Abstract
Background and purpose:
Pulmonary embolism (PE) represents a real diagnostic challenge. PE is associated with pulmonary hypertension due to pulmonary vascular obstruction and vasoconstriction. We recently reported that pulmonary gas embolism transiently increases exhaled nitric oxide (FENO), but it is not known whether solid emboli may alter FENO, and whether an intact endogenous NO synthesis has a beneficial effect in experimental solid pulmonary embolism.
Experimental approach:
We used anaesthetised and ventilated rabbits in these experiments. To mimic PE, a single intravenous infusion of homogenized autologous skeletal muscle tissue (MPE) was given to rabbits with intact NO production (MPE of 60, 15, or 7.5 mg kg−1; group 1) and to another group (group 2) with inhibited NO synthesis (L-NAME 30 mg kg−1; MPE of 7.5, 15 or 30 mg kg−1).
Key results:
In group 1, after MPE, FENO increased rapidly and dose-dependently and FENO was still significantly elevated after 60 min with the two highest emboli doses. All these animals survived more than 60 min after embolization. In group 2, MPE of 7.5, 15 and 30 mg kg−1, in combination with NO synthesis inhibition, resulted in 67%, 50% and 25% survival at 60 min respectively, representing a statistically significant decrease in survival. Cardiovascular and blood-gas changes after MPE were intensified by pre-treatment with NO synthesis inhibitor.
Conclusions and implications:
We conclude that solid PE causes a sustained, dose-dependent increase in FENO, giving FENO a diagnostic potential in PE. Furthermore, intact NO production appears critical for tolerance to acute PE.
Keywords: nitric oxide, pulmonary embolism, cardiovascular system, lung diseases, nitric oxide synthase, respiratory system
Introduction
Venous thrombo-embolic disease is the third most common cardiovascular disease after ischaemic coronary heart disease and stroke. In 90% of the cases of pulmonary embolism (PE), the source is deep venous thrombosis. Prevention of deep venous thrombosis and early diagnosis of PE are of great value, because mortality rate of untreated PE is 30% compared to 8% when treated (Olin, 2002). The signs and symptoms of PE are nonspecific and mimic those of other both common and uncommon diseases. The now used diagnostic tests have low specificity and sensitivity or are time-consuming and expensive (Olin, 2002). The underlying pathophysiology of PE is pulmonary macro- or micro-obstruction together with reactive arterial vasoconstriction, depending on emboli size, leading to pulmonary hypertension of varying severity. Acute pulmonary hypertension may cause right ventricular failure (acute cor pulmonale) and eventually cardiogenic shock (Via and Braschi, 2004). Vasoactive mediators have been shown to be involved in the pulmonary vasoconstriction during experimental PE, including thromboxane A2 (Reeves et al., 1983), 5-hydroxytryptamine (serotonin) (Utsunomiya et al., 1981) and endothelin-1 (Schmeck et al., 1998). Impaired blood gases are another feature of PE, indicating ventilation–perfusion mismatch, but these are not a conclusive diagnostic sign for PE (Meyer et al., 2003).
Endogenous generation of the gaseous molecule NO plays an important role in the modulation of pulmonary vascular tone to optimise ventilation–perfusion matching (Persson et al., 1990). In healthy human adults, NO is of importance in regulation of both basal pulmonary and systemic vascular resistance (Stamler et al., 1994) and local regulation of pulmonary blood flow is attenuated by administration of an NO synthase inhibitor in healthy human subjects (Rimeika et al., 2004). Measuring NO in exhaled breath is a simple, non-invasive method of monitoring changes in endogenous NO production and scavenging in the lung (Gustafsson et al., 1991; Ricciardolo et al., 2004).
There are several ways of inducing experimental PE including intravenous (i.v.) infusion of autologous blood clots (Utsunomiya et al., 1981; Reeves et al., 1983), gas (Schmeck et al., 1998), microspheres (Bottiger et al., 1996) and autologous muscle (Feigin et al., 1983; Murray et al., 1987; Priebe, 1988; Tajimi et al., 1989; Tanaka et al., 1990, 1991). Recently, we reported that venous gas embolism, which induces transient PE, led to a temporary increase in exhaled nitric oxide (FENO) and that a part of this increase was due to a decrease in end-tidal CO2 (ETCO2) (Agvald et al., 2006). It has been shown that pulmonary gas embolism will resolve by itself within 30 min in a similar experimental model (Hlastala et al., 1979), which might explain why the increase in FENO was short-lived in our recent study. This raises the question whether a more prolonged acute PE leads to a sustained increase in FENO. The issue is important in order to evaluate the potential utility of performing a clinical study on acute PE and measurement of FENO in humans.
Thus, the aim of the present study was to investigate whether acute PE with solid emboli causes a sustained increase in FENO and also to investigate whether this change in production or scavenging of endogenous NO is beneficial in a PE setting, as a recent study indicated that L-arginine administration in vivo in an animal model did not alter the effects of acute PE (Souza-Silva et al., 2005). We therefore examined: (1) FENO by studying the response of intact endogenous NO generation in an experimental rabbit model of PE and (2) the effects on systemic haemodynamics and mortality in this model during inhibition of the endogenous NO production. We used i.v. infusion of autologous muscle homogenate to induce acute PE because pilot experiments showed that haemoglobin release during autologous blood clot infusion interfered with FENO measurements, that is, free haemoglobin scavenged NO produced in the lung. Autologous muscle infusion is suggested to mimic the acute phase of pulmonary embolization (Priebe, 1988) and to produce immediate and consistent haemodynamic changes comparable with those occurring in humans with massive and diffuse PE (Murray et al., 1987).
In this study, we demonstrate that FENO increases following PE and that NO synthesis is essential for tolerance to PE challenge.
