Abstract
Background and Purpose:
There is increasing evidence to suggest that cannabis can ameliorate muscle-spasticity in multiple sclerosis, as was objectively shown in experimental autoimmune encephalomyelitis models. The purpose of this study was to investigate further the involvement of CB1 and CB2 cannabinoid receptors in the control of experimental spasticity.
Experimental approach:
Spasticity was induced in wildtype and CB1-deficient mice following the development of relapsing, experimental autoimmune encephalomyelitis. Spastic-hindlimb stiffness was measured by the resistance to flexion against a strain gauge following the administration of CB1 and CB2 agonists.
Key Results:
As previously suggested, some CB2-selective agonists (RWJ400065) could inhibit spasticity. Importantly, however, the anti-spastic activity of RWJ400065 and the therapeutic effect of non-selective CB1/CB2 agonists (R(+)WIN55,212–2 and CP55, 940) was lost in spastic, CB1-deficit mice.
Conclusions and Implications:
The CB1 receptor controls spasticity and cross-reactivity to this receptor appears to account for the therapeutic action of some CB2 agonists. As cannabinoid-induced psychoactivity is also mediated by the CB1 receptor, it will be difficult to truly dissociate the therapeutic effects from the well-known, adverse effects of cannabinoids when using cannabis as a medicine. The lack of knowledge on the true diversity of the cannabinoid system coupled with the lack of total specificity of current cannabinoid reagents makes interpretation of in vivo results difficult, if using a purely pharmacological approach. Gene knockout technology provides an important tool in target validation and indicates that the CB1 receptor is the main cannabinoid target for an anti-spastic effect.
Keywords: cannabinoids, CB1 receptor, CB2 receptor, experimental autoimmune encephalomyelitis, spasticity, multiple sclerosis
Introduction
There has been recent interest in the therapeutic potential of cannabis for control for a number of symptoms, notably spasticity that often develops as a consequence of multiple sclerosis (MS. Consroe et al., 1997; Pertwee, 2002). Using cannabinoid agonists and antagonists, we were the first to provide objective, experimental evidence for the tonic control of spasticity by the cannabinoid system in the experimental autoimmune encephalomyelitis (EAE) model of MS (Baker et al., 2000, 2001). This supported patient claims for the use of medicinal cannabis (Consroe et al., 1997) and has been validated by the modest improvements of symptoms in more recent clinical trials of cannabinoids in MS (Zajicek et al., 2003, 2005; Vaney et al., 2004; Brady et al., 2004; Wade et al., 2004; Freeman et al., 2006). Although the exact cause of spasticity is not definitively known, it is clear that this results from alterations in the balance, possibly secondary to selective neuronal loss, between excitatory and inhibitory neural circuits (Brown, 1994; Dutta et al., 2006). This results in loss of control of neurotransmission between the muscles and the central nervous system resulting in uncontrolled spastic movements, which in some instances can be treated using GABA receptor agonists (Brown, 1994; Ivanhoe and Reistetter, 2004). After the initial observations in EAE (Baker et al., 2000), the cannabinoid type 1 (CB1) receptor and endocannabinoid system has been shown to regulate synaptic neurotransmission (Howlett et al., 2002; Wilson and Nicoll, 2002) and this action would be consistent with the cannabinoid control of spasticity. In contrast to CB1, there is limited evidence to indicate that normal nerve tissues express cannabinoid type 2 (CB2) receptors (Van Sickle et al., 2005) and they appear to be restricted to leucocytes (Munro et al., 1993; Galiegue et al., 1995; Howlett et al., 2002), although they are expressed by glial cells and may be upregulated in inflamed brain tissue (Maresz et al., 2005; Wotherspoon et al., 2005) and therefore may not be anticipated to control problems of neurotransmission. Surprisingly, however, a CB2 agonist ameliorated and an antagonist transiently worsened spasticity in EAE (Baker et al., 2000), suggesting that CB2 agonists could provide therapies that avoid the psychoactive effects associated with CB1 agonism (Baker et al., 2000; Howlett et al., 2002; Varvel et al., 2005). In animals, cannabimimetic potential is determined by activity in ‘tetrad' (hypomotility, hypothermia, ring catelepsy analgesia) tests, which show no response owing to CB2 agonism (Howlett et al., 2002). However, currently there are no absolutely specific cannabinoid reagents (agonists or antagonists) available, which solely act on either of the CB1 or CB2 receptors, and although they may be selective to one or other of the cannabinoid receptors in vitro, at the doses used in vivo, there is the potential for cannabinoids to crossreact with the other CB receptor (Pertwee, 1999; Howlett et al., 2002). Furthermore, there is increasing evidence for additional receptors that mediate cannabimemetic effects (Hajos et al., 2001; Howlett et al., 2002; Begg et al., 2005; Baker et al., 2006), which further complicates the interpretation of pharmacological data. Therefore, receptor–deletion using transgenic technology (Zimmer et al., 1999, Brooks et al., 2002) provides a level of certainty of the role of the CB receptor subtype that is not provided by the CB receptor antagonism alone. This was used to re-evaluate the CB2-mediated control of spasticity during EAE.
