Abstract
Background and purpose:
Although vascular smooth muscle cells are known to express the Na+–Ca2+ exchanger (NCX), its functional role has remained unclear, mainly because of its relatively low expression. We thus investigated the involvement of NCX in the mechanism for the forskolin-induced vaso-relaxation, using wild type (WT) and transgenic (TG) mice that specifically over-express NCX1.3 in smooth muscle.
Experimental approach:
We examined the relaxing effect of forskolin during the pre-contraction induced by 100 nM U46619, a thromboxane A2 analogue in the mouse isolated thoracic aorta. We also measured the intracellular Ca2+ concentration ([Ca2+]i) in fura-PE3-loaded aortic strips.
Key results:
The forskolin-induced decreases in [Ca2+]i and tension were much greater in aortas from TG mice than in those from WT mice. In a low Na+ solution, forskolin-induced decreases in [Ca2+]i and tension were greatly inhibited in both groups of aortas. In WT aortas, the presence of 100 nM SEA0400, an NCX inhibitor, had only a little effect on the forskolin-induced decreases in [Ca2+]i, but inhibited the forskolin-induced relaxation. However, in TG aortas, the presence of SEA0400 greatly inhibited the forskolin-induced decreases in [Ca2+]i and tension.
Conclusions and Implications:
The NCX was involved in the forskolin-induced reduction of [Ca2+]i and tension in the mouse thoracic aorta. Measurement of [Ca2+]i and tension in aortas of the TG mouse is thus considered to be a useful tool for evaluating the role of NCX in vascular tissue.
Keywords: Na+–Ca2+ exchanger, transgenic mouse, cAMP
Introduction
Forskolin induces the relaxation of the vascular smooth muscle through the production of adenosine 3′,5′-cyclic monophosphate (cAMP). The relaxant effects mediated by cAMP are thought to be due to a decrease in intracellular Ca2+ concentration [Ca2+]i, as well as a decrease in Ca2+ sensitivity of the contractile apparatus in smooth muscle cells (Nishimura and van Breemen 1989; Ushio-Fukai et al., 1993). To explain the cAMP-induced decrease in [Ca2+]i, several mechanisms have been proposed, including an inhibition of Ca2+ influx owing to a hyperpolarization via stimulation of Ca2+-activated K+ channels (Sadoshima et al., 1988), a stimulation of Ca2+ uptake into the intracellular stores (Mueller and van Breemen 1979), an increase in Ca2+ extrusion from cells through the sarcolemmal Ca2+ pump (Bulbring and Tomita 1987) and Ca2+ efflux through the forward mode of the Na+–Ca2+ exchanger (NCX) (Yamanaka et al., 2003).
In many mammalian tissues, NCX has been shown to be involved in the maintenance of Ca2+ homeostasis (Schulze et al., 1993; Lee et al., 2001). The NCX can transport Ca2+ either into (reverse mode) or out of (forward mode) the cells, depending on the electrochemical driving force on the exchanger. Three isoforms (NCX1, NCX2 and NCX3) of NCX have been identified in mammalian cells (Nicoll et al., 1990, 1996). Of these three isoforms, NCX1-specific transcripts are most widely expressed in many tissues (Komuro et al., 1992; Nicoll et al., 1996) including heart and vascular smooth muscle. NCX1 has been reported to be activated by external monovalent cations (Gadsby et al., 1991) and [Ca2+]i (Hilgemann, 1990), although it is inhibited by low cytoplasmic pH (Doering and Lederer 1994), high cytoplasmic Na+ concentration (Hilgemann, 1990; Gadsby et al., 1991) and ATP depletion (Condrescu et al., 1995; Iwamoto et al., 1996a, 1996b). In addition, it has been reported that NCX is phosphorylated by cAMP-dependent protein kinase (PKA) (Ruknudin et al., 2000; Wei et al., 2003) and the phosphorylation of PKA increases the activity of NCX1 (Ruknudin et al., 2000). We recently reported that isoproterenol induces a relaxation of the vascular smooth muscle, at least in part, by decreasing [Ca2+]i through the activation of NCX in the porcine coronary artery (Yamanaka et al., 2003). The activated NCX was thus thought to contribute to Ca2+ extrusion by its forward mode of action.
Recently, genetically engineered mouse models for human diseases have been produced and a number of new findings have thus been accumulated. However, the physiological approaches for these mouse models sometimes have limitations owing to their small sizes. Especially, the measurements of [Ca2+]i of the mouse vascular smooth muscle strips have been performed only in a few reports (Iwamoto et al., 2004). In addition, to our knowledge, there has been no report in which the [Ca2+]i of mouse thoracic aorta strips were measured. Concerning the NCX, we have recently reported that NCX transgenic (TG) mice that specifically overexpress NCX1.3 in smooth muscle are hypersensitive to salt intake in terms of inducing hypertension, thus indicating that salt-sensitive hypertension is triggered by Ca2+ entry through NCX in vascular smooth muscle (Iwamoto et al., 2004). It is thus clear that NCX is operating in a reverse mode during the pathogenesis of salt-sensitive hypertension. However the role of the NCX in its forward mode under normal conditions has not been explored in this model.
In the present study, we measured the [Ca2+]i and tension of the thoracic aorta from wild-type (WT) and TG mice that specifically overexpress NCX1.3 in smooth muscle. By monitoring the [Ca2+]i and tension, we examined the involvement of NCX in the forskolin-induced decreases in the [Ca2+]i and tension in aortas from WT and TG mice. As we have reported previously (Nishimura et al., 1987), β-adrenoceptors are not abundantly expressed in aorta, compared with the coronary artery, we used forskolin instead of isoproterenol in the present study. Our working hypothesis was that forskolin-induced decreases in [Ca2+]i and tension should be enhanced in the TG mice, in comparison to WT mice.
Methods
NCX TG mice
The generation of TG mice overexpressing canine NCX 1.3 in smooth muscle under the control of human smooth muscle α actin promoter on a C57BL/6J background has been previously described (Iwamoto et al., 2004). Western blot analysis showed NCX1.3 protein to be overexpressed in the aortas, but not in the hearts, of these TG mice at 6- to 8-fold the level of endogenous NCX1. On the other hand, no difference was found in the protein expression levels of Na+, K+-ATPase (α2 and α3), L-type Ca2+ channel (α1C) and sarcolemmal Ca2+ ATPase in aortas (Iwamoto et al., 2004). To confirm the transgene in the TG mice, we routinely performed PCR, using tissue from their tails.