Methods
Anaesthesia and initial surgical procedures
The experiments were approved by the local animal ethics committee. Male white New Zealand rabbits (n=32, body weight 2.7±0.1 kg) were anaesthetised via an ear vein with sodium pentobarbital (6 mg ml−1 in saline, 40–60 mg kg−1). The animals were placed in the supine position and tracheotomised to allow mechanical ventilation, using a constant volume ventilator (model 683, Harvard Apparatus, South Natick, MA, USA). The ventilator was supplied with NO-free air using a charcoal filter (110 × 11 cm). Respiratory rate was 40 min−1, and tidal volume was initially adjusted to keep the ETCO2 close to 5.0% as determined by a gas analyser (Oscar Oxy, Datex, Helsinki, Finland), which sampled gas (150 ml min−1, 15–20% of minute ventilation) from one of two sidearms connected to the tracheal cannula, and by using a Naphion sampling catheter. To the other sidearm, a pressure transducer (Statham, Hato Rey, Puerto Rico) was connected to monitor the insufflation pressure (IP). The gas from the ventilator outlet was led through a switching valve to one of two beakers creating a positive end-expiratory pressure (PEEP) of 1–2 or 4–5 cm H2O. During the experiment, the gas flow was altered between the lower PEEP (9 min) and the higher PEEP (1 min), with an interval of 10 min in total, in order to optimise ventilation and prevent atelectasis formation. A continuous infusion containing glucose (24.3 g l−1), dextran 70 (Macrodex 26.5 g l−1), NaHCO3 (6.2 g l−1), sodium pentobarbital (4.1 g l−1) and pancuronium bromide (98 mg l−1) was administered at a rate of 5 ml kg−1 h−1 via the ear vein by means of a Terumo STC-521 syringe pump (Terumo Corp., Tokyo, Japan). A heparinised catheter was inserted in the left common carotid artery for mean arterial blood pressure (MAP) and heart rate (HR) recordings (Staham pressure transducer), and arterial blood sampling. Another catheter was inserted in the right jugular vein for administration of drugs and PE material. Body temperature was maintained at 38–38.5°C by means of a heating pad connected to a thermostat. Muscles from the anterior compartment of the right lower hind limb were resected and placed in saline. After the surgery, the animals were allowed a 30–60 min intervention-free period to reach stable circulatory conditions and FENO values.
NO measurements in exhaled breath
FENO was continuously measured by means of a chemiluminescence-based system (NIOX, Aerocrine AB, Solna, Sweden) sampling at 40–100 ml min−1 through a Naphion sampling catheter at the end of a mixing chamber (size: 5 tidal volumes) connected to the ventilator exhaust. The completeness of the mixing of expired air was intermittently checked by monitoring CO2 concentration in the same chamber. Calibration was performed using certified NO standard gas in nitrogen (AGA Specialgas, Lidingö, Sweden).
Preparation of muscle emboli
Material for muscle tissue pulmonary embolisation (MPE) was prepared by modification of a technique described previously (Priebe, 1988). Thus, resected anterior tibial skeletal muscle was cleared from visible connective tissue and then homogenized and dissolved in normal saline to a concentration of 0.1 g muscle ml−1 and 50 IE heparin ml−1 was added to the mixture. The homogenate was passed through a 0.5-mm mesh to prevent obstruction in the three-way stopcock of the venous catheter.
Experimental protocol
The animals were divided into two groups: (1) animals with intact endogenous NO production receiving MPE of either (1a) 60 mg kg−1, (1b) 15 mg kg−1 or (1c) 7.5 mg kg−1 and (2) animals with inhibited NO production (NG-nitro-L-arginine methyl ester (L-NAME) 30 mg kg−1 administered 40 min before MPE)) receiving MPE of (2a) 30 mg kg−1, (2b) 15 mg kg−1 or (2c) 7.5 mg kg−1. Group 2 animals received the lower doses of MPE as initial experiments indicated a marked enhancement of emboli effects after L-NAME pre-treatment. Blood samples were collected and analysed for blood gases and acid–base status (ABL 300, Radiometer A/S, Copenhagen, Denmark) before L-NAME administration and shortly before MPE. MPE was infused by means of an infusion pump (CMA/100, Carnegie Medicin AB, Stockholm, Sweden) with a flow of 150 μl kg−1 min−1 via a three-way stopcock into a saline carrier flow (864 Syringe Pump, Univentor LTD, Zejtun, Malta) of 150 μl kg−1 min−1 through the jugular vein catheter. Arterial blood samples were collected and analysed at 10, 20, 40 and 60 min after embolization. FENO, ETCO2, HR, MAP and IP were continuously monitored on a Grass Polygraph (Grass Instruments Co, Quincy, MA, USA) during the experiments.
Statistical analysis
Data are expressed as means±s.e.m. Statistical significance was considered at P<0.05. The effect of L-NAME infusion was analysed by paired t-test. The effect of MPE within the same group over time was analysed by means of one-way repeated-measures analysis of variance (ANOVA) or repeated-measures ANOVA on ranks (when normality test failed) and the comparisons of MPE effects between the different groups were made with two-way ANOVA where time after embolization, pretreatment or emboli dose were the factors. Student–Newman–Keul's post hoc test was used for multiple comparisons. Survival at 60 min was analysed with Fischer's exact test and survival fraction was calculated according to the Kaplan–Meier method. All statistical tests were performed by use of commercial software (SigmaStat, Jandel, San Rafael, CA, USA).
Drugs
The drugs purchased were: Heparin (Kabi Vitrum, Stockholm, Sweden), pancuronium bromide (Pavulon, Organon, Oss, Holland), dextran 70 (Macrodex, Pharmalink, Spånga, Sweden) and sodium pentobarbital (Apoteksbolaget, Stockholm, Sweden). L-NAME and routine chemicals were from Sigma Chemical Co (St Louis, MO, USA).
Results
Status of all animals before muscle emboli challenge
After the intervention-free period, the different groups showed similar baseline values for haemodynamic, blood-gas and FENO parameters (Table 1). The animals receiving L-NAME (30 mg kg−1 i.v.) exhibited significant increments in MAP and a small or nonsignificant decrease in HR after the drug administration, whereas the IP did not change (Table 1).
Table 1.
