Abstract
Background and purpose:
Muraglitazar, a dual PPARα/γ agonist, caused a robust increase in body weight in db/db mice. The purpose of the study was to see if this increase in weight was due to oedema and/or adipogenesis.
Experimental approach:
The affinity of muraglitazar at PPARα/γ receptors was characterized using transactivation assays. Pre-adipocyte differentiation, expression of genes for adipogenesis (aP2), fatty acid oxidation (ACO) and sodium reabsorption (ENaCγ and Na+, K+-ATPase); haemodilution parameters and serum electrolytes were measured to delineate the role of muraglitazar in causing weight gain vis a vis rosiglitazone.
Key Results:
Treatment with muraglitazar (10 mg kg−1) for 14 days significantly reduced plasma glucose and triglycerides. Reduction in plasma glucose was significantly greater than after similar treatment with rosiglitazone (10 mg kg−1). A marked increase in weight was also observed with muraglitazar that was significantly greater than with rosiglitazone. Muraglitazar increased aP2 mRNA and caused adipocyte differentiation in 3T3-L1 cells similar to rosiglitazone. It also caused a marked increase in ACO mRNA in the liver of the treated mice. Expression of mRNA for ENaCγ and Na+, K+-ATPase in kidneys was up-regulated after either treatment. Increased serum electrolytes and decreased RBC count, haemoglobin and haematocrit were observed with both muraglitazar and rosiglitazone.
Conclusions and implications:
Although muraglitazar has a better glucose lowering profile, it also has a greater potential for weight gain than rosiglitazone. In conclusion, muraglitazar causes both robust adipogenesis and oedema in a 14-day treatment of db/db mice as observed in humans.
Keywords: PPAR dual agonist, antidiabetic, hypolipidaemic, weight gain, adipogenesis, oedema, db/db mice
Introduction
Diabetes mellitus is a metabolic disease characterized by hyperglycaemia resulting from defects in insulin secretion, insulin action or both. Over the last decade, hyperglycaemia has been shown to be only one component of a series of anomalies afflicting patients with type II diabetes mellitus (T2DM). Concurrent maladies including insulin resistance, obesity, hypertension and dyslipidaemia define diabetes as a metabolic syndrome.
The peroxisome proliferator-activated receptor (PPAR) family stands out as a well-validated target in search for new and improved treatment options for diabetic patients. PPARs are members of the nuclear hormone superfamily of ligand-activated transcription factors and include three distinct PPAR subtypes that are products of different genes and are commonly designated as PPARα, γ and δ (Desvergne and Wahli, 1999). The knowledge that PPARα and PPARγ are the molecular targets for the actions of fibrates and thiazolidinediones, respectively, has led to a renaissance in nuclear receptor research to develop drugs for diabetes and associated dyslipidaemia. Specific PPARγ agonists such as rosiglitazone and pioglitazone are effective compounds but have an unattractive side effect profile that includes weight gain, oedema, neutropenia and haemodilution (Mudaliar et al., 2003). Clinical experience shows that thiazolidinediones induce oedema in 10–15% of patients, sometimes predisposing them to reversible congestive heart failure, requiring discontinuation of therapy (Nesto et al., 2003). Activation of PPARγ, expressed in adipose tissue, lower intestine and immune system, regulates glucose and lipid homeostasis and triggers insulin sensitization in addition to robust pre-adipocyte differentiation leading to weight gain (Desvergne and Wahli, 1999). PPARα, predominantly expressed in the liver, specifically regulates the transcription of genes involved in hepatic fatty acid uptake and oxidation, mainly acyl CoA synthetase, acyl CoA oxidase (ACO) and carnitine palmitoyl-transferase-1 (CPT-1) (Desvergne and Wahli, 1999) and thus, may have a positive effect on body weight through increased catabolism of fat in addition to its lipid-lowering effects (Staels and Fruchart, 2005). PPARα−/− mice show a phenotype with monogenic, late-onset, spontaneous obesity with a stable caloric intake, implicating the PPARα in resistance to obesity (Costet et al., 1998).
Given the importance of simultaneously controlling glucose homeostasis, insulin sensitization and lipid metabolism in T2DM, activation of both PPAR α and γ subtypes is beneficial (Staels and Fruchart, 2005). This had led to the development of a number of dual α/γ agonists such as muraglitazar, tesaglitazar and several others, which were shelved subsequently (Hegarty et al., 2004; Barlocco, 2005; Oakes et al., 2005). This is because although dual PPARα/γ activators seem to have a favourable pharmacodynamic–pharmacokinetic profile, several serious concerns have arisen in preclinical and clinical studies. Ragaglitazar has been discontinued owing to bladder tumours in rodents and KRP-297 followed a similar fate (Egerod et al., 2005). Thus, application of dual PPAR agonists requires further scrutiny. Muraglitazar, a dual PPAR agonist, with its high efficacy and no tumorigenic potential could not be developed further owing to safety concerns of oedema and induction of congestive cardiac failure in susceptible patients (FDA advisory committee transcript on muraglitazar, 2005; FDA statistical review, 2005; BMY-News, 2006) and tesaglitazar owing to anticipated nephrotoxicity (Astra-Zeneca press release, 2006).
PPARγ activators are known to cause both oedematous weight gain and adipogenesis (Mudaliar et al., 2003; Cock et al., 2004). Recent reports have implicated the activation of PPAR γ receptors in oedema owing to their presence in the distal nephron epithelium, in the renal medullary ducts (Guan et al., 2005; Zhang et al., 2005). Oedema is postulated to be caused by stimulation of the epithelial sodium channel (ENaC) and Na+, K+-ATPase system in the collecting ducts as observed in rodents (Guan et al., 2005; Zhang et al., 2005). Specific deletion of PPARγ in the collecting ducts has been observed to block thiazolidinedione-induced fluid retention (Guan et al., 2005; Zhang et al., 2005).
