Abstract
Background and purpose:
Mitochondrial aldehyde dehydrogenase (ALDH-2) has been shown to provide a pathway for bioactivation of organic nitrates and to be prone to desensitization in response to highly potent, but not to less potent, nitrates. We therefore sought to support the hypothesis that bioactivation by ALDH-2 critically depends on the number of nitrate groups within the nitrovasodilator.
Experimental approach:
Nitrates with one (PEMN), two (PEDN; GDN), three (PETriN; glyceryl trinitrate, GTN) and four (pentaerithrityl tetranitrate, PETN) nitrate groups were investigated. Vasodilatory potency was measured in isometric tension studies using isolated aortic segments of wild type (WT) and ALDH-2−/− mice. Activity of the cGMP-dependent kinase-I (reflected by levels of phosphorylated VAsodilator Stimulated Phosphoprotein, P-VASP) was quantified by Western blot analysis, mitochondrial dehydrogenase activity by HPLC. Following incubation of isolated mitochondria with PETN, PETriN-chromophore and PEDN, metabolites were quantified using chemiluminescence nitrogen detection and mass spectrometry.
Key results:
Compared to WT, vasorelaxation in response to PETN, PETriN and GTN was attenuated about 10fold in ALDH-2−/− mice, identical to WT vessels preincubated with inhibitors of ALDH-2. Reduced vasodilator potency correlated with reduced P-VASP formation and diminished biotransformation of the tetranitrate- and trinitrate-compounds. None of these findings were observed for PEDN, GDN and PEMN.
Conclusions and implications:
Our results support the crucial role of ALDH-2 in bioactivating highly reactive nitrates like GTN, PETN and PETriN. ALDH-2-mediated relaxation by organic nitrates therefore depends mainly on the number of nitrate groups. Less potent nitrates like PEDN, GDN and PEMN are apparently biotransformed by other pathways.
Keywords: nitric oxide, nitrovasodilators, pentaerithrityl tetranitrate, mitochondrial aldehyde dehydrogenase
Introduction
For more than 100 years, organic nitrates have been playing an important role in the treatment of coronary artery disease, myocardial infarction, heart failure and arterial hypertension (Abrams, 1995).
Until recently, the exact mechanism of bioactivation of nitroglycerin (glyceryl trinitrate, GTN) was unknown. In 2002, Chen et al. showed, that the mitochondrial isoform of aldehyde dehydrogenase (ALDH-2) bioactivates GTN and that this process involves redox-sensitive thiol groups; they attributed thereby a reductase function to this enzyme, in addition to the established dehydrogenase and esterase functions. Further studies revealed that tolerance to GTN is accompanied by an inhibition of the enzyme due to stimulation of reactive oxygen species within mitochondria leading to an oxidation of these SH groups (Sydow et al., 2004). In 2005, studies with mice with genetic deletion of ALDH-2 (ALDH-2−/−) proved the concept of the central role for ALDH-2 in bioactivating nitrates and also in nitrate tolerance development (Chen et al., 2005). The clinical relevance of this concept was demonstrated by studies with Asian individuals with a point mutation of ALDH-2 (glutamate to lysine at Glu504–Glu487 in the mature enzyme) rendering the enzyme inactive, who show a significantly decreased responsiveness to GTN (Mackenzie et al., 2005; Li et al., 2006).
Recently, by using inhibitors of aldehyde dehydrogenases such as daidzin and benomyl, we showed that, similarly to GTN, the organic nitrate pentaerithrityl tetranitrate (PETN) with four nitrate (–ONO2) groups and its metabolite pentaerithrityl trinitrate (PETriN) is bioactivated by this nitrate reductase in Wistar rats (Daiber et al., 2004b). In contrast, the concentration–relaxation relationship to acute challenges to pentaerithrityl dinitrate (PEDN), pentaerithrityl mononitrate (PEMN) and isosorbide dinitrate (ISDN) was not at all modified by ALDH-inhibitors. Also Chen et al. (2005) did not observe impaired vasorelaxation in response to ISDN in ALDH-2−/− mice. It remained unclear although, whether the molecular structure of the dinitrate or the number of nitrate groups determined susceptibility to the ALDH-2-dependent bioactivation pathway.
To elucidate the bioactivation process of organic nitrates, we used organic nitrates with the identical core structure and differing number of nitrate groups – PETN, PETriN, PEDN and PEMN as well as GTN and glyceryl dinitrate (1,3-GDN) (see Figure 1) – and traced the metabolites of PETN (PETriN, PEDN and PEMN) through the bioactivation process. In the present study with the use of ALDH-2−/− mice, we have shown that the nitrate reductase activity of ALDH-2 is important for the bioactivation of highly potent organic nitrates in general and that the number of nitrate groups rather than other structural features of the nitrovasodilator determined the bioactivation pathway.
Figure 1.
Structures of PETN, PETriN, PEDN, PEMN, GTN, 1,3-GDN and spermine NONOate.
Materials and methods
Animals
Young adult male ALDH-2−/− mice (8-week-old) were matched with respect to age and gender with C57B6 wild-type mice (WT) representing the genetic background of the ALDH-2−/− mice; animals were housed under a 12 h light/dark cycle and had free access to standard chow and water ad libitum. The generation of the ALDH-2 null mutant was described previously (Kitagawa et al., 2000). A total number of 88 animals were used in this study. Animals were killed by anaesthesia with 5% isoflurane in room air and subsequent exsanguination. Aortas and hearts were prepared as described previously (Daiber et al., 2005). All animal treatment was carried out in accordance with the Declaration of Helsinki and with the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the US. National Institutes of Health and has been approved by the Ethics Committee of the University Hospital Mainz.