Baseline data and effect of L-NAME treatment
| FENO (p.p.b) | ETCO2 (%) | MAP (mm Hg) | HR (bpm) | IP (cm H2O) | SaO2 (%) | PaCO2 (kPa) | pH | |
|---|---|---|---|---|---|---|---|---|
| Group 1a | ||||||||
| MPE 60 mg kg−1 (n=6) | ||||||||
| Baseline | 22±2 | 5.1±0.1 | 78±4 | 280±10 | 9.9±0.4 | 94±2 | 4.6±0.1 | 7.48±0.02 |
| Group 1b | ||||||||
| MPE 15 mg kg−1 (n=5) | ||||||||
| Baseline | 20±1 | 5.0±0.1 | 83±4 | 300±10 | 10.3±0.4 | 98±1 | 4.8±0.1 | 7.49±0.01 |
| Group 1c | ||||||||
| MPE 7.5 mg kg−1 (n=5) | ||||||||
| Baseline | 19±1 | 5.0±0.1 | 87±4 | 300±10 | 9.4±0.3 | 97±1 | 4.8±0.1 | 7.51±0.01 |
| Group 2a | ||||||||
| L-NAME+MPE 30 mg kg−1 (n=4) | ||||||||
| Baseline | 23±3 | 5.1±0.1 | 72±3 | 320±10 | 8.5±1.1 | 95±1 | 4.5±0.2 | 7.50±0.02 |
| After L-NAME | <1 | 4.9±0.1 | 91±7* | 310±10 | 8.8±1.0 | 95±1 | 4.4±0.1 | 7.50±0.03 |
| Group 2b | ||||||||
| L-NAME+MPE 15 mg kg−1 (n=6) | ||||||||
| Baseline | 18±1 | 4.9±0.1 | 81±2 | 310±20 | 7.5±1.2 | 96±1 | 4.5±0.1 | 7.51±0.01 |
| After L-NAME | <1 | 5.0±0.1 | 100±3** | 260±20* | 7.9±1.2 | 95±1 | 4.4±0.1 | 7.51±0.01 |
| Group 2c | ||||||||
| L-NAME+MPE 7.5 mg kg−1 (n=6) | ||||||||
| Baseline | 19±2 | 5.0±0.1 | 81±2 | 320±10 | 7.9±0.7 | 95±1 | 4.4±0.1 | 7.48±0.01 |
| After L-NAME | <1 | 4.9±0.1 | 95±4** | 320±20 | 8.0±0.7 | 94±1 | 4.3±0.1 | 7.49±0.01 |
Abbreviations: ETCO2, end-tidal carbon dioxide; FENO, nitric oxide concentration in mixed exhaled gas; HR, heart rate; IP, insufflation pressure; L-NAME, NG-nitro-L-arginine methyl ester; MAP, mean arterial blood pressure; MPE, muscle tissue pulmonary embolization; PaCO2, arterial partial pressure of carbon dioxide; SaO2, arterial oxygen saturation.
Artificially ventilated pentobarbital anesthetized rabbits. The table shows baseline values and the effects of L-NAME treatment (30 mg kg−1 i.v.) in different groups. * and ** indicate P<0.05 and P<0.01, respectively, when comparing values before and after L-NAME treatment.
P<0.05
P<0.01
Effects of MPE challenge in animals with no pretreatment
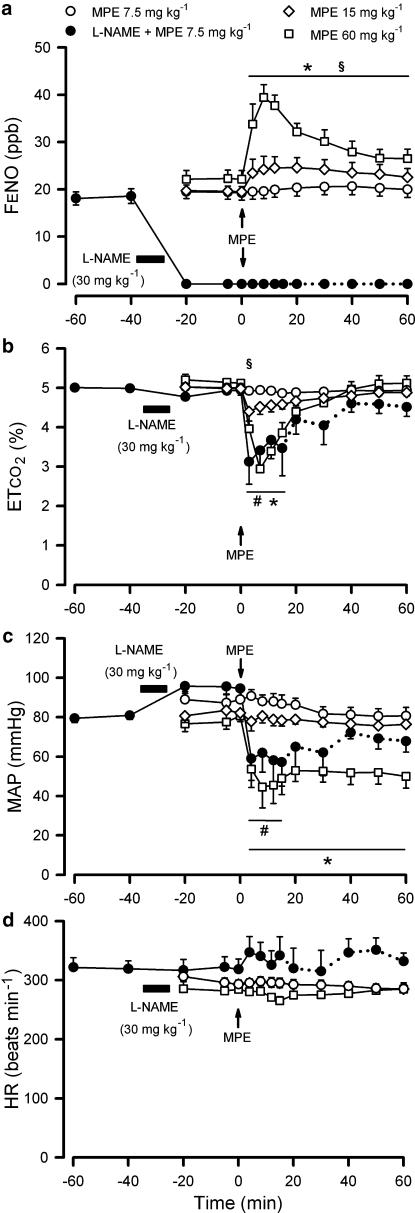
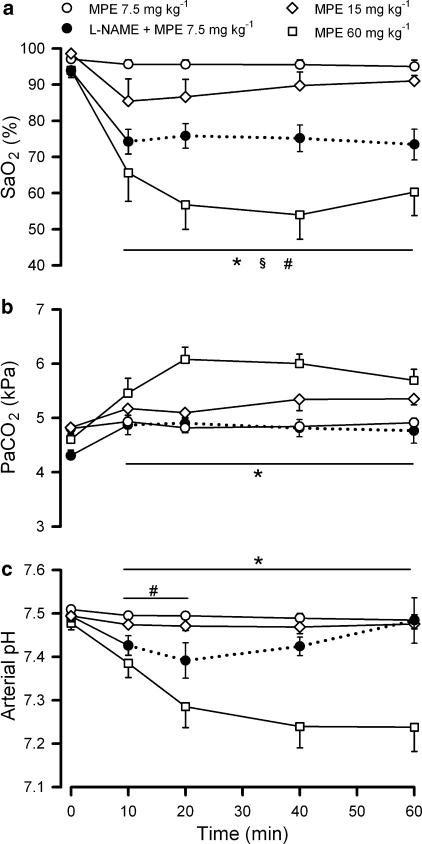
A representative original experimental recording of MPE effects on FENO, ETCO2 and MAP can be seen in Figure 1. Upon embolization, FENO increased within minutes and the NO increase was dependent on emboli dose (P<0.001, Figure 2). In group 1a (MPE 60 mg kg−1, n=6) and 1b (MPE 15 mg kg−1, n=5), FENO was still raised 60 min after MPE (P<0.05), whereas no change in FENO could be detected in group 1c (MPE 7.5 mg kg−1, n=5). Parallel to the NO increase in group 1a and 1b, there was a significant decrease in ETCO2 (Figure 2), which was more pronounced in group 1a. MAP was decreased only in group 1a (P<0.05, Figure 2). HR did not change significantly in any group (Figure 2). Arterial oxygen saturation (SaO2) was decreased after MPE in all groups (P<0.05, Figure 3) and the effect was more pronounced in group 1a when compared with the other two groups (P<0.05). After MPE in group 1a, arterial partial pressure of carbon dioxide (PaCO2) increased (P<0.05) and arterial pH was significantly decreased (Figure 3).