Tissue preparation
The study protocol was approved by the Animal Care and the Ethical Committee of the Research Institute of Angiocardiology, Faculty of Medicine, Kyushu University. Male WT mice (C57BL/6J; Kyudo, Fukuoka, Japan) and male TG mice 3–5 weeks old, weighing 10–25 g were used. After the mice were killed by cervical dislocation, segments of the thoracic aortas measuring approximately 1.5 cm in length were excised. The surrounding connective tissues were then carefully dissected away under a binocular microscope. The aortas were cut into circular strips (1.0 × 1.0 mm) for the measurements of tension and cut open and made into the sheets of 1.5 × 4.0 mm for the measurements of [Ca2+]i. We were obliged to measure tension and [Ca2+]i separately, because we could not obtain a sufficient fura-PE3 signal from the small strips used to measure the tension. To remove the endothelium, the inner surface of the aortas were rubbed with a cotton swab as previously described (Kanaide, 1999).
Measurement of tension
The circular strips were mounted vertically to a force transducer (TB-612T Nihon Kohden, Tokyo, Japan) in an organ bath and equilibrated in normal physiological salt solution (PSS) at 37°C for at least 0.5 h before starting measurements, as described previously (Miyagi et al., 1996). During the 0.5 h equilibration period, the strips were stimulated with 118 mM K+-PSS every 5 min and the resting load was increased in a stepwise manner to get the maximal tension (around 150 mg). The tension was expressed as a percentage, by assigning the values in normal PSS (5.9 mM K+) and that at the steady state of contraction induced by 100 nM U46619 to be 0 and 100%, respectively.
Fura-PE3 loading
For the fura-PE3 loading, the aortic sheets were incubated in Dulbecco's-modified Eagle's medium gassed with 5% CO2 and 95% O2 containing 50 μM fura-PE3 in the form of acetoxymethyl ester (fura-PE3/AM), 1 μM probenecid and 5% fetal bovine serum for 3 h at 37°C (Kanaide, 1999). The sheets were equilibrated in normal PSS for at least 1 h before the measurements and then were fixed on a black rubber plate with fine needles.
Front-surface fluorimetry
Changes in the fluorescence intensity of the fura-PE3-Ca2+ complex were monitored by the use of a front-surface fluorimeter specifically designed for fura-PE3 fluorimetry (CAM-OF3, Japan Spectroscopic Co., Tokyo, Japan), as previously described (Hirano et al., 1990). The fluorescence intensities at 500 nm obtained with 340 nm (F340) and 380 nm excitation (F380) and their ratio (F340/F380) were monitored as an indication of [Ca2+]i (Kanaide, 1999). The fluorescence ratio was expressed as a percentage, by assigning the values at rest in normal PSS (5.9 mM K+) and that at the steady state of [Ca2+]i elevation induced by 100 nM U46619 to be 0 and 100%, respectively.
Solutions
The composition of normal PSS for fura-PE3 studies was as follows (mM): NaCl 121.8, KCl 4.7, NaHCO3 15.5, KH2PO4 1.2, MgCl2 1.2, CaCl2 1.25 and D-glucose 11.5. 118 mM K+-PSS was prepared by replacing NaCl with KCl and thus contained 25.2 mM Na+. Solutions of low Na+ (25.2 mM) PSS were made by a substitution of LiCl for NaCl (Yamanaka et al., 2003). PSS was bubbled with a mixture of 95% O2 and 5% CO2 and the resulting pH was 7.4.
Data analysis
All data from the separate measurements of [Ca2+]i and tension were collected by a computerized data acquisition system (Mac Lab, AD Instruments, Castle Hill, Australia, running on an Apple Macintosh computer). The data for the representative traces shown in the figures were printed directly from the computer using a laser printer (Cannon, Japan). The data are expressed as the mean±s.e.m. of the indicated number of experiments. One strip obtained from one animal was used for each experiment, and therefore the number of experiments (n value) indicates the number of animals. The statistical analysis was performed using Student's unpaired t-test and P-values of less than 0.05 were considered to be significant.
Materials
Fura PE3/AM was purchased from Texas Fluorescence Laboratory (Austin, TX, USA). Prostaglandin F2α (PGF2α) was purchased from Cayman Chemical (Ann Arbor, MI, USA). Fetal bovine serum, forskolin, diltiazem, serotonin, histamine, phenylephrine and U46619 (9,11-dideoxy-9a,11a-methanoepoxy PGF2α) were purchased from Sigma (St Louis, MO, USA). SEA0400 (2-(4-(2,5-difluorophenyl)methoxy)-5-ethoxyaniline) and KB-R7943 (2-(2-(4-(4-nitrobenzyloxy) phenyl) ethyl) isothiourea methanesulphonate) were kindly donated by Taisho Pharmaceutical Co., Ltd (Saitama, Japan), and Organon Co., Ltd (Osaka, Japan), respectively. All other chemicals were from Wako (Osaka, Japan).
Results
Comparison of the contractile response of aortic strips to U46619 between WT and NCX-TG mice
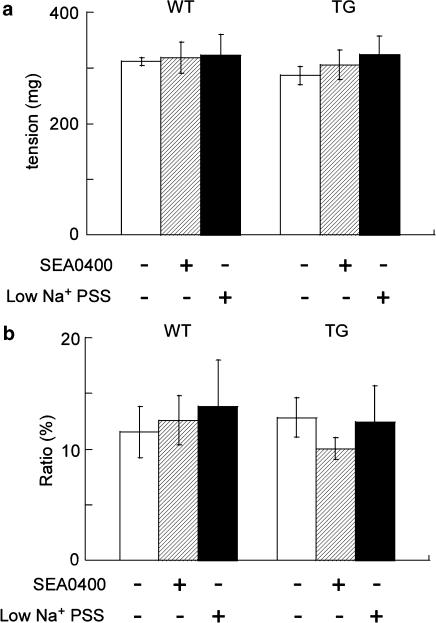
We first evaluated any differences in the [Ca2+]i elevation and tension development induced by 100 nM U46619, a thromboxane A2 analog, between WT and TG (Figure 1). In terms of the absolute value of tension, there was no difference in the U46619-induced tension between WT and TG aortas, or with and without NCX inhibition (Figure 1). The elevation of the [Ca2+]i induced by U46619 in the aortic strips was expressed relative to that induced by 50 μM ionomycin as a standard (100%) response. As ionomycin is a Ca2+ ionophore, the absolute value of the ionomycin-induced [Ca2+]i elevation was considered to be constant under all our experimental conditions. On this basis, there was no difference in the elevation of [Ca2+]i induced by U46619, between WT and TG aortas and also with and without NCX inhibition (Figure 1). Accordingly, there was no difference in the contractile response to U46619 between aortic tissue from WT and TG mice.