Methods
Animals
Biozzi ABH and ABH mice lacking the CB1 receptor (Cnr1) gene were generated as described previously (Brooks et al., 2002, Pryce et al., 2003). The congenic ABH.Cnr1−/−used for breeding were produced by intercrossing after seven generations of backcrossing. Genomic screening for the absence of wild-type Cnr1, using polymerase chain reaction (Brooks et al., 2002), was performed on parental animals, and the functional deletion of CB1 protein was confirmed in animals used in these studies by the resistance to sedative doses (20 mg kg−1 intraperitoneal (i.p.)) of R(+)WIN-55,212–2. These were from in-house bred stock that was maintained in a 12 h-light/dark cycle with controlled humidity and temperature. Animals were fed RM-1E diet and water ad libitum. All animal studies conformed to the United Kingdom Animals (Scientific Procedures) Act 1986.
Chemicals
The full CB1/CB2 agonists R(+)WIN-55,212–2 ((R)-(+)-[2,3-dihydro-5-methyl-3-(-4-morpholinylmethyl)pyrrolo-[1,2,3-de]-1,4-benzoxazin-6-yl]-1-naphthale-nylmethanone mesylate) (Ki CB1=9.9 nM, Ki CB2=16.2 nM (Rinaldi-Carmona et al., 1994)) and CP55,940 ((1R,3R,4R)-3-[2-hydroxy-4-(1,1-dimethylheptyl)phenyl]-4-(3-hydroxypropyl)cyclohexan-1-ol) (Ki CB1=1.4 nM, Ki CB2=1.4 nM (Rinaldi-Carmona et al., 1994)) were purchased from RBI/Sigma (Poole, UK) and Tocris Ltd (Bristol, UK). The CB2-selective agonist JWH056 (1-deoxy-δ-8-tetrahydrocannabinol; receptor affinity. Ki CB1=8770 nM, Ki CB2=32 nM) was provided by Dr J Huffman, Clemson University, South Carolina, USA (Huffman et al., 1996). The CB2-selective agonist RWJ400065 (binding affinity. Ki CB1=600 nM, Ki CB2=10 nM), forskolin-stimulated cyclic adenosine monophosphate (cAMP) agonism half-maximal inhibitory concentration (IC50) CB1=6600 nM, IC50 CB2=6.6 nM (Dr D Argentieri and Dr D Ritchie, unpublished observations) and non-selective CB1/CB2 agonist RWJ352303 (Ki CB1=0.6 nM Ki CB2=0.3 nM), forskolin-stimulated cAMP agonism in SKN cells IC50 CB1=0.64 nM, IC50 CB2=0.14 nm (Dr D Argentieri and Dr D Ritchie, unpublished observations) compounds were provided by RW Johnson] (Raritan, NJ, USA). These were suspended in intralipid 30% (Pharmacia, Milton Keynes, UK) before i.p. or intravenous (i.v.) injection in 0.1 ml. Unanaesthetized animals were placed in a 87 × 112 × 60 mm box (Alpha laboratories, Eastleigh, UK) that had a slot cut to allow exit of the tail from the box. The tail was transiently immersed in warm water (about 45°C) to induce vasodilatation, before injection into the tail vein using 30 g needles. The RWJ compounds were initially injected i.v. to avoid effects of first-pass metabolism encountered with some compounds, such as tetrahydrocannabinol (Baker et al., 2000), using dose ranges based on pharmacokinetic data (Dr D Argentieri and Dr D Ritchie, unpublished observations). The i.p. route was selected for CP55,950 and R(+)WIN-55,212–2 as it was already known that these agents were rapidly active following i.p. delivery (Baker et al., 2000).
Induction of spasticity
Young adult mice were injected subcutaneously with 1 mg of freeze-dried mouse spinal cord homogenate in Freund's adjuvant on days 0 and 7 to induce EAE as described previously (Baker et al., 1990). Animals developed relapsing-remitting episodes of limb paralysis and spasticity typically developed after 2–3 relapses, about 80–100 days postinduction. This was assessed during remission from active paralytic episodes by the force required to bend the hind limb to full flexion against a strain gauge (Baker et al., 2000).