In earlier studies with diet-induced obese C57BL/6 mice and db/db mice, muraglitazar showed either no weight gain (Hariharan et al., 2002) or a nonsignificant trend towards a weight gain (Harrity et al., 2006). As we observed a robust increase in body weight with muraglitazar treatment in db/db mice, our major aim was to examine if this increase in weight was oedematous and/or due to increased adipogenesis.
Methods
In vitro studies
PPAR transactivation assays
HEK-293 cells (ATCC, Manassas, VA, USA) were maintained in Dulbecco modified Eagle's medium (DMEM) containing 10% charcoal stripped serum. The cells were plated in six-well plates and transiently transfected with vectors containing full-length hPPARγ, hPPARα or hPPARδ and Rat-PPRE-pTAL-Luc plasmids (Chakrabarti et al., 2004). pAdvantage vector was used as a luciferase enzyme translation enhancer and pRL-cytomegalovirus vector as a transfection efficiency control. Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA, USA) was used for the transfections. The plasmid constructs were prepared in-house. After 24 h, the cells were harvested and plated into 96-well plates at a density of 3 × 104 cells per well and allowed to attach for 1 h. The cells were then incubated with test compounds for 24 h. Dimethylsulphoxide (DMSO) (0.1%) was used as a vehicle control. After 24 h, cells were lysed and assayed for luciferase activity using the Steady-Glo luciferase system according to the manufacturer's instructions (Promega, Madison, WI, USA). The activity of the compounds as PPAR agonists was related to the transcription of the reporter (Luciferase) gene. Plates were read in a Wallac scintillation counter.
Mouse pre-adipocyte differentiation assay
Compounds were assayed for their ability to induce PPARγ-mediated differentiation of mouse 3T3L-1 pre-adipocytes (Shibata et al., 1999). 3T3-L1 pre-adipocytes (ATCC) were seeded at 5 × 104 cells ml−1 per well in a 24-well collagen-coated plate. Two days post-confluent cells were induced for 48 h with a mixture of 1 μM dexamethasone, 10 μg ml−1 insulin and 0.5 mM isobutylmethylxanthine (IBMX) in addition to different concentrations of the test compound. After 48 h, the media was replaced with DMEM supplemented with 10% fetal bovine serum (FBS) and 10 μg ml−1 insulin along with different concentrations of test compound. This treatment continued for 3 days, after which the cells were re-fed every 48 h with DMEM supplemented with 10% FBS. At the end of the incubation, wells were washed with phosphate-buffered saline (PBS) and lysed by 100 μl 1% (v/v) NP40 in PBS. Cell lysates were used to quantitate triglycerides and protein using GPO reagent kit (Pointe Scientific Inc., Canton, MI, USA) and BCA method (Sigma, St Louis, MO, USA) respectively. Triglyceride content was expressed as mM. The activity of the reference PPARγ agonist, rosiglitazone was defined to be 100% in this assay.
Gene expression studies
In vitro gene expression studies
To delineate the role of muraglitazar in adipocyte differentiation and subsequent adipogenesis, expression of aP2 was measured in 3T3-L1 cells. For this, 3T3-L1 cells were seeded (5 × 104 cells/well) in 24-well tissue culture plates and grown to confluence in DMEM with 100 U ml−1 penicillin, 100 μg ml−1 streptomycin and 1 mM sodium pyruvate, supplemented with 10% fetal calf serum. Cells were grown at 37°C in 5% CO2 in a humidified chamber and re-fed fresh media every 2 days. The PPAR ligands were dissolved in DMSO and diluted with DMEM that contained 10% fetal bovine serum, 1 μM dexamethasone, and 150 nM insulin. The final media concentration of DMSO was 0.1% in the dilutions. Cells treated with 0.1% DMSO in media served as vehicle controls. The cells were incubated with PPAR ligands for 5 days and were re-fed with fresh dilutions on the third day of the treatment period, except where noted. Each compound concentration was tested in duplicate or triplicate wells.
In vivo gene expression studies
Administration of PPARα agonists increase the expression of peroxisomal (ACO) and mitochondrial β-oxidation enzymes (carnitine acetyl-transferase, CPT-1) which facilitate fatty acid uptake and catabolism. In this study, db/db mice were treated either with rosiglitazone or muraglitazar at 10 mg kg−1 for 14 days and liver tissue was harvested on day 14 and snap frozen for analysing the ACO expression. To explore the role of muraglitazar in renal electrolyte homeostasis, the mRNA expression of selected genes in the kidney were determined using real-time-polymerase chain reaction (RT-PCR). To measure expression of the mRNA for ENaCγ and Na+, K+-ATPase-α, db/db mice were treated with rosiglitazone (10 mg kg−1) or muraglitazar at 10 mg kg−1 for 14 days and whole kidneys were harvested and snap frozen.
Total RNA extraction and RT-PCR
Total RNA from 3T3–L1 cells (for ap2 mRNA expression), mice liver (ACO mRNA expression) and kidney (for ENaCγ and Na+-K+-ATPase-α mRNA expression) were isolated from frozen kidney tissues of treated mice using Trizol reagent (Invitrogen, Carlsbad, CA, USA) and purified using RNeasy Mini kits (Qiagen, Valencia, CA, USA). Total RNA (1–2 μg) was reverse-transcribed in a 50 μl reaction using Taqman reverse transcription reagents (ABI). RT-PCR multiplex reaction was performed using gene specific Taqman primers and probes and analysed using ABI 7900 HT Sequence Detection System. A quantitative mRNA expression level of aP2, ACO, ENaCγ subunit (ENaCγ) and Na+-K+-ATPase-α was determined. Data were normalized to the expression of 18 s endogenous control mRNA and presented as fold of vehicle control. mRNA expression data were analysed using the relative quantitation (RQ) study SDS software (Applied Biosystems, CA, USA).