Isometric tension studies
Vasodilator responses to organic nitrates and other vasodilators were assessed as previously described (Munzel et al., 1995). Precontraction by prostaglandin F2α (PGF2α; 3 μM) resulted in a similar tone in aortae from WT and knockout mice (1.7–1.9 g). In separate experiments, vessels from mice were preincubated with benomyl (10 μM), an irreversible inhibitor of ALDH as described (Sydow et al., 2004; Daiber et al., 2005), or with 1H-[1,2,4]oxodiazolo[4,3-a]quinoxalin-1-one (ODQ, 3 μM), an inhibitor of soluble guanylyl cyclase (sGC).
Western blot analysis and activity assay for cGMP-dependent kinase I
Aortic segments (1 cm) from ALDH-2−/− and WT mice were frozen and homogenized in liquid nitrogen. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and electroblotting was performed as described (Mulsch et al., 2001; Schulz et al., 2002). Immunoblotting was performed using specific antibodies for ALDH-2 (1:2000, polyclonal) as previously characterized (McMichael et al., 1986) to measure protein expression. To assess the activity of sGC and its downstream target cGK-1, immunoblotting was performed with a mouse monoclonal antibody (clone 16C2, 0.5 μg ml−1, Calbiochem, La Jolla, CA, USA) specific for vasodilator stimulated phosphoprotein (VASP) phosphorylated at serine239, as described (Oelze et al., 2000). The extent of VASP phosphorylation (P-VASP) reflects the activity of cGK-1 and was tested in the absence or presence of either solvent (1% DMSO), 1 μM PETN or 1 mM PEDN, respectively (incubation of isolated mouse aortic rings at 37°C for 10 min).
Biotransformation of organic nitrates in isolated heart mitochondria
Mouse heart mitochondria were prepared according to a published method (Raha et al., 2000) which was slightly modified (Daiber et al., 2004a). For sample preparation, we used the protocol as recently published (Daiber et al., 2005). The mitochondrial fraction was incubated with 1 μM and 0.25 μM PETN or 1 μM and 10 μM PEDN for 30 min at 37°C. Suspensions were then snap frozen and stored at –80°C. Levels of PETN and PEDN were measured by an HPLC-based method with chemiluminescence nitrogen detection (CLND) as described (Seeling and Lehmann, 2006). For detailed protocol of analysis, values in table format and chromatograms see supplementary information (Supplementary Figure 4S and Supplementary Table 2S).
ALDH-2 dehydrogenase activity in isolated mouse heart mitochondria
The activity of ALDH-2 in isolated mitochondria was determined by measuring the conversion of 2-hydroxy-3-nitro-benzaldehyde to 2-hydroxy-3-nitro-benzoic acid using a modified published protocol (Daiber et al., 2004b). For detailed protocol of analysis, characterization of highly specific nitrated substrate and chromatograms see Supplementary information (Supplementary Figures 2S and 3S).
Statistical analysis
Results are expressed as mean±s.e.m. One-way ANOVA (with Bonferroni's or Dunn's correction for comparison of multiple means) was used for comparisons of vascular responses, protein expression levels and ALDH-2 dehydrogenase activity, paired t-test was used for CLND analysis. In tension recordings, the EC50 value for each experiment was obtained by log-transformation. P<0.05 was considered significant.
Materials
GTN (1 mg ml−1) was provided by G Pohl-Boskamp (Hohenlockstedt, Germany). PETN (with 75 w/w% lactose), PETriN, PEDN and PEMN were kindly donated by Actavis Deutschland GmbH (Langenfeld, Germany). 1,3-GDN (115 mg, >95% purity) was prepared by Kathrin Lange, Institute of Pharmacy, University of Jena, as described in the literature (Shan et al., 2004), for NMR spectrum see Supplementary Figure 9S (online supplement). 1,2-GDN (1 mg ml−1 in acetonitrile) was purchased from Cerilliant Co. (Round Rock, TX, USA). Spermine NONOate was from Cayman Chemical (Ann Arbor, MI, USA), benomyl (methyl-1-(butylcarbamoyl)-2-benzimidazole carbamate) from Chem Service (West Chester, PA, USA) and daidzin (7-glucoside of 4′,7-dihydroxy-isoflavone) from Indofine Chemical Company Inc. (Hillsborough, NJ, USA). All other chemicals were of analytical grade and obtained from Sigma-Aldrich, Fluka or Merck. For structures of vasodilators used in these experiments see Figure 1.