Assessment of body temperature
Temperature was monitored using a thermocouple placed under the hindlimb as previously described (Brooks et al., 2002).
Data analysis and statistical procedures
Each group contained a minimum of five different animals and the results represent the mean±s.e. resistance to flexion force (N) or individual limbs, which were compared using repeated measures analysis of variance or paired t tests using Sigmastat software (Baker et al., 2001). Changes in temperature were compared using paired t tests using Sigmastat Software (Brooks et al., 2002).
Results
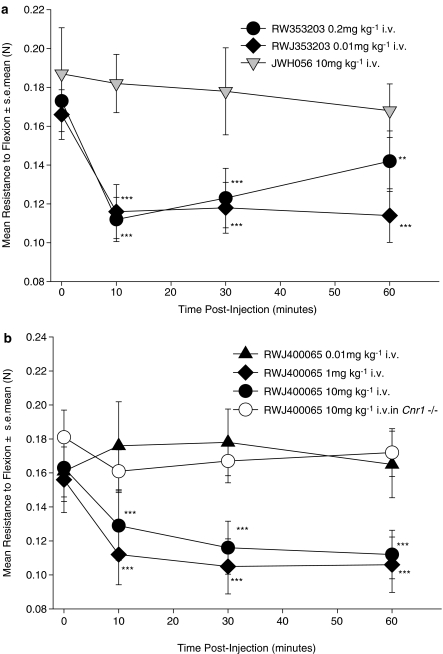
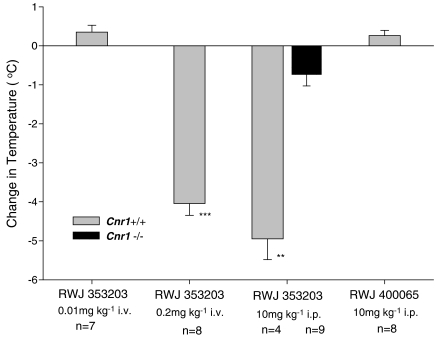
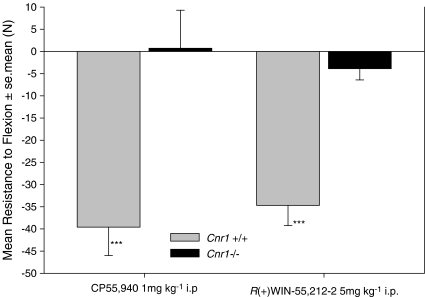
In an attempt to validate our previous studies showing an anti-spastic activity of CB2 agonists (JWH133 receptor affinity. Ki CB1=680 nM, CB2=3 nM. Baker et al., 2000), additional compounds were investigated. Surprisingly, 10 mg kg−1 i.v. JWH056, which is less potent at CB2, but with a lower affinity for CB1 (Ki>8 μM) than JWH133, failed to inhibit spasticity at 10–60 min after injection i.v. (Figure 1a), whereas RWJ352303, a potent non-selective CB1 agonist, inhibited spasticity (Figure 1a). However, a dose-dependent anti-spastic activity was detectable following injection i.v. of a potent CB2 agonist RWJ400065 (Figure 1b). This compound has similar binding affinities to JWH133 and failed to induce observable sedation (unpublished observations) and hypothermia (Figure 2), indicative of CB1 receptor-mediated effects. In contrast, RWJ352303 had the potential to induce ‘tetrad-like' effects (Figure 2), but was still active as an anti-spastic agent (Figure 1a), at doses that did not induce ‘tetrad-type' effects, shown here by hypothermic responses (Figure 2). However, when 10 mg kg−1 i.v. RWJ400065 was injected into Cnr1−/− mice, there was no apparent anti-spastic activity (Figure 1B). To clarify this further, commonly used high-affinity CB1/CB2 non-selective agonists were examined. However, there was no evidence of inhibition of spasticity in CB1-deficient mice with either CP55,940 or R(+)WIN-55, 212–2 compared to significant (P<0.001) inhibitory activity in wild-type mice (Figure 3). This suggested that CB1 and not the CB2 receptors were actually mediating the inhibitory effects of some CB2 agonists.
Figure 1.
Inhibition of spasticity, with CB2 agonists is CB1-mediated. Following the development of spasticity ABH mice were injected i.v. with either: (a) the non-selective agonist RWJ35320 or the CB2-selective agonist JWH056 or (b) the CB2-selective agonist, RWJ400065. These received 0.2 mg kg−1 (n=17 limbs), 0.01 mg kg−1 (n=13 limbs) RWJ353203 or 10 mg kg−1 JWH056 (n=7 limbs) or 0.01 mg kg−1 (n=12 limbs), 1 mg kg−1 (n=16 limbs) or 10 mg kg−1 (n=16 limbs) RWJ400065 in wild-type or CB1-deficient mice (n=12 limbs) in intralipid. The resistance to flexion was measured against a strain gauge. **P<0.01, ***P<0.001 compared to baseline.