In vivo studies
C57BL/KsJ-db/db mice (10–12 weeks old, either sex, from Jackson Laboratory, Bar Harbor, ME, USA) were from the animal facility of Ranbaxy Laboratories Ltd, Gurgaon, India. Animals were maintained on a 12-h day/night schedule with ad libitum access to standard mouse diet (Harlan Teklad, Oxon, UK) and water. All animal experiments were conducted according to the Guidelines of Experimental Animal Care issued by the Committee for Purpose of Control & Supervision of Experiments on Animals (CPCSEA). At the end of the acclimatization period, the mice were bled under light ether anaesthesia by a retro-orbital puncture and plasma samples were analysed for glucose levels. Animals were grouped on the basis of random plasma glucose values so that the average glucose level of one group was not significantly different from that of the other.
Drug treatment and blood sampling
Drugs were administered orally by gavage, once a day at a volume of 10 ml kg−1 of body weight for 14 days starting from day 1. Blood sampling was performed retro-orbitally under the fed condition on the 14th day 1 h after dosing and plasma was separated to measure the plasma glucose and triglyceride levels. The animals were grouped as follows: Group 1: control; Group 2: rosiglitazone (10 mg kg−1); Group 3: muraglitazar (10 mg kg−1); Group 4: fenofibrate (100 mg kg−1). Another group of animals were treated with increasing doses (0.3, 1, 3 and 10 mg kg−1) of muraglitazar for 14 days and glucose and triglyceride levels were measured.
Plasma levels of glucose and triglycerides in db/db mice
Plasma samples were analysed for biochemical parameters using commercial diagnostic kits for triglyceride and glucose procured from Ranbaxy Diagnostics. Quality control samples (for determination of inter and intra-assay variability) were co-analysed.
Liver and body weight changes after drug treatment in db/db mice
The animals were weighed weekly to monitor any change in body weight. Increase in the body weight after 14 days treatment with various treatments and muraglitazar (at 0.3, 1, 3 and 10 mg kg−1) was determined by subtracting the weight of each mouse on day 1 from its weight on day 14. On day 15, the liver was weighed and the change in weight was expressed as a percentage change with respect to the body weight.
Oedema parameters in treated db/db mice
To study the role of muraglitazar on renal electrolyte homeostasis, plasma electrolyte concentrations were measured in plasma. The blood collected was also analysed for haemodilution markers such as RBC count, haemoglobin and haematocrit.
Statistical analysis
Nonlinear regression analysis was used to determine the EC50 of the agonists. Efficacy was calculated as % maximum of a standard response. Rosiglitazone was used as standard for hPPARγ. Data are presented as mean±s.e.m. Calculations of the % reduction in plasma triglyceride and fed glucose levels were performed using MS Excel and Graph Pad Prism software (version 4.02). Difference between groups was analysed by performing one-way analysis of variance followed by Dunnett's or Newman Keul's multiple comparison test for various parameters of vehicle- and drug-treated groups.
Chemicals
Muraglitazar was synthesized in the Department of Medicinal Chemistry, New Drug Discovery Research, Ranbaxy Laboratories Ltd. Rosiglitazone was procured from Sun Pharmaceuticals Ltd (Mumbai, India) and fenofibrate from Sigma (St Louis, MO, USA). Muraglitazar and rosiglitazone were freshly suspended in 0.25% carboxy methylcellulose just before use. All other chemicals used in the study were of analytical grade.
Results
Transactivation assays
Muraglitazar, synthesized in our chemical division, showed a significant activation of both hPPARα and hPPARγ in an in vitro transactivation assay. Potent dose-dependent agonistic activity of both PPARα and PPARγ was demonstrated in transiently transfected HEK-293 cells with EC50 values of 0.28 and 0.16 μM, respectively (Table 1). The potency of muraglitazar as prepared in our lab was comparable to the reported values of muraglitazar (Barlocco, 2005) with EC50 values of 0.24 and 0.12 μM for PPARα and PPARγ, respectively. No significant PPARδ activity was observed at >30 μM (data not shown). Rosiglitazone was used as a primary standard for PPARγ activity. Muraglitazar shows functional activity comparable to that of rosiglitazone at PPARγ; rosiglitazone has negligible potency at PPARα.
Table 1.
In vitro transactivation activity profile of rosiglitazone and muraglitazar
| Compound |
Transactivation Ec50 (μM) |
||
|---|---|---|---|
| hPPARγ | hPPARα | hPPARδ | |
| Rosiglitazone (BRL 49653) | 0.056±0.017 | >30 | >30 |
| Muraglitazar (BMS 298585) | 0.16±0.04 | 0.28±0.03 | >30 |
EC50 values for PPARα, γ or δ agonist activity were calculated as the concentration of the test ligand (μM) required for the half-maximal fold induction of luciferase activity. Data shown are means±s.e.m. from three experiments for each treatment.
Differentiation of mouse 3T3L-1 pre-adipocytes
In a pre-adipocyte differentiation assay, which measures the extent of predominantly PPARγ-mediated differentiation of pre-adipocytes into triglyceride loaded adipocytes, muraglitazar-induced dose-dependent differentiation at potency similar to rosiglitazone. The EC50 values for rosiglitazone and muraglitazar in adipogenesis assays were 94±58.1 and 131±46.8 nM, respectively, which were not significantly different from each other and showed potent activation of PPARγ.
