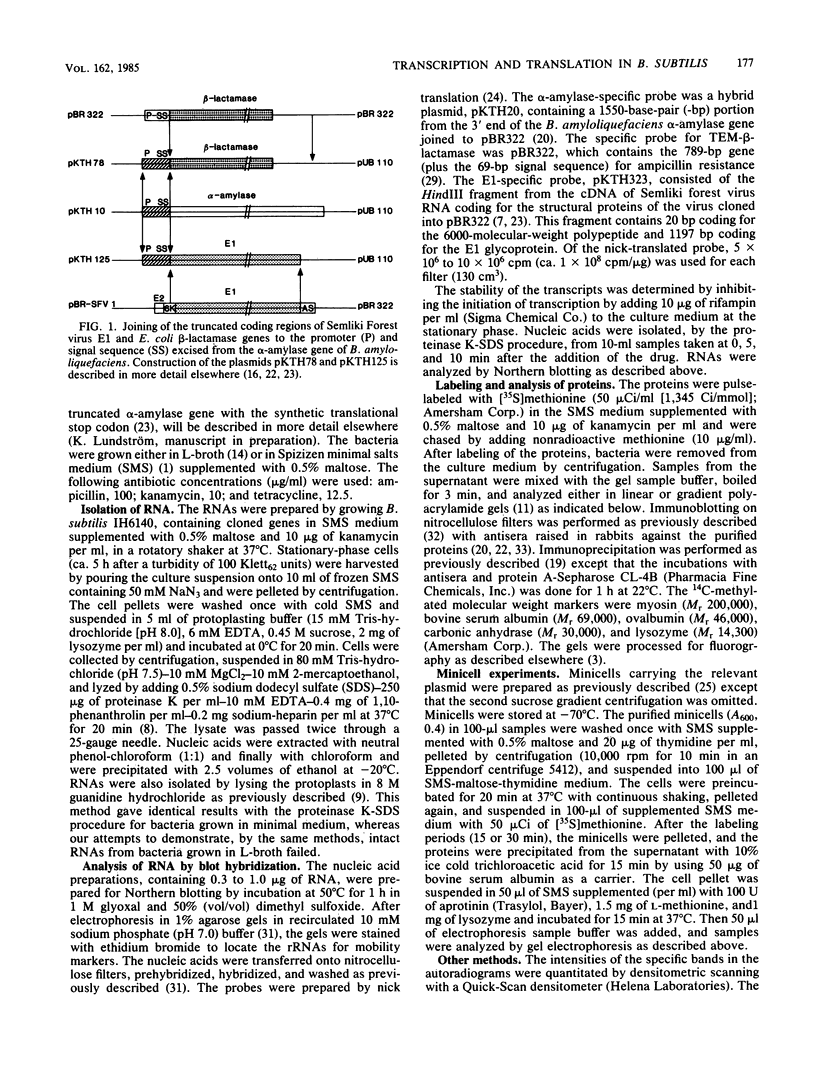
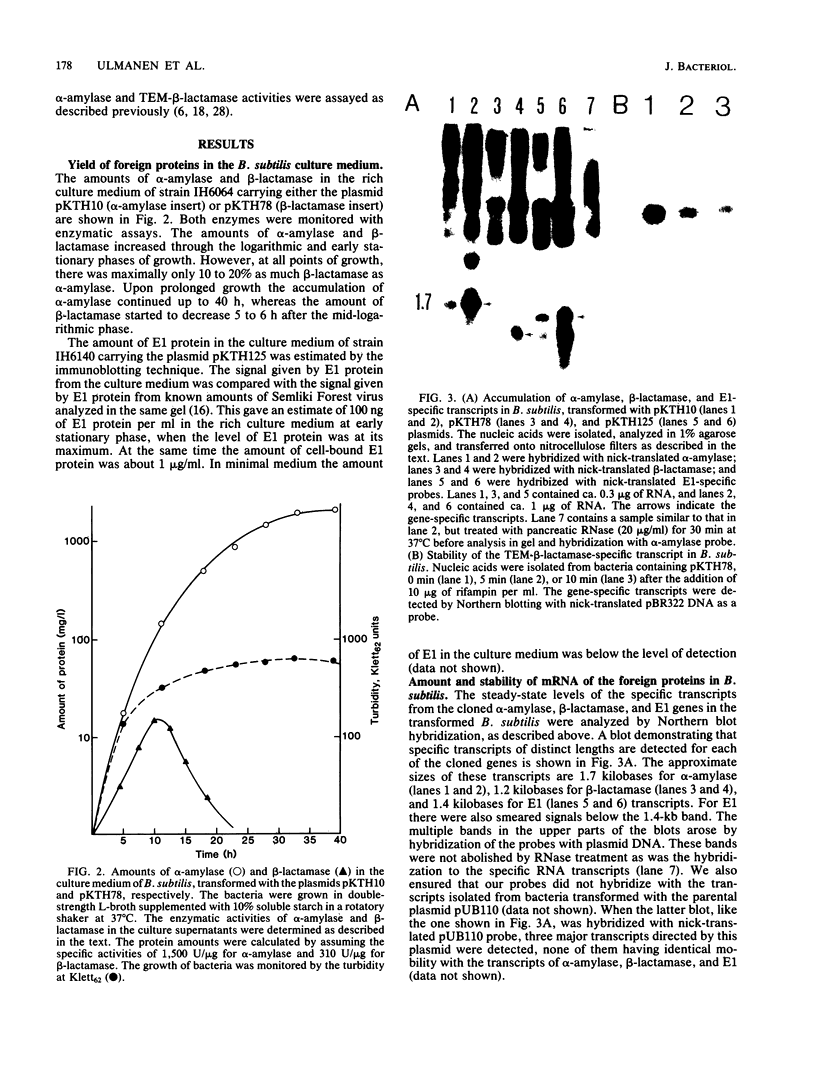
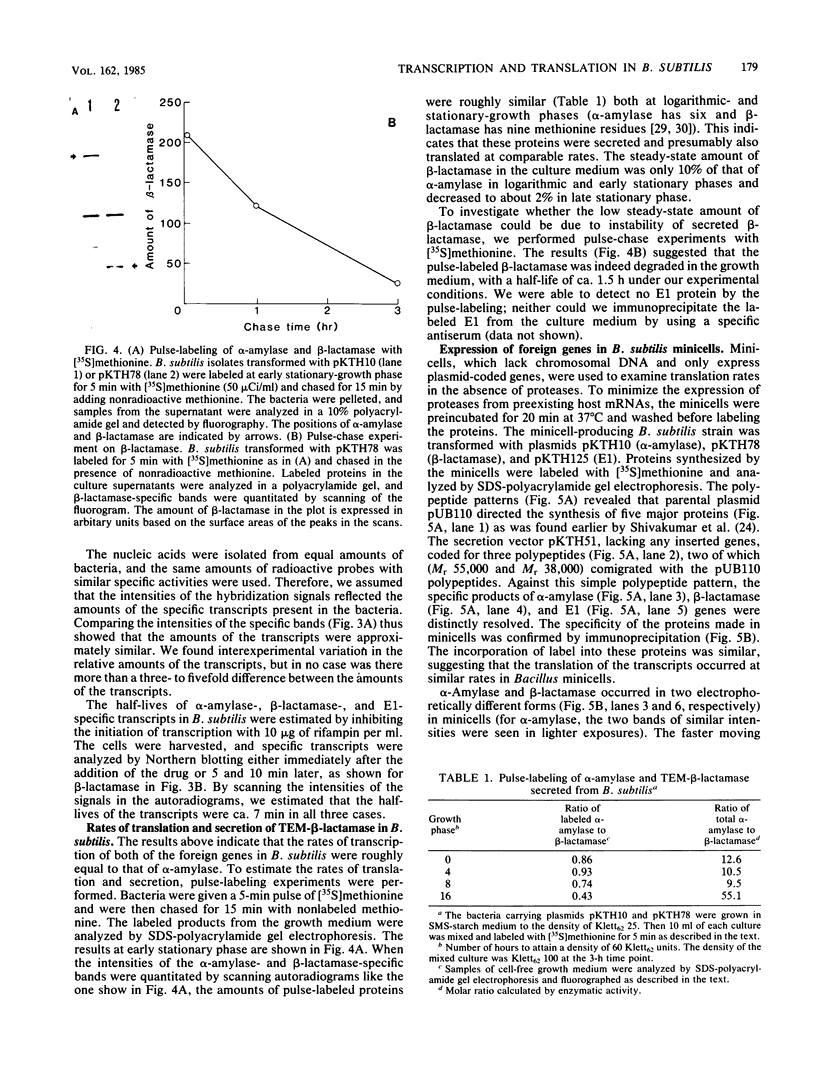
Abstract
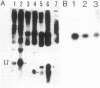
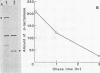
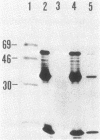
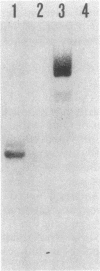
Expression levels of Bacillus amyloliquefaciens alpha-amylase, Escherichia coli TEM-beta-lactamase, and Semliki Forest virus glycoprotein E1 genes were compared in Bacillus subtilis. All three model genes were expressed by using a secretion vector, constructed by joining the B. amyloliquefaciens alpha-amylase promoter and signal sequence with plasmid pUB110 (I. Palva, M. Sarvas, P. Lehtovaara, M. Sibakov, and L.Kääriäinen, Proc. Natl. Acad. Sci. U.S.A. 79:5582-5586, 1982). When transformed B. subtilis cells were grown to early stationary phase, the amount of beta-lactamase in the culture medium was ca. 10% and that of E1 was ca. 0.01% of the amount of alpha-amylase. The amounts of specific, full-length transcripts of the cloned genes were estimated by Northern blot hybridization to be roughly equal. The half-lives of these transcripts in B. subtilis were also similar. Pulse-chase experiments with [35S]methionine showed that alpha-amylase and beta-lactamase were translated and secreted at comparable rates but that beta-lactamase was degraded during the chase periods. In transformed minicells from B. subtilis, the products of alpha-amylase, beta-lactamase, and E1 genes accumulated at similar rates. We conclude that the expression of the three genes cloned in the secretion vector was similar at the levels of transcription and translation in B. subtilis. In the case of beta-lactamase, the low-yield could be explained by proteolytic degradation of the secreted product by B. subtilis exoproteases, whereas with E1 we could not determine whether the low yield was due to proteolytic degradation, inefficient secretion, or both.