Abstract
Background and purpose:
Sphingosine 1-phosphate (S1P) is a serum-borne naturally occurring sphingolipid, specifically enriched in high-density lipoprotein (HDL) fractions. S1P binds to G-protein-coupled S1P1 receptors to activate endothelial NO synthase (eNOS) in vascular endothelial cells. We explored whether and how statins, 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors, modulate expression of S1P1 receptors and endothelial responses for subsequent stimulation with S1P or with HDL.
Experimental approach:
Protein expression and phosphorylation and mRNA expression in cultured bovine aortic endothelial cells (BAEC) were determined using immunoblots and reverse transcription PCR analyses, respectively. NO synthesis was assessed as nitrite production.
Key results:
Stimulation of BAEC with pitavastatin or atorvastatin led to significant increases in S1P1-receptors, at levels of protein and mRNA, in a dose-dependent manner. When BAEC were treated with pitavastatin prior to stimulation with S1P or with normal human HDL, phosphorylation and activation of eNOS evoked by S1P or by HDL was enhanced. These effects of statins were counteracted by L-mevalonate and were mimicked by an inhibitor of geranylgeranyl transferase I, suggesting that inhibition of HMG-CoA reductase activity and subsequent decreases in protein geranylgeranylation may contribute to these actions of statins. Specific knock down of S1P1 receptors by small interfering RNA led to attenuation of eNOS responses to HDL.
Conclusions and implications:
Statins induce S1P1 receptors and potentiate responses of endothelial cells to HDL-associated sphingolipids, identifying a novel aspect of the pleiotropic actions of statins through which they may exert NO-dependent vascular protective effects.
Keywords: sphingolipids, G-protein-coupled receptors, statins, lipid metabolism, signal transduction, nitric oxide synthase
Introduction
Hyperlipidemic disorders are strongly associated with the progression of atherogenic vascular diseases, including ischemic heart diseases and strokes, which comprise a major cause of mortality in industrialized countries (Durrington, 2003). 3-Hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors, or statins, are important agents in the treatment of hypercholesterolemic patients and they have been shown to exert favorable clinical outcomes (Ridker, 2003). Interestingly, at least some of these beneficial actions of statins appear to be mediated by their ‘pleiotropic' effects, independently of cholesterol-lowering actions. Such pleiotropic effects include improvement of endothelial dysfunction, increased bioavailability of NO and antioxidant effects (reviewed in Davignon, 2004), indicating that statins not only attenuate cholesterol synthesis in the liver but also directly modulate functions of cardiovascular cells, notably those of vascular endothelial cells. Inhibition of HMG-CoA reductase activity by statins results in decreased cellular content of its product, L-mevalonate, which in turn is metabolized to cholesterol as well as to other important lipid intermediates, including isoprenoids (for review, see Liao, 2002). These isoprenoids play pivotal roles in regulating subcellular localizations, and thereby physiological functions of several key signaling proteins, including small G-proteins. Thus, statins, by affecting protein isoprenylation, may modulate protein expression levels within cardiovascular cells to ultimately exert their pleiotropic effects.
Sphingosine 1-phosphate (S1P) is a serum-borne, naturally occurring sphingolipid metabolite and is present in submicromolar concentrations in normal human sera (Yatomi et al., 1997). Recent studies have revealed that this lipid is capable of modulating a very wide variety of biological activities in numerous organs in mammals (reviewed in (Hla, 2003)). Specifically, in vascular endothelial cells, S1P mediates important effects such as migration, survival, proliferation, vasorelaxation and angiogenic morphogenesis (Hla, 2003). Many of these effects of S1P are mediated by its binding to and activation of G-protein-coupled S1P receptors, which are expressed at the endothelial cell surface (reviewed in Hla, 2001). Five independent receptor subtypes, S1P1–S1P5 have been identified in mammals, of which S1P1 and S1P3 represent major receptors for S1P expressed in endothelial cells (Lee et al., 1999; Morales-Ruiz et al., 2001). Effector molecules that mediate S1P receptor activation to physiological responses of vascular endothelial cells include the endothelial isoform of nitric oxide synthase (eNOS), which in turn is modulated by its upstream protein kinase cascades, phosphoinositide 3′-OH kinase (PI3-K)-Akt (Igarashi et al., 2001a, 2001b, 2003). Interestingly, S1P, which was found to be enriched in high-density lipoprotein (HDL) fractions of normal human sera, may play key roles in mediating HDL-induced vascular endothelial responses (reviewed in Okajima, 2002). Thus, alterations in expression of S1P1 receptors could potentially influence the responses of vascular endothelial cells to serum lipoprotein constituents.