Gene expression studies
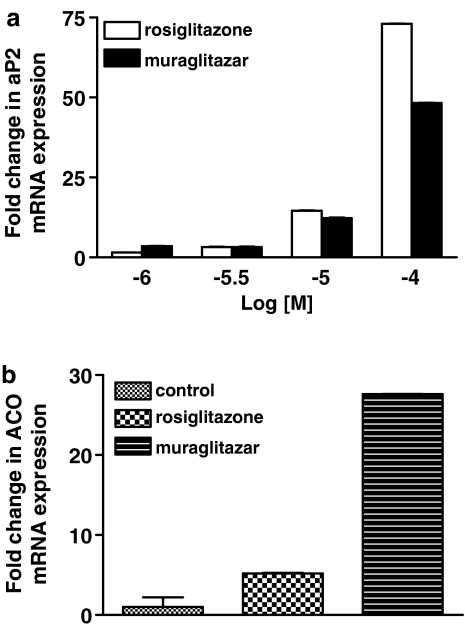
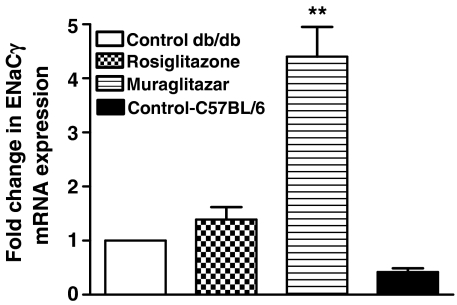
aP2 mRNA expression data were analysed using the relative quantitation (RQ) study SDS software (Applied Biosystems). At 100 μM concentration, aP2 mRNA expression by rosiglitazone was higher than muraglitazar (Figure 1a). However, at lower concentrations, both had similar levels of expression. These findings are suggestive of a similar mode and extent of lipid sequestration into fat cells by both the drugs and support the observed weight gain in in vivo experiments. Induction of ACO mRNA was observed with muraglitazar suggesting its role in fatty acid uptake and mitochondrial oxidation, whereas rosiglitazone was devoid of this effect. ACO mRNA expression was 4.3-fold higher in livers of muraglitazar-treated db/db mice as compared to rosiglitazone treatment at the same dose (Figure 1b). To determine if muraglitazar induced any fluid retention/oedema, the mRNA expression levels of ENaCγ and Na+, K+-ATPase-α were studied by RT-PCR in tissues from db/db mice treated with muraglitazar for 14 days. The gene expression results showed that there is an increasing trend in the mRNA expression level of ENaCγ and Na+, K+-ATPase-α (data not shown) in both muragiltazar and rosigiltazone-treated groups as compared to vehicle-treated db/db mice. However, the mRNA expression level of ENaCγ was significantly elevated (4.4-fold) in mice treated with muragiltazar compared to control db/db mice (Figure 2).
Figure 1.
(a) Relative quantitation of aP2 mRNA expression by rosiglitazone and muraglitazar as determined by semiquantitative RT-PCR. Confluent 3T3-L1 cells were incubated for 5 days with various concentrations of PPAR-γ ligands, rosiglitazone, muraglitazar or 0.1% DMSO (as vehicle control) and RNA was isolated as described under ‘Methods'. aP2 mRNA levels were normalized by endogenous 18S control. Data shown are means±s.d. of duplicate determinations. A representative bar graph is shown. In (b) mRNA expression of ACO after 14 days treatment with muraglitazar (10 mg kg−1) and rosiglitazone (10 mg kg−1); n=2.
Figure 2.
mRNA expression of ENaCγ in db/db mice and C57BL/6 mice after 14 days treatment with muraglitazar (10 mg kg−1) and rosiglitazone (10 mg kg−1). **P<0.01 vs control; n=4.
Glucose and triglyceride lowering activity
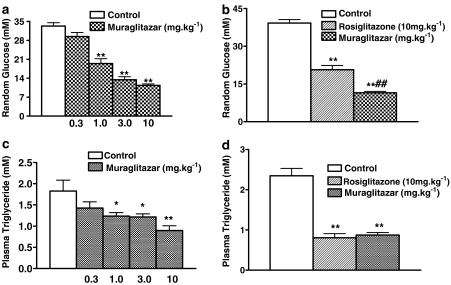
Muraglitazar showed a dose-dependent reduction in random glucose levels and triglycerides in db/db mice treated for 14 days (Figure 3a and c). Rosiglitazone and muraglitazar at 10 mg kg−1 showed significant reduction in plasma glucose levels under fed conditions in comparison with vehicle control after 14 days of treatment. The glucose-lowering activity was significantly more in the muraglitazar-treated group compared to rosiglitazone-treated group (Figure 3b). Rosiglitazone and muraglitazar at 10 mg kg−1 also showed significant reduction in plasma triglycerides in comparison with vehicle control after 14 days of treatment (Figure 3d).
Figure 3.
Dose-dependent effect of muraglitazar on random glucose (a) and triglyceride levels (c). Effect of rosiglitazone and muraglitazar on random glucose (b) and triglyceride levels (d) after 14 days treatment in db/db mice. *P<0.05,**P<0.01 vs control; ##P<0.01 vs rosiglitazone group; n=12 per group.