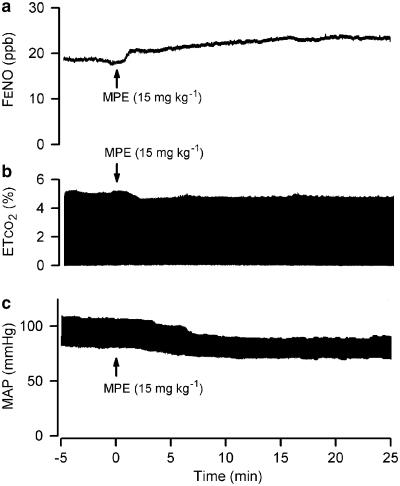
Figure 1.
Experimental recordings of the effects of MPE. Records are from artificially ventilated pentobarbital anaesthetised rabbits. The panels show values for mixed FENO (a), ETCO2 (b) and MAP (c) during MPE challenge (15 mg kg−1 at time 0) in a group 1b animal.
Figure 2.
Changes in FENO, ETCO2, MAP and HR upon MPE. Records are from artificially ventilated pentobarbital anaesthetised rabbits. Changes in mixed FENO (a), ETCO2 (b), MAP (c) and HR (d) upon MPE (time 0). The horizontal filled bar shows the infusion of L-NAME. The dotted lines indicate that some animals in group 2c have died and therefore are removed from the statistics (n=4–5 for remaining animals). * and § Indicate P<0.05 for changes induced by MPE 60 mg kg−1 and by MPE 15 mg kg−1, compared to that before MPE. #Indicates P<0.05 for the comparison of MPE 7.5 mg kg−1 without and with L-NAME pretreatment. Data are given as means± s.e.m.; n=5–6 for each group.
Figure 3.
Changes in blood–gas values upon MPE. Records are from artificially ventilated pentobarbital anaesthetised rabbits. Changes in SaO2 (a), PaCO2 (b)and arterial pH (c) upon MPE (time 0). The dotted lines indicate that some animals in group 2c have died and therefore are removed from the statistics (n=4–5 for remaining animals). * and § indicate P<0.05 for changes induced by MPE 60 mg kg−1 and by MPE 15 mg kg−1, compared to that before MPE. #indicates P<0.05 for the comparison of MPE 7.5 mg kg−1 without and with L-NAME pretreatment. Data are given as means±s.e.m.; n=5–6 for each group.
Effects of MPE in the presence of NO synthase inhibition
In all L-NAME pretreated groups, FENO remained below detection level, even after MPE challenge. After MPE in group 2c (L-NAME+MPE 7.5 mg kg−1, n=6), ETCO2 and MAP decreased (P<0.05, Figure 2), and were significantly lower in group 2c compared to group 1c (which received the same embolus dose and was not pretreated with L-NAME). HR (Figure 2) did not change significantly. In group 2c, SaO2 was significantly decreased, and PaCO2 was significantly increased (Figure 3). The SaO2 and pH effects were more pronounced in group 2c (L-NAME treated) compared to group 1c. In general, there were clear significant differences in the measured parameters that indicated worsening with L-NAME pretreatment in combination with MPE compared to no pretreatment and the same MPE dose. Owing to significant early mortality, group 2a (L-NAME+MPE 30 mg kg−1, n=4) and 2b (L-NAME+MPE 15 mg kg−1, n=6) were considered unsuitable for detailed statistical comparison, regarding measured parameters beyond 10 min, although these groups showed similar, but more severe, responses to MPE compared to the other groups.
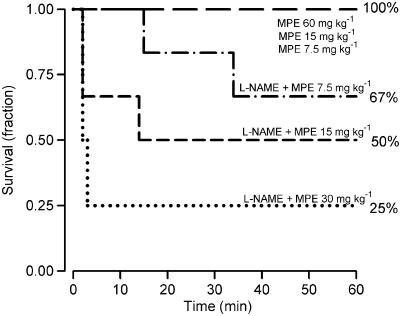
Comparison of survival in the different groups
The animals with intact endogenous NO formation tolerated a higher MPE dose than the L-NAME-treated animals, as seen from survival curves (Figure 4). When comparing survival at 60 min in all L-NAME-pretreated animals (n=16, mean MPE dose of 16±2 mg kg−1) with all animals with no pretreatment (n=16; mean MPE dose 30±6 mg kg−1), it is evident that L-NAME pretreatment in combination with MPE, significantly increased mortality (P=0.002 in Fischer's exact test; after L-NAME eight out of 16 animals survived the MPE challenge whereas 16 of 16 survived among the non-pretreated animals). Moreover, when evaluating survival in the two lowest MPE dose groups (MPE 7.5 and 15 mg kg−1; n=22) exhibiting an equal mean MPE dose of 11.25 mg kg−1, it was clear that the animals with L-NAME pretreatment had a higher mortality at 60 min (P=0.04; five non-survivors out of 12) compared to animals with no pretreatment (10 out of 10 survived).
Figure 4.
