Abstract
Background and purpose:
Exogenously administered thyrotropin-releasing hormone (TRH) is known to exert potent but short-acting centrally-mediated antinociceptive effects. We sought to investigate the mechanisms underlying these effects using the synthetic TRH analogue taltirelin, focusing on the descending monoaminergic systems in mice.
Experimental approach:
The mice received systemic or local injections of taltirelin combined with either central noradrenaline (NA) or 5-hydroxytryptamine (5-HT) depletion by 6-hydroxydopamine (6-OHDA) or DL-p-chlorophenylalanine (PCPA), respectively, or blockade of their receptors. The degree of antinociception was determined using the tail flick and tail pressure tests.
Key results:
Subcutaneously (s.c.) administered taltirelin exhibited dose-dependent antinociceptive effects in the tail flick and tail pressure tests. These effects appeared to be primarily supraspinally mediated, since intracerebroventricularly (i.c.v.) but not intrathecally (i.t.) injected taltirelin generated similar effects. Depletion of central NA abolished only the analgesic effect of taltirelin (s.c. and i.c.v.) on mechanical nociception. By contrast, depletion of central 5-HT abolished only its analgesic effect on thermal nociception. Intraperitoneal (i.p.) and i.t. injection of the α2-adrenoceptor antagonist yohimbine respectively reduced the analgesic effect of taltirelin (s.c. and i.c.v.) on mechanical nociception. By contrast, the 5-HT1A receptor antagonist WAY-100635 (i.p. and i.t.) reduced the effect of taltirelin (s.c. and i.c.v.) on thermal nociception. Neither the 5-HT2 receptor antagonist ketanserin nor the opioid receptor antagonist naloxone altered the antinociceptive effect of taltirelin.
Conclusions and Implications:
These findings suggest that taltirelin activates the descending noradrenergic and serotonergic pain inhibitory systems, respectively, to exert its analgesic effects on mechanical and thermal nociception.
Keywords: thyrotropin-releasing hormone, taltirelin, descending noradrergic system, descending serotonergic system, α2-adrenergic receptors, 5-HT receptors
Introduction
Thyrotropin-releasing hormone (TRH), a neuropeptide discovered originally as a pituitary hormone, has been shown to be distributed widely in the central nervous system (CNS), where it acts as a neurotransmitter or a neuromodulator (Winokur and Utiger, 1974; Hökfelt et al., 1975; Wu et al., 1992) and exerts a variety of CNS effects that are not related to its endocrine activity in releasing thyroid-stimulating hormone. When administered exogenously, TRH produces various behavioral changes including an increase in locomotor activity (Andrews and Sahgal, 1983; Yamamura et al., 1991), appearance of serotonin syndrome-like activities (Fone et al., 1989; Funk et al., 1997) and antinociceptive effects against noxious stimuli (Boschi et al., 1983; Webster et al., 1983; Kawamura et al., 1985; Reny-Palasse et al., 1989). In addition, clinical and preclinical studies have demonstrated a protective role of TRH against epilepsy (Matsumoto et al., 1987; Ujihara et al., 1991; Broberger and McCormick, 2005) and neurodegeneration (Pizzi et al., 1999; Jaworska-Feil et al., 2001, Urayama et al., 2002). Thus, TRH is profoundly involved in the regulation of motor activity (see also Ono and Fukuda, 1982), pain perception and neuronal excitability, and TRH and its analogues have been used clinically for the treatment of patients with motor disturbance owing to neurological disorders such as spinocerebellar degeneration (spinocerebellar ataxia) (Sobue et al., 1980; Takeuchi et al., 1989).
The antinociceptive activity of TRH may be beneficial for patients suffering from spinocerebellar ataxia, as pain – including generalized muscle and joint pain – is reported to be a common feature of Machado–Joseph disease, the most common spinocerebellar ataxia that is inherited in an autosomal dominant manner (Løkkegaard et al., 1998; Takei et al., 2004). However, the mechanisms underlying the analgesic effects of TRH are poorly understood. In the study presented here, we characterized the antinociceptive effects of TRH by using its stable analogue taltirelin, which has more potent CNS activity with lower endocrine activity than TRH itself (Suzuki et al., 1990), focusing on the relationship with the monoaminergic system descending the spinal cord. We found that TRH exhibits modality-specific antinociception mediated via distinct descending monoaminergic systems; taltirelin activates the descending noradrenergic and 5-hydroxytryptaminergic pain inhibitory systems to generate analgesic effects on mechanical and thermal nociception, respectively. Some preliminary data have been published elsewhere in abstract format (Tokuda et al., 2005).
Methods
All of the experimental protocols used here were approved by the Animal Care and Use Committee of Nagoya City University and were carried out according to the guidelines of the National Institutes of Health and the Japanese Pharmacological Society.
Effects on acute nociception
The degree of antinociception was determined using the tail flick test and the tail pressure test in 5-week-old male ICR mice.
In the tail flick test, a radiant heat tail flick analgesia meter (MK-330; Muromachi Kikai, Tokyo, Japan) was used to measure response latencies, following a modification of the method of D'Amour and Smith (1941). Focused heat was applied to the ventral surface of the tail at 2.5 cm from its distal end, and the latency to reflexive removal of the tail from the heat (tail flick latency) was recorded. The tail flick latency was measured in duplicate, and the mean of the two values was used for analysis. A cutoff latency of 15 s was imposed to avoid tissue damage.
Following the tail flick test, mice were subjected to the tail pressure test (Pressure Analgesy-Meter, Muromachi Kikai) to assess their threshold for acute mechanical nociception. Pressure was applied about 1.5 cm from the base of the tail via a blunt probe. The pressure level was increased at a rate of 10 mm Hg/s, and the pressure (mm Hg) required to elicit a response was determined for each mouse; this pressure was defined as the nociceptive threshold. Tail pressure measurements were made in duplicate, and the mean of the two values was used for calculations. The cutoff pressure was 200 mm Hg.
The 198 mice used in the present study (except monoamine-depleted mice) exhibited a mean tail flick latency of 5.1±0.1 s (range, 3.0–8.9 s) and a mean nociceptive threshold of 68.9±0.8 mm Hg (range, 46.5–108.5 mm Hg in the tail pressure test).
Assessment of locomotor activities
Locomotor activities during exploratory behavior in an open arena (18 × 28 cm floor with 13-cm-high walls) were measured in an automated behavioral experimental apparatus (Animex IIIA, Shimazu, Kyoto, Japan). In this equipment, the movement detector operates by counting the number of times an animal elicits a capacitance change. Mice were injected intracerebroventricularly (i.c.v.) with either saline (as a control) or taltirelin. Assessment of locomotor activities was carried out for 1 h post injection, locomotion was measured in 5-min windows during that time.
Depletion of noradrenaline and 5-hydroxytryptamine
Under anesthesia with intraperitoneal (i.p.) administration of pentobarbital sodium (60 mg kg−1), some groups of mice were injected intracisternally with 50 μg of the catecholaminergic neurotoxin 6-hydroxydopamine hydrobromide (6-OHDA) to cause damage to central noradrenergic neurons. 6-OHDA was dissolved in 5 μl of 0.9% saline containing ascorbic acid (100 μg ml−1). Control animals were injected with the vehicle alone. The assessment of acute nociception was made 7 days after treatment with either 6-OHDA or vehicle.
To deplete central 5-hydroxytryptamine (5-HT), p-chlorophenylalanine (PCPA) 300 mg kg−1 day−1, suspended in 0.5% carboxymethylcellulose (CMC) sodium solution, was administered i.p. for 5 consecutive days. Control animals were injected with CMC alone. The assessment of acute nociception was made 1 day after the last treatment with either PCPA or vehicle.