Figure 1.
Tension development (a) and [Ca2+]i elevation (b) induced by 100 nM U46619 in WT and NCX-TG mouse, with and without NCX inhibition by SEA0400. Tension development was measured as the absolute value (mg); elevation of [Ca2+]i was expressed relative to that obtained with 50 μM ionomycin (set at 100%). In the [Ca2+]i measurement, the strips were first stimulated with 100 nM U46619, and then exposed to 50 μM ionomycin. The strips were exposed to 100 nM SEA0400 or low Na+ PSS 5 min before and during the stimulation with U46619. Data shown are mean values±s.e.m. (n=4–6).
Effect of NCX overexpression on the forskolin-induced decreases in [Ca2+]i and tension
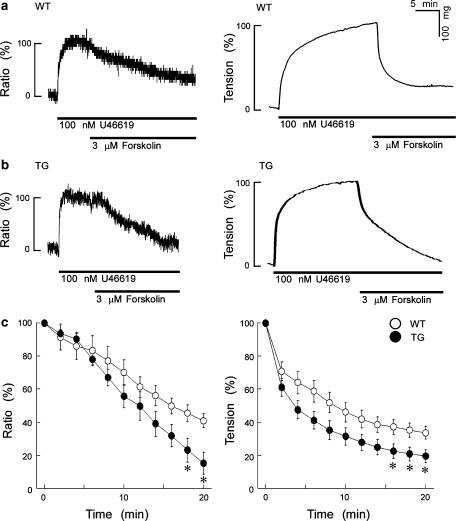
Figure 2a and b show a time course of the effect of 3 μM forskolin on [Ca2+]i and tension induced by 100 nM U46619 in WT or TG mouse thoracic aortas. In both the WT and TG tissues, the application of 100 nM U46619 induced rapid increases in both [Ca2+]i and tension. The level of [Ca2+]i reached a steady-state value within 10 min and tension reached a steady-state value within 20 min. The steady-state levels of [Ca2+]i and tension induced by 100 nM U46619 remained at the same levels at least for 30 min in both the WT and TG aortas. In both groups, the application of 3 μM forskolin during the steady state of [Ca2+]i elevation or contraction induced by 100 nM U46619 markedly decreased [Ca2+]i and tension, which reached a new steady state within 20 min. Figure 2c shows the summary (n=6) of the time course of the effect of 3 μM forskolin on the levels of [Ca2+]i and tension induced by 100 nM U46619 in the WT and TG mouse thoracic aortas. The levels of [Ca2+]i and tension after the application of 3 μM forskolin in the TG mouse aorta were significantly lower than those in the WT mouse thoracic aortas.
Figure 2.
The effect of 3 μM forskolin on [Ca2]i and tension induced by 100 nM U46619 in the WT (a) and TG (b) mouse thoracic aortas. In both groups, the application of 3 μM forskolin induced sustained decreases in [Ca2+]i and tension, when forskolin was applied during the steady-state contraction induced by 100 nM U46619. The levels of [Ca2+]i and tension were measured separately, using the aortic preparations obtained from the same mouse. The levels of [Ca2+]i and tension induced by 100 nM U46619 were set at 100% and the resting levels at 0%. Panels (c) shows a summary of the results obtained from six similar experiments (six different mice) as in panels a and b. Compared with the WT mouse, forskolin induced the greater decreases in the [Ca2]i and tension in the TG mouse thoracic aortas. Data shown are mean values±s.e.m. *P<0.05 vs WT.
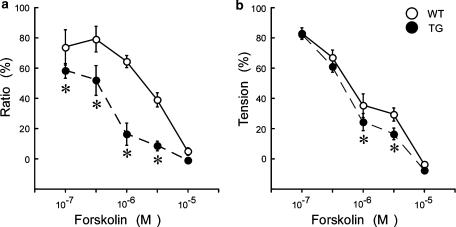
In order to construct a dose–response curve for forskolin on the levels of [Ca2+]i and tension induced by 100 nM U46619 in the WT and TG aortas, experiments similar to those shown in Figure 2 using the various concentrations of forskolin were performed and the data measured at the time point of 20 min after the application of 3 μM forskolin were plotted (Figure 3). The decreases in [Ca2+]i and tension induced by forskolin were much greater in the TG mouse than those in the WT mouse thoracic aortas. The effect of NCX overexpression on the decrease in [Ca2+]i was much more apparent than that in the decrease in tension.
Figure 3.
The concentration response curves of the effect of forskolin on [Ca2]i (a) and tension (b) induced by 100 nM U46619 in the WT and TG mouse thoracic aortas. We measured the levels of [Ca2+]i and tension at 20 min after the application of forskolin. Compared with tissues from WT mouse, forskolin induced greater decreases in [Ca2]i and tension in the TG mouse thoracic aortas. Data shown are mean values±s.e.m. (n=4–6). *P<0.05 vs WT.
Effect of the NCX inhibitor on the forskolin-induced decreases in [Ca2+]i and tension
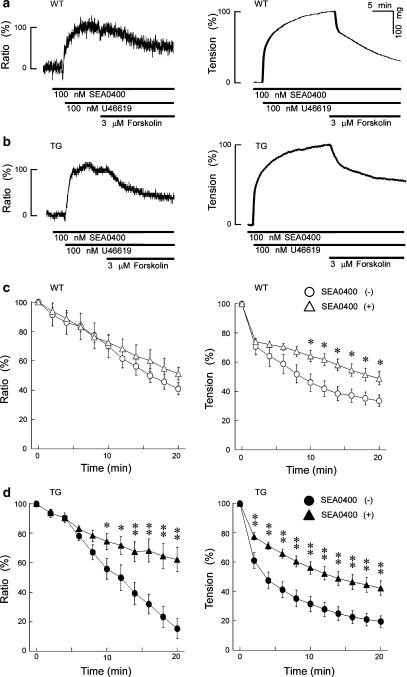
To confirm the involvement of the NCX in the forskolin-induced vaso-relaxation, we measured the effect of an NCX inhibitor and of a low extracellular Na+ concentration ([Na+]o) on the responses of WT and TG mouse thoracic aortas. We first examined the effect of SEA0400, a selective NCX inhibitor (Matsuda et al., 2001, 2005; Tanaka et al., 2002). The presence of 100 nM SEA0400 5 min before and during the application of 100 nM U46619 had no effect on the increases in [Ca2+]i and tension in the WT and TG tissues. Figure 4a and b show representative time courses of the effect of 3 μM forskolin on the levels of [Ca2+]i and the tension induced by 100 nM U46619 in the presence of 100 nM SEA0400 applied 5 min before and during the application of 100 nM U46619 in the WT or TG aortic strips. Figure 4c and d show the summary of 5 to 6 independent experiments performed as in Figure 4a and b. In this plot, the data points shown in Figure 2c were also included as a control (in the absence of SEA0400) in order to show clearly the effect of SEA0400. In the WT aortas (Figure 4c), 100 nM SEA0400 had little effect on the 3 μM forskolin-induced decreases in [Ca2+]i and tension. On the other hand, in the aortas from TG mice (Figure 4d), 100 nM SEA0400 strongly inhibited the 3 μM forskolin-induced decreases in [Ca2+]i and tension. We also tried to use KB-R7943, another NCX inhibitor but found application of 3 μM KB-R7943 to decrease the sustained elevation of [Ca2+]i induced by U46619 in mouse thoracic aortas.