Survival curves resulting from MPE in different groups. The lines represent the survival proportion during the first 60 min after MPE (administered at time 0) for the non-pretreated groups; MPE of 60 (group 1a, n=6), 15 (group 1b, n=5) or 7.5 mg kg−1 (group 1c, n=5) and the L-NAME (30 mg kg−1 i.v.) pretreated groups (MPE of 30 (group 2a, n=4), 15 (group 2b, n=6) or 7.5 mg kg−1 (group 2c, n=6)).
Discussion
The present study demonstrates that FENO is rapidly and dose-dependently increased upon acute PE and that this increase is sustained for at least 60 min. Furthermore, we show that endogenous NO production is essential for tolerance to PE challenge.
Recently, we found that FENO was increased in pulmonary gas embolism and that this increase was abolished after 20 min (Agvald et al., 2006). The short-lasting effect on FENO might be a consequence of a pulmonary gas embolism of this kind resolving within 30 min (Hlastala et al., 1979). Our laboratory has also previously shown that fatal, massive venous infusions of air or helium, which completely stops the blood circulation in the lung, increased FENO (Gustafsson et al., 1991), but from that study, it cannot be inferred whether FENO would be increased in conditions compatible with survival. When examining the effects of haemodilution in pulmonary gas embolism, Deem et al. (1999) noted that FENO is increased in haemodiluted rabbits but not in controls upon venous gas embolization. These three previous studies were made on pulmonary gas embolism and only one of them (Agvald et al., 2006) was designed exclusively to investigate the relationship between FENO and acute PE. In this study, we used a model of solid pulmonary emboli, which is more similar to pulmonary thromboembolism in humans. With the present model, we found that the more severe the PE challenge, the larger the increase in FENO. Because of these considerations, we are of the opinion that the present study is more relevant for the clinical situation of PE and may be valuable in order to initiate studies in humans with acute PE.
The ability of our model to mimic acute PE was demonstrated by the following parameters; decreased ETCO2, decreased MAP and impaired blood–gas values compared to normal, which are some of the key features of PE (McIntyre and Sasahara, 1974). We believe that i.v. infusion of homogenized muscle tissue induced PE and pulmonary hypertension, both by mechanical obstruction of the pulmonary circulation and by stimulating pulmonary thromboembolism and subsequent activation of platelets and the coagulation system. The persistence of the PE effect in our model is supported by the sustained impairment of the blood gases (Figure 3).
In the rabbit lung, the endogenous and FENO is generated by NO synthases in a calcium-dependent fashion (Gustafsson et al., 1991; Persson et al., 1994). Most of FENO in humans arises from distal airways (Persson et al., 1993). It has been suggested that a considerable part of the FENO in the rabbit is formed by the isoenzyme endothelial NO synthase (eNOS), which is localized throughout the vascular endothelium, and the alveolar and airway epithelia (Vaughan et al., 2003). During states of inflammation in the airways, for example asthma in the human, the inducible isoenzyme of NO synthase (iNOS) is upregulated (Hamid et al., 1993) and contributes to part of the increased FENO in this condition (Ricciardolo et al., 2004).
Several mechanisms might contribute to the increase in FENO following PE, including increased production and/or decreased elimination: (1) Reduced inhibition of CO2 on NO synthase (NOS): pulmonary CO2 has been shown to continuously regulate NO production from NOS (Adding and Gustafsson, 2002). Thus, decreased CO2 (as detected by lowered ETCO2) will exert less inhibition of NOS and therefore lead to increase in FENO (Strömberg et al., 1997; Adding et al., 1999). Recently, we showed that a part of the increase in FENO after pulmonary gas embolism was due to this mechanism (Agvald et al., 2006). (2) Release of NOS stimulating substances: PE is also known to lead to the release of bioactive substances (Smulders, 2000; Stratmann and Gregory, 2003), several of which have been shown to modulate FENO (Adding and Gustafsson, 2002). An interesting observation is that polymorphonuclear leukocytes, during pulmonary thrombo-embolization, increase their release of NO as a response to neutrophil-derived relaxing factor (Dikshit et al., 1993). (3) Reduced scavenging of NO by Hb: reduced binding of pulmonary NO to Hb in the red blood cells, owing to reduced blood flow and diminished blood volume in the lung capillaries, may increase FENO (Berg et al., 2000). In support of this, completely stopping the pulmonary blood flow has been shown to increase FENO (Gustafsson et al., 1991). (4) Induction of iNOS: upregulating the iNOS-gene, as in asthma, might be of importance in a later phase of PE. However, iNOS is not likely to be important in the acute phase owing to the time lag in induction of the enzyme (Di Rosa et al., 1990). (5) Effects of changes in blood gases: despite profound changes in blood gases during PE, the lowered PaO2 (Ide et al., 1999; Agvald et al., 2002) and the decreased arterial pH (Adding et al., 1999) are not within the ranges needed to alter FENO.
The results in this study indicate that FENO has the potential to be a diagnostic marker for acute PE in humans, although there are a few problems associated with FENO measurements, these have to be overcome. First, in most instances there are no individual baseline values for FENO in the clinic and there is a need for normative group data and cutoff values. This has been addressed by establishing recommendations for standardized procedures of measurements (ATS/ERS, 2005) and in asthma research it has been shown that it is possible to establish adequate cutoff values, which are more specific and sensitive than several other diagnostic tests for asthma (Berkman et al., 2005). In addition, in some situations such as in planned surgery, it is possible to obtain individual baseline values of FENO. Second, as already mentioned above, some other disease conditions are also associated with increased FENO, but the symptoms of these diseases might not mimic the symptoms of PE.