Figure 2.
Hypothermia induced by cannabinoids. Wild-type or CB1-deficient mice were injected either i.v. or i.p. with the non-selective agonist RWJ35320 or CB2-selective agonist RWJ400065 in intralipid. The change in body temperature (mean±s.e.mean (vertical lines)) 20 min following injection compared to baseline was assessed. **P<0.01, ***P<0.001 compared to baseline by paired t tests.
Figure 3.
Spasticity is controlled by the CB1 receptor. Wild-type or CB1-deficient mice (Cnr1−/−) were injected intraperitoneally with the full CB1/CB2 agonists CP-55,940 (n=8/group) or R(+)WIN-55,212–2 (n=14/group). To facilitate visualization of differences between groups, results are expressed as the mean±s.e.mean (vertical lines) percentage change in the resistance to hindlimb flexion compared to baseline, 10 min after the injection of compound. ***P<0.001 compared to baseline by paired t tests.
Discussion and conclusions
Although this study confirms our previous observation (Baker et al., 2000) that ‘tetrad inactive' apparent CB2 agonists can show anti-spastic activity, this does not appear to be owing to the direct activity of CB2 receptors. This most likely occurs because CB2 agonists/antagonists (Baker et al., 2000), or possibly their in vivo metabolites, have some affinity for CB1 receptors that may actually mediate the inhibitory effects. The biology of cannabis and the cannabinoid system now indicates that both tetrahydrocannabinol and CB1 receptors are the major mediators for both therapy in spasticity and also the adverse side effects (Howlett et al., 2002; Wilkinson et al., 2003; Varvel et al., 2005). It will be virtually impossible to truly dissociate these two effects, using cannabis. Clinical studies indicate that there is a substantial variability of individuals to tolerate cannabis and tetrahydrocannabinol (Zajicek et al., 2003; Brady et al., 2004; Wade et al., 2004). The apparent therapeutic window, before psychoactive effects, appears to be very small and is consistent with the modest effects in symptom control observed so far (Zajicek et al., 2003, 2005; Brady et al., 2004; Wade et al., 2004; Freeman et al., 2006), which nevertheless validate our original observations in animal models (Baker et al., 2000, 2001; Wilkinson et al., 2003). This variability of individuals to tolerate cannabinoids means that it will be difficult to dose–titrate adequately with potent CB1 agonists and that weak CB1 agonists, such as at the level found in some CB2 agonists, may be preferable for clinical use.
Currently there are two recognized cannabinoid receptors, but there is pharmacological evidence (Breivogel et al., 2001; Hajos et al., 2001; Howlett et al., 2002; Baker et al., 2006; Oz, 2006), some of which is disputed (Kawamura et al., 2006; Takahashi and Castillo 2006), for additional receptors or pathways that mediate cannabimimetic effects. Although the use of gene knockout technology is not without its own limitations, it provides an important tool in target validation. The loss of anti-spastic activity of R(+) WIN55,212–2 and CP55,940, both full CB1/CB2 agonists, in CB1-deficient mice supports the indication that CB1 and not CB2 is mediating the therapeutic anti-spastic effect. Nevertheless, anti-spastic control is feasible in CB1-deficient animals as shown previously with arvanil (Brooks et al., 2002). Arvanil, a potent transient receptor potential vanilloid type 1 (TRPV1) receptor and weak CB1 agonist, can also inhibit spasticity in the presence of CB1/CB2 antagonists and high doses of the TRPV1 antagonist capsazepine (Brooks et al., 2002). It can also induce cannabimemetic ‘tetrad-type' responses, such as hypothermia, and hypomotility, in wild-type and Cnr1−/− mice (Brooks et al., 2002). However, capsazepine is a weak TRPV1 antagonist in mice (Correll et al., 2004) and the hypothermia and marked hypomotility induced by 0.5 mg kg−1 i.v. arvanil is lost in Trpv1−/− mice (unpublished observations), further indicating the value of receptor knockout animals in target validation. However, cannabinoid receptors can exist as homodimers, and novel heterodimer formations between CB1 receptors and other G-protein-coupled receptors are assumed or are generated (Kearn et al., 2005; Wager-Miller et al., 2002; Rios et al., 2006). Therefore, CB1/CB2 receptor heterodimers or heterodimers between CB1 and any other molecule to which the CB2 agonists may bind would not exist in Cnr1−/− mice and this may have accounted for the loss of activity of RWJ400065 in CB1-deficient mice. Therefore, similar studies in Cnr2−/− mice are required to exclude definitively a role for CB2 in the control of spasticity.