In vascular endothelial cells, expression levels of S1P1 receptors are subject to dynamic regulation by extracellular stimuli, including phorbol esters (Hla and Maciag, 1990) as well as vascular endothelial growth factor (VEGF) (Igarashi et al., 2003). It seemed therefore plausible to us that statins might modulate S1P1 receptor expression levels and subsequent sphingolipid signaling of endothelial cells. In the present studies, we provide evidence that statins increase expression levels of S1P1 receptors and augment eNOS responses to S1P as well as to HDL in cultured vascular endothelial cells.
Methods
Cell culture and drug treatments
Bovine aortic endothelial cells (BAEC) were commercially obtained from Cell Systems (Kirkland, WA, USA), maintained in culture as described, and used for experiments between passages 5 and 7 (Igarashi et al., 2003).
Human umbilical vein endothelial cells (HUVEC) were commercially obtained from Kurabo (Osaka, Japan). They were grown on culture plates that had been coated with gelatin (0.1% w v−1) using HuMedia-EG2 culture medium (Kurabo), split at a ratio of 1:4 every 4 days, and were used for experiments between passages 4 and 6.
Both cell types had been serum-starved overnight before being used for experiments to exclude the effects of residual S1P in fetal bovine serum. Pitavastatin and atorvastatin were dissolved in dimethylsulfoxide and pravastatin was dissolved in methanol. L-mevalonate was prepared as described (Zamir et al., 1991). All other drug treatments were performed exactly as described previously (Igarashi et al., 2003). Final concentrations of the solvents did not exceed 0.1 % (w v−1) in any experiment.
Immunoblot analyses in cultured cells
Immunoblot analyses were performed as described previously (Igarashi et al., 2003). Equal amounts of protein were loaded in each lane.
Semiquantitative reverse transcription-PCR (RT-PCR) analyses
Total RNA was isolated from BAEC using RNeasy mini column (Qiagen, Valencia, CA, USA) following the supplier's protocol. cDNA was synthesized from 1 μg of cellular RNA using oligo(dT)18 primer and reverse transcriptase exactly following the supplier's instructions in a total volume of 20 μl. Enzymatic amplification was conducted with a 1 μl aliquot of cDNA mix essentially as described (Hla and Maciag, 1990). Polymerase chain reaction was performed in 20 mM Tris-HCl (pH 9.0 at 25°C), 50 mM KCl, 0.1% Triton X-100, 1.5 mM MgCl2, 0.2 mM dNTPs, 0.4 mM each of primer pair and 25 U ml−1 of Taq DNA polymerase. The reaction mixture was heated at 94°C for 1 min, annealed for 2 min and extended at 72°C for 3 min. The primer sequences, annealing temperature and PCR cycles in each assay condition are summarized in Table 1 (Supplementary Figure 1). The resulting PCR product was separated on a 2% agarose gel and visualized with ethidium bromide under ultraviolet light. Gel images were captured with a CCD camera system and subjected to densitometric analyses using NIH image software 1.63. We optimized the assay conditions and verified that increasing amounts of a starting mRNA sample yield increasing amounts of RT-PCR product under these conditions in each primer pair.
Table 1.
Primer sequences and assay conditions of RT-PCR
| Genes | Sense primer (5′–3′) | Antisense primer (5′–3′) | Annealing temperature (°C) | Cycles |
|---|---|---|---|---|
| S1P1 | AAGACCTGTGACATCCTCTTC | ATGAACCCTTTAGGAGCTTGACAA | 55 | 22 |
| S1P3 | GACTGCTCTACCATCTTGCCC | ATGAACACGCTCACCACAAT | 55 | 32 |
| SR-BI | CACTACGCGCAGTATGTGCT | TGGCACTGGTGGGCTGTC | 57 | 28 |
| GAPDH | ACCACAGTCCATGCCATCAC | TCCACCACCCTGTTGCTGTA | 55 | 21 |
Abbreviations: GAPDH, glyceraldehyde 3-phosphate dehydrogenase; S1P, Sphingosine 1-phosphate.