After the assessment of acute nociception, the mice were killed by inhalation of ether. The brainstem and spinal cord were dissected out, weighed and then frozen on dry ice. Each brainstem or spinal cord was homogenized in 450 μl of 0.1 M perchloric acid containing the synthetic monoamine dihydroxybenzylamine (0.01 μg ml−1) as an internal standard. The contents of noradrenaline (NA), 5-HT and dopamine (DA) were measured using reverse-phase high-performance liquid chromatography with electrochemical detection.
Drugs
In the present study, the experimenters were aware of the drugs injected. All drugs except 6-OHDA and PCPA were dissolved in 0.9% w/v physiological saline. When given either subcutaneously (s.c.) or i.p., the drugs were administered in a volume of 0.1 ml (10 g body weight)−1. For intrathecal (i.t.) injection, the drugs were administered in a volume of 5 μl via a disposable 27-gauge needle, which was inserted into the subarachnoid space through the intervertebral foramen between L5 and L6 according to the method described by Hylden and Wilcox (1980). For i.c.v. injection, taltirelin was also administered in a volume of 5 μl via a disposable 27-gauge needle that was inserted into the lateral ventricle (Haley and McCormick, 1957). The α2-adrenergic receptor antagonist or 5-HT receptor antagonists were administered 15 min before taltirelin injection.
Statistical analysis
All data are expressed as the mean±s.e.m. The effects of taltirelin on the tail flick latency in the tail flick test and the nociceptive threshold in the tail pressure test were evaluated in a time course study, where taltirelin was administered at time zero. The nociceptive threshold at each time point was normalized to the predrug value. Two-tailed non-parametric multiple comparisons with Bonferroni correction following the Kruskal–Wallis test (Glantz, 1992) were used for comparisons between the control and treated groups. The Mann–Whitney U-test was used for comparisons between two groups. Differences at P<0.05 (two-tailed) were considered significant.
Materials
Taltirelin hydrate was donated by Tanabe Seiyaku (Osaka, Japan). Yohimbine HCl, ketanserin tartarate, WAY-100635 maleate, naloxone HCl and 6-OHDA were purchased from Sigma Chemical (St Louis, MO, USA). DL-PCPA was obtained from Nakarai (Kyoto, Japan).
Results
Systemically and supraspinally administered taltirelin produces antinociceptive effects against a thermal or mechanical stimulus
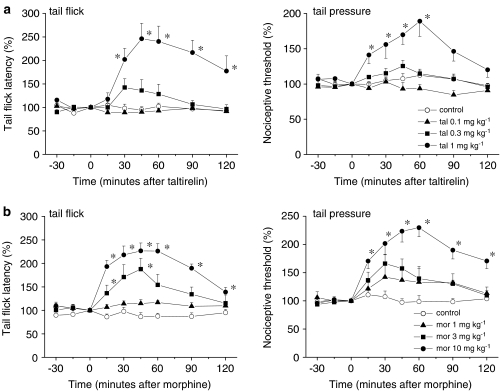
We first examined whether systemically administered taltirelin produced antinociceptive effects against a thermal or mechanical stimulus. As the time course graphs in Figure 1a illustrate, taltirelin hydrate (0.1, 0.3 and 1 mg kg−1, s.c.) generated dose-dependent antinociceptive effects against a thermal and a mechanical stimulus (in the tail flick and tail pressure tests, respectively). In particular, the analgesic effects elicited at 1 mg kg−1 were nearly equivalent to those of morphine HCl (10 mg kg−1, s.c. Figure 1b).
Figure 1.
TRH analogue taltirelin generates analgesic effects against acute thermal and mechanical nociception. Thermal and mechanical nociception was assessed in the tail flick (left) and tail pressure (right) tests, respectively. Taltirelin hydrate ((a); tal, 0.1, 0.3 and 1 mg kg−1) was administered s.c. at time zero. The analgesic effects elicited at 1 mg kg−1 were nearly equivalent to those of morphine HCl ((b); mor, 10 mg kg−1, s.c., administered at time zero). Each point represents the mean (±s.e.m.) result from six mice. Ordinates: mean normalized tail flick latencies (tail flick test; left) and nociceptive thresholds (tail pressure test; right). Abscissae: time in minutes after taltirelin or morphine application. The asterisks indicate data points for which a significant difference between the control and taltirelin- or morphine-treated groups was observed, as determined by two-tailed non-parametric Bonferroni-type multiple comparisons following the Kruskal–Wallis test (three comparisons in four groups, *P<0.05).
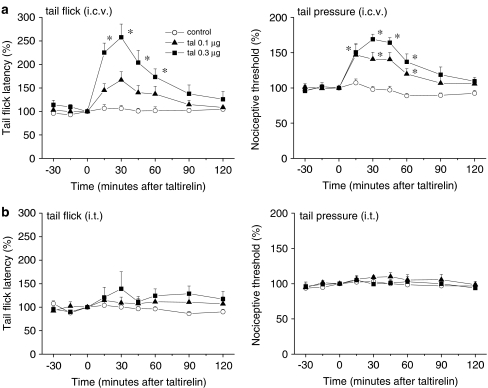
We then assessed the possible sites at which taltirelin exerted its antinociceptive effects by locally injecting taltirelin hydrate either i.c.v. or i.t. (0.1 and 0.3 μg). Figure 2 demonstrates that i.c.v.-injected taltirelin exerted potent antinociceptive effects in the tail flick and tail pressure tests, but lacked an analgesic effect after i.t. injection. Thus, supraspinal sites primarily contribute to the antinociceptive effects of systemically administered taltirelin.
Figure 2.
Effects of local injection of taltirelin. I.c.v. (a) but not i.t. (b) administration of taltirelin hydrate (tal, 0.1 and 0.3 μg) generated antinociceptive effects in the tail flick (left) and tail pressure (right) tests, indicating that taltirelin has supraspinal sites of action. Taltirelin was injected at time zero. Each point represents the mean (±s.e.m.) result from six mice. Ordinates: mean normalized tail flick latencies (tail flick test; left) and nociceptive thresholds (tail pressure test; right). Abscissae: time in minutes after taltirelin application. The asterisks indicate data points for which a significant difference between the control and taltirelin-treated groups was observed, as determined by two-tailed non-parametric Bonferroni-type multiple comparisons following the Kruskal–Wallis test (two comparisons in three groups, *P<0.05).