Figure 4.
The effect of 100 nM SEA0400 on the forskolin-induced decreases in [Ca2]i and tension in the WT (a) and TG (b) mouse thoracic aortas. Forskolin (3 μM) was applied during the contraction induced by U46619, in the presence of 100 nM SEA0400, a potent and selective inhibitor of NCX. (c) and (d) show the summary of 5–6 independent experiments performed as in panels a and b. In this plot, the data points shown in Figure 2c were also included as a control (in the absence of SEA0400) for comparison. In the aortas from WT mice, the presence of 100 nM SEA0400 had no significant effect on the forskolin-induced decreases in [Ca2]i, whereas it inhibited the forskolin-induced decreases in tension. In the aortas from TG mice, the presence of 100 nM SEA0400 inhibited the forskolin-induced decreases in both [Ca2]i and tension. Data shown are mean values±s.e.m. *P<0.05 vs SEA0400 (−); **P<0.01 vs SEA0400 (−).
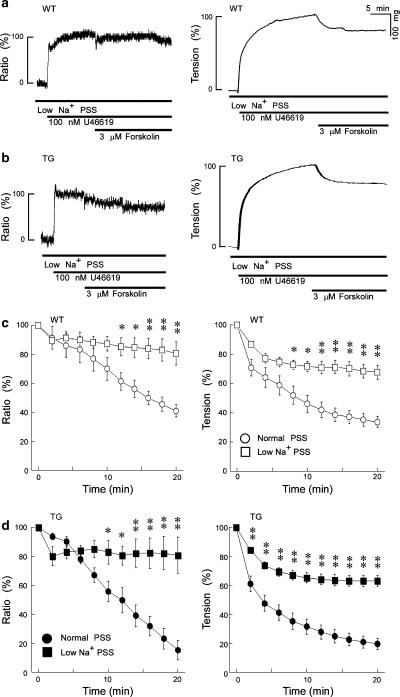
Figure 5 shows a representative time course of the effect of 3 μM forskolin on the levels of [Ca2+]i and tension induced by 100 nM U46619 in a low Na+ (25.2 mM) PSS, in which most of the Na+ was replaced with Li+. The increases in the [Ca2+]i and tension induced by 100 nM U46619 in low Na+ PSS were not significantly different from those in normal PSS, as shown in Figure 5a and b. However, the decreases in [Ca2+]i and tension induced by 3 μM forskolin under the low [Na+]o condition were markedly inhibited in both groups, relative to the effects in normal PSS (Figure 5c and d). In these graphs, the data points shown in Figure 2c (normal PSS) have been included in order to show clearly the effect of low Na+ PSS.
Figure 5.
Effects of low Na+ PSS on forskolin-induced decreases in [Ca2+]i and tension in the WT (a) and TG (b) mouse thoracic aortas. Forskolin (3 μM) was applied during the contraction induced by U46619 in low Na+ PSS ([Na+]: 25.2 mM, [K+]: 5.9 mM, [Li+]: 121.8 mM). (c) and (d) show the summary of 5–6 independent experiments performed as in panels a and b. In this plot, the data points shown in Figure 2c were also included as a control (in normal PSS) for comparison. The forskolin-induced decreases in [Ca2]i and tension were greatly inhibited in the low Na+ PSS in both groups. Data shown are mean values±s.e.m. *P<0.05 vs Normal PSS; **P<0.01 vs Normal PSS.
In order to exclude the possibility that 100 nM SEA0400 might have an effect on voltage-operated Ca2+ channels, we examined the effect of diltiazem, a calcium channel blocker, on the levels of the [Ca2+]i and tension induced by 100 nM U46619. We chose diltiazem as an inhibitor of voltage-operated Ca2+ channels, because dihydropyridines are known to be photosensitive and interfere with the performance of fluorimetry (Kanaide et al., 1988). We measured the [Ca2+]i and tension at 20 min after the application of 100 μM diltiazem, because the application of 100 μM diltiazem during the steady state of [Ca2+]i elevation or contraction induced by 100 nM U46619 decreased the [Ca2+]i and tension, which reached a new steady state within 20 min. As shown in Figure 6, the presence of 100 nM SEA0400 5 min before and during the application of 100 nM U46619 had no effect on the 100 μM diltiazem-induced decreases in [Ca2+]i and tension. Additionally, there were no difference between the WT and TG mouse thoracic aortas in terms of the 100 μm diltiazem-induced decreases in the [Ca2+]i and tension.
Figure 6.
The effect of 100 μM diltiazem on [Ca2+]i (a) and tension (b) induced by 100 nM U46619 in the absence or presence of 100 nM SEA0400 in the WT and TG mouse thoracic aortas. The levels of [Ca2+]i and tension seen 20 min after the application of 100 μM diltiazem were plotted. There was no difference in the effect of diltiazem between the SEA-treated and non-treated groups. In addition, there was no difference in the 100 μM diltiazem-induced decreases in [Ca2+]i and tension between the WT mice and TG mice. Data are the mean±s.e.m. (n=4–6). P<0.05 vs WT SEA0400 (−).