Effect on body weight
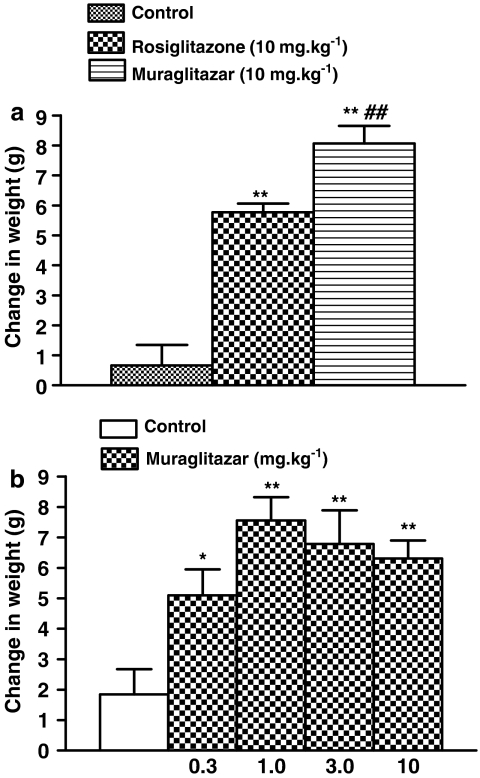
There was dose-dependent increase in body weight gain in grams (g) in muraglitazar (0.3, 1, 3 and 10 mg kg−1)-treated groups in comparison with vehicle control group (Figure 4a). Also a significant increase in body weight gain was observed in rosiglitazone group. The effect, however, was more prominent in the muraglitazar-treated group and was significantly greater than in rosiglitazone (Figure 4b).
Figure 4.
Weight gain in db/db mice. (a) Effect of rosiglitazone (10 mg kg−1) and muraglitazar (10 mg kg−1) on body weight change after a 14-day treatment. (b) Dose–response for muraglitazar on body weight change in db/db mice after a 14-day treatment. *P<0.5; **P<0.01 vs control; ##P<0.01 vs rosiglitazone group; n=8 per group.
Change in liver weight owing to PPARα-induced activity
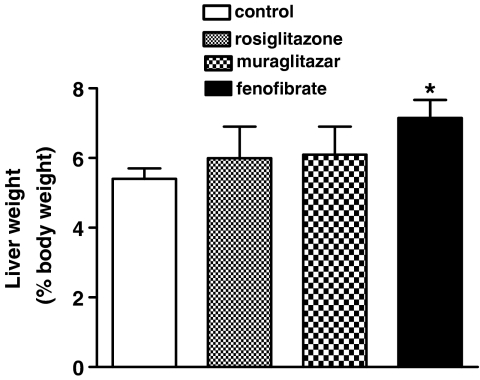
Muraglitazar (10 mg kg−1) did not show any increase in liver weight after a 14-day treatment (Figure 5). Fenofibrate (100 mg kg−1) induced a significant increase in the liver weight as was expected owing to its PPARα activity (Lee et al., 1995; Figure 5). Rosiglitazone serving as a negative control showed no increase in liver weight (Figure 5).
Figure 5.
Effect of rosiglitazone (10 mg kg−1), muraglitazar (10 mg kg−1) and fenofibrate (100 mg kg−1) on liver weight (shown as % body weight) after 14-day treatment in db/db mice. *P<0.05 vs control; n=6 per group.
Effect of muraglitazar and rosiglitazone on oedema parameters
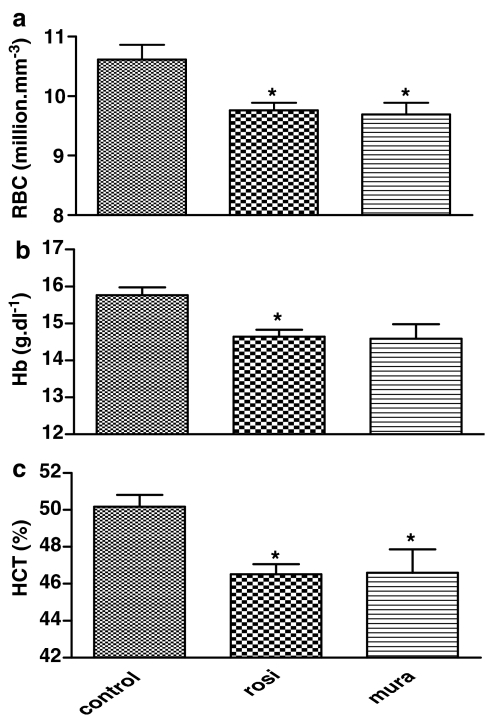
An increase in sodium and chloride concentrations (control values: 147±0.8 and 100±0.8 mmol l−1, respectively) was observed in plasma of mice treated with both muraglitazar (150±0.5 mmol l−1, P<0.05 vs control for Na+ and 102±0.5 mmol l−1, P<0.05 vs control for Cl−) and rosiglitazone (150±0.5 mmol l−1, P<0.05 vs control for Na+ and 102.5±0.6 mmol l−1, P<0.05 for Cl−). As sodium retention is always followed by an increase in water reabsorption via collecting ducts, a haemodilution is expected. Muraglitazar (10 mg kg−1) showed a marked decrease in RBC count, haemoglobin and haematocrit in treated db/db mice (Figure 6a–c). Similar changes were seen with 10 mg kg−1 rosiglitazone (Figure 6–c).
Figure 6.
Haemodilution parameters after 14 days treatment with muraglitazar (10 mg kg−1) and rosiglitazone (10 mg kg−1). Mean (±s.e.m.) values for RBC count (a), haemoglobin (Hb, b) and % haematocrit (%HCT, c) are shown. *P<0.05 vs control; n=8 per group.