Depletion of central NA and 5-HT decreases the antinociceptive effect of taltirelin on mechanical and thermal nociception, respectively
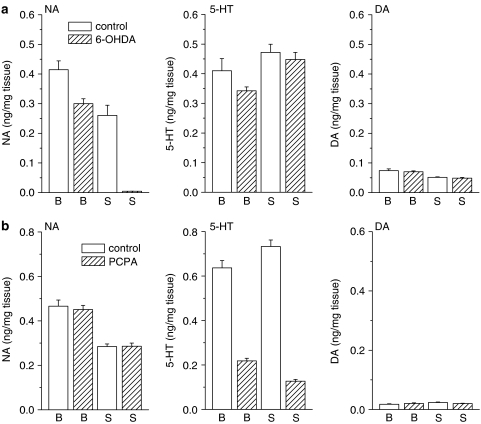
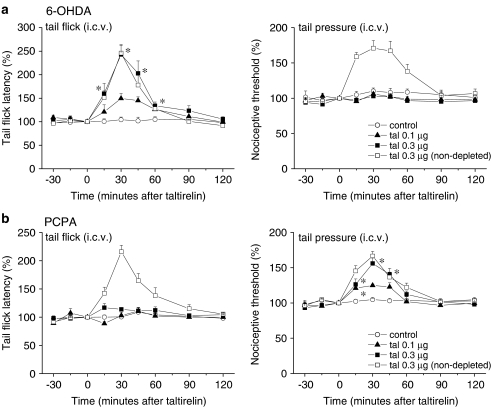
As the monoaminergic pathways descending to the lumbar spinal cord have a crucial influence on spinal nociceptive transmission (Millan, 2002), we next evaluated the role of these pathways in the supraspinally mediated antinociceptive effects of taltirelin. We first assessed the effects of i.c.v.-injected taltirelin 1 week after intracisternal injection of 6-OHDA to deplete central NA. Consistent with our previous study (Tanabe et al., 2005), treatment with 6-OHDA reduced the NA contents of the brainstem and spinal cord to 72.4 and 1.7%, respectively, of those in control mice treated with vehicle (ascorbic acid) alone, whereas the simultaneously measured 5-HT and DA contents of both areas were unchanged (Figure 3a, data obtained from six vehicle-treated and 18 6-OHDA-treated animals). In mice treated with 6-OHDA, the mean tail flick latency (3.5±0.1 s, n=18) was shorter than that in mice treated with vehicle alone (4.1±0.2 s, n=6, P<0.05), whereas depletion of NA did not affect the mean withdrawal threshold in the tail pressure test (70.1±2.3 mm Hg in 6-OHDA-treated vs 73.9±4.0 mm Hg in vehicle-treated, n=18 and 6, respectively). After depletion of NA, taltirelin hydrate (0.1 and 0.3 μg, i.c.v.) exhibited an analgesic effect on thermal nociception that was almost equivalent to the effect in vehicle-treated mice. By contrast, the analgesic effect of taltirelin on mechanical nociception was completely abolished in 6-OHDA-treated mice (Figure 4a).
Figure 3.
Depletion of descending NA and 5-HT. To deplete NA or 5-HT, 6-OHDA or PCPA was injected intracisternally once or administered i.p. for 5 consecutive days, respectively. After assessment of thermal and mechanical nociception, the contents of NA, 5-HT and DA in the brainstem (B) and spinal cord (S) obtained from vehicle control (n=6 each) and 6-OHDA (a)- or PCPA (b)-treated mice (n=18 each) were measured using reverse-phase high-performance liquid chromatography with electrochemical detection.
Figure 4.
Depletion of descending NA and 5-HT levels almost abolishes the analgesic effects of supraspinally administered taltirelin on mechanical and thermal nociception, respectively. To deplete NA (a) or 5-HT (b), 6-OHDA or PCPA was injected intracisternally once or administered i.p. for 5 consecutive days, respectively. Taltirelin hydrate (tal, 0.1 and 0.3 μg, i.c.v.) was administered at time zero. Each point represents the mean (±s.e.m.) result from six mice. Ordinates: mean normalized tail flick latencies (tail flick test; left) and nociceptive thresholds (tail pressure test; right). Abscissae: time in minutes after taltirelin application. The asterisks indicate data points for which a significant difference between the control and taltirelin-treated groups was observed, as determined by two-tailed non-parametric Bonferroni-type multiple comparisons following the Kruskal–Wallis test (two comparisons in three groups, *P<0.05). Pretreatment with vehicle (ascorbic acid in (a) and CMC in (b)) alone did not affect the antinociceptive effects of taltirelin hydrate (0.3 μg, i.c.v.), as shown superimposed in the graphs (n=6, clear squares).
We next evaluated the effects of i.c.v.-injected taltirelin using mice in which central 5-HT levels had been depleted by i.p. injection of PCPA for 5 consecutive days. This treatment with PCPA reduced the 5-HT contents of the brainstem and spinal cord to 34.3 and 17.3%, respectively, of those in control mice treated with vehicle (CMC) alone, whereas the simultaneously measured NA and DA contents of both areas were unchanged (Figure 3b, data obtained from six vehicle-treated and 18 PCPA-treated animals). PCPA did not affect either the mean tail flick latency (4.2±0.1 s in PCPA-treated vs 4.1±0.1 s in vehicle-treated, n=18 and 6, respectively) or nociceptive threshold in the tail pressure test (75.8±2.0 mm Hg in PCPA-treated vs 73.5±3.8 mm Hg in vehicle-treated, n=18 and 6, respectively). In mice treated with PCPA, taltirelin hydrate (0.1 and 0.3 μg, i.c.v.) exhibited an analgesic effect on mechanical nociception that was almost equivalent to the effect in vehicle-treated mice. By contrast, the analgesic effect of taltirelin on thermal nociception was completely abolished in PCPA-treated mice (Figure 4b).
Together, these results indicate that taltirelin supraspinally activates the descending noradrenergic and serotonergic pain inhibitory systems to generate analgesic effects on mechanical and thermal nociception respectively. We then performed pharmacological experiments to investigate the spinal adrenoceptor and 5-HT receptor subtypes mediating the supraspinal analgesic actions of taltirelin.
Spinal α2-adrenergic and 5-HT1A-receptors mediate the supraspinal actions of taltirelin on mechanical and thermal nociception, respectively
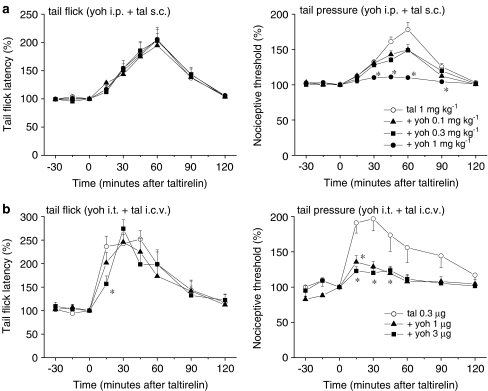
The noradrenergic endogenous pain-inhibitory system and α2-adrenoceptors in the lumbar spinal cord have been demonstrated to be sequentially activated to generate analgesic effects (Sagen and Proudfit, 1984; Jones, 1991; Tanabe et al., 2005; Takasu et al., 2006). In support of this, the α2-adrenergic receptor antagonist yohimbine HCl (0.1, 0.3 and 1 mg kg−1, i.p.) dose-dependently suppressed the analgesic effect of systemically administered taltirelin hydrate (1 mg kg−1, s.c.) on mechanical nociception, whereas its effect on thermal nociception was unaffected (Figure 5a). These results were reproduced when both yohimbine HCl (1 and 3 μg, i.t.) and taltirelin hydrate (0.3 μg, i.c.v.) were locally injected (Figure 5b).
Figure 5.
α2-adrenergic receptor antagonist yohimbine reduces the analgesic effect of taltirelin on mechanical nociception. Yohimbine HCl (yoh) was administered either i.p. ((a); 0.1, 0.3 and 1 mg kg−1) or i.t. ((b); 1 and 3 μg) 15 min before the administration of taltirelin hydrate (tal, 1 mg kg−1, s.c. in (a) and 0.3 μg, i.c.v. in (b), administered at time zero). Each point represents the mean (±s.e.m.) result from six mice. Ordinates: mean normalized tail flick latencies (tail flick test; left) and nociceptive thresholds (tail pressure test; right). Abscissae: time in minutes after taltirelin application. The asterisks indicate data points for which a significant difference between the taltirelin-only and yohimbine-treated groups was observed, as determined by two-tailed non-parametric Bonferroni-type multiple comparisons following the Kruskal–Wallis test (three comparisons in four groups in (a), and two comparisons in three groups in (b), *P<0.05).