Relaxant effect of forskolin during PGF2α-induced contraction
We examined whether the contribution of the NCX to the forskolin-induced relaxation might depend on the agent used for pre-contraction. In the mouse aorta, phenylephrine, histamine or serotonin had negligible contractile effects in comparison to that seen with 100 nM U46619 (data not shown), whereas PGF2α induced a sustained contraction (Figure 7a). The level of the maximal tension development obtained with 10 μM PGF2α in WT (61.5±12.3 mg; n=4) was approximately 20% of that obtained with 100 nM U46619 (Figure 1). However, PGF2α induced only a negligible increase in [Ca2+]i compared to that seen with U46619 (Figure 7c). The PGF2α-induced contraction thus appeared to depend on the increase in the Ca2+ sensitivity of the contractile apparatus to a greater extent than the U46619-induced contraction. The contractile effect of PGF2α in TG aortas was similar to that in WT aortas and SEA0400 had no effect on these PGF2α-induced pre-contractions (Figure 7b). Forskolin induced a concentration-dependent relaxation during the PGF2α-induced contraction both in WT and TG tissues (Figure 7d). The relaxant effect of 3 μM forskolin in TG aortas was slightly but significantly greater than that seen in WT aortas (Figure 7d). Furthermore, SEA0400 had no significant effect on the forskolin-induced relaxation during the PGF2α-induced contraction in aortas from both WT and TG mice (Figure 7e).
Figure 7.
Forskolin-induced relaxation during the PGF2α-induced precontraction. (a) A representative trace of the tension showing the contraction induced by 10 μM PGF2α and the subsequent relaxation induced by 3 μM forskolin. (b) A comparison of the contractile effect of 10 μM PGF2α in tissues from WT and TG mice and between the presence and absence of 100 nM SEA0400. (c) Representative traces showing the effect of 10 μM PGF2α on [Ca2+]i in comparison to that seen with 100 nM U46619. The levels of [Ca2+]i obtained at rest and at the peak elevation obtained with 50 μM ionomycin were assigned values of 0 and 100%, respectively. (d) The concentration-dependent relaxant effects of forskolin on PGF2α-induced contractions in aortas from WT and TG mice. The level of precontraction seen just before the application of forskolin was assigned a value of 100%. (e) The effect of 100 nM SEA0400 on the relaxation induced by 300 nM and 3 μM forskolin during the 10 μM PGF2α-induced contraction. Data are the mean s.e.m. (n=4 in (b), 3–4 in (d), 3–4 in (e)). *P<0.05, n.s., not significantly different.
Discussion and conclusions
By using a genetically engineered mouse that overexpressed the NCX in smooth muscle, we have recently reported that salt-sensitive hypertension could be triggered by the Ca2+ entry through the NCX in vascular smooth muscle cells (Iwamoto et al., 2004). It follows that the NCX may thus be operating in a reverse mode during the pathogenesis of the salt-sensitive hypertension. However the role of the forward mode of the NCX under normal conditions has not been explored in this model. In addition, we have also reported that the forward mode NCX may be involved in the mechanism underlying the isoproterenol- or forskolin-induced reduction of the [Ca2+]i and tension in the porcine coronary artery under the normal condition (Yamanaka et al., 2003). In the present study, we have tested the hypothesis that the forskolin-induced reduction of the [Ca2+]i and tension might be enhanced in the aortas from the NCX overexpressing TG mice. The reason why we used forskolin instead of isoproterenol in the present study is that the aorta expresses β-adrenoceptors much less abundantly than the coronary artery as we have reported previously (Nishimura et al., 1987). In fact, the application of 100 μM isoproterenol after 100 nM U46619-induced contraction did not induce any significant relaxation in both WT and TG mouse thoracic aortas (data not shown). The data presented here clearly showed that forskolin-induced decreases in the [Ca2+]i and tension were enhanced in the TG mice in comparison to the WT mice. The results using the low Na+ PSS and SEA0400, an NCX inhibitor, also confirmed this hypothesis.
We used the TG mouse overexpressing canine NCX1.3 in the smooth muscle driven by the smooth muscle α-actin promoter (Iwamoto et al., 2004). Gene expression of NCX has been reported in blood vessels (Nakasaki et al., 1993; Juhaszova et al., 1996; Quednau et al., 1997; Wakimoto et al., 2000) and functional studies indicated that the NCX plays an important role in the regulation of cytosolic Ca2+ in vascular myocytes (Zhu et al., 1994; Nazer and van Breemen 1998; Slodzinski and Blaustein 1998; Blaustein and Lederer 1999). The NCX-knockout mouse is an alternative and complementary model to further examine the role of the NCX in vascular smooth muscle. However, we could not use the NCX-knockout mouse, because it has been reported from several laboratories that the NCX-knockout mouse is embryonically lethal (Cho et al., 2000; Wakimoto et al., 2000; Koushik et al., 2001; Reuter et al., 2002a, 2002b). For these reasons, we believe that the TG mouse overexpressing NCX1 in the smooth muscle may thus be the best model for an investigation of the role of NCX in vascular smooth muscle, at present.
Forskolin was used to increase the cellular cAMP content. If our working hypothesis that cAMP-mediated pathways would increase the forward mode of NCX activity was correct, forskolin should induce greater decreases in [Ca2+]i and tension in the TG mice than in the WT mice. This prediction was clearly proven in the experiments shown in Figures 2 and 3. In addition, the effect of NCX overexpression on the decrease in [Ca2+]i was much greater than that on the decrease in tension. This observation could be explained by the notion that the cAMP-induced relaxation is regulated by decreases in both [Ca2+]i and Ca2+ sensitivity and that the latter plays a more predominant role in relaxation than the former. It is thus concluded that the NCX overexpression results in the enhancement of the forskolin-induced decreases in the [Ca2+]i and tension under normal condition.
To further confirm the involvement of NCX in the forskolin-induced decreases in [Ca2+]i and tension, we used NCX inhibitors and low Na+ PSS. Although KB-R7943 was synthesized as a potent inhibitor of NCX (IC50 is 2–4 μM) (Iwamoto et al., 1996b), previous studies did not necessarily support the selectivity of KB-R7943 for NCX (Sobolevsky and Khodorov 1999; Arakawa et al., 2000; Pintado et al., 2000; Ouardouz et al., 2005). In fact, the application of 3 μM KB-R7943 decreased the sustained elevation of [Ca2+]i induced by U46619 in mouse thoracic aorta. Recently, SEA0400 has been reported to be a more potent and selective inhibitor of NCX (IC50 is 5–33 nM)(Matsuda et al., 2001, 2005; Tanaka et al., 2002) and this compound had no significant effect on the U46619-induced [Ca2+]i elevation and tension. Matsuda et al. (2001) showed that SEA0400 in the concentration range that inhibits NCX exhibits negligible affinities for Ca2+ channels, Na+ channels, K+ channels, noradrenaline transporter and various receptors and that it does not affect the activities of the store-operated Ca2+ channel, Na+–H+ exchanger and several enzymes including Na+, K+-ATPase and Ca2+-ATPase. We thus used this compound in the present study. Based on the observation that SEA0400 significantly inhibited the forskolin-induced decreases in [Ca2+]i and tension in aortas from TG mice, we concluded that NCX is involved in the forskolin-induced decreases in [Ca2+]i and tension. In the WT aortas, SEA0400 tended to inhibit the forskolin-induced decreases in [Ca2+]i and tension, although this effect did not reach statistical significance. The NCX activity in WT mouse thoracic aortas is thus possibly lower than that of the pig coronary artery (Yamanaka et al., 2003). SEA0400 has also been reported to exert non-specific effect (Reuter et al., 2002a, 2002b). We therefore examined the effect of lowering the extracellular Na+ concentrations on the forskolin-induced decrease in [Ca2+]i and tension. The forward mode of NCX was inhibited in the low Na+ PSS. The observations with low Na+ PSS provided additional support for the involvement of NCX in the forskolin-induced decreases in [Ca2+]i and tension. Thus, overexpression of the NCX results in enhancement of forskolin-induced decreases in [Ca2+]i and tension under normal conditions through the activation of NCX.