Discussion
Muraglitazar, with a 25-fold greater affinity at PPARα receptors than fenofibrate (EC50 10 μM), or WY 14643 (EC50 8.1 μM), was expected to cause no weight gain in rodents. Potent PPARα agonist activity is normally associated with, and has been shown to cause, a lipid-lowering effect as well as body weight reduction in the hamster model of dyslipidaemia (Glaxo Wellcome group, 1997; Minnich et al., 2001) and in rodents (Chaput et al., 2000; Guerre-Millo et al., 2000). In a recent study in ob/ob mice, fenofibrate prevented the rosiglitazone-induced weight gain (Carmona et al., 2005) in addition to correction of their dyslipidaemic profile. Fenofibrate and its combination groups showed a decrease in the adipose tissue mass and an increase in hepatic fatty acid oxidation (Carmona et al., 2005) validating the role of PPARα in clinical settings where the clinical efficacy of fibrates is accompanied with ‘on treatment' weight loss in humans.
It thus seems clear that PPARα/PPARγ dual agonists with a higher propensity towards PPARα activation should ideally show a weight decreasing, or a weight neutralizing, tendency to counteract PPARγ activity. However, in our study, a marked increase in body weight was observed with muraglitazar that was significantly greater than the weight gain observed with rosiglitazone after a 14-day treatment in db/db mice. A similar weight gain has also been reported with tesaglitazar in Zucker fa/fa rats treated for 21 days (Hegarty et al., 2004; Oakes et al., 2005), and this has been attributed to its PPARγ agonist activity. In the present study, in mouse pre-adipocyte differentiation assay, both rosiglitazone and muraglitazar were observed to have similar EC50 values indicating that muraglitazar through its PPARγ activation has a similar propensity as rosiglitazone in causing adipogenesis. Moreover, both rosiglitazone and muraglitazar had similar levels of the adipocyte gene, aP2, mRNA expressed in 3T3 L1 cells suggesting a similar mode and extent of lipid sequestration into fat cells by both compounds (Bernlohr et al., 1985; Kletzien et al., 1992; Gregoire et al., 1998). In earlier reports, rosiglitazone had shown a potential for adipogenesis by activating aP2 mRNA in high-fat-fed rats (Chakrabarti et al., 2004). The increased weight gaining potential of muraglitazar in our study was accompanied by a better glucose-lowering ability than rosiglitazone bringing to mind the adage, ‘the paradox of PPARγ: The greater the weight gain, the better the drug works'. The triglyceride-lowering efficacy of muraglitazar, however, was similar to that of rosiglitazone.
The robust weight gain and glucose lowering by muraglitazar in db/db mice demonstrated its activation of PPARγ, a trend similar to the rosiglitazone group of animals, whereas the fenofibrate-treated group with only activation of PPARα did not show such changes. Fenofibrate caused a significant increase in liver weight owing to its PPARα activation; however, no such increase was observed with muraglitazar implying that somehow the PPARα agonist activity of muraglitazar does not translate into increased liver weight. This is an obvious offset of direct PPARα activation as has been observed with fenofibrate and tesaglitazar (Hegarty et al., 2004; Oakes et al., 2005; Harrity et al., 2006). PPARα activators have been found to significantly increase the expression of hepatic genes such as ACO in Zucker rats and db/db mice (Schoonjans et al., 1996). Muraglitazar has been previously shown to increase ACO mRNA in the liver of db/db mice and has shown weight neutrality in DIO C57BL/6J and db/db mice (Hariharan et al., 2002). However, in our study, in spite of a significant increase in ACO mRNA in muraglitazar-treated db/db livers, no weight neutrality or weight loss was observed showing that a high agonistic potency at PPARα receptors is not enough to neutralize the weight gain caused by PPARγ activation. This is supported by observations with DRF 2655, which has a PPARα activity 10-fold weaker than muraglitazar, is a potent anti-obesity and lipid-lowering and glucose-lowering molecule, perhaps, because of a 10-fold lesser activity at PPARγ receptors than rosiglitazone (Vikramadithyan et al., 2003). Based on our observations with muraglitazar, we hypothesize that the increased body weight gain observed could be due to the utilization and siphoning of the fatty acids generated as a result of the PPARα-mediated effect, towards PPARγ mediated lipogenesis. Pre-clinical data are now being corroborated with clinical studies. Muraglitazar has shown oedema and weight gain in patients undergoing phase II clinical trials and has presented problems of precipitating congestive cardiac failure in susceptible patients (Defronzo et al., 2005).
The weight gaining tendency of muraglitazar is postulated to be due to either enhanced adipogenesis or to water retention, shown as increased plasma or extracellular volume (Harrity et al., 2006). Normally, thiazolidinediones are associated with dose-related fluid retention, believed to be a re-feeding oedema owing to enhanced insulin action significantly compounded by direct PPARγ activation leading to increased sodium reabsorption in the distal renal tubule (Chen et al., 2005). Data from the muraglitazar trial show that muraglitazar also shares these presumed PPARγ-mediated effects (Belder, 2005). Patients with peripheral and pulmonary oedema associated with thiazolidinedione therapy fail to respond to thiazide and loop diuretics. Clinical improvement ensues only after discontinuation of thiazolidinedione therapy (Mudaliar et al., 2003). A recent report, however, observes complete inhibition of the early weight gain in C57BL/6J mice, induced by pioglitazone, on pretreatment with amiloride, a K+-sparing diuretic which is a selective inhibitor of ENaC (Guan et al., 2005). These results point towards a requirement for ENaC-mediated Na+ absorption in the collecting duct in mediating pioglitazone-associated fluid accumulation (Guan et al., 2005). Selective genetic deletion of Pparg gene from the renal collecting ducts using Ppargflox/flox mice prevented weight gain and thus confirmed the PPARγ-ENaC-dependent mechansism of thiazolidinedione-induced oedema (Zhang et al., 2005).