In this study, inhibition of endogenous NO synthesis, in combination with MPE, was associated with decreased survival and with more pronounced haemodynamic and blood–gas disturbances. Inhibition of endogenous NO formation is acutely well tolerated in the rabbit and, apart from causing an increase in systemic blood pressure with, sometimes, a drop in HR, does not cause acute effects on the airways (Persson et al., 1990), which is in agreement with the present minor effects of L-NAME before MPE challenge, shown in this study. Thus, effects of L-NAME per se cannot explain the mortality observed upon MPE. Previously, we found that rabbits with inhibited endogenous NO production show decreased tolerance to pulmonary gas embolism (Agvald et al., 2006) and in this study, this is also true for solid PE and that the inhibition of NO synthesis incurs a decreased tolerance to embolism, expressed as dose of MPE tolerated. Previously, it has been shown that endogenous NO synthesis inhibition does not affect basal pulmonary arterial pressure in dogs (Sander et al., 2003) and moderately increases basal pulmonary vascular resistance in rabbits (Persson et al., 1990), and severely augments hypoxic vasoconstriction in rabbits (Persson et al., 1990) and dogs (Sander et al., 2003). Another example of a condition where inhibited NO synthesis enhances pulmonary hypertension is during thromboxane A2 mimetic-induced pulmonary hypertension in rabbits (Wall et al., 1999). It is also known that endogenous NO is a regulator of heart performance (Paulus and Bronzwaer, 2004) and that acute NO synthase inhibition reduces myocardial oxygen consumption and myocardial contractility (Sherman et al., 1997). In a PE model evaluating the anti-thrombotic actions of NO, Emerson et al. (1999) have previously shown that mortality is increased upon PE in L-NAME pretreated mice compared to controls.
The beneficial role of endogenous NO synthesis in PE may therefore be due to one or several of the following: (1) decreasing the degree of pulmonary hypertension (Cherry and Gillis, 1987; Persson et al., 1990), (2) modulation of perfusion in systemic vascular beds (Stamler et al., 1994), for example, increased oxygen supply to the coronary circulation (Smith et al., 1991), (3) increased heart contractility (Paulus and Bronzwaer, 2004) and (4) inhibition of platelet aggregation (Radomski et al., 1990; Emerson et al., 1999). However, this study does not aim to completely elucidate the precise mechanisms involved in our findings.
In conclusion, following PE, FENO increases rapidly and dose-dependently, and remains increased for at least 1 h; thus, FENO has a potential as a diagnostic tool in PE. At least, increased FENO could strengthen the indication for further examinations (spiral CT or pulmonary-arterial angiography) in patients with signs and symptoms, arousing suspicions of PE. The utility of FENO measurements in human PE will, however, not be possible to define until appropriate clinical studies have been performed and these should employ standardization of exhalation rates according to published guidelines (ATS/ERS, 2005). We believe that such studies are important to perform in the light of this study, showing sustained increments in FENO after PE with solid emboli. Furthermore, this study suggests that intact endogenous NO production is essential for tolerance to PE. These observations might be of importance in pharmaceutical development of compounds altering the endogenous NO system.
Acknowledgments
Supported by the European Space Agency, the Swedish National Space Board, Fraenckel's Foundation for Medical Research, the Lars Hierta Foundation, the Magnus Bergvalls Foundation, the Swedish Science Council proj. 07919 and the Swedish Heart-Lung Foundation.
Abbreviations
- eNOS
endothelial nitric oxide synthase
- ETCO2
end-tidal carbon dioxide
- FENO
NO concentration in mixed exhaled gas
- HR
heart rate
- iNOS
inducible nitric oxide synthase
- IP
insufflation pressure
- L-NAME
NG-nitro-L-arginine methyl ester
- MAP
mean arterial blood pressure
- MPE
muscle tissue pulmonary embolisation
- NO
nitric oxide
- NOS
nitric oxide synthase
- PaCO2
arterial partial pressure of carbon dioxide
- PE
pulmonary embolism
- PEEP
positive end-expiratory pressure
- SaO2
arterial oxygen saturation
Conflict of Interest
Lars E Gustafsson holds patents on exhaled nitric oxide technologies and is a minority shareholder (<1%, no current market value) in Aerocrine AB (publ.).
References
- Adding LC, Agvald P, Persson MG, Gustafsson LE. Regulation of pulmonary nitric oxide by carbon dioxide is intrinsic to the lung. Acta Physiol Scand. 1999;167:167–174. doi: 10.1046/j.1365-201x.1999.00585.x. [DOI] [PubMed] [Google Scholar]
- Adding LC, Gustafsson LE.Physiology of exhaled nitric oxide Disease Markers in Exhaled Breath 2002Mercel Dekker Inc: New York; 29–72.In: Marczin N, Kharitonov S, Yacoub MH, Barnes PJ (eds) [Google Scholar]
- Agvald P, Adding LC, Artlich A, Persson MG, Gustafsson LE. Mechanisms of nitric oxide generation from nitroglycerin and endogenous sources during hypoxia in vivo. Br J Pharmacol. 2002;135:373–382. doi: 10.1038/sj.bjp.0704489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agvald P, Adding LC, Nilsson KF, Gustafsson LE, Linnarsson D. Increased expired NO and roles of CO2 and endogenous NO after venous gas embolism in rabbits. Eur J Appl Physiol. 2006;97:210–215. doi: 10.1007/s00421-006-0179-8. [DOI] [PubMed] [Google Scholar]