KB-R7943 has been reported to inhibit the L-type Ca2+ channels (Ouardouz et al., 2005). In order to exclude the possibility that SEA0400 might also inhibit L-type Ca2+ channels, we examined the effect of diltiazem, a Ca2+ channel blocker, on the U46619-induced sustained increase in [Ca2+]i and tension in the presence or absence of SEA0400. As the presence of SEA0400 had no effect on the diltiazem-induced decreases in [Ca2+]i and tension in the WT aortas, we concluded that SEA0400 may therefore not affect the opening of L-type Ca2+ channels. We considered that if SEA0400 inhibited L-type Ca2+ channels, then diltiazem should no longer induce any decrease in [Ca2+]i and tension in the presence of this compound, because L-type Ca2+ channels had already been inhibited by SEA0400. In addition, as there was no difference between the WT and TG tissues regarding the diltiazem-induced decreases in [Ca2+]i and tension, we considered that there would be no difference in the expression of the L-type Ca2+ channel between the WT and TG mouse thoracic aortas. This finding is in close agreement with a previous study which showed no difference in the protein levels of the L-type Ca2+ channel (α1C) in aortas from the TG mouse by Western blotting (Iwamoto et al., 2004).
The present study also suggested that the contribution of the NCX to the cAMP-mediated relaxation could differ with the type of the precontraction. The forskolin-induced relaxation seen during U46619-induced precontraction was enhanced in TG. However, such enhancement was small during PGF2α-induced precontraction. Furthermore, SEA0400 had no significant inhibitory effect on the forskolin-induced relaxation during PGF2α-induced precontraction. As PGF2α contracted mouse aorta with a negligible elevation of [Ca2+]i, its contractile mechanism may depend more on the increase in the Ca2+ sensitivity of the contractile apparatus than on increased [Ca2+]i. It may also be that when the increase in the Ca2+ sensitivity is the major component of the contractile mechanism, the cAMP-mediated relaxation becomes largely dependent on that component and much less affected by changes in [Ca2+]i, mediated by the NCX.
In a previous report (Iwamoto et al., 2004), the reverse mode of NCX was considered to exert a dominant effect in DOCA-salt hypertension model. The Na+/K+-ATPase activity was considered to be inhibited by the endogenous cardiotonic steroid under this situation, which in turn induced a localized elevation of [Na+]i in the submembranous regions and decreased the resting membrane potential. It was conceivable that this situation favored the reverse mode of NCX. The functional consequences of activation of the reverse mode of NCX became apparent in TG mice overexpressing NCX in vascular smooth muscle cells, by increasing vascular tone and thereby causing hypertension. On the other hand, the forward mode of NCX should be favored under the present experimental conditions; cAMP-mediated signaling was activated during the U46619-induced sustained contractions that was associated with an increase in [Ca2+]i. The functional consequences of activation of the forward mode of NCX was augmented in TG aortas. All these observations suggest that the overexpression of NCX in TG mice could result in the augmentation of the NCX activity in either forward or reverse direction, depending on the local conditions.
In summary, the present results indicated that forskolin-induced decreases in [Ca2+]i and tension were enhanced in aortas from TG mice in comparison to those from WT mice, thus confirming that the cAMP-mediated pathway activates the forward mode of the NCX, enhancing vaso-relaxation. The measurement of [Ca2+]i and tension of the aortic tissue from TG mice is thus considered to be a useful tool to evaluate the role of the NCX in vascular tissue.
Acknowledgments
We thank Mr Brian Quinn for linguistic comments and help with the manuscript. This study was supported in part by the grant from the 21st Century COE Program and Grants-in-Aid for Scientific Research (nos. 17590222, 17590744, 17790493) from the Ministry of Education, Culture, Sports, Science and Technology, Japan, and Mochida Memorial Foundation for Medical and Pharmaceutical Research.
Abbreviations
- [Ca2+]i
intracellular Ca2+ concentration
- [Na+]o
extracellular Na+ concentration
- NCX
Na+–Ca2+ exchanger
- PGF2α
prostaglandin F2α
- PKA
cAMP dependent protein kinase
- PSS
physiological saline solution
- TG
transgenic
Conflict of interest
The authors state no conflict of interest.