However, the results from this study suggest that our previous data showing control of spasticity with endocannabinoid degradation inhibitors may need to be more cautiously interpreted (Baker et al., 2001). Many of these inhibitors, often based on the structural modifications of anandamide, have low affinity for CB1 receptors and are inactive in ‘tetrad' tests, just as CB2-selective agonists appear to be. Compounds believed to inhibit the anandamide transporter, including AM404, VDM11 (Baker et al., 2001), OMDM-1, OMDM-2 (de Lago et al., 2004), UCM707 (de Lago et al., 2006) 0–2093 and 0–3246 (Ligresti et al., 2006) all exhibit anti-spastic activity. However, many of these agents have activity on additional molecules such as TRPV1 vanilloid receptors and the cannabinoid degrading enzyme: fatty acid amide hydrolase (FAAH), which could account for their biological activity (Ralevic et al., 2001; Fowler et al., 2004). Although a site for membranous diffusion of endocannabinoids has been suggested (Moore et al., 2005), the existence of a specific transporter for anandamide, independent of FAAH, has been questioned (Glaser et al., 2003; Ortega-Gutierrez et al., 2004; Kaczocha et al., 2006). Therefore, until the putative endocannabinoid transporter(s) are identified and cloned, it must be considered possible that the therapeutic, anti-spastic effect of cannabinoid re-uptake inhibitors may be explained by alternative mechanisms. However, using FAAH gene knockout mice (ABH.Faah−/−), we have been able to verify the activity of some FAAH inhibitors (Boger et al., 2000) as anti-spastic agents (unpublished observations). The lack of true understanding of diversity of the cannabinoid system and importantly the lack of absolute specificity of current cannabinoid agonists and antagonists (Pertwee, 1999) means that it may be difficult to correctly interpret results, particularly in vivo, if using a purely pharmacological approach.
Acknowledgments
We thank the support of the Multiple Sclerosis Society of Great Britain and Northern Ireland and Aims2Cure we also thank Dr C Ledent for supplying the founders for the CB1 knockout line, Dr Huffman for supplying compounds, Dr D Argentieri and Dr D Ritchie (RWJ Johnson, Raritan) for providing access and supply of the RWJ compounds. RW Johnson, Raritan, NJ, USA, in part funded experiments involving RWJ compounds.
Abbreviations
- CB1
cannabinoid type 1 receptor
- CB2
cannabinoid type 2 receptor
- EAE
experimental autoimmune encephalomyelitis
- FAAH
fatty acid amide hydrolase
- MS
multiple sclerosis
- TRPV1
transient receptor potential vanilloid type 1
Conflict of interest
The authors state no conflict of interest.
References
- Baker D, O'Neill JK, Gschmeissner SE, Wilcox CE, Butter C, Turk JL. Induction of chronic relapsing experimental allergic encephalomyelitis in Biozzi mice. J Neuroimmunol. 1990;28:261–270. doi: 10.1016/0165-5728(90)90019-j. [DOI] [PubMed] [Google Scholar]
- Baker D, Pryce G, Croxford JL, Brown P, Pertwee RG, Huffman JW, et al. Cannabinoids control spasticity and tremor in a multiple sclerosis model. Nature. 2000;404:84–87. doi: 10.1038/35003583. [DOI] [PubMed] [Google Scholar]
- Baker D, Pryce G, Croxford JL, Brown P, Pertwee RG, Makriyannis A, et al. Endocannabinoids control spasticity in a multiple sclerosis model. FASEB J. 2001;15:300–302. doi: 10.1096/fj.00-0399fje. [DOI] [PubMed] [Google Scholar]