Results
Isometric tension studies
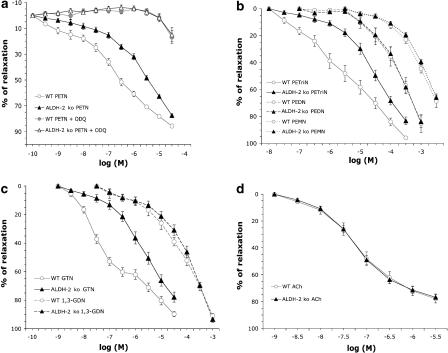
To test the responsiveness of the animals to organic nitrates and other vasodilators, isolated mouse aortic segments (pre-contracted with 3 μM PGF2α) were exposed to increasing concentrations of PETN and its metabolites as well as GTN, 1,3-GDN, spermine NONOate, isosorbide-5-mononitrate and ACh to obtain concentration relaxation relationships (Figure 2a–d and Supplementary information, Supplementary Figure 1S; the structures of the nitrates used are shown in Figure 1). In aortic rings from WT control mice, there was a clear relationship between organic nitrate potency and the number of nitrate groups (the exact –log(EC50)-values are given in Table 1). In ALDH-2−/− mice, concentration relaxation relationship for PETN and PETriN showed a clear shift to the right, the potency being decreased by approximately 10-fold (Figure 2a and b). In contrast, the concentration-relaxation curves in response to PEDN and PEMN in ALDH-2−/− mice were identical to those from WT mice (Figure 2b). We also observed an identical concentration–relaxation-response for isosorbide mononitrate, yielding an EC50-value of 2.45 mM for WT and 2.88 mM for ALDH-2−/− mice (see Supplementary information, Supplementary Figure 1S and Supplementary Table 1S).
Figure 2.
Concentration–relaxation curves of isolated aortic rings from C57B6 (wild type, WT) and ALDH-2−/− mice using different vasodilators after preconstriction with prostaglandin F2α (3 μM): (a) Effects of inhibition of sGC with ODQ on responses to PETN (b) Comparison between PETriN, PEDN and PEMN (dotted line); (c) Comparison between GTN and 1,3-GDN (d) Effects of ACh. Results are statistically summarized in Table 1.
Table 1.
Potency and efficacy of vasodilator-induced relaxation in isolated aortic rings from WT- vs ALDH-2−/− mice
| PETN | PETN+10 μM Benomyl | PETriN | PEDN | PEDN+10 μM Benomyl | PEMN | PETN+3 μM ODQ | GTN | 1,3-GDN | ACh | |
|---|---|---|---|---|---|---|---|---|---|---|
| WTi | 6.77±0.09 (n=7) | 6.12±0.23a,b (n=8) | 5.51±0.20 (n=10) | 3.89±0.07a (n=11) | 3.98±0.18c (n=7) | 3.24±0.03 (n=12) | 4.83±0.03a (n=8) | 7.24±0.08 (n=8) | 4.26±0.11 (n=8) | 7.19±0.07 (n=17) |
| ALDH-2−/−i | 5.84±0.09a (n=12) | 5.77±0.27a,b (n=6) | 4.69±0.08d (n=12) | 3.84±0.08c (n=12) | 4.25±0.17c (n=6) | 3.17±0.03e (n=11) | 4.86±0.03a (n=8) | 5.87±0.08f (n=7) | 3.92±0.16 g (n=8) | 7.22±0.05 h (n=19) |
| WTj | 85.6±1.14 (n=7) | 77.2±2.32a,b (n=8) | 95.8±1.21 (n=10) | 85.2±2.19a (n=11) | 76.8±0.18c (n=7) | 68.7±6.76 (n=12) | 10.9±5.72a (n=12) | 89.8±1.85 (n=8) | 91.03±1.64 (n=8) | 78.0±3.01 (n=17) |
| ALDH-2−/−j | 77.5±1.10a (n=12) | 75.3±3.21a,b (n=6) | 83.2±2.80d (n=12) | 83.9±1.29c (n=12) | 78.2±8.51c (n=6) | 65.9±2.66e (n=11) | 15.8±5.61a (n=12) | 77.9±3.49f (n=7) | 93.44±2.10 g (n=8) | 76.8±2.43 h (n=19) |
Abbreviations: ALDH, aldehyde dehydrogenase; 1,3-GDN, 1,3-glyceryl dinitrate; NS, not significant; PEDN, pentaerithrityl dinitrate; WT, wild type.
P<0.05 vs WT PETN.
n.s. vs ALDH-2−/− PETN.
n.s. vs WT PEDN.
P<0.05 vs WT PETriN.
n.s. vs WT PEMN.
P<0.05 vs WT GTN.
n.s. vs WT 1,3-GDN.
n.s. vs WT Ach; (n) indicates number of experiments.
The values shown are –log(EC50) [M] of concentration–relaxation curves±s.e.m.
Maximal relaxation (% relaxation) of concentration–relaxation-curves±s.e.m.
In a second series of experiments, aortic rings were preincubated with the potent ALDH-2-inhibitor (but non-selective for ALDH-2), benomyl. In rings from WT mice, benomyl shifted the concentration–relaxation relationship in response to PETN, to the right, comparable to that observed in the ALDH-2−/− mice. Pretreatment of vessels from ALDH-2−/− mice with benomyl did not cause a further modification of the PETN concentration–relaxation response. Additionally, in WT mice, the concentration–relaxation relationship for PEDN (which was not impaired in the knockout animals) was not affected by pharmacological inhibition of ALDH-2 (see Supplementary information, Supplementary Figure 1S and Table 1S).
To assess the importance of the sGC/cGMP pathway for the expression of PETN bioactivity, we incubated aortic rings with an inhibitor of sGC, ODQ. In these tissues, relaxation in response to PETN was essentially absent in the WT as well as in the ALDH-2−/− mice (Figure 2a).