- ATS/ERS ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med. 2005;171:912–930. doi: 10.1164/rccm.200406-710ST. [DOI] [PubMed] [Google Scholar]
- Berg JT, Deem S, Kerr ME, Swenson ER. Hemoglobin and red blood cells alter the response of expired nitric oxide to mechanical forces. Am J Physiol Heart Circ Physiol. 2000;279:H2947–H2953. doi: 10.1152/ajpheart.2000.279.6.H2947. [DOI] [PubMed] [Google Scholar]
- Berkman N, Avital A, Breuer R, Bardach E, Springer C, Godfrey S. Exhaled nitric oxide in the diagnosis of asthma: comparison with bronchial provocation tests. Thorax. 2005;60:383–388. doi: 10.1136/thx.2004.031104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottiger BW, Motsch J, Dorsam J, Mieck U, Gries A, Weimann J, et al. Inhaled nitric oxide selectively decreases pulmonary artery pressure and pulmonary vascular resistance following acute massive pulmonary microembolism in piglets. Chest. 1996;110:1041–1047. doi: 10.1378/chest.110.4.1041. [DOI] [PubMed] [Google Scholar]
- Cherry PD, Gillis CN. Evidence for the role of endothelium-derived relaxing factor in acetylcholine-induced vasodilatation in the intact lung. J Pharmacol Exp Ther. 1987;241:516–520. [PubMed] [Google Scholar]
- Deem S, McKinney S, Polissar NL, Hedges RG, Swenson ER. Hemodilution during venous gas embolization improves gas exchange, without altering V(A)/Q or pulmonary blood flow distributions. Anesthesiology. 1999;91:1861–1872. doi: 10.1097/00000542-199912000-00041. [DOI] [PubMed] [Google Scholar]
- Di Rosa M, Radomski M, Carnuccio R, Moncada S. Glucocorticoids inhibit the induction of nitric oxide synthase in macrophages. Biochem Biophys Res Commun. 1990;172:1246–1252. doi: 10.1016/0006-291x(90)91583-e. [DOI] [PubMed] [Google Scholar]
- Dikshit M, Kumari R, Srimal RC. Pulmonary thromboembolism-induced alterations in nitric oxide release from rat circulating neutrophils. J Pharmacol Exp Ther. 1993;265:1369–1373. [PubMed] [Google Scholar]
- Emerson M, Momi S, Paul W, Alberti PF, Page C, Gresele P. Endogenous nitric oxide acts as a natural antithrombotic agent in vivo by inhibiting platelet aggregation in the pulmonary vasculature. Thromb Haemost. 1999;81:961–966. [PubMed] [Google Scholar]
- Feigin DS, Bookstein JJ, Alazraki NP. The effect of vasospasm on perfusion radionuclide imaging in experimental pulmonary embolism. Invest Radiol. 1983;18:463–469. doi: 10.1097/00004424-198309000-00012. [DOI] [PubMed] [Google Scholar]
- Gustafsson LE, Leone AM, Persson MG, Wiklund NP, Moncada S. Endogenous nitric oxide is present in the exhaled air of rabbits, guinea pigs and humans. Biochem Biophys Res Commun. 1991;181:852–857. doi: 10.1016/0006-291x(91)91268-h. [DOI] [PubMed] [Google Scholar]
- Hamid Q, Springall DR, Riveros-Moreno V, Chanez P, Howarth P, Redington A, et al. Induction of nitric oxide synthase in asthma. Lancet. 1993;342:1510–1513. doi: 10.1016/s0140-6736(05)80083-2. [DOI] [PubMed] [Google Scholar]
- Hlastala MP, Robertson HT, Ross BK. Gas exchange abnormalities produced by venous gas emboli. Respir Physiol. 1979;36:1–17. doi: 10.1016/0034-5687(79)90011-2. [DOI] [PubMed] [Google Scholar]
- Ide H, Nakano H, Ogasa T, Osanai S, Kikuchi K, Iwamoto J. Regulation of pulmonary circulation by alveolar oxygen tension via airway nitric oxide. J Appl Physiol. 1999;87:1629–1636. doi: 10.1152/jappl.1999.87.5.1629. [DOI] [PubMed] [Google Scholar]
- McIntyre KM, Sasahara AA. Hemodynamic and ventricular responses to pulmonary embolism. Prog Cardiovasc Dis. 1974;17:175–190. doi: 10.1016/0033-0620(74)90042-5. [DOI] [PubMed] [Google Scholar]
- Meyer G, Roy PM, Sors H, Sanchez O. Laboratory tests in the diagnosis of pulmonary embolism. Respiration. 2003;70:125–132. doi: 10.1159/000070056. [DOI] [PubMed] [Google Scholar]
- Murray IP, Mikhail MS, Banner MJ, Modell JH. Pulmonary embolism: high-frequency jet ventilation offers advantages over conventional mechanical ventilation. Crit Care Med. 1987;15:114–117. [PubMed] [Google Scholar]
- Olin JW. Pulmonary embolism. Rev Cardiovasc Med. 2002;3 Suppl 2:S68–S75. [PubMed] [Google Scholar]
- Paulus WJ, Bronzwaer JG. Nitric oxide's role in the heart: control of beating or breathing. Am J Physiol Heart Circ Physiol. 2004;287:H8–H13. doi: 10.1152/ajpheart.01147.2003. [DOI] [PubMed] [Google Scholar]
- Persson MG, Gustafsson LE, Wiklund NP, Moncada S, Hedqvist P. Endogenous nitric oxide as a probable modulator of pulmonary circulation and hypoxic pressor response in vivo. Acta Physiol Scand. 1990;140:449–457. doi: 10.1111/j.1748-1716.1990.tb09021.x. [DOI] [PubMed] [Google Scholar]
- Persson MG, Midtvedt T, Leone AM, Gustafsson LE. Ca(2+)-dependent and Ca(2+)-independent exhaled nitric oxide, presence in germ-free animals, and inhibition by arginine analogues. Eur J Pharmacol. 1994;264:13–20. doi: 10.1016/0014-2999(94)90629-7. [DOI] [PubMed] [Google Scholar]
- Persson MG, Wiklund NP, Gustafsson LE. Endogenous nitric oxide in single exhalations and the change during exercise. Am Rev Respir Dis. 1993;148:1210–1214. doi: 10.1164/ajrccm/148.5.1210. [DOI] [PubMed] [Google Scholar]
- Priebe HJ. Efficacy of vasodilator therapy in canine model of acute pulmonary hypertension. Am J Physiol. 1988;255:H1232–H1239. doi: 10.1152/ajpheart.1988.255.5.H1232. [DOI] [PubMed] [Google Scholar]