- Baker D, Pryce G, Davies WL, Hiley CR. In silico patent searching reveals a new cannabinoid receptor. Trends Pharmacol Sci. 2006;27:1–4. doi: 10.1016/j.tips.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Begg M, Pacher P, Batkai S, Osei-Hyiaman D, Offertaler L, Mo FM, et al. Evidence for novel cannabinoid receptors. Pharmacol Ther. 2005;106:133–145. doi: 10.1016/j.pharmthera.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Boger DL, Sato H, Lerner AE, Hedrick MP, Fecik RA, Miyauchi H, et al. Exceptionally potent inhibitors of fatty acid amide hydrolase: the enzyme responsible for degradation of endogenous oleamide and anandamide. Proc Natl Acad Sci USA. 2000;97:5044–5049. doi: 10.1073/pnas.97.10.5044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady CM, DasGupta R, Dalton C, Wiseman OJ, Berkley KJ, Fowler CJ. An open-label pilot study of cannabis-based extracts for bladder dysfunction in advanced multiple sclerosis. Mult Scler. 2004;10:425–433. doi: 10.1191/1352458504ms1063oa. [DOI] [PubMed] [Google Scholar]
- Breivogel CS, Griffin G, Di Marzo V, Martin BR. Evidence for a new G protein-coupled cannabinoid receptor in mouse brain. Mol Pharmacol. 2001;60:155–163. [PubMed] [Google Scholar]
- Brooks JW, Pryce G, Bisogno T, Jagger SI, Hankey DJR, Brown P, et al. Arvanil-induced inhibition of spasticity and persistent pain: further evidence for additional therapeutic non-CB1 cannabinoid receptors. Eur J Pharmacol. 2002;439:83–92. doi: 10.1016/s0014-2999(02)01369-9. [DOI] [PubMed] [Google Scholar]
- Brown P. Pathophysiology of spasticity. J Neurol Neurosurg Psychiatry. 1994;57:773–777. doi: 10.1136/jnnp.57.7.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consroe P, Musty R, Rein J, Tillery W, Pertwee R. The perceived effects of smoked cannabis on patients with multiple sclerosis. Eur Neurol. 1997;38:44–48. doi: 10.1159/000112901. [DOI] [PubMed] [Google Scholar]
- Correll CC, Phelps PT, Anthes JC, Umland S, Greenfeder S. Cloning and pharmacological characterization of mouse TRPV1. Neurosci Lett. 2004;370:55–60. doi: 10.1016/j.neulet.2004.07.058. [DOI] [PubMed] [Google Scholar]
- de Lago E, Fernández-Ruiz J, Ortega-Gutiérrez S, Cabranes A, Pryce G, Baker D, et al. UCM707, an inhibitor of anandamide uptake, behaves as a symptom control agent in models of Huntington's disease and multiple sclerosis, but fails to delay/arrest the progression of different motor-related disorders. Eur Neuropsychopharmacol. 2006;16:7–18. doi: 10.1016/j.euroneuro.2005.06.001. [DOI] [PubMed] [Google Scholar]
- de Lago E, Ligresti A, Ortar G, Morera E, Cabranes A, Pryce G, et al. In vivo pharmacological actions of two novel inhibitors of anandamide cellular uptake. Eur J Pharmacol. 2004;484:249–257. doi: 10.1016/j.ejphar.2003.11.027. [DOI] [PubMed] [Google Scholar]
- Dutta R, McDonough J, Yin X, Peterson J, Chang A, Torres T, et al. Mitochondrial dysfunction as a cause of axonal degeneration in multiple sclerosis patients. Ann Neurol. 2006;59:478–489. doi: 10.1002/ana.20736. [DOI] [PubMed] [Google Scholar]
- Fowler CJ, Tiger G, Ligresti A, Lopez-Rodriguez ML, Di Marzo V. Selective inhibition of anandamide cellular uptake versus enzymatic hydrolysis – a difficult issue to handle. Eur J Pharmacol. 2004;492:1–11. doi: 10.1016/j.ejphar.2004.03.048. [DOI] [PubMed] [Google Scholar]
- Freeman RM, Adekanmi O, Waterfield MR, Waterfield AE, Wright D, Zajicek J. The effect of cannabis on urge incontinence in patients with multiple sclerosis: a multicentre, randomised placebo-controlled trial (CAMS-LUTS) Int Urogynecol J Pelvic Floor Dysfunct. 2006;17:636–641. doi: 10.1007/s00192-006-0086-x. [DOI] [PubMed] [Google Scholar]
- Galiegue S, Mary S, Marchand J, Dussossoy D, Carriere D, Carayon P, et al. Expression of central and peripheral cannabinoid receptors in human immune tissues and leukocyte subpopulations. Eur J Biochem. 1995;232:54–61. doi: 10.1111/j.1432-1033.1995.tb20780.x. [DOI] [PubMed] [Google Scholar]
- Glaser ST, Abumrad NA, Fatade F, Kaczocha M, Studholme KM, Deutsch DG. Evidence against the presence of an anandamide transporter. Proc Natl Acad Sci USA. 2003;100:4269–4274. doi: 10.1073/pnas.0730816100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajos N, Ledent C, Freund TF. Novel cannabinoid-sensitive receptor mediates inhibition of glutamatergic synaptic transmission in the hippocampus. Neuroscience. 2001;106:1–4. doi: 10.1016/s0306-4522(01)00287-1. [DOI] [PubMed] [Google Scholar]