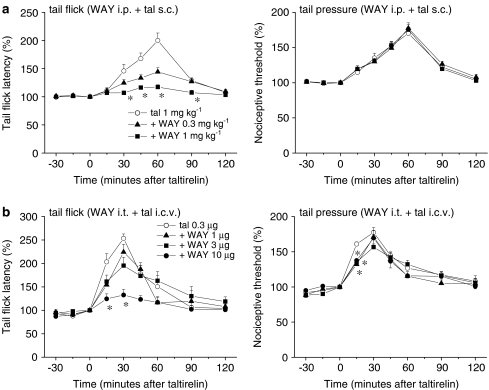
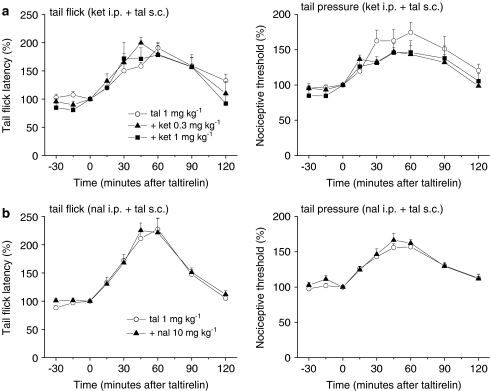
By contrast, the analgesic effect of systemic taltirelin hydrate (1 mg kg−1, s.c.) on thermal nociception was reduced by the 5-HT1A receptor antagonist WAY-100635 (0.3 and 1 mg kg−1, i.p.), whereas its effect on mechanical nociception was hardly affected (Figure 6a). Again, i.t. injection of WAY-100635 (1, 3 and 10 μg) selectively reduced the analgesic effect of i.c.v.-injected taltirelin hydrate (0.3 μg) on thermal nociception in a dose-dependent manner (Figure 6b). Although several 5-HT receptor subtypes in the spinal dorsal horn have been shown to participate in the spinal modulation of nociception (Hamon and Bourgoin, 1999; Millan, 2002), it appears that spinal 5-HT1A receptors mediate the supraspinally produced analgesic effect of taltirelin on thermal nociception. In line with this conclusion, the 5-HT2A receptor antagonist ketanserin (0.3 and 1 mg kg−1, i.p.) did not affect the analgesic effect of taltirelin hydrate (1 mg kg−1, i.p.) on thermal and mechanical nociception (Figure 7a).
Figure 6.
5-HT1A receptor antagonist WAY-100635 reduces the analgesic effect of taltirelin on thermal nociception. WAY-100635 (WAY) was administered either i.p. ((a); 0.3 and 1 mg kg−1) or i.t. ((b); 1, 3 and 10 μg) 15 min before the administration of taltirelin hydrate (tal, 1 mg kg−1, s.c. in (a) and 0.3 μg, i.c.v. in (b), administered at time zero). Each point represents the mean (±s.e.m.) result from six mice. Ordinates: mean normalized tail flick latencies (tail flick test; left) and nociceptive thresholds (tail pressure test; right). Abscissae: time in minutes after taltirelin application. The asterisks indicate data points for which a significant difference between the taltirelin-only and WAY-100635-treated groups was observed, as determined by two-tailed non-parametric Bonferroni-type multiple comparisons following the Kruskal–Wallis test (two comparisons in three groups in (a), and three comparisons in four groups in (b), *P<0.05).
Figure 7.
Neither 5-HT2A receptors nor opioid receptors mediate the antinociceptive effects of taltirelin. The 5-HT2A receptor antagonist ketanserin (ket; 0.3 and 1 mg kg−1 in (a)) or the opioid receptor antagonist naloxone HCl (nal; 10 mg kg−1 in (b)) was administered i.p. 15 min before the administration of taltirelin hydrate (1 mg kg−1,administered at time zero). Each point represents the mean (±s.e.m.) result from six mice. Ordinates: mean normalized tail flick latencies (tail flick test; left) and nociceptive thresholds (tail pressure test; right). Abscissae: time in minutes after taltirelin application.
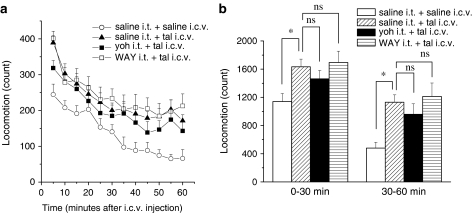
Consistent with the previous studies (Andrews and Sahgal, 1983; Yamamura et al., 1991), taltirelin hydrate (0.3 μg, i.c.v.) significantly increased locomotor activity (Figure 8, n=9). Blockade of spinal α2-adrenergic receptors with yohimbine HCl (3 μg, i.t.) or 5-HT1A receptors with WAY-100635 (10 μg, i.t.), which respectively resulted in selective inhibition of the supraspinally mediated analgesic effects of taltirelin on mechanical and thermal nociception, did not change the effects of taltirelin on locomotor activity (Figure 8). Hence, it appears that the analgesic effects of taltirelin can be separated from its effects on the motor system.
Figure 8.
Neither blockade of spinal α2-adrenergic nor 5-HT1A receptors affects the increased locomotor activity elicited by i.c.v. administered taltirelin. Mice were injected i.c.v. with taltirelin (tal, 0.3 μg) 15 min after i.t. injection of either yohimbine HCl (yoh, 3 μg) or WAY-100635 (WAY, 10 μg), and the assessment of locomotor activities was carried out for 1 h post injection (n=9 each). Ordinates: locomotion measured in 5-min periods (a) and between 0–30 and 30–60 min after injection (b). Taltirelin was administered at time zero. In (b), the significance of differences between the saline i.t.+taltirelin i.c.v. group and other groups (saline i.t.+saline i.c.v., yoh i.t.+tal i.c.v., WAY i.t.+tal i.c.v.) was determined by two-tailed non-parametric Bonferroni-type multiple comparisons following the Kruskal–Wallis test (three comparisons in four groups, *P<0.05). ns; not significant.
Taltirelin acts on targets other than opioid receptors in supraspinal structures
The descending monoaminergic pathways mediating the supraspinal action of taltirelin that we have demonstrated so far could be shared by opioids acting on the supraspinal structures (Kuraishi et al., 1983; Sawynok and Reid, 1987). Therefore, we finally assessed whether opioid receptors were involved in the antinociceptive effects of taltirelin. However, the opioid receptor antagonist naloxone HCl (10 mg kg−1, i.p.) did not alter the analgesic effects of systemically administered taltirelin hydrate (1 mg kg−1, s.c.) on thermal and mechanical nociception (Figure 7b). Thus, we conclude that the supraspinally mediated analgesic action of taltirelin is independent of opioid receptor activation.
Discussion
TRH and its analogues, used clinically in the treatment of motor disturbance owing to neurological disorders, such as spinocerebellar degeneration (Sobue et al., 1980; Takeuchi et al., 1989), have various CNS effects that are not related to their endocrine activity. In the present study, we focused on their antinociceptive activity whose mechanisms have not been fully determined. Our results indicated that the stable TRH analogue taltirelin, which has been shown to pass the blood–brain barrier and be more resistant to enzymatic degradation than TRH (Chishima, 1994), produces supraspinally mediated antinociceptive effects, which are characterized by their modality specificity recruiting distinct descending monoaminergic pathways to exert analgesic effects on thermal and mechanical nociception (5-hydroxytryptaminergic and noradrenergic pathways, respectively) and independent of its effects on the motor system.