References
- Arakawa N, Sakaue M, Yokoyama I, Hashimoto H, Koyama Y, Baba A, et al. KB-R7943 inhibits store-operated Ca2+ entry in cultured neurons and astrocytes. Biochem Biophys Res Commun. 2000;279:354–357. doi: 10.1006/bbrc.2000.3968. [DOI] [PubMed] [Google Scholar]
- Blaustein MP, Lederer WJ. Sodium/calcium exchange: its physiological implications. Physiol Rev. 1999;79:763–854. doi: 10.1152/physrev.1999.79.3.763. [DOI] [PubMed] [Google Scholar]
- Bulbring E, Tomita T. Catecholamine action on smooth muscle. Pharmacol Rev. 1987;39:49–96. [PubMed] [Google Scholar]
- Cho CH, Kim SS, Jeong MJ, Lee CO, Shin HS. The Na+–Ca2+ exchanger is essential for embryonic heart development in mice. Mol Cells. 2000;10:712–722. doi: 10.1007/s10059-000-0712-2. [DOI] [PubMed] [Google Scholar]
- Condrescu M, Gardner JP, Chernaya G, Aceto JF, Kroupis C, Reeves JP. ATP-dependent regulation of sodium–calcium exchange in Chinese hamster ovary cells transfected with the bovine cardiac sodium–calcium exchanger. J Biol Chem. 1995;270:9137–9146. doi: 10.1074/jbc.270.16.9137. [DOI] [PubMed] [Google Scholar]
- Doering AE, Lederer WJ. The action of Na+ as a cofactor in the inhibition by cytoplasmic protons of the cardiac Na+–Ca2+ exchanger in the guinea-pig. J Physiol. 1994;480 Pt 1:9–20. doi: 10.1113/jphysiol.1994.sp020336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadsby DC, Noda M, Shepherd RN, Nakao M. Influence of external monovalent cations on Na–Ca exchange current-voltage relationships in cardiac myocytes. Ann N Y Acad Sci. 1991;639:140–146. doi: 10.1111/j.1749-6632.1991.tb17297.x. [DOI] [PubMed] [Google Scholar]
- Hilgemann DW. Regulation and deregulation of cardiac Na+–Ca2+ exchange in giant excised sarcolemmal membrane patches. Nature. 1990;344:242–245. doi: 10.1038/344242a0. [DOI] [PubMed] [Google Scholar]
- Hirano K, Kanaide H, Abe S, Nakamura M. Effects of diltiazem on calcium concentrations in the cytosol and on force of contractions in porcine coronary arterial strips. Br J Pharmacol. 1990;101:273–280. doi: 10.1111/j.1476-5381.1990.tb12700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamoto T, Kita S, Zhang J, Blaustein MP, Arai Y, Yoshida S, et al. Salt-sensitive hypertension is triggered by Ca2+ entry via Na+/Ca2+ exchanger type-1 in vascular smooth muscle. Nat Med. 2004;10:1193–1199. doi: 10.1038/nm1118. [DOI] [PubMed] [Google Scholar]
- Iwamoto T, Pan Y, Wakabayashi S, Imagawa T, Yamanaka HI, Shigekawa M. Phosphorylation-dependent regulation of cardiac Na+/Ca2+ exchanger via protein kinase C. J Biol Chem. 1996a;271:13609–13615. doi: 10.1074/jbc.271.23.13609. [DOI] [PubMed] [Google Scholar]
- Iwamoto T, Watano T, Shigekawa M. A novel isothiourea derivative selectively inhibits the reverse mode of Na+/Ca2+ exchange in cells expressing NCX1. J Biol Chem. 1996b;271:22391–22397. doi: 10.1074/jbc.271.37.22391. [DOI] [PubMed] [Google Scholar]
- Juhaszova M, Shimizu H, Borin ML, Yip RK, Santiago EM, Lindenmayer GE, et al. Localization of the Na+–Ca2+ exchanger in vascular smooth muscle, and in neurons and astrocytes. Ann N Y Acad Sci. 1996;779:318–335. doi: 10.1111/j.1749-6632.1996.tb44804.x. [DOI] [PubMed] [Google Scholar]
- Kanaide H. Measurement of [Ca2+]i in smooth muscle strips using front-surface fluorimetry. Methods Mol Biol. 1999;114:269–277. doi: 10.1385/1-59259-250-3:269. [DOI] [PubMed] [Google Scholar]
- Kanaide H, Kobayashi S, Nishimura J, Hasegawa M, Shogakiuchi Y, Matsumoto T, et al. Quin2 microfluorometry and effects of verapamil and diltiazem on calcium release from rat aorta smooth muscle cells in primary culture. Circ Res. 1988;63:16–26. doi: 10.1161/01.res.63.1.16. [DOI] [PubMed] [Google Scholar]
- Komuro I, Wenninger KE, Philipson KD, Izumo S. Molecular cloning and characterization of the human cardiac Na+/Ca2+ exchanger cDNA. Proc Natl Acad Sci USA. 1992;89:4769–4773. doi: 10.1073/pnas.89.10.4769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koushik SV, Wang J, Rogers R, Moskophidis D, Lambert NA, Creazzo TL, et al. Targeted inactivation of the sodium-calcium exchanger (Ncx1) results in the lack of a heartbeat and abnormal myofibrillar organization. FASEB J. 2001;15:1209–1211. doi: 10.1096/fj.00-0696fje. [DOI] [PubMed] [Google Scholar]
- Lee CH, Poburko D, Sahota P, Sandhu J, Ruehlmann DO, Van Breemen C. The mechanism of phenylephrine-mediated [Ca2+]i oscillations underlying tonic contraction in the rabbit inferior vena cava. J Physiol. 2001;534:641–650. doi: 10.1111/j.1469-7793.2001.t01-1-00641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda T, Arakawa N, Takuma K, Kishida Y, Kawasaki Y, Sakaue M, et al. SEA0400, a novel and selective inhibitor of the Na+–Ca2+ exchanger, attenuates reperfusion injury in the in vitro and in vivo cerebral ischemic models. J Pharmacol Exp Ther. 2001;298:249–256. [PubMed] [Google Scholar]
- Matsuda T, Koyama Y, Baba A. Functional proteins involved in regulation of intracellular Ca2+ for drug development: pharmacology of SEA0400, a specific inhibitor of the Na+–Ca2+ exchanger. J Pharmacol Sci. 2005;97:339–343. doi: 10.1254/jphs.fmj04007x2. [DOI] [PubMed] [Google Scholar]
- Miyagi Y, Kobayashi S, Nishimura J, Fukui M, Kanaide H. Dual regulation of cerebrovascular tone by UTP: P2U receptor-mediated contraction and endothelium-dependent relaxation. Br J Pharmacol. 1996;118:847–856. doi: 10.1111/j.1476-5381.1996.tb15477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller E, van Breemen C. Role of intracellular Ca2+ sequestration in β-adrenergic relaxation of a smooth muscle. Nature. 1979;281:682–683. doi: 10.1038/281682a0. [DOI] [PubMed] [Google Scholar]