In the present study, marked haemodilution was observed after muraglitazar treatment for 14 days. A significant decrease in RBC count, haemoglobin and haematocrit was also observed, indicating an increase in plasma volume. As water reabsorption is secondary to increase in sodium retention, we measured plasma electrolytes wherein a significant increase was observed in plasma Na+ and Cl+ ions. Na+-reabsorption is enhanced by upregulation of expression of ENaC and Na+, K+-ATPase. We observed a significant increase in mRNA expression of ENaCγ and a trend towards increase in Na+, K+-ATPase in whole kidneys of db/db mice treated with muraglitazar. A similar trend was also observed with rosiglitazone. This indicated that oedema was also seen with treatment with muraglitazar as is reported with rosiglitazone.
To summarize, it is evident that molecules can be ascribed as PPARα or PPARγ agonists by receptor binding, co-activation studies and gene expression analyses (Oakes et al., 2005). However, on translating these activities in vivo, it is difficult to ascribe metabolic effects to distinct activation of PPARα vs PPARγ, as the patterns of effect resulting from stimulation of selective agonists show considerable overlap. It can be inferred from our observations that the PPARα activity of muraglitazar does not translate effectively so as to counteract the PPARγ-induced weight gain in rodents. This study clearly indicates that muraglitazar causes both significant oedema and adipogenesis with a 14-day treatment in db/db mice, as it does in humans.
Acknowledgments
This study was supported by NDDR Division, R&D, Ranbaxy Research Laboratories. We gratefully acknowledge the help given to us by Dr VM Shingatgeri and Dr SM Rajaram.
Abbreviations
- ACO
acyl CoA oxidase
- CPT-1
carnitine palmitoyl-transferase-1
- ENaC
epithelial sodium channel
- PPAR
peroxisome proliferator-activated receptors
Conflicts of interest
The authors state no conflict of interest.
References
- Astra-Zeneca Press release AstraZeneca discontinues development of GALIDA TM (tesaglitazar) May 6, 2006.
- Barlocco D. Muraglitazar. Curr Opin Invest Drugs. 2005;6:427–434. [PubMed] [Google Scholar]
- Belder R.FDA advisory committee transcript on muraglitazar 200564Available at
- Bernlohr DA, Bolanowski MA, Kelly TJJ, Lane MD. Evidence for an increase in transcription of specific mRNAs during differentiation of 3T3-L1 preadipocytes. J Biol Chem. 1985;260:5563–5567. [PubMed] [Google Scholar]
- Bristol-Meyer Squibb Company News BMY announces discontinuation of development of muraglitazar, an investigational oral treatment for Type 2 Diabetes. May 18, 2006.
- Carmona MC, Couche K, Nibbelink M, Prunet B, Bross A, Desbazeille M, et al. Fenofibrate prevents rosiglitazone-induced weight gain in ob/ob mice. Int J Obes Relat Metals Disord. 2005;29:864–871. doi: 10.1038/sj.ijo.0802943. [DOI] [PubMed] [Google Scholar]
- Chaput E, Saladin R, Silvestre M, Edgar AD. Fenofibrate and rosiglitazone lower serum triglycerides with opposing effects on body weight. Biochem Biophys Res Commun. 2000;271:445–450. doi: 10.1006/bbrc.2000.2647. [DOI] [PubMed] [Google Scholar]
- Chakrabarti R, Misra P, Vikramadithyan RK, Premkumar M, Hiriyan J, Datla SR, et al. Antidiabetic and hypolipidemic potential of DRF 2519- a dual activator of PPAR-α and PPAR-γ. Eur J Pharmacol. 2004;491:195–206. doi: 10.1016/j.ejphar.2004.03.034. [DOI] [PubMed] [Google Scholar]
- Chen L, Yang B, Mcnulty JA, Clifton LG, Binz JG, Grimes AM, et al. GI262570, a peroxisome proliferators-activated receptor γ agonist, changes electrolytes and water reabsorption from the distal nephron in rats. J Pharmacol Exp Ther. 2005;312:718–725. doi: 10.1124/jpet.104.074088. [DOI] [PubMed] [Google Scholar]
- Cock TA, Houten SM, Auwerx J. PPARγ: too much of a good thing causes harm. EMBO Repo. 2004;5:142–147. doi: 10.1038/sj.embor.7400082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costet P, Legendre C, More J, Edgar A, Galtizer P, Pineau T. PPAR-alpha isoform deficiency leads to progressive dyslipidemia with sexually dimorphic obesity and steatosis. J Biol Chem. 1998;273:29577–29585. doi: 10.1074/jbc.273.45.29577. [DOI] [PubMed] [Google Scholar]
- De Fronzo RA, Rubin CJ, Mohideen P.Improvement of glycemic control with Muraglitazar, a novel dual PPAR alpha/gamma agonist, in combination with metformin in patients with type 2 diabetes: a double blind, randomized, pioglitazone-controlled study Diabetes 200554Suppl 114-OR [Google Scholar]
- Desvergne B, Wahli W. Peroxisome proliferators activated receptors: nuclear control of metabolism. Endocr Rev. 1999;20:649–688. doi: 10.1210/edrv.20.5.0380. [DOI] [PubMed] [Google Scholar]
- Egerod FL, Nielsen HS, Iversen LL, Thorup I, Storgaard TT, Oleksiewicz MB. Biomarkers for early effects of carcinogenic dual-acting PPAR agonists in rat urinary bladder urothelium in-vivo. Biomarkers. 2005;10:295–309. doi: 10.1080/13547500500218682. [DOI] [PubMed] [Google Scholar]
- FDA advisory committee transcript on muraglitazar September 9 2005. pp. 47–49, 64. Available at
- FDA statistical review 2005.