The concentration–relaxation curves for GTN was significantly shifted to the right in the knockout mice, in agreement with previous reports (Chen et al., 2005), whereas the response to 1,3-GDN was identical in WT and in ALDH-2−/− mice (Figure 2c).
Vasorelaxation in response to the endothelium-dependent vasodilator ACh was not altered in ALDH-2−/− mice as compared to WT, showing that ALDH-2 deletion did not influence endothelium-dependent NO-signalling in these mice (Figure 2d). These findings were further supported by identical concentration-relaxation curves in rings from WT and ALDH-2 knockout mice in response to the direct NO-donor spermine NONOate, yielding an EC50 of 549 nM for WT and 501 nM for ALDH-2−/− mice (see Supplementary information, Supplementary Figure 1S and Supplementary Table 1S).
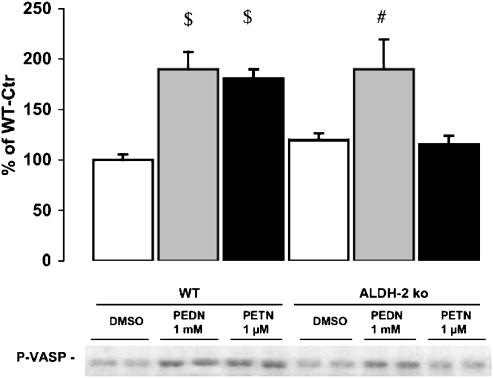
NO/cGMP/cGK-I signalling pathway monitored by P-VASP formation
Phosphorylation of the vasodilator stimulated phosphoprotein (P-VASP) is a surrogate parameter for the activity of the cGMP-dependent kinase I (cGK-I) and is a suitable measure of NO bioactivity derived from organic nitrates, inside the vessel wall (Figure 3). For ex vivo incubation of isolated aortic rings with the organic nitrates, the chosen concentrations were based on the results from the vasorelaxation studies described above. PEDN (1 mM) caused maximal vasorelaxation in both WT and ALDH-2−/− tissues, 1 μM PETN caused 61±2% in the WT tissues and 32±2% relaxation in tissues from the knockout mice.
Figure 3.
Activity of cGK-1 as reflected by levels of phosphorylated VAsodilator Stimulated Phosphoprotein at serine 239 (P-VASP) in response to acute challenges with organic nitrates. Aortic segments of C57B6 (WT) and ALDH-2−/− (ALDH-2 ko) were incubated with PEDN, PETN or solvent (DMSO). Bars reflect densitometric analysis. The Western blot is representative of five independent experiments. $P<0.05 vs WT DMSO; #P<0.05 vs WT DMSO, ALDH-2 ko DMSO and ALDH-2 ko PETN, NS vs WT PETN and WT PEDN.
Basal levels of P-VASP after incubation with solvent (1% DMSO) were not significantly different between WT and ALDH-2−/− mice. Stimulation with 1 mM PEDN induced a similar increase in P-VASP levels in tissues from both strains of mice. Incubation with 1 μM PETN resulted in no increase of P-VASP levels in aortic rings from the knockout strain, compared to solvent incubation, whereas it was significantly increased in those from WT mice, compared to solvent.
In additional experiments, we measured the amount of nitric oxide by electron paramagnetic resonance using colloid iron(II)-dithiocarbamate complex, Fe(DETC)2, as a spin trap. Isolated whole aortas of WT and ALDH-2−/− mice were incubated with 20 μM PETN. We observed no difference in NO production between the two groups (see Supplementary information, Supplementary Figure 8S).
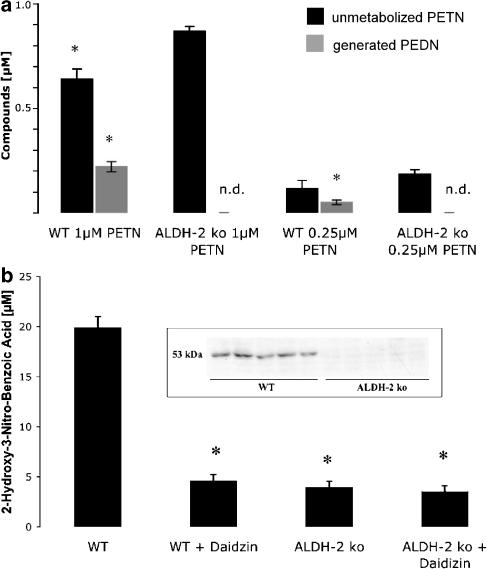
Assessment of biotransformation of organic nitrates
The conversion of PETN and PEDN in mitochondria from WT and ALDH-2−/− mice was measured by CLND analysis. We chose these compounds based on the organ bath experiments, because of their apparently differing susceptibility to the ALDH-2-dependent bioactivation pathway.
In mitochondria from WT, 36 and 48% of PETN starting concentrations were biotransformed to PEDN, whereas in ALDH-2−/− mice levels of PEDN were below the limit of detection (Figure 4a). After incubation with PEDN (10 and 1 μM), levels of PEDN were not significantly different in WT (8.1±0.3 and 0.78±0.12 μM, respectively) and ALDH-2−/− mice (8.4±0.0 and 0.82±0.13 mM, respectively; n=2–3) (see Supplementary Table 2S). The rate of recovery was 87% for PEDN and 94% for PETN using this method.
Figure 4.