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anagnostopoulos C., Spizizen J. REQUIREMENTS FOR TRANSFORMATION IN BACILLUS SUBTILIS. J Bacteriol. 1961 May;81(5):741–746. doi: 10.1128/jb.81.5.741-746.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Brown S., Coleman G. Stability of rapidly labelled messenger ribonucleic acid in Bacillus amyloliquefaciens during the phases of minimum and maximum extracellular enzyme formation. J Mol Biol. 1975 Aug 5;96(2):335–344. doi: 10.1016/0022-2836(75)90352-6. [DOI] [PubMed] [Google Scholar]
- Garoff H., Frischauf A. M., Simons K., Lehrach H., Delius H. Nucleotide sequence of cdna coding for Semliki Forest virus membrane glycoproteins. Nature. 1980 Nov 20;288(5788):236–241. doi: 10.1038/288236a0. [DOI] [PubMed] [Google Scholar]
- Gopalakrishna Y., Langley D., Sarkar N. Detection of high levels of polyadenylate-containing RNA in bacteria by the use of a single-step RNA isolation procedure. Nucleic Acids Res. 1981 Jul 24;9(14):3545–3554. doi: 10.1093/nar/9.14.3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grisolia V., Carlomagno M. S., Bruni C. B. Cloning and expression of the distal portion of the histidine operon of Escherichia coli K-12. J Bacteriol. 1982 Aug;151(2):692–700. doi: 10.1128/jb.151.2.692-700.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gryczan T. J., Contente S., Dubnau D. Characterization of Staphylococcus aureus plasmids introduced by transformation into Bacillus subtilis. J Bacteriol. 1978 Apr;134(1):318–329. doi: 10.1128/jb.134.1.318-329.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lai J. S., Sarvas M., Brammar W. J., Neugebauer K., Wu H. C. Bacillus licheniformis penicillinase synthesized in Escherichia coli contains covalently linked fatty acid and glyceride. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3506–3510. doi: 10.1073/pnas.78.6.3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehtovaara P., Ulmanen I., Palva I. In vivo transcription initiation and termination sites of an alpha-amylase gene from Bacillus amyloliquefaciens cloned in Bacillus subtilis. Gene. 1984 Oct;30(1-3):11–16. doi: 10.1016/0378-1119(84)90099-4. [DOI] [PubMed] [Google Scholar]