Measurement of nitrite levels in culture media
Release of NO from BAEC for 6 min after addition of HDL was assayed by measurement of nitrite accumulation in the culture media (phenol red-free Dulbecco's modified Eagle's medium) with an automated NO detector high-performance liquid chromatography system (ENO-20, Eicom Co., Kyoto, Japan). Nitrite was separated on a column (NO-PAK packed with polystyrene polymer, 4.6 × 50 mm; Eicom, Kyoto, Japan), mixed with a Griess reagent to form a purple azo dye in a reaction coil, and was detected by absorption at 540 nm as a single peak. The mobile phase, which was delivered by a pump at a rate of 0.33 ml min−1, comprised 10% methanol containing 0.15 M NaCl-NH4Cl and 0.5 g l−1 tetrasodium ethylenediaminetetraacetic acid. The Griess reagent, which is 1.25 M HCl containing 5 g l−1 sulfanilamide and 0.25 g l−1 N-naphthylethylenediamine, was delivered at a rate of 0.1 ml min−1. Tubes had been washed with distilled water before sampling. The rates of nitrite accumulation were expressed as picomol mg protein−1 min−1.
Transfection with siRNA
Bovine S1P1 receptor (Lee et al., 2000) silencing target starts at the 114 nucleotides upstream of the start codon. The sequences of the sense and anti-sense small interfering RNA (siRNAs) are 5′-ACGCCUGGAUCUCUCUCCUdTdT-3′ and 5′-AGGAGAGAGAUCCAGGCGUdTdT-3′; and those of scrambled siRNA are used as negative control: 5′-UCGCCGUCUCCCUUUGACAdTdT-3′ and 5′-UGUCAAAGGGAGACGGCGAdTdT-3′. One day after the cultures were split, BAEC were transfected with 1 nM of siRNA using Lipofectamine 2000 as described previously (Gonzalez et al., 2004). After the transfection, cells were incubated as described above, serum-starved overnight and then subjected to experiments.
Other methods
All experiments were performed at least three times. Protein was determined with the BioRad (Hercules, CA, USA) Protein Assay Kit using bovine serum albumin as a standard.
Data analysis
Mean values for individual experiments are expressed as mean±s.e.m. Statistical differences were analyzed by analysis of variances followed by Scheffe's F-test using STAT VIEW II (Abacus Concepts, Berkeley, CA, USA). A P-value less than 0.05 was considered to be statistically significant.
Materials
Statins were provided by Kowa Company, Ltd (Tokyo, Japan). S1P was from BioMol (Plymouth Meeting, PA, USA). GGTI 286 and FTI-277 were from Calbiochem (San Diego, CA, USA). Normal human HDL was from ICN (Irvine, CA, USA). SuperScript RNase H− reverse transcriptase was from Invitrogen (Carlsbad, CA, USA). Taq DNA polymerase was from Promega (Madison, WI, USA). siRNA was from PROLIGO (St Louis, MS, USA). Lipofectamine 2000 was from Invitrogen. All other materials were obtained as described previously (Igarashi et al., 2001a, 2001b, 2003) unless otherwise stated.
Results
Statins upregulate S1P1 receptor expression
In most of the current experiments, we exploited BAEC, an archetypal endothelial cell culture, as a model. We first explored whether or not treatment with statins alters the expression of S1P1 receptors of vascular endothelial cells. BAEC were treated with pitavastatin, a recently developed HMG-CoA reductase inhibitor, and resulting cell lysates were subjected to immunoblot analyses. As shown in Figure 1, when cells were treated with pitavastatin (3 μM), the expression of S1P1 receptor protein increased within 8 h of drug addition. Increases in S1P1 receptor protein induced by pitavastatin appeared to reach the maximum at 16 h after drug addition, and persisted at least until 24 h (Figure 1). Pitavastatin, within these time periods, did not modulate expression of several other signaling proteins, including those of eNOS (Figure 1), of a protein kinase Akt, of caveolin-1 or of the principal VEGF receptor subtype KDR (data not shown). The dose–dependency of the induction of the S1P1 receptor is shown in Figure 2a. In these dose–response experiments, BAEC were treated with various concentrations of pitavastatin for 16 h, and the lysates derived from these cells were analyzed in immunoblots probed with the S1P1 receptor antibody. As can be seen in Figure 2a, pitavastatin-mediated induction of S1P1 receptor protein occurred in a dose-dependent fashion between 1 and 10 μM. The effects of other statins were also examined in Figure 2b, demonstrating that pitavastatin and atorvastatin, but not pravastatin, were able to increase amounts of S1P1 receptor protein under these conditions. We also studied whether or not statins promoted expression of S1P1 receptor protein in HUVEC. Figure 2c demonstrates that pitavastatin increased the amount of S1P1 receptor protein 1.7±0.1-fold vs vehicle-treated cells (P<0.01).