(a) Chemiluminescence nitrogen detection (CLND) of PETN and PEDN in suspensions of isolated mitochondria. Bars reflect the amount of recovered organic nitrate by CLND analysis after incubation of isolated mouse liver mitochondria with the concentration shown on the x-axis. *P<0.05 vs ALDH-2 ko; n.d.: not detected, e.g. peaks were below the level of quantification and therefore not elegible for analysis. (b) Activity of ALDH-2 was assessed by measuring the conversion of 2-hydroxy-3-nitro-benzaldehyde to the benzoic acid product (2H3N-BA) in isolated mitochondria. Insert shows a Western blot of ALDH-2 in extracts of aortic tissue. *P<0.05 vs WT.
In another set of experiments, we assessed the capability of isolated liver mitochondria to biotransform the organic nitrate KL-61, a PETriN derivate, to its dinitrate metabolite using mass spectrometric analysis. KL-61 bioactivation was significantly reduced in ALDH-2−/− mice compared to WT (see Supplementary information, Supplementary Figures 5S, 6S and 7S).
Measurement of ALDH-2 dehydrogenase activity
Aldehyde dehydrogenase activity was measured in isolated mitochondria to verify that ALDH-2 activity was absent in knockout animals and to test whether other isoforms could compensate for its absence. Aldehyde dehydrogenase activity was dramatically reduced (only background activity remained) in the ALDH-2−/− mice as compared to WT controls. This effect could be mimicked by preincubation of WT mitochondria with the selective ALDH-2 inhibitor, daidzin (Figure 4b). Western blots of extracts of aortic tissue showed that the enzyme was absent in extracts from ALDH-2 knockout mice (Figure 4b, insert). The original HPLC chromatograms are shown in the Supplementary information (supplementary Figures 2S and 3S).
Discussion
Using ALDH-2−/− animals and PETN as nitrovasodilator and model substance, the results of our study indicate that ALDH-2 is, in general, essential for the bioactivation of organic nitrates with high potency/reactivity within the clinical concentration range and therefore provides the molecular proof of our recently proposed concept based on experiments with inhibitors of ALDH-2 (Daiber et al., 2004b).
Chen et al. (2002) showed in 2002 that mitochondrial ALDH provides a pathway of nitroglycerin bioactivation. In 2005 the same authors extended their concept by demonstrating, that genetic deletion of ALDH-2 leads to a striking impairment of the vascular responsiveness to GTN, but not to ISDN or sodium nitroprusside (SNP) (Chen et al., 2005).
It remained unclear, however, whether the ALDH-2-independent transformation of a dinitrate like ISDN to a vasoactive metabolite was due to the different chemical structure of the molecule, compared to GTN, or to the different number of nitrate groups. This uncertainty also raised the question whether the moderate potency of ISDN compared to GTN is secondary to steric reasons and/or a consequence of a hindered access to the active site of ALDH-2. By choosing PETN and its metabolites PETriN, PEDN and PEMN as well as GTN and its dinitrate-metabolite 1,3-GDN as organic nitrates, which differ only in the number of nitrate groups adjacent to the core structure, we could explore these questions in our experiments. PETN is an antianginal drug that is widely used across Europe in the treatment of stable coronary artery disease. It is reported to clinically provoke less tolerance than other nitrovasodilators such as GTN or ISDN (Jurt et al., 2001; Schwemmer and Bassenge, 2003).
Our results clearly show, that bioactivation of these organic nitrates by ALDH-2 does not depend on different molecular structures but rather on the number of nitrate groups and, consequently, on reactivity (positive charge on the nitrogen in the –ONO2 group, electrophilicity). It is important to note, that only the highly reactive and therefore highly potent nitrates PETN, GTN, PETriN but not the less potent ones 1,3-GDN, PEDN, PEMN and ISMN showed an impaired potency in ALDH-2 knockout mice, clearly indicating that only tri- and tetranitrates, but not di- and mononitrates, were bioactivated by ALDH-2. 1,2-GDN (data not shown) was tested in a pilot study and yielded the same concentration–relaxation-response (up to 30%) as 1,3-GDN.
In 2004, our group demonstrated in rat aortic rings, that the concentration–relaxation curves in response to the potent organic nitrates PETN and PETriN were shifted to higher concentrations after preincubation with the potent ALDH-2 inhibitor benomyl (Daiber et al., 2004b). Here we show, that a genetic deletion of ALDH-2 leads to results comparable to those of pharmacological inhibition of the enzyme by benomyl. Indeed, benomyl yields no additional effect when given to tissues from the knockout strain, indicating that there are no other ALDH-isoforms involved in the bioactivation of organic nitrates in the deficient mice, as benomyl is not ALDH-2-specific. This assumption is supported by the observation, that aldehyde dehydrogenase activity, determined by measuring the conversion of 2-hydroxy-3-nitro-benzaldehyde to 2-hydroxy-3-nitro-benzoic acid was completely absent in the mitochondria of ALDH-2−/− mice, suggesting that no other isoform of ALDH was present in the mitochondria to take over the function of the deleted enzyme.
Owing to the high lipophilicity of organic nitrates – GTN has an oil/water coefficient of more than 100 – one expects these drugs to accumulate in mitochondria, known to be a sink for lipophilic substances (Bell et al., 1963). Transformation by other ALDH isoforms located in aqueous phases of the cell, for instance,cytosolic ALDH-1, does not seem very likely. In addition to our organ bath data, these considerations are supported by recent data from Oyama et al. (2005) who did not find higher levels of ALDH-1 in tissue of ALDH-2−/− mice compared to controls.