- Howlett AC, Barth F, Bonner TI, Cabral G, Casellas P, Devane WA, et al. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol Rev. 2002;54:161–202. doi: 10.1124/pr.54.2.161. [DOI] [PubMed] [Google Scholar]
- Huffman JW, Yu S, Showalter V, Abood ME, Wiley JL, Compton DR, et al. Synthesis and pharmacology of a very potent cannabinoid lacking a phenolic hydroxyl with high affinity for the CB2 receptor. J Med Chem. 1996;39:3875–3877. doi: 10.1021/jm960394y. [DOI] [PubMed] [Google Scholar]
- Ivanhoe CB, Reistetter TA. Spasticity: the misunderstood part of the upper motor neuron syndrome. Am J Phys Med Rehabil. 2004;83 Suppl:S3–S9. doi: 10.1097/01.phm.0000141125.28611.3e. [DOI] [PubMed] [Google Scholar]
- Kaczocha M, Hermann A, Glaser ST, Bojesen IN, Deutsch DG. Anandamide uptake is consistent with rate-limited diffusion and is regulated by the degree of its hydrolysis by fatty acid amide hydrolase. J Biol Chem. 2006;281:9066–9075. doi: 10.1074/jbc.M509721200. [DOI] [PubMed] [Google Scholar]
- Kawamura Y, Fukaya M, Maejima T, Yoshida T, Miura E, Watanabe M, et al. The CB1 cannabinoid receptor is the major cannabinoid receptor at excitatory presynaptic sites in the hippocampus and cerebellum. J Neurosci. 2006;26:2991–3001. doi: 10.1523/JNEUROSCI.4872-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearn CS, Blake-Palmer K, Daniel E, Mackie K, Glass M. Concurrent stimulation of cannabinoid CB1 and dopamine D2 receptors enhances heterodimer formation: a mechanism for receptor cross-talk. Mol Pharmacol. 2005;67:1697–1704. doi: 10.1124/mol.104.006882. [DOI] [PubMed] [Google Scholar]
- Ligresti A, Cascio MG, Pryce G, Kulasegram S, Beletskaya I, De Petrocellis L, et al. New potent and selective inhibitors of anandamide reuptake with antispastic activity in a mouse model of multiple sclerosis. Br J Pharmacol. 2006;147:83–91. doi: 10.1038/sj.bjp.0706418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maresz K, Carrier EJ, Ponomarev ED, Hillard CJ, Dittel BN. Modulation of the cannabinoid CB2 receptor in microglial cells in response to inflammatory stimuli. J Neurochem. 2005;95:437–445. doi: 10.1111/j.1471-4159.2005.03380.x. [DOI] [PubMed] [Google Scholar]
- Moore SA, Nomikos GG, Dickason-Chesterfield AK, Schober DA, Schaus JM, Ying BP. Identification of a high-affinity binding site involved in the transport of endocannabinoids. Proc Natl Acad Sci USA. 2005;102:17852–17857. doi: 10.1073/pnas.0507470102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- Ortega-Gutierrez S, Hawkins EG, Viso A, Lopez-Rodriguez ML, Cravatt BF. Comparison of anandamide transport in FAAH wild-type and knockout neurons: evidence for contributions by both FAAH and the CB1 receptor to anandamide uptake. Biochemistry. 2004;43:8184–8190. doi: 10.1021/bi049395f. [DOI] [PubMed] [Google Scholar]
- Oz M. Receptor-independent actions of cannabinoids on cell membranes: focus on endocannabinoids. Pharmaco Ther. 2006;111:114–144. doi: 10.1016/j.pharmthera.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Pertwee RG. Pharmacology of cannabinoid receptor ligands. Curr Med Chem. 1999;6:635–664. [PubMed] [Google Scholar]
- Pertwee RG. Cannabinoids and multiple sclerosis. Pharmacol Ther. 2002;95:165–174. doi: 10.1016/s0163-7258(02)00255-3. [DOI] [PubMed] [Google Scholar]
- Pryce G, Ahmed Z, Hankey DJ, Jackson SJ, Croxford JL, Pocock JM, et al. Cannabinoids inhibit neurodegeneration in models of multiple sclerosis. Brain. 2003;126:2191–2202. doi: 10.1093/brain/awg224. [DOI] [PubMed] [Google Scholar]
- Ralevic V, Kendall DA, Jerman JC, Middlemiss DN, Smart D. Cannabinoid activation of recombinant and endogenous vanilloid receptors. Eur J Pharmacol. 2001;424:211–219. doi: 10.1016/s0014-2999(01)01153-0. [DOI] [PubMed] [Google Scholar]