Figure 1.
Effects of pitavastatin on expression of S1P1 receptor protein. The upper panel shows a protein immunoblot assay of cell lysates derived from BAEC treated with pitavastatin (3 μM) for the times indicated. Equal quantities of cellular protein (20 μg per lane) were resolved by SDS-PAGE, transferred to a nitrocellulose membrane and subjected to immunoblot analyses probed with antibodies directed against S1P1 receptor or eNOS as indicated. The experiment shown is representative of four independent experiments that produced similar results. The lower panel shows the results of densitometric analyses from pooled data, as a time course of the increase of expression of S1P1 receptor protein, relative to the signals obtained in the absence of pitavastatin. Each data point represents the mean±s.e.m. derived from four independent experiments. *indicates P<0.05 vs the initial values (t=0).
Figure 2.
Characterization of increased S1P1 receptor protein induced by statins in cultured endothelial cells. (a) Results of immunoblot analyses of dose–response experiments with pitavastatin for S1P1 receptor protein in BAEC. Cells were treated with pitavastatin or vehicle for 16 h at the indicated concentrations. Immunoblots were probed for S1P1 receptor as above. The data shown in the upper panel are representative of four independent experiments that yielded equivalent results. The lower panel shows the results of densitometric analyses from pooled data, plotting the fold increase of S1P1 receptor at the pitavastatin-concentration indicated, relative to the signals obtained in the absence of pitavastatin. *indicates P<0.05 vs vehicle. (b) BAEC were treated with various statins (or vehicle) at the indicated concentration for 16 h. The data shown are representative of four independent experiments that yielded equivalent results. (c) Effects of pitavastain on S1P1 receptor protein expression were examined in HUVEC. HUVEC were treated with pitavastatin (3 μM for 16 h) or vehicle; cell lysates were prepared and studied in immunoblot assays as above. The data shown are representative of five independent experiments that yielded equivalent results.
Characterization of statin-elicited increases in S1P1 receptors
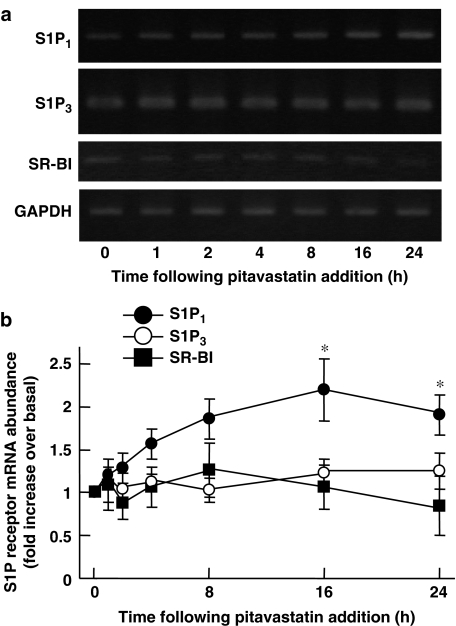
Because pitavastatin seemed the most potent of the statins tested in increasing S1P1 receptor protein (Figure 2b), we focused on this agent in our subsequent experiments in which we sought to explore the mechanisms whereby statins induce S1P1 receptor in BAEC. We studied whether or not pitavastatin alters S1P1 receptor expression at the level of mRNA steady-state abundance using RT-PCR analyses. As shown in Figure 3, abundance of S1P1 transcripts derived from BAEC treated with pitavastatin (3 μM for 16–24 h) was significantly higher than those from untreated cells. In contrast to these results, abundance of transcripts encoding S1P3, another major receptor subtype for S1P in endothelial cells (Lee et al., 1999), or that of those encoding scavenger receptor class B type I (SR-BI), classical HDL receptors expressed in numerous tissues including vascular endothelium (Krieger, 2001), did not change over time on pitavastatin challenge under these conditions (Figure 3). Statins inhibit HMG-CoA reductase, which produces L-mevalonate, a key intermediate metabolite of cholesterol biosynthetic pathway. We tested the effect of added L-mevalonate on the increased S1P1 protein and mRNA, induced by pitavastatin. As shown in Figure 4a and b, increases in S1P1 protein as well as mRNA were completely reversed when cells were co-treated with L-mevalonate. In contrast, addition of squalene, a metabolite that occurs immediately before cholesterol, did not affect pitavastatin-promoted augmentation of S1P1 protein and mRNA expression (Figure 4b and data not shown). The involvement of isoprenoid compounds were tested by using pharmacological inhibitors of protein isoprenylation pathways. GGTI-286, an inhibitor of geranylgeranyltransferase I, but not FTI-277, an inhibitor of fernesyltransferase (Liao, 2002) mimicked the effects of the statins in increasing expression of S1P1 receptor protein (Figure 4c), suggesting that induction of S1P1 receptors by statins may involve attenuation of protein geranylgeranylation rather than farnesylation.