The present finding that the antinociception produced by taltirelin is mediated by its action on the supraspinal structures is consistent with previous studies using TRH and its analogues (Boschi et al., 1983; Webster et al., 1983; Zhukov et al., 1988; Reny-Palasse et al., 1989). When administered systemically in rodents, taltirelin has about 30–100 times more potent CNS activity and 50 times weaker endocrine activity than TRH (Suzuki et al., 1990; Yamamura et al., 1990). Its high resistance to enzymatic degradation also may contribute to the longer lasting analgesic effect of systemically and supraspinally administered taltirelin. Various lines of evidence suggest that TRH and its analogues exhibit excitatory effects on spinal nociceptive processing and motor output, including a facilitatory influence on N-methyl-D-aspartate receptor-mediated nociceptive responses in spinal dorsal horn neurons (Chizh and Headley, 1994, 1996) and the spinal reflex (Ono and Fukuda, 1982; Ono et al., 1990; Kinoshita et al., 1994). However, we demonstrated that i.t.-injected taltirelin did not change the nociceptive threshold to a thermal or mechanical stimulus. Although it remains unclear whether TRH and its analogues modulate the excitability of inhibitory interneurons in the spinal superficial layers, we suppose that these interneurons could be excited to generate no net changes in the nociceptive thresholds by counteracting the facilitatory influence of TRH and its analogues on spinal sensory transmission (Chizh and Headley, 1994, 1996). Together, TRH and its analogues appear to act primarily at supraspinal sites to generate analgesic effects.
Several studies have indicated that TRH and its analogues enhance the release of monoamines in the brain (Heal and Green, 1979; Heal et al., 1987; Itoh et al., 1994; Fukuchi et al., 1998). Moreover, Funk et al. (1997) have demonstrated that spinal 5-HT plays an essential role in the behavioral responses induced by TRH. As the descending noradrenergic and 5-hydroxytryptaminergic pathways constitute a major component of the endogenous pain-inhibitory system (Jones, 1991; Millan, 2002), it is likely that taltirelin supraspinally activates these descending pathways to release NA and 5-HT in the spinal cord. This was evident in the present study, as the antinociceptive effects of i.c.v.-injected taltirelin were markedly inhibited after depletion of spinal monoamines. More importantly, our results indicated that taltirelin recruits different monoaminergic pathways to exert its antinociceptive effects on thermal and mechanical nociception. Depletion of spinal NA abolished only the analgesic effect of taltirelin on mechanical nociception, whereas depletion of spinal 5-HT eliminated the effect on thermal nociception. Such modality specificity has been demonstrated in the antinociceptive effect of morphine (Kuraishi et al., 1983; Sawynok and Reid, 1987) and spinally applied NA and 5-HT (Kuraishi et al., 1985), suggesting that spinal NA and 5-HT may play a predominant role in the regulation of mechanical and thermal nociception, respectively. In our pharmacological experiments, the analgesic effects of supraspinally administered taltirelin on mechanical and thermal nociception were reduced selectively by i.t. injection of the α2-adreno-ceptor antagonist yohimbine and the 5-HT1A receptor antagonist WAY-100635, respectively. These results are consistent with our results obtained in mice in which the descending noradrenergic or 5-hydroxytryptaminergic pain inhibitory system was expected to be non-functional after depletion of NA or 5-HT. Hence, taltirelin, acting on the supraspinal structures, elicits increased release of NA and 5-HT in the lumbar spinal cord, which results in consequential activation of α2-adrenergic and 5-HT1A receptors, respectively, and produces modality-specific antinociception.
Intrathecally applied α2-adrenergic receptor agonists exert an analgesic effect on acute thermal (Reddy et al., 1980; Hunter et al., 1997; Stone et al., 1997) as well as mechanical nociception (Ochi and Goto, 2000). Moreover, electrical stimulation of noradrenergic nuclei in the brainstem can generate spinal α2-adrenoceptor-mediated analgesic effects on acute thermal nociception (Jones and Gebhart, 1986; Yeomans et al., 1992). Hence, the descending noradrenergic system coupled with spinal α2-adrenergic receptors can potentially influence both thermal and mechanical nociception. Although we suppose that taltirelin activates only a limited population of the descending noradrenergic pathways, which regulate mechanical nociception in the spinal dorsal horn, we have now no evidence to support this hypothesis. By contrast, the descending 5-hydroxytryptaminergic pathways have been demonstrated to exert both pro- and antinociceptive actions, which may be partly attributable to multiple classes of 5-HT receptors activated by released 5-HT in the spinal cord (Millan, 2002). Although spinal 5-HT1A receptors are likely to mediate the pro-nociceptive effects of exogenous or endogenous 5-HT on acute mechanical nociception (Bardin and Colpaert, 2004; Bonnefont et al., 2005), their analgesic role in thermal nociceptive processing demonstrated by others (Eide and Hole, 1991; Xu et al., 1994) argues for our current results. As supraspinally administered taltirelin, during blockade of spinal 5-HT1A receptors with WAY-100635, did not promote the antinociceptive effect in the tail pressure test, taltirelin seems to activate the descending 5-hydroxytryptaminergic pathways that affect only the neuronal circuitry invoked by the thermal stimulus.
The periaqueductal gray matter (PAG) has excitatory projections to the 5-hydroxytryptaminergic nucleus raphe magnus and the noradrenergic locus coeruleus, A5 and A7 cell groups (Basbaum and Fields, 1979; Willis et al., 1984; Cameron et al., 1995), which send descending 5-hydroxytryptaminergic and noradrenergic projections to the spinal cord, respectively. Injection of TRH and its analogues to the PAG generates antinociception (Webster et al., 1983). Consistently, stimulation of the PAG inhibits nociceptive dorsal horn neurons concomitantly with the release of NA and 5-HT (Cui et al., 1999). TRH receptor subtype 2 (termed TRHR2) has been shown to be highly expressed in the pontine nucleus, thalamus and cerebellar cortex. Moreover, the PAG and several brainstem nuclei also express TRHR2 (Cao et al., 1998; Heuer et al., 2000; O'Dowd et al., 2000). By contrast, the receptor type expressed in the pituitary gland is TRHR1 (Cao et al., 1998). Taltirelin has been demonstrated to exhibit a lower affinity for TRH receptors in the anterior pituitary than TRH (Asai et al., 1999). These lines of evidence, together with our present findings, suggest that TRHR2 receptors, presumably in the PAG, are possible sites of action through which TRH and its analogues can initiate their antinociceptive effects.
The descending monoaminergic pathways mediating the supraspinal action of taltirelin could be shared by opioids acting on the supraspinal structures (Kuraishi et al., 1983; Sawynok and Reid, 1987). However, the finding that the opioid receptor antagonist naloxone, at a sufficient dose, did not alter the antinociceptive effects of taltirelin indicates that the antinociceptive effect of TRH and its analogues is independent of the opioidergic system. This argues for the finding by Kawamura et al. (1985) that the TRH-induced antinociception was not antagonized by naloxone (but see Webster et al., 1983; Reny-Palasse et al., 1989).
It has been reported that there is a highly negative correlation between the tail skin temperature and tail flick latency; when the tail skin temperature is increased, the tail flick latency is reduced (Tjolsen et al., 1988; Lund et al., 1989). Moreover, depletion of spinal 5-HT increases the tail skin temperature, that results in a reduced tail flick latency (Tjolsen et al., 1988). Hence, spinal 5-HT released after supraspinal injection of taltirelin may have influence on the tail skin temperature. However, TRH and its analogues have been shown to produce hyperthermia (Metcalf et al., 1981). Consistently in our preliminary experiments in anesthetized mice, taltirelin produced thermogenic effects and slightly increased tail skin temperature (data not shown), indicating that taltirelin increases tail flick latency without lowering of tail skin temperature.