- Radomski MW, Palmer RM, Moncada S. Characterization of the L-arginine:nitric oxide pathway in human platelets. Br J Pharmacol. 1990;101:325–328. doi: 10.1111/j.1476-5381.1990.tb12709.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves WC, Demers LM, Wood MA, Skarlatos S, Copenhaver G, Whitesell L, et al. The release of thromboxane A2 and prostacyclin following experimental acute pulmonary embolism. Prostaglandins Leukot Med. 1983;11:1–10. doi: 10.1016/0262-1746(83)90104-x. [DOI] [PubMed] [Google Scholar]
- Ricciardolo FL, Sterk PJ, Gaston B, Folkerts G. Nitric oxide in health and disease of the respiratory system. Physiol Rev. 2004;84:731–765. doi: 10.1152/physrev.00034.2003. [DOI] [PubMed] [Google Scholar]
- Rimeika D, Nyren S, Wiklund NP, Koskela Renström L, Törring A, Gustafsson LE, et al. Regulation of regional lung perfusion by nitric oxide. Am J Respir Crit Care Med. 2004;170:450–455. doi: 10.1164/rccm.200312-1663OC. [DOI] [PubMed] [Google Scholar]
- Sander M, Welling KL, Ravn JB, Boberg B, Amtorp O. Endogenous NO does not regulate baseline pulmonary pressure, but reduces acute pulmonary hypertension in dogs. Acta Physiol Scand. 2003;178:269–277. doi: 10.1046/j.1365-201X.2003.01140.x. [DOI] [PubMed] [Google Scholar]
- Schmeck J, Koch T, Patt B, Heller A, Neuhof H, van Ackern K. The role of endothelin-1 as a mediator of the pressure response after air embolism in blood perfused lungs. Intensive Care Med. 1998;24:605–611. doi: 10.1007/s001340050622. [DOI] [PubMed] [Google Scholar]
- Sherman AJ, Davis CA, III, Klocke FJ, Harris KR, Srinivasan G, Yaacoub AS, et al. Blockade of nitric oxide synthesis reduces myocardial oxygen consumption in vivo. Circulation. 1997;95:1328–1334. doi: 10.1161/01.cir.95.5.1328. [DOI] [PubMed] [Google Scholar]
- Smith RE, Palmer RM, Moncada S. Coronary vasodilatation induced by endotoxin in the rabbit isolated perfused heart is nitric oxide-dependent and inhibited by dexamethasone. Br J Pharmacol. 1991;104:5–6. doi: 10.1111/j.1476-5381.1991.tb12375.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smulders YM. Pathophysiology and treatment of haemodynamic instability in acute pulmonary embolism: the pivotal role of pulmonary vasoconstriction. Cardiovasc Res. 2000;48:23–33. doi: 10.1016/s0008-6363(00)00168-1. [DOI] [PubMed] [Google Scholar]
- Souza-Silva AR, Dias-Junior CA, Uzuelli JA, Moreno H, Jr, Evora PR, Tanus-Santos JE. Hemodynamic effects of combined sildenafil and L-arginine during acute pulmonary embolism-induced pulmonary hypertension. Eur J Pharmacol. 2005;524:126–131. doi: 10.1016/j.ejphar.2005.09.022. [DOI] [PubMed] [Google Scholar]
- Stamler JS, Loh E, Roddy MA, Currie KE, Creager MA. Nitric oxide regulates basal systemic and pulmonary vascular resistance in healthy humans. Circulation. 1994;89:2035–2040. doi: 10.1161/01.cir.89.5.2035. [DOI] [PubMed] [Google Scholar]
- Stratmann G, Gregory GA. Neurogenic and humoral vasoconstriction in acute pulmonary thromboembolism. Anesth Analg. 2003;97:341–354. doi: 10.1213/01.ANE.0000068983.18131.F0. [DOI] [PubMed] [Google Scholar]
- Strömberg S, Lönnqvist PA, Persson MG, Gustafsson LE. Lung distension and carbon dioxide affect pulmonary nitric oxide formation in the anaesthetized rabbit. Acta Physiol Scand. 1997;159:59–67. doi: 10.1046/j.1365-201X.1997.568335000.x. [DOI] [PubMed] [Google Scholar]
- Tajimi K, Tanaka H, Kasai T, Kobayashi K, Okuaki A. Selective pulmonary vasodilatory effect of ZSY-27 in dogs with pulmonary hypertension due to pulmonary embolism. Crit Care Med. 1989;17:163–165. doi: 10.1097/00003246-198902000-00012. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Tajimi K, Matsumoto A, Kobayashi K. Vasodilatory effects of milrinone on pulmonary vasculature in dogs with pulmonary hypertension due to pulmonary embolism: a comparison with those of dopamine and dobutamine. Clin Exp Pharmacol Physiol. 1990;17:681–690. doi: 10.1111/j.1440-1681.1990.tb01267.x. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Tajimi K, Moritsune O, Kobayashi K, Okada K. Effects of milrinone on pulmonary vasculature in normal dogs and in dogs with pulmonary hypertension. Crit Care Med. 1991;19:68–74. doi: 10.1097/00003246-199101000-00017. [DOI] [PubMed] [Google Scholar]
- Utsunomiya T, Krausz MM, Shepro D, Hechtman HB. Prostaglandin control of plasma and platelet 5-hydroxytryptamine in normal and embolized animals. Am J Physiol. 1981;241:H766–H771. doi: 10.1152/ajpheart.1981.241.5.H766. [DOI] [PubMed] [Google Scholar]
- Vaughan DJ, Brogan TV, Kerr ME, Deem S, Luchtel DL, Swenson ER. Contributions of nitric oxide synthase isozymes to exhaled nitric oxide and hypoxic pulmonary vasoconstriction in rabbit lungs. Am J Physiol Lung Cell Mol Physiol. 2003;284:L834–L843. doi: 10.1152/ajplung.00341.2002. [DOI] [PubMed] [Google Scholar]
- Via G, Braschi A. Pathophysiology of severe pulmonary hypertension in the critically ill patient. Minerva Anestesiol. 2004;70:233–237. [PubMed] [Google Scholar]
- Wall MH, Patterson KW, Kavanagh BP, Pearl RG. Inhibition of endogenous nitric oxide synthesis potentiates the effects of sodium nitroprusside but not of adenosine in experimental pulmonary hypertension. Pharmacology. 1999;58:34–43. doi: 10.1159/000028266. [DOI] [PubMed] [Google Scholar]