Figure 3.
Effects of pitavastatin on S1P1 transcript in BAEC. (a) Representative result of RT-PCR analyses of transcripts derived from BAEC treated with pitavastatin (3 μM) for the times indicated. Total RNA was isolated, reverse transcribed and amplified with specific oligonucleotide primers directed to S1P1, S1P3, SR-BI or glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as described in detail in the text. (b) Results of densitometric analyses from pooled data, plotting the fold increase of S1P1, S1P3 or SR-BI transcripts relative to those of GAPDH under the conditions indicated, relative to the signals obtained in the absence of drug treatments. *indicates P<0.05 vs t=0; n=5.
Figure 4.
Pharmacological characterization of pitavastatin-induced upregulation of S1P1 protein and mRNA. The upper panel of the (a) shows the effects of L-mevalonate on the pitavastatin-induced increases in S1P1 receptor protein. BAEC were treated with pitavastatin (3 μM for 16 h) or vehicle; some cells were also treated with L-mevalonate (400 μM) or vehicle, as indicated. Immunoblots were probed for S1P1 receptor as above. (b) Total RNA was isolated from cells treated with pitavastatin (3 μM for 16 h) in conjunction with L-mevalonate or squalene as indicated (400 μM), subjected to RT-PCR analyses as described above for S1P1 or for GAPDH. *indicates P<0.05 vs vehicle-treated cells. †Indicates P<0.05 vs pitavastatin-treated cells. (c) Result of an immunoblot analysis of cell lysates derived from BAEC, which were treated with GGTI-286 or with FTI-277 (10 μM for 16 h, respectively) is indicated. n=4 in each panel.
Statins promote eNOS responses to subsequent stimulation with S1P or with HDL
The functional consequences of the induction of S1P1 receptors in pitavastatin-treated BAEC were explored in a series of experiments. S1P has been previously shown to promote the phosphorylation of eNOS at Ser1179 (Igarashi et al., 2001a). We performed immunoblot analyses in lysates prepared from pitavastatin-pretreated BAEC that had been subsequently exposed to S1P, using a phospho-specific antibody as probe (Figure 5). BAEC were first incubated with pitavastatin (3 μM for 16 h) or its vehicle, then treated with S1P (100 nM for up to 60 min). S1P induced eNOS phosphorylation in BAEC that had not been pretreated with pitavastatin (Figure 5; also see Igarashi et al., 2001a). However, when BAEC had been first pre-incubated with pitavastatin for 16 h, the subsequent phosphorylation of eNOSSer1179 induced by S1P was significantly augmented (Figure 5), demonstrating the association of S1P1 receptor induction by pitavastatin with the enhanced BAEC responses to S1P. In the same cell lysates, we confirmed that the S1P-evoked activation of Akt, which serves as an upstream protein kinase that phosphorylates eNOSSer1179, was also increased in pitavastatin-pretreated groups (phospho-Western assays using an antibody specific to phospho-AktSer473 (Igarashi et al., 2001a); data not shown). We examined the effects of pitavastatin on S1P-evoked eNOS responses in HUVEC and found that pretreatment with pitavastatin (3 μM for 16 h) led to augmented phosphorylation of eNOSSer1177 (human sequence) elicited by S1P (100 nM for 5 min; data not shown).
Figure 5.