In summary, using the TRH analogue taltirelin, we have demonstrated that the antinociceptive effect of TRH (and its analogues) was initiated by its action on the supraspinal structures and mediated by the descending monoaminergic pain-inhibitory system coupled with spinal α2-adrenergic and 5-HT1A receptors. The antinociception was characterized by modality specificity observed in the noradrenergic effects on mechanical nociceptive processing and 5-hydroxytryptaminergic effects on thermal nociceptive processing. Although the precise mechanisms underlying the modality-specific antinociceptive effect remain to be determined, our study provides new insights into the analgesic action of TRH.
Acknowledgments
We thank Tanabe Seiyaku for the kind gift of taltirelin.
Abbreviations
- CMC
carboxymethylcellulose
- DA
dopamine
- 5-HT
5-hydroxytryptamine (serotonin)
- NA
noradrenaline
- 6-OHDA
6-hydroxydopamine hydrobromide
- PAG
periaqueductal gray matter
- PCPA
DL-p-chlorophenylalanine
- TRH
thyrotropin-releasing hormone
Conflict of interest
The authors state no conflict of interest.
References
- Andrews JS, Sahgal A. The effects of thyrotropin-releasing hormone, metabolites and analogues on locomotor activity in rats. Regul Pept. 1983;7:97–109. doi: 10.1016/0167-0115(83)90302-6. [DOI] [PubMed] [Google Scholar]
- Asai H, Kinoshita K, Yamamura M, Matsuoka Y. Diversity of thyrotropin-releasing hormone receptors in the pituitary and discrete brain regions of rats. Jpn J Pharmacol. 1999;79:313–317. doi: 10.1254/jjp.79.313. [DOI] [PubMed] [Google Scholar]
- Bardin L, Colpaert FC. Role of spinal 5-HT1A receptors in morphine analgesia and tolerance in rats. Eur J Pain. 2004;8:253–261. doi: 10.1016/j.ejpain.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Basbaum AI, Fields HL. The origin of descending pathways in the dorsolateral funiculus of the spinal cord of the cat and rat: further studies on the anatomy of pain modulation. J Comp Neurol. 1979;187:513–531. doi: 10.1002/cne.901870304. [DOI] [PubMed] [Google Scholar]
- Bonnefont J, Chapuy E, Clottes E, Alloui A, Eschalier A. Spinal 5-HT1A receptors differentially influence nociceptive processing according to the nature of the noxious stimulus in rats: effect of WAY-100635 on the antinociceptive activities of paracetamol, venlafaxine and 5-HT. Pain. 2005;114:482–490. doi: 10.1016/j.pain.2005.01.019. [DOI] [PubMed] [Google Scholar]
- Boschi G, Desiles M, Reny V, Rips R, Wrigglesworth S. Antinociceptive properties of thyrotropin releasing hormone in mice: comparison with morphine. Br J Pharmacol. 1983;79:85–92. doi: 10.1111/j.1476-5381.1983.tb10499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broberger C, McCormick DA. Excitatory effects of thyrotropin-releasing hormone in the thalamus. J Neurosci. 2005;25:1664–1673. doi: 10.1523/JNEUROSCI.3198-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron AA, Khan IA, Westlund KN, Willis WD. The efferent projections of the periaqueductal gray in the rat: a Phaseolusvulgaris-leucoagglutinin study. II. Descending projections. J Comp Neurol. 1995;351:585–601. doi: 10.1002/cne.903510408. [DOI] [PubMed] [Google Scholar]
- Cao J, O'Donnell D, Vu H, Payza K, Pou C, Godbout C, et al. Cloning and characterization of a cDNA encoding a novel subtype of rat thyrotropin-releasing hormone receptor. J Biol Chem. 1998;273:32281–32287. doi: 10.1074/jbc.273.48.32281. [DOI] [PubMed] [Google Scholar]
- Chishima S. A radioimmunoassay for TA-0910, a new metabolically stable thyrotropin-releasing hormone analogue. J Pharm Biomed Anal. 1994;12:795–804. doi: 10.1016/0731-7085(94)e0007-n. [DOI] [PubMed] [Google Scholar]
- Chizh BA, Headley PM. Thyrotropin-releasing hormone (TRH)-induced facilitation of spinal neurotransmission: a role for NMDA receptors. Neuropharmacology. 1994;33:115–121. doi: 10.1016/0028-3908(94)90105-8. [DOI] [PubMed] [Google Scholar]
- Chizh BA, Headley PM. Thyrotropin-releasing hormone facilitates spinal nociceptive responses by potentiating NMDA receptor-mediated transmission. Eur J Pharmacol. 1996;300:183–189. doi: 10.1016/0014-2999(95)00869-1. [DOI] [PubMed] [Google Scholar]
- Cui M, Feng Y, McAdoo DJ, Willis WD. Periaqueductal gray stimulation-induced inhibition of nociceptive dorsal horn neurons in rats is associated with the release of norepinephrine, serotonin, and amino acids. J Pharmacol Exp Ther. 1999;289:868–876. [PubMed] [Google Scholar]
- D'Amour FE, Smith DL. A method for determining loss of pain sensation. J Pharmacol Exp Ther. 1941;72:74–79. [Google Scholar]
- Eide PK, Hole K. Different role of 5-HT1A and 5-HT2 receptors in spinal cord in the control of nociceptive responsiveness. Neuropharmacology. 1991;30:727–731. doi: 10.1016/0028-3908(91)90180-j. [DOI] [PubMed] [Google Scholar]
- Fone KC, Johnson JV, Bennett GW, Marsden CA. Involvement of 5-HT2 receptors in the behaviours produced by intrathecal administration of selected 5-HT agonists and the TRH analogue (CG 3509) to rats. Br J Pharmacol. 1989;96:599–608. doi: 10.1111/j.1476-5381.1989.tb11858.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuchi I, Asahi T, Kawashima K, Kawashima Y, Yamamura M, Matsuoka Y, et al. Effects of taltirelin hydrate (TA-0910), a novel thyrotropin-releasing hormone analog, on in vivo dopamine release and turnover in rat brain. Arzneimittelforschung. 1998;48:353–359. [PubMed] [Google Scholar]
- Funk D, Post RM, Pert A. Role of central dopaminergic and 5-hydroxytryptaminergic projections in the behavioral responses elicited by thyrotropin-releasing hormone in rats. Psychopharmacology. 1997;133:356–362. doi: 10.1007/s002130050414. [DOI] [PubMed] [Google Scholar]
- Glantz SA.Alternatives to analysis of variance and the t test based on ranks Primer of Biostatistics 1992McGraw-Hill: New York; 320–371.In: Jeffers JD, Englis MR (eds).3rd edn. [Google Scholar]
- Haley TJ, McCormick WG. Pharmacological effects produced by intracerebral injection of drugs in the conscious mouse. Br J Pharmacol Chemother. 1957;12:12–15. doi: 10.1111/j.1476-5381.1957.tb01354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamon M, Bourgoin S.Serotonin and its receptors in pain control Novel aspects of pain management – opioids and beyond 1999Wiley-Liss: New York; 203–228.In: Sawynok J, Cowan A (eds). [Google Scholar]