- Lundström K., Palva I., Käriäinen L., Garoff H., Sarvas M., Pettersson R. F. Secretion of Semliki Forest virus membrane glycoprotein E1 from Bacillus subtilis. Virus Res. 1985 Feb;2(1):69–83. doi: 10.1016/0168-1702(85)90061-9. [DOI] [PubMed] [Google Scholar]
- Nielsen J. B., Caulfield M. P., Lampen J. O. Lipoprotein nature of Bacillus licheniformis membrane penicillinase. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3511–3515. doi: 10.1073/pnas.78.6.3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Callaghan C. H., Morris A., Kirby S. M., Shingler A. H. Novel method for detection of beta-lactamases by using a chromogenic cephalosporin substrate. Antimicrob Agents Chemother. 1972 Apr;1(4):283–288. doi: 10.1128/aac.1.4.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oker-Blom C., Kalkkinen N., Käriäinen L., Pettersson R. F. Rubella virus contains one capsid protein and three envelope glycoproteins, E1, E2a, and E2b. J Virol. 1983 Jun;46(3):964–973. doi: 10.1128/jvi.46.3.964-973.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palva I., Lehtovaara P., Käriäinen L., Sibakov M., Cantell K., Schein C. H., Kashiwagi K., Weissmann C. Secretion of interferon by Bacillus subtilis. Gene. 1983 May-Jun;22(2-3):229–235. doi: 10.1016/0378-1119(83)90107-5. [DOI] [PubMed] [Google Scholar]
- Palva I. Molecular cloning of alpha-amylase gene from Bacillus amyloliquefaciens and its expression in B. subtilis. Gene. 1982 Jul-Aug;19(1):81–87. doi: 10.1016/0378-1119(82)90191-3. [DOI] [PubMed] [Google Scholar]