Effects of pitavastatin on eNOS phosphorylation responses elicited by S1P. In the upper panel is shown the results of a protein immunoblot assay probed with an antibody directed against eNOS, phosphorylated at serine 1179. BAEC had been incubated with pitavastatin (3 μM for 16 h) or vehicle, then they were treated with S1P (100 nM). Following addition of S1P, cells were harvested at the times indicated, and equal quantities of cell lysate (20 μg per lane) were resolved by SDS-PAGE, transferred to a nitrocellulose membrane, and probed with antibodies directed against phospho-eNOS and (total) eNOS. In the lower panel, a time course of this effect is shown, using pooled data. *Indicates P<0.05 compared to values determined in the absence of pretreatment with pitavastatin; n=5.
S1P is specifically enriched in serum HDL fractions (Okajima, 2002) and S1P in HDL plays key roles in exerting HDL-elicited endothelial responses (Kimura et al., 2001). We therefore hypothesized that pitavastatin-pretreated BAEC that express higher amounts of S1P1 receptor would exhibit elevated levels of responses to HDL. Like purified S1P, normal human HDL (100 μg ml−1) exerted similar phosphorylation and activation responses of eNOS in endothelial cells (Figure 6; see also Kimura et al., 2001; Nofer et al., 2004). The HDL concentration of 100 μg ml−1 was chosen as a submaximal concentration utilized in a previous study with cultured vascular endothelial cells (Kimura et al., 2001) to obtain maximum eNOS responses. We have also confirmed that purified normal human apolipoprotein-I (100 μg ml−1 up to 10 min), in contrast to native HDL, failed to induce eNOS phosphorylation (data not shown). Pretreatment with pitavastatin significantly increased phosphorylation of eNOS (Figure 6a and b). We also measured the production of nitrite as an index of NO production by BAEC. Although HDL by itself induced robust nitrite production, pretreatment with pitavastatin led to a significant increase of HDL-elicited nitrite accumulation (Figure 6c). Thus, pretreatment with pitavastatin leads to elevated levels of eNOS responses to subsequent HDL stimulation at both levels of eNOS phosphorylation at a putative Akt site (Ser1179) and of eNOS enzyme activation. We studied the effects of L-mevalonate on endothelial responses to HDL and as shown in Figure 7, L-mevalonate abolished the effects of pitavastatin on HDL-elicited eNOS phosphorylation.
Figure 6.
Effects of pitavastatin on eNOS phosphorylation and activation responses elicited by HDL. (a) Results of a protein immunoblot assay probed with an antibody directed against the phosphorylated form of eNOS. BAEC had been incubated with pitavastatin (3 μM for 16 h) or vehicle, then they were treated with HDL (100 μg ml−1). Following addition of HDL, cells were harvested at the times indicated, and equal quantities of cell lysate (20 μg per lane) were resolved by SDS-PAGE, transferred to a nitrocellulose membrane, and probed with antibodies directed against phospho-eNOS and (total) eNOS. (b) Results of densitometric analyses from pooled data, plotting the fold increase of eNOS phosphorylation at the time following HDL addition as indicated, relative to the signals obtained in the absence of HDL. *Indicates P<0.05 vs values determined in the absence of pretreatment with pitavastatin; n=5. (c) Rate of nitrite production. BAEC were treated with HDL (100 μg ml−1 for 5 min); some cells had been pretreated with pitavastatin (3 μM for 16 h). Culture media were then collected and nitrite measured as described in Methods. Each data point represents the mean±s.e.m. of pooled data derived from four independent experiments. *Indicates P<0.05 compared to values determined in the absence of HDL; †Indicates P<0.05 vs values obtained from cells treated with HDL but without pitavastatin.
Figure 7.
Effects of L-mevalonate on pitavastatin-induced enhancement of HDL-mediated phosphorylation of eNOS. In the upper panel are shown immunoblots probed with antibody directed against Ser1179-phosphorylated eNOS (p-eNOS) or eNOS. BAEC were pre-treated for 16 h with pitavastatin (3 μM) and L-mevalonate (400 μM), then treated with HDL (100 μg ml−1 for 5 min). Cell lysates (20 μg per lane) were resolved by SDS-PAGE, transferred to a nitrocellulose membrane and probed with an antibody specific to phospho-eNOS. Equal loading of samples was confirmed by re-probing the immunoblots with antibodies against (total) eNOS. Results shown are from an experiment representative of four. The lower panel shows pooled data derived from four independent experiments, expressed as the mean±s.e.m.