The inability of other ALDH-isoforms to compensate for ALDH-2-deficiency in terms of bioactivating organic nitrates could also explain the clinical observation by Mackenzie et al. (2005) who described an impaired GTN- but not SNP-dependent relaxation in individuals of eastern Asian background, carrying a defective variant of ALDH-2. This assumption was also supported by recent clinical studies in Chinese patients with coronary artery disease (Li et al., 2006).
The crucial role of ALDH-2 in bioactivating reactive (potent) organic nitrates was proven in the present studies by CLND analysis of the products formed in isolated mitochondria upon incubation with PETN and PEDN. PETN was virtually unmetabolized in the knockout strain, even at the pharmacological concentration of 1 μM. Interestingly, the same concentration induced approximately 50% vasorelaxation in the aortic rings, suggesting that there must be a site for non-selective bioactivation of organic nitrates, outside the mitochondria. The biotransformation of PEDN, although, was unchanged in the ALDH-2−/− mice compared to control, showing that ALDH-2 was not important for the bioactivation of organic nitrates with low potency, as already reported for ISDN (Chen et al., 2005). Here we have provided evidence, that this phenomenon described by Chen is more likely to be determined by the number of nitrate groups attached to the core structure of the molecule rather than by other structural features of the molecule.
Bioactivation of organic nitrates beyond ALDH-2
Intriguingly, Oyama et al. (2005) found higher levels of the cytochorome P 450 isoform, CYP2E1, in liver of ALDH-2−/− mice compared to WT, whereas levels of CYP1A1 and CYP4B1 were not different. This favours the possibility that members of the cytochrome P450 family could play a role in bioactivating organic nitrates, especially in the lower part (>1 μM) of the concentration-relaxation curve of highly potent organic nitrates, as well as in less potent organic nitrates (Mulsch et al., 1995; Minamiyama et al., 1999; Kleschyov et al., 2003; Munzel et al., 2005). Kleschyov et al. (2003) demonstrated that GTN released NO only after incubation in concentrations exceeding 1 μM, not below (Kleschyov et al., 2003). Our EPR data, showing no differences between WT and ALDH-2−/− mice regarding the release of NO from PETN above that threshold of 1 μM support the idea of a non-specific site for organic nitrate bioactivation. Moreover, maximal relaxation was not altered for highly reactive organic nitrates like PETN, PETriN or GTN either in ALDH deficiency (Figure 2) or after inhibition of ALDH with benomyl (Daiber et al., 2004b). The potential role of cytochrome P450 or other enzymatic systems plus possible non-specific bioactivation involving low molecular weight thiols like cysteine (Kozlov et al., 2003; Gorren et al., 2005) is made more likely by the observations in this study and in others, that inhibition of ALDH-2 did not lead to a total loss of vasorelaxation in response to organic nitrates. either in humans or in animal models, (Chen et al., 2002, 2005; Sydow et al., 2004; Daiber et al., 2004b; Mackenzie et al., 2005).
Summary and conclusion
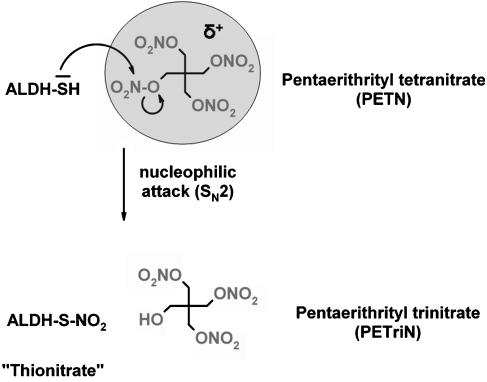
The high affinity pathway for organic nitrates via ALDH-2 can be explained, at least in part, by the fact that ALDH-2 contains two highly reactive thiol groups in its active site, which interact with highly reactive organic nitrates by nucleophilic attack (Munzel et al., 2005). Thereby, three or more nitrate groups create a positively polarized centre with an electrophilicity high enough to allow a nucleophilic attack from one of the ALDH-2 thiol groups. Organic nitrates with less than three nitrate groups are not as reactive and therefore not as susceptible to bioactivation by ALDH-2 (see Figure 5).
Figure 5.
Hypothetical mechanism for the reaction of a cysteine-thiol group at the active-site of ALDH-2 with a nitrate group of PETN explaining the role of the number of nitrate (–ONO2) groups in the organic nitrate for its bioactivation. Three or more nitrate groups create a positively charged (electrophilic) environment at the nitrogens which facilitates the nucleophilic attack of an ALDH-2 thiol group with subsequent formation of a thionitrate intermediate.
Taken together, there appears to be for a highly potent organic nitrate, a threshold of about 1–10 μM that determines the pathway of bioactivation: below this threshold, the drug is predominantly metabolized by ALDH-2; above, non-specific bioactivation pathways involving enzymatic systems such as cytochrome P450s or reducing agents like low molecular weight thiols are likely to dominate.
External data objects
Acknowledgments
The expert technical assistance of Jörg Schreiner is gratefully acknowledged. We thank Dr Dirk Stalleicken for helpful discussions and providing PETN and its metabolites. This paper contains results that are part of the thesis of Lena Steinhoff. The present work was supported by Vascular Biology grants from Actavis Deutschland GmbH (TM and JL) as well as continuous funding by the German Research Foundation (DFG) (SFB 553 – C17 to TM and AD).