- Palva I., Sarvas M., Lehtovaara P., Sibakov M., Käriäinen L. Secretion of Escherichia coli beta-lactamase from Bacillus subtilis by the aid of alpha-amylase signal sequence. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5582–5586. doi: 10.1073/pnas.79.18.5582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersson R. F., Lundström K., Chattopadhyaya J. B., Josephson S., Philipson L., Käriäinen L., Palva I. Chemical synthesis and molecular cloning of a STOP oligonucleotide encoding an UGA translation terminator in all three reading frames. Gene. 1983 Sep;24(1):15–27. doi: 10.1016/0378-1119(83)90127-0. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- STEIN H. [Cholecystography in young infants]. Arch Kinderheilkd. 1961 Nov;165:27–33. [PubMed] [Google Scholar]
- Shivakumar A. G., Hahn J., Dubnau D. Studies on the synthesis of plasmid-coded proteins and their control in Bacillus subtilis minicells. Plasmid. 1979 Apr;2(2):279–289. doi: 10.1016/0147-619x(79)90046-5. [DOI] [PubMed] [Google Scholar]
- Smith W. P., Tai P. C., Davis B. D. Bacillus licheniformis penicillinase: cleavages and attachment of lipid during cotranslational secretion. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3501–3505. doi: 10.1073/pnas.78.6.3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcliffe J. G. Nucleotide sequence of the ampicillin resistance gene of Escherichia coli plasmid pBR322. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3737–3741. doi: 10.1073/pnas.75.8.3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takkinen K., Pettersson R. F., Kalkkinen N., Palva I., Söderlund H., Käriäinen L. Amino acid sequence of alpha-amylase from Bacillus amyloliquefaciens deduced from the nucleotide sequence of the cloned gene. J Biol Chem. 1983 Jan 25;258(2):1007–1013. [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vänänen P. The use of red cells with fused Semliki Forest virus envelope proteins in antibody determinations by hemolysis in gel. J Virol Methods. 1982 Mar;4(2):117–126. doi: 10.1016/0166-0934(82)90081-7. [DOI] [PMC free article] [PubMed] [Google Scholar]