- Nakasaki Y, Iwamoto T, Hanada H, Imagawa T, Shigekawa M. Cloning of the rat aortic smooth muscle Na+/Ca2+ exchanger and tissue-specific expression of isoforms. J Biochem (Tokyo) 1993;114:528–534. doi: 10.1093/oxfordjournals.jbchem.a124211. [DOI] [PubMed] [Google Scholar]
- Nazer MA, van Breemen C. Functional linkage of Na+–Ca2+ exchange and sarcoplasmic reticulum Ca2+ release mediates Ca2+ cycling in vascular smooth muscle. Cell Calcium. 1998;24:275–283. doi: 10.1016/s0143-4160(98)90051-3. [DOI] [PubMed] [Google Scholar]
- Nicoll DA, Longoni S, Philipson KD. Molecular cloning and functional expression of the cardiac sarcolemmal Na+–Ca2+ exchanger. Science. 1990;250:562–565. doi: 10.1126/science.1700476. [DOI] [PubMed] [Google Scholar]
- Nicoll DA, Quednau BD, Qui Z, Xia YR, Lusis AJ, Philipson KD. Cloning of a third mammalian Na+–Ca2+ exchanger, NCX3. J Biol Chem. 1996;271:24914–24921. doi: 10.1074/jbc.271.40.24914. [DOI] [PubMed] [Google Scholar]
- Nishimura J, Kanaide H, Nakamura M. Characteristics of adrenoceptors and [3H]nitrendipine receptors of porcine vascular smooth muscle: differences between coronary artery and aorta. Circ Res. 1987;60:837–844. doi: 10.1161/01.res.60.6.837. [DOI] [PubMed] [Google Scholar]
- Nishimura J, van Breemen C. Direct regulation of smooth muscle contractile elements by second messengers. Biochem Biophys Res Commun. 1989;163:929–935. doi: 10.1016/0006-291x(89)92311-5. [DOI] [PubMed] [Google Scholar]
- Ouardouz M, Zamponi GW, Barr W, Kiedrowski L, Stys PK. Protection of ischemic rat spinal cord white matter: Dual action of KB-R7943 on Na+/Ca2+ exchange and L-type Ca2+ channels. Neuropharmacology. 2005;48:566–575. doi: 10.1016/j.neuropharm.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Pintado AJ, Herrero CJ, Garcia AG, Montiel C. The novel Na+/Ca2+ exchange inhibitor KB-R7943 also blocks native and expressed neuronal nicotinic receptors. Br J Pharmacol. 2000;130:1893–1902. doi: 10.1038/sj.bjp.0703519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quednau BD, Nicoll DA, Philipson KD. Tissue specificity and alternative splicing of the Na+/Ca2+ exchanger isoforms NCX1, NCX2, and NCX3 in rat. Am J Physiol. 1997;272:C1250–C1261. doi: 10.1152/ajpcell.1997.272.4.C1250. [DOI] [PubMed] [Google Scholar]
- Reuter H, Henderson SA, Han T, Matsuda T, Baba A, Ross RS, et al. Knockout mice for pharmacological screening: testing the specificity of Na+–Ca2+ exchange inhibitors. Circ Res. 2002a;91:90–92. doi: 10.1161/01.res.0000027529.37429.38. [DOI] [PubMed] [Google Scholar]
- Reuter H, Henderson SA, Han T, Ross RS, Goldhaber JI, Philipson KD. The Na+–Ca2+ exchanger is essential for the action of cardiac glycosides. Circ Res. 2002b;90:305–308. doi: 10.1161/hh0302.104562. [DOI] [PubMed] [Google Scholar]
- Ruknudin A, He S, Lederer WJ, Schulze DH. Functional differences between cardiac and renal isoforms of the rat Na+–Ca2+ exchanger NCX1 expressed in Xenopus oocytes. J Physiol. 2000;529:599–610. doi: 10.1111/j.1469-7793.2000.00599.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadoshima J, Akaike N, Kanaide H, Nakamura M. Cyclic AMP modulates Ca-activated K channel in cultured smooth muscle cells of rat aortas. Am J Physiol. 1988;255:H754–H759. doi: 10.1152/ajpheart.1988.255.4.H754. [DOI] [PubMed] [Google Scholar]
- Schulze D, Kofuji P, Hadley R, Kirby MS, Kieval RS, Doering A, et al. Sodium/calcium exchanger in heart muscle: molecular biology, cellular function, and its special role in excitation–contraction coupling. Cardiovasc Res. 1993;27:1726–1734. doi: 10.1093/cvr/27.10.1726. [DOI] [PubMed] [Google Scholar]
- Slodzinski MK, Blaustein MP. Physiological effects of Na+/Ca2+ exchanger knockdown by antisense oligodeoxynucleotides in arterial myocytes. Am J Physiol. 1998;275:C251–C259. doi: 10.1152/ajpcell.1998.275.1.C251. [DOI] [PubMed] [Google Scholar]
- Sobolevsky AI, Khodorov BI. Blockade of NMDA channels in acutely isolated rat hippocampal neurons by the Na+/Ca2+ exchange inhibitor KB-R7943. Neuropharmacology. 1999;38:1235–1242. doi: 10.1016/s0028-3908(99)00040-4. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Nishimaru K, Aikawa T, Hirayama W, Tanaka Y, Shigenobu K. Effect of SEA0400, a novel inhibitor of sodium–calcium exchanger, on myocardial ionic currents. Br J Pharmacol. 2002;135:1096–1100. doi: 10.1038/sj.bjp.0704574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ushio-Fukai M, Abe S, Kobayashi S, Nishimura J, Kanaide H. Effects of isoprenaline on cytosolic calcium concentrations and on tension in the porcine coronary artery. J Physiol. 1993;462:679–696. doi: 10.1113/jphysiol.1993.sp019576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakimoto K, Kobayashi K, Kuro OM, Yao A, Iwamoto T, Yanaka N, et al. Targeted disruption of Na+/Ca2+ exchanger gene leads to cardiomyocyte apoptosis and defects in heartbeat. J Biol Chem. 2000;275:36991–36998. doi: 10.1074/jbc.M004035200. [DOI] [PubMed] [Google Scholar]
- Wei SK, Ruknudin A, Hanlon SU, McCurley JM, Schulze DH, Haigney MC. Protein kinase A hyperphosphorylation increases basal current but decreases beta-adrenergic responsiveness of the sarcolemmal Na+–Ca2+ exchanger in failing pig myocytes. Circ Res. 2003;92:897–903. doi: 10.1161/01.RES.0000069701.19660.14. [DOI] [PubMed] [Google Scholar]
- Yamanaka J, Nishimura J, Hirano K, Kanaide H. An important role for the Na+–Ca2+ exchanger in the decrease in cytosolic Ca2+ concentration induced by isoprenaline in the porcine coronary artery. J Physiol. 2003;549:553–562. doi: 10.1113/jphysiol.2002.037135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z, Tepel M, Neusser M, Zidek W. Role of Na+–Ca2+ exchange in agonist-induced changes in cytosolic Ca2+ in vascular smooth muscle cells. Am J Physiol. 1994;266:C794–C799. doi: 10.1152/ajpcell.1994.266.3.C794. [DOI] [PubMed] [Google Scholar]