Abbreviations
- ACh
acetylcholine
- ALDH-2
mitochondrial aldehyde dehydrogenase
- cGK-I
cGMP-dependent kinase I
- CLND
chemiluminescence nitrogen detection
- Ctr
control animals (C57B6)
- 1,3-GDN
1,3-glyceryl dinitrate
- GTN
glyceryl trinitrate (nitroglycerin)
- ISDN
isosorbide dinitrate
- NO
nitric oxide
- ODQ
1H-[1,2,4]oxodiazolo[4,3-a]quinoxalin-1-one
- PETN
pentaerithrityl tetranitrate
- PETriN
pentaerithrityl trinitrate
- PEDN
pentaerithrityl dinitrate
- PEMN
pentaerithrityl mononitrate
- sGC
soluble guanylyl cyclase
- SNP
sodium nitroprusside
- VASP
vasodilator stimulated phosphoprotein
Conflict of interest
The authors state no conflict of interest.
Supplementary Information accompanies the paper on British Journal of Pharmacology website (http://www.nature.com/bjp)
References
- Abrams J. The role of nitrates in coronary heart disease. Arch Intern Med. 1995;155:357–364. [PubMed] [Google Scholar]
- Bell FK, O'Neill JJ, Burgison RM. Determination of the oil/water distribution coefficients of glyceryl trinitrate and two similar nitrate esters. J Pharm Sci. 1963;52:637–639. doi: 10.1002/jps.2600520706. [DOI] [PubMed] [Google Scholar]
- Chen Z, Foster MW, Zhang J, Mao L, Rockman HA, Kawamoto T, et al. An essential role for mitochondrial aldehyde dehydrogenase in nitroglycerin bioactivation. Proc Natl Acad Sci USA. 2005;102:12159–12164. doi: 10.1073/pnas.0503723102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Zhang J, Stamler JS. Identification of the enzymatic mechanism of nitroglycerin bioactivation. Proc Natl Acad Sci USA. 2002;99:8306–8311. doi: 10.1073/pnas.122225199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daiber A, Oelze M, August M, Wendt M, Sydow K, Wieboldt H, et al. Detection of superoxide and peroxynitrite in model systems and mitochondria by the luminol analogue L-012. Free Radic Res. 2004a;38:259–269. doi: 10.1080/10715760410001659773. [DOI] [PubMed] [Google Scholar]
- Daiber A, Oelze M, Coldewey M, Bachschmid M, Wenzel P, Sydow K, et al. Oxidative stress and mitochondrial aldehyde dehydrogenase activity: a comparison of pentaerythritol tetranitrate with other organic nitrates. Mol Pharmacol. 2004b;66:1372–1382. doi: 10.1124/mol.104.002600. [DOI] [PubMed] [Google Scholar]
- Daiber A, Oelze M, Sulyok S, Coldewey M, Schulz E, Treiber N, et al. Heterozygous deficiency of manganese superoxide dismutase in mice (Mn-SOD+/−): a novel approach to assess the role of oxidative stress for the development of nitrate tolerance. Mol Pharmacol. 2005;68:579–588. doi: 10.1124/mol.105.011585. [DOI] [PubMed] [Google Scholar]
- Gorren AC, Russwurm M, Kollau A, Koesling D, Schmidt K, Mayer B. Effects of nitroglycerin/L-cysteine on soluble guanylate cyclase: evidence for an activation/inactivation equilibrium controlled by nitric oxide binding and haem oxidation. Biochem J. 2005;390:625–631. doi: 10.1042/BJ20050565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurt U, Gori T, Ravandi A, Babaei S, Zeman P, Parker JD. Differential effects of pentaerythritol tetranitrate and nitroglycerin on the development of tolerance and evidence of lipid peroxidation: a human in vivo study. J Am Coll Cardiol. 2001;38:854–859. doi: 10.1016/s0735-1097(01)01414-0. [DOI] [PubMed] [Google Scholar]
- Kitagawa K, Kawamoto T, Kunugita N, Tsukiyama T, Okamoto K, Yoshida A, et al. Aldehyde dehydrogenase (ALDH) 2 associates with oxidation of methoxyacetaldehyde; in vitro analysis with liver subcellular fraction derived from human and Aldh2 gene targeting mouse. FEBS Lett. 2000;476:306–311. doi: 10.1016/s0014-5793(00)01710-5. [DOI] [PubMed] [Google Scholar]
- Kleschyov AL, Oelze M, Daiber A, Huang Y, Mollnau H, Schulz E, et al. Does nitric oxide mediate the vasodilator activity of nitroglycerin. Circ Res. 2003;93:e104–e112. doi: 10.1161/01.RES.0000100067.62876.50. [DOI] [PubMed] [Google Scholar]
- Kozlov AV, Dietrich B, Nohl H. Various intracellular compartments cooperate in the release of nitric oxide from glycerol trinitrate in liver. Br J Pharmacol. 2003;139:989–997. doi: 10.1038/sj.bjp.0705323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Zhang D, Jin W, Shao C, Yan P, Xu C, et al. Mitochondrial aldehyde dehydrogenase-2 (ALDH2) Glu504Lys polymorphism contributes to the variation in efficacy of sublingual nitroglycerin. J Clin Invest. 2006;116:506–511. doi: 10.1172/JCI26564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie IS, Maki-Petaja KM, McEniery CM, Bao YP, Wallace SM, Cheriyan J, et al. Aldehyde dehydrogenase 2 plays a role in the bioactivation of nitroglycerin in humans. Arterioscler Thromb Vasc Biol. 2005;25:1891–1895. doi: 10.1161/01.ATV.0000179599.71086.89. [DOI] [PubMed] [Google Scholar]