- Rinaldi-Carmona M, Barth F, Héaulme M, Shire D, Calandra B, Congy C, et al. SR141716A, a potent and selective antagonist of the brain cannabinoid receptor. FEBS Lett. 1994;350:240–244. doi: 10.1016/0014-5793(94)00773-x. [DOI] [PubMed] [Google Scholar]
- Rios C, Gomes I, Devi LA. mu opioid and CB1 cannabinoid receptor interactions: reciprocal inhibition of receptor signaling and neuritogenesis. Br J Pharmacol. 2006;148:387–395. doi: 10.1038/sj.bjp.0706757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi KA, Castillo PE. The CB1 cannabinoid receptor mediates glutamatergic synaptic suppression in the hippocampus. Neuroscience. 2006;139:795–802. doi: 10.1016/j.neuroscience.2006.01.024. [DOI] [PubMed] [Google Scholar]
- Van Sickle MD, Duncan M, Kingsley PJ, Mouihate A, Urbani P, Mackie K, et al. Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science. 2005;310:329–332. doi: 10.1126/science.1115740. [DOI] [PubMed] [Google Scholar]
- Vaney C, Heinzel-Gutenbrunner M, Jobin P, Tschopp F, Gattlen B, Hagen U, et al. Efficacy, safety and tolerability of an orally administered cannabis extract in the treatment of spasticity in patients with multiple sclerosis: a randomized, double-blind, placebo-controlled, crossover study. Mult Scler. 2004;10:417–424. doi: 10.1191/1352458504ms1048oa. [DOI] [PubMed] [Google Scholar]
- Varvel SA, Bridgen DT, Tao Q, Thomas B, Martin B, Lichtman A. Δ9-Tetrahydrocannabinol accounts for the antinociceptive, hypothermic, and cataleptic effects of marijuana in mice. J Pharmacol Exp Ther. 2005;314:329–337. doi: 10.1124/jpet.104.080739. [DOI] [PubMed] [Google Scholar]
- Wade DT, Makela P, Robson P, House H, Bateman C. Do cannabis based medicinal extracts have general or specific effects on symptoms in multiple sclerosis? A double-blind, randomized, placebo-controlled study on 160 patients. Mult Scler. 2004;10:434–441. doi: 10.1191/1352458504ms1082oa. [DOI] [PubMed] [Google Scholar]
- Wager-Miller J, Westenbroek R, Mackie K. Dimerization of G protein-coupled receptors: CB1 cannabinoid receptors as an example. Chem Phys Lipids. 2002;121:83–89. doi: 10.1016/s0009-3084(02)00151-2. [DOI] [PubMed] [Google Scholar]
- Wilkinson JD, Whalley BJ, Baker D, Pryce G, Constanti A, Gibbons S, et al. Medicinal cannabis: is Δ9-tetrahydrocannabinol necessary for all its effects? J Pharm Pharmacol. 2003;55:1687–1694. doi: 10.1211/0022357022304. [DOI] [PubMed] [Google Scholar]
- Wilson RI, Nicoll RA. Endocannabinoid signalling in the brain. Science. 2002;296:678–682. doi: 10.1126/science.1063545. [DOI] [PubMed] [Google Scholar]
- Wotherspoon G, Fox A, McIntyre P, Colley S, Bevan S, Winter J. Peripheral nerve injury induces cannabinoid receptor 2 protein expression in rat sensory neurons. Neuroscience. 2005;135:235–245. doi: 10.1016/j.neuroscience.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Zajicek J, Fox P, Sanders H, Wright D, Vickery J, Nunn A, et al. Cannabinoids for the treatment of other symptoms related to multiple sclerosis (CAMS study): multicentre, randomized, placebo-controlled trial. Lancet. 2003;362:1517–1526. doi: 10.1016/S0140-6736(03)14738-1. [DOI] [PubMed] [Google Scholar]
- Zajicek JP, Sanders HP, Wright DE, Vickery PJ, Ingram WM, Reilly SM, et al. Cannabinoids in multiple sclerosis (CAMS) study: safety and efficacy data for 12 months follow up. J Neurol Neurosurg Psychiatry. 2005;76:1664–1669. doi: 10.1136/jnnp.2005.070136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer A, Zimmer AM, Hohmann AG, Herkenham M, Bonner TI. Increased mortality, hypoactivity, and hypoalgesia in cannabinoid CB1 receptor knockout mice. Proc Natl Acad Sci USA. 1999;96:5780–5785. doi: 10.1073/pnas.96.10.5780. [DOI] [PMC free article] [PubMed] [Google Scholar]