- Heal DJ, Green AR. Administration of thyrotropin releasing hormone (TRH) to rats releases dopamine in n. accumbens but not n. caudatus. Neuropharmacology. 1979;18:23–31. doi: 10.1016/0028-3908(79)90005-4. [DOI] [PubMed] [Google Scholar]
- Heal DJ, Stoodley N, Elliott JM, Marsden CA, Bennett GW, Youdim MB. Behavioural and biochemical evidence for the release of noradrenaline in mouse brain by TRH and some of its biologically stable analogues. Neuropharmacology. 1987;26:313–322. doi: 10.1016/0028-3908(87)90183-3. [DOI] [PubMed] [Google Scholar]
- Heuer H, Schafer MK, O'Donnell D, Walker P, Bauer K. Expression of thyrotropin-releasing hormone receptor 2 (TRH-R2) in the central nervous system of rats. J Comp Neurol. 2000;428:319–336. [PubMed] [Google Scholar]
- Hökfelt T, Fuxe K, Johansson O, Jeffcoate S, White N. Distribution of thyrotropin-releasing hormone (TRH) in the central nervous system as revealed with immunohistochemistry. Eur J Pharmacol. 1975;34:389–392. doi: 10.1016/0014-2999(75)90269-1. [DOI] [PubMed] [Google Scholar]
- Hunter JC, Fontana DJ, Hedley LR, Jasper JR, Lewis R, Link RE, et al. Assessment of the role of α2-adrenoceptor subtypes in the antinociceptive, sedative and hypothermic action of dexmedetomidine in transgenic mice. Br J Pharmacol. 1997;122:1339–1344. doi: 10.1038/sj.bjp.0701520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hylden JL, Wilcox GL. Intrathecal morphine in mice: a new technique. Eur J Pharmacol. 1980;67:313–316. doi: 10.1016/0014-2999(80)90515-4. [DOI] [PubMed] [Google Scholar]
- Itoh Y, Ogasawara T, Yamazaki A, Ukai Y, Miura A, Kimura K. Enhancement of noradrenaline release from rat frontal cortex by thyrotropin releasing hormone and its analog, (3R,6R)-6-methyl-5-oxo-3-thiomorpholinylcarbonyl-L-histidyl-L-prolinamide, as studied by intracerebral microdialysis. J Pharmacol Exp Ther. 1994;268:255–261. [PubMed] [Google Scholar]
- Jaworska-Feil L, Kajta M, Budziszewska B, Leskiewicz M, Lason W. Protective effects of TRH and its stable analogue, RGH-2202, on kainate-induced seizures and neurotoxicity in rodents. Epilepsy Res. 2001;43:67–73. doi: 10.1016/s0920-1211(00)00178-9. [DOI] [PubMed] [Google Scholar]
- Jones SL. Descending noradrenergic influences on pain. Prog Brain Res. 1991;88:381–394. doi: 10.1016/s0079-6123(08)63824-8. [DOI] [PubMed] [Google Scholar]
- Jones SL, Gebhart GF. Characterization of coeruleospinal inhibition of the nociceptive tail-flick reflex in the rat: mediation by spinal α2-adrenoceptors. Brain Res. 1986;364:315–330. doi: 10.1016/0006-8993(86)90844-9. [DOI] [PubMed] [Google Scholar]
- Kawamura S, Sakurada S, Sakurada T, Kisara K, Sasaki Y, Suzuki K. The antinociceptive effects of histidyl-proline diketopiperazine and thyrotropin-releasing hormone in the mouse. Eur J Pharmacol. 1985;112:287–294. doi: 10.1016/0014-2999(85)90774-5. [DOI] [PubMed] [Google Scholar]
- Kinoshita K, Nagao T, Ono H. Effects of TA-0910, an orally active TRH analog, on the spinal reflex in spinal rats. Neuropharmacology. 1994;33:1183–1188. doi: 10.1016/s0028-3908(05)80008-5. [DOI] [PubMed] [Google Scholar]
- Kuraishi Y, Harada Y, Aratani S, Satoh M, Takagi H. Separate involvement of the spinal noradrenergic and serotonergic systems in morphine analgesia: the differences in mechanical and thermal algesic tests. Brain Res. 1983;273:245–252. doi: 10.1016/0006-8993(83)90849-1. [DOI] [PubMed] [Google Scholar]
- Kuraishi Y, Hirota N, Satoh M, Takagi H. Antinociceptive effects of intrathecal opioids, noradrenaline and serotonin in rats: mechanical and thermal algesic tests. Brain Res. 1985;326:168–171. doi: 10.1016/0006-8993(85)91398-8. [DOI] [PubMed] [Google Scholar]
- Løkkegaard T, Nielsen JE, Hasholt L, Fenger K, Werdelin L, Tranebjaerg L, et al. Machado-Joseph disease in three Scandinavian families. J Neurol Sci. 1998;156:152–157. doi: 10.1016/s0022-510x(98)00081-1. [DOI] [PubMed] [Google Scholar]
- Lund A, Tjolsen A, Hole K. The apparent antinociceptive effect of desipramine and zimelidine in the tail flick test in rats is mainly caused by changes in tail skin temperature. Pain. 1989;38:65–69. doi: 10.1016/0304-3959(89)90074-2. [DOI] [PubMed] [Google Scholar]
- Matsumoto A, Kumagai T, Takeuchi T, Miyazaki S, Watanabe K. Clinical effects of thyrotropin-releasing hormone for severe epilepsy in childhood: a comparative study with ACTH therapy. Epilepsia. 1987;28:49–55. doi: 10.1111/j.1528-1157.1987.tb03622.x. [DOI] [PubMed] [Google Scholar]
- Metcalf G, Dettmar PW, Lynn AG, Brewster D, Havler ME. Thyrotropin-releasing hormone (TRH) analogues show enhanced CNS selectivity because of increased biological stability. Regul Pept. 1981;2:277–284. doi: 10.1016/0167-0115(81)90032-x. [DOI] [PubMed] [Google Scholar]
- Millan MJ. Descending control of pain. Prog Neurobiol. 2002;66:355–474. doi: 10.1016/s0301-0082(02)00009-6. [DOI] [PubMed] [Google Scholar]
- Ochi T, Goto T. The antinociceptive effect of FR140423 in mice: involvement of spinal α2-adrenoceptors. Eur J Pharmacol. 2000;400:199–203. doi: 10.1016/s0014-2999(00)00402-7. [DOI] [PubMed] [Google Scholar]
- O'Dowd BF, Lee DK, Huang W, Nguyen T, Cheng R, Liu Y, et al. TRH-R2 exhibits similar binding and acute signaling but distinct regulation and anatomic distribution compared with TRH-R1. Mol Endocrinol. 2000;14:183–193. doi: 10.1210/mend.14.1.0407. [DOI] [PubMed] [Google Scholar]
- Ono H, Fukuda H. Ventral root depolarization and spinal reflex augmentation by a TRH analog in rat spinal cord. Neuropharmacology. 1982;21:739–744. doi: 10.1016/0028-3908(82)90058-2. [DOI] [PubMed] [Google Scholar]
- Ono H, Nagano N, Yamada M, Fukuda H. Effects of a new analog of thyrotropin-releasing hormone, Nα-[{(S)-4-oxo-2-azetidinyl carbonyl]-L-histidyl-L-prolinamide dehydrate (YM-14673) on spinal reflex potentials and flexor reflexes in spinalized rats. Neuropharmacology. 1990;29:69–74. doi: 10.1016/0028-3908(90)90085-6. [DOI] [PubMed] [Google Scholar]
- Pizzi M, Boroni F, Benarese M, Moraitis C, Memo M, Spano P. Neuroprotective effect of thyrotropin-releasing hormone against excitatory amino acid-induced cell death in hippocampal slices. Eur J Pharmacol. 1999;370:133–137. doi: 10.1016/s0014-2999(99)00139-9. [DOI] [PubMed] [Google Scholar]