- McMichael M, Hellstrom-Lindahl E, Weiner H. Identification and selective precipitation of human aldehyde dehydrogenase isozymes using antibodies raised to horse liver aldehyde dehydrogenase isozymes. Alcohol Clin Exp Res. 1986;10:323–329. doi: 10.1111/j.1530-0277.1986.tb05098.x. [DOI] [PubMed] [Google Scholar]
- Minamiyama Y, Takemura S, Akiyama T, Imaoka S, Inoue M, Funae Y, et al. Isoforms of cytochrome P450 on organic nitrate-derived nitric oxide release in human heart vessels. FEBS Lett. 1999;452:165–169. doi: 10.1016/s0014-5793(99)00612-2. [DOI] [PubMed] [Google Scholar]
- Mulsch A, Bara A, Mordvintcev P, Vanin A, Busse R. Specificity of different organic nitrates to elicit NO formation in rabbit vascular tissues and organs in vivo. Br J Pharmacol. 1995;116:2743–2749. doi: 10.1111/j.1476-5381.1995.tb17236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulsch A, Oelze M, Kloss S, Mollnau H, Topfer A, Smolenski A, et al. Effects of in vivo nitroglycerin treatment on activity and expression of the guanylyl cyclase and cGMP-dependent protein kinase and their downstream target vasodilator-stimulated phosphoprotein in aorta. Circulation. 2001;103:2188–2194. doi: 10.1161/01.cir.103.17.2188. [DOI] [PubMed] [Google Scholar]
- Munzel T, Daiber A, Mulsch A. Explaining the phenomenon of nitrate tolerance. Circ Res. 2005;97:618–628. doi: 10.1161/01.RES.0000184694.03262.6d. [DOI] [PubMed] [Google Scholar]
- Munzel T, Sayegh H, Freeman BA, Tarpey MM, Harrison DG. Evidence for enhanced vascular superoxide anion production in nitrate tolerance. A novel mechanism underlying tolerance and cross-tolerance. J Clin Invest. 1995;95:187–194. doi: 10.1172/JCI117637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oelze M, Mollnau H, Hoffmann N, Warnholtz A, Bodenschatz M, Smolenski A, et al. Vasodilator-stimulated phosphoprotein serine 239 phosphorylation as a sensitive monitor of defective nitric oxide/cGMP signaling and endothelial dysfunction. Circ Res. 2000;87:999–1005. doi: 10.1161/01.res.87.11.999. [DOI] [PubMed] [Google Scholar]
- Oyama T, Isse T, Kagawa N, Kinaga T, Kim YD, Morita M, et al. Tissue-distribution of aldehyde dehydrogenase 2 and effects of the ALDH2 gene-disruption on the expression of enzymes involved in alcohol metabolism. Front Biosci. 2005;10:951–960. doi: 10.2741/1589. [DOI] [PubMed] [Google Scholar]
- Raha S, McEachern GE, Myint AT, Robinson BH. Superoxides from mitochondrial complex III: the role of manganese superoxide dismutase. Free Radic Biol Med. 2000;29:170–180. doi: 10.1016/s0891-5849(00)00338-5. [DOI] [PubMed] [Google Scholar]
- Schulz E, Tsilimingas N, Rinze R, Reiter B, Wendt M, Oelze M, et al. Functional and biochemical analysis of endothelial (dys)function and NO/cGMP signaling in human blood vessels with and without nitroglycerin pretreatment. Circulation. 2002;105:1170–1175. doi: 10.1161/hc1002.105186. [DOI] [PubMed] [Google Scholar]
- Schwemmer M, Bassenge E. New approaches to overcome tolerance to nitrates. Cardiovasc Drugs Ther. 2003;17:159–173. doi: 10.1023/a:1025343919959. [DOI] [PubMed] [Google Scholar]
- Seeling A, Lehmann J. NO-donors, part X [1] : investigations on the stability of pentaerythrityl tetranitrate (PETN) by HPLC-chemoluminescence-N-detection (CLND) versus UV-detection in HPLC. J Pharm Biomed Anal. 2006;40:1131–1136. doi: 10.1016/j.jpba.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Shan R, Velazquez C, Knaus EE. Syntheses, calcium channel agonist-antagonist modulation activities, and nitric oxide release studies of nitrooxyalkyl 1, 4-dihydro-2, 6-dimethyl-3-nitro-4-(2, 1, 3-benzoxadiazol-4-yl)pyridine-5-ca rboxylate racemates, enantiomers, and diastereomers. J Med Chem. 2004;47:254–261. doi: 10.1021/jm030333h. [DOI] [PubMed] [Google Scholar]
- Sydow K, Daiber A, Oelze M, Chen Z, August M, Wendt M, et al. Central role of mitochondrial aldehyde dehydrogenase and reactive oxygen species in nitroglycerin tolerance and cross-tolerance. J Clin Invest. 2004;113:482–489. doi: 10.1172/JCI19267. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.