- Glaxo Wellcome Group Use of agonists of peroxisome proliferator activated receptor alpha for treating obesity 1997(WO 9736579)
- Gregoire FM, Smas CM, Sul HS. Understanding adipocyte differentiation. Physiol Rev. 1998;78:783–809. doi: 10.1152/physrev.1998.78.3.783. [DOI] [PubMed] [Google Scholar]
- Guan Y, Hao C, Cha DR, Rao R, Lu W, Kohan DE, et al. Thiazolidinone expand body fluid volume through PPAR gamma stimulation of EnaC-mediated renal salt absorption. Nat Med. 2005;11:861–866. doi: 10.1038/nm1278. [DOI] [PubMed] [Google Scholar]
- Guerre-Millo M, Gervois MP, Raspe E, Madsen L, Poulain P, Derudas B, et al. PPARα activators improve insulin sensitivity and reduce adiposity. J Biol Chem. 2000;275:16638–16642. doi: 10.1074/jbc.275.22.16638. [DOI] [PubMed] [Google Scholar]
- Hariharan N, Chen S, Cheng P, Chu C, Devasthale P, Farelly D, et al. BMS-298585, a novel uniquely balanced dual activator of PPAR alpha and gamma, shows robust glucose and lipid lowering effects in genetic and diet-induced diabetic and hyperlipidemic mouse models. Diabetes. 2002;512:A405-P. [Google Scholar]
- Harrity T, Farrelly D, Tieman A, Chu C, Kunselman L, Gu L, et al. Muraglitazar, a novel dual (alpha/gamma) PPAR activator, improves diabetes and other metabolic abnormalities and preserves beta-cell function in db/db mice. Diabetes. 2006;55:240–248. [PubMed] [Google Scholar]
- Hegarty BD, Furler SM, Oakes ND, Kraegen EW, Cooney GJ. PPAR activation induces tissue-specific effects on fatty acid uptake and metabolism in vivo – a study using the novel PPAR alpha/gamma agonist tesaglitazar. Endocrinology. 2004;145:3158–3164. doi: 10.1210/en.2004-0260. [DOI] [PubMed] [Google Scholar]
- Kletzien RF, Foellmi LA, Harris PK, Wyse BM, Clarke SD. Adipocyte fatty acid binding protein: regulation of gene expression in vivo and in vitro by an insulin-sensitizing agent. Mol Pharmacol. 1992;42:558–562. [PubMed] [Google Scholar]
- Lee SS, Pineau T, Drago J, Lee EJ, Owens JW, Kroetz DL, et al. Targeted disruption of the alpha isoform of the PPAR gene in mice results in abolishment of the pleotropic effects of peroxisome proliferators. Mol Cell Biol. 1995;15:3012–3022. doi: 10.1128/mcb.15.6.3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minnich A, Tian N, Byan L, Bilder G. A potent PPAR alpha agonist stimulates mitochondrial fatty acid beta-oxidation in liver and skeletal muscles. Am J Physiol Endocrinol Metab. 2001;280:E270–E279. doi: 10.1152/ajpendo.2001.280.2.E270. [DOI] [PubMed] [Google Scholar]
- Mudaliar S, Cheng AR, Henry RR. TZD's, peripheral oedema and type 2 diabetes: incidence, pathophysiology and clinical implications. Endocr Pract. 2003;9:406–416. doi: 10.4158/EP.9.5.406. [DOI] [PubMed] [Google Scholar]
- Nesto RW, Bell D, Bonow RO, Fonseca V, Grundy SM, Horton ES, et al. TZD use, fluid retention and CHF: a consensus statement from the AHA and ADA. Circulation. 2003;108:2941–2948. doi: 10.1161/01.CIR.0000103683.99399.7E. [DOI] [PubMed] [Google Scholar]
- Oakes ND, Thalen PD, Hultstrand T, Jacinto S, Camejo G, Wallin B, et al. Tesaglitazar, a dual PPAR alpha/gamma agonist ameliorates glucose and lipid intolerance in obese Zucker rats. Am J Physiol Regul Integr Comp Physiol. 2005;289:R938–R946. doi: 10.1152/ajpregu.00252.2005. [DOI] [PubMed] [Google Scholar]
- Shibata T, Matsui K, Nagao K, Shinkai H, Yonemori F, Wakitani K. Pharmacological profiles of a novel oral antidiabetic agent, JTT-501, an isoxazolidinedione derivative. Eur J Pharmacol. 1999;364:211–219. doi: 10.1016/s0014-2999(98)00832-2. [DOI] [PubMed] [Google Scholar]
- Schoonjans K, Staels B, Auwerx J. Role of the peroxisome proliferators-activated receptor (PPAR) in mediating the effects of fibrates and fatty acids on gene expression. Lipid Res. 1996;37:907–925. [PubMed] [Google Scholar]
- Staels B, Fruchart JC. Therapeutic roles of PPAR agonists. Diabetes. 2005;54:2460–2470. doi: 10.2337/diabetes.54.8.2460. [DOI] [PubMed] [Google Scholar]
- Vikramadithyan RK, Hiriyan J, Suresh J, Genshome C, Babu RK, Misra P, et al. DRF-2655: a unique molecule that reduces body weight and ameliorates metabolic abnormalities. Obes Res. 2003;11:292–303. doi: 10.1038/oby.2003.44. [DOI] [PubMed] [Google Scholar]
- Zhang H, Zhang A, Kohan DE, Nelson RD, Gonazalez FG, Yang T. Collecting duct-specific deletion of peroxisome proliferators-activated receptor gamma blocks thiazolidinedione-induced fluid retention. Proc Natl Acad Sci USA. 2005;102:9406–9411. doi: 10.1073/pnas.0501744102. [DOI] [PMC free article] [PubMed] [Google Scholar]