- Reddy SV, Maderdrut JL, Yaksh TL. Spinal cord pharmacology of adrenergic agonist-mediated antinociception. J Pharmacol Exp Ther. 1980;213:525–533. [PubMed] [Google Scholar]
- Reny-Palasse V, Monier C, Rips R. Opioid involvement in TRH-induced antinociception in the rat following intracerebral administration. Pain. 1989;38:193–201. doi: 10.1016/0304-3959(89)90238-8. [DOI] [PubMed] [Google Scholar]
- Sagen J, Proudfit HK. Effect of intrathecally administered noradrenergic antagonists on nociception in the rat. Brain Res. 1984;310:295–301. doi: 10.1016/0006-8993(84)90152-5. [DOI] [PubMed] [Google Scholar]
- Sawynok J, Reid A. Effect of 6-hydroxydopamine-induced lesions to ascending and descending noradrenergic pathways on morphine analgesia. Brain Res. 1987;419:156–165. doi: 10.1016/0006-8993(87)90579-8. [DOI] [PubMed] [Google Scholar]
- Sobue I, Yamamoto H, Konagaya M, Iida M, Takayanagi T. Effect of thyrotropin-releasing hormone on ataxia of spinocerebellar degeneration. Lancet. 1980;1:418–419. doi: 10.1016/s0140-6736(80)90963-0. [DOI] [PubMed] [Google Scholar]
- Stone LS, MacMillan LB, Kitto KF, Limbird LE, Wilcox GL. The α2a adrenergic receptor subtype mediates spinal analgesia evoked by α2 agonists and is necessary for spinal adrenergic-opioid synergy. J Neurosci. 1997;17:7157–7165. doi: 10.1523/JNEUROSCI.17-18-07157.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M, Sugano H, Matsumoto K, Yamamura M, Ishida R. Synthesis and central nervous system actions of thyrotropin-releasing hormone analogues containing a dihydroorotic acid moiety. J Med Chem. 1990;33:2130–2137. doi: 10.1021/jm00170a014. [DOI] [PubMed] [Google Scholar]
- Takasu K, Honda M, Ono H, Tanabe M. Spinal α2-adrenergic and muscarinic receptors and the NO release cascade mediate supraspinally produced effectiveness of gabapentin at decreasing mechanical hypersensitivity in mice after partial nerve injury. Br J Pharmacol. 2006;148:233–244. doi: 10.1038/sj.bjp.0706731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takei A, Fukazawa T, Hamada T, Sohma H, Yabe I, Sasaki H, et al. Effects of tandospirone on ‘5-HT1A receptor-associated symptoms' in patients with Machado–Josephe disease: an open-label study. Clin Neuropharmacol. 2004;27:9–13. doi: 10.1097/00002826-200401000-00005. [DOI] [PubMed] [Google Scholar]
- Takeuchi Y, Fujiwara K, Ishimura K, Shimada Y, Ochi M, Yoshioka H, et al. Efficacy of thyrotropin-releasing hormone in the treatment of cerebellar ataxia. Pediatr Neurol. 1989;5:107–110. doi: 10.1016/0887-8994(89)90035-0. [DOI] [PubMed] [Google Scholar]
- Tanabe M, Takasu K, Kasuya N, Shimizu S, Honda M, Ono H. Role of descending noradrenergic system and spinal α2-adrenergic receptors in the effects of gabapentin on thermal and mechanical nociception after partial nerve injury in the mouse. Br J Pharmacol. 2005;144:703–714. doi: 10.1038/sj.bjp.0706109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjolsen A, Berge OG, Eide PK, Broch OJ, Hole K. Apparent hyperalgesia after lesions of the descending serotonergic pathways is due to increased tail skin temperature. Pain. 1988;33:225–231. doi: 10.1016/0304-3959(88)90094-2. [DOI] [PubMed] [Google Scholar]
- Tokuda Y, Tanabe M, Honda M, Ono H. Descending serotonergic and noradrenergic mechanisms mediate taltirelin-induced analgesic effects on acute thermal and mechanical nociception, respectively. J Pharmacol Sci. 2005;97 Suppl I:205P. [Google Scholar]
- Ujihara H, Xie RM, Sasa M, Ishihara K, Fujita Y, Yoshimura M, et al. Inhibition by thyrotropin-releasing hormone of epileptic seizures in spontaneously epileptic rats. Eur J Pharmacol. 1991;196:15–19. doi: 10.1016/0014-2999(91)90403-d. [DOI] [PubMed] [Google Scholar]
- Urayama A, Yamada S, Kimura R, Zhang J, Watanabe Y. Neuroprotective effect and brain receptor binding of taltirelin, a novel thyrotropin-releasing hormone (TRH) analogue, in transient forebrain ischemia of C57BL/6J mice. Life Sci. 2002;72:601–607. doi: 10.1016/s0024-3205(02)02268-3. [DOI] [PubMed] [Google Scholar]
- Webster VA, Griffiths EC, Slater P. Antinociceptive effects of thyrotrophin-releasing hormone and its analogues in the rat periaqueductal grey region. Neurosci Lett. 1983;42:67–70. doi: 10.1016/0304-3940(83)90423-8. [DOI] [PubMed] [Google Scholar]
- Willis WD, Gerhart KD, Willcockson WS, Yezierski RP, Wilcox TK, Cargill CL. Primate raphe- and reticulospinal neurons: effects of stimulation in periaqueductal gray or VPLc thalamic nucleus. J Neurophysiol. 1984;51:467–480. doi: 10.1152/jn.1984.51.3.467. [DOI] [PubMed] [Google Scholar]
- Winokur A, Utiger RD. Thyrotropin-releasing hormone: regional distribution in rat brain. Science. 1974;185:265–267. doi: 10.1126/science.185.4147.265. [DOI] [PubMed] [Google Scholar]
- Wu W, Elde R, Wessendorf MW, Hökfelt T. Identification of neurons expressing thyrotropin releasing-hormone receptor mRNA in spinal cord and lower brainstem of rat. Neurosci Lett. 1992;142:143–146. doi: 10.1016/0304-3940(92)90359-f. [DOI] [PubMed] [Google Scholar]
- Xu W, Qiu XC, Han JS. Serotonin receptor subtypes in spinal antinociception in the rat. J Pharmacol Exp Ther. 1994;269:1182–1189. [PubMed] [Google Scholar]
- Yamamura M, Kinoshita K, Nakagawa H, Tanaka T, Maeda K, Ishida R. Pharmacological study of TA-0910, a new thyrotropin-releasing hormone (TRH) analog, (I): effects on the central nervous system by oral administration. Jpn J Pharmacol. 1990;53:451–461. doi: 10.1254/jjp.53.451. [DOI] [PubMed] [Google Scholar]
- Yamamura M, Kinoshita K, Nakagawa H, Ishida R. Pharmacological study of TA-0910, a new thyrotropin-releasing hormone (TRH) analog (II): involvement of the DA system in the locomotor stimulating action of TA-0910. Jpn J Pharmacol. 1991;55:57–68. doi: 10.1254/jjp.55.57. [DOI] [PubMed] [Google Scholar]
- Yeomans DC, Clark FM, Paice JA, Proudfit HK. Antinociception induced by electrical stimulation of spinally projecting noradrenergic neurons in the A7 catecholamine cell group of the rat. Pain. 1992;48:449–461. doi: 10.1016/0304-3959(92)90098-V. [DOI] [PubMed] [Google Scholar]
- Zhukov VN, Yakimova KS, Shamyakina IY. Hyperthermia and antinociceptive activity of thyrotropin-releasing hormone and morphine following central administration in rats. Acta Physiol Pharmacol Bulg. 1988;14:18–23. [PubMed] [Google Scholar]