Specific knockdown of S1P1 receptor by siRNA attenuates eNOS responses to HDL
We used another approach to examine the roles of S1P1 receptors in the responses of eNOS to HDL. Transfection of BAEC with siRNA specific to S1P1 mRNA led to decreases of S1P1 receptor at both protein and mRNA levels, whereas having no substantial effects on mRNA levels of S1P3 receptors or those of SR-BI (Figure 8a). Under these conditions, BAEC which had been transfected with S1P1 siRNA exhibited attenuated eNOS responses to HDL (Figure 8b and c), indicating that S1P1 receptors play a major role in HDL-elicited eNOS responses of BAEC. siRNA specific to S1P1 mRNA did not attenuate pitavastatin-induced upregulation of S1P1 receptor protein (3 μM of pitavastatin for 16 h, data not shown).
Figure 8.
Effects of siRNA on S1P1 receptor expression and HDL-elicited eNOS responses in BAEC. (a) Expression of S1P1 receptors was studied as above at protein level (left half, immunoblot analysis) and at mRNA level (right half, RT-PCR assay), using BAEC that had been transfected with siRNA as described in Methods. Experiments were repeated three times with identical data. The upper part of panel (b) shows immunoblots probed with antibody directed against Ser1179-phosphorylated eNOS (p-eNOS). Cells had been transfected with various siRNAs as indicated, then they were treated with HDL (100 μg ml−1) for the times indicated and subjected to immunoblot analyses. The lower part shows results of densitometric analyses of pooled data derived from four independent experiments, plotting the fold increase of eNOS phosphorylation at the time following HDL addition as indicated, relative to the signals obtained in the absence of HDL. *Indicates P<0.05 compared to values determined in the absence of HDL; †indicates P<0.05 vs values obtained from cells treated with HDL but without pitavastatin. (c) Results of nitrite measurement. BAEC that had been transfected with siRNA were treated with HDL (100 μg ml−1) for 6 min. Culture media were then collected and nitrite measured as described in Methods. Each data point represents the mean±s.e.m. derived from four independent experiments.
Discussion
These studies demonstrate that statins (HMG-CoA reductase inhibitors) induced upregulation of S1P1 receptor expression and increased eNOS responses to stimulation with S1P or to that with HDL in cultured vascular endothelial cells. Immunoblot analyses revealed that treatment with pitavastatin, a recently developed HMG-CoA reductase inhibitor, increases S1P1 receptor protein in BAEC (Figure 1). In dose–response studies, we found that both pitavastatin and atorvastatin were able to increase S1P1 receptor protein, over a concentration range of 1–10 μM (Figure 2), comparable to the concentrations modulating expression of many other endothelial genes (Liao, 2002). Pitavastatin, which was the most potent in increasing S1P1 receptor protein expression among the statins tested, increases expression of the mRNA for the S1P1 receptor as well (Figure 3). We also studied the effects of statins on the expression of two other receptors that are potentially relevant with endothelial responses to S1P and HDL. S1P3 receptors represent another major receptor subtype for S1P expressed in vascular endothelial cells (Lee et al., 1999); SR-BI receptors are the scavenger receptors that bind HDL and mediate selective lipid uptake, ubiquitously expressed in various mammalian cells (Krieger, 2001) including vascular endothelial cells (Yuhanna et al., 2001). Our RT-PCR assays revealed that pitavastatin did not affect the mRNAs for S1P3 or of SR-BI under these experimental conditions (Figure 3). Thus, in endothelial cells, induction of S1P1 receptor by statins is limited to this subtype, among the receptors tested. Notably, a recent report demonstrated that pitavastatin is able to enhance expression of SR-BI in a macrophage cell line (Han et al., 2004), suggesting that the effects of statins on expression of various lipid receptor subtypes may depend on the cell type involved. FTY720 is a recently developed immunosuppressive agent (Mandala et al., 2002) and because this agent acts through S1P receptor systems in various organs, it is tempting to speculate that statins may mediate responses to this agent by affecting expression of S1P receptors.
S1P1 receptor induction by pitavastatin became apparent 8 h after drug addition and persisted at least for 24 h (Figures 1 and 3), in contrast to the time course observed in S1P1 receptor induction by PMA or by VEGF, both of which occur within an hour following cell stimulation (Hla and Maciag, 1990; Igarashi et al., 2003). Induction of S1P1 receptor elicited by statins may involve mechanisms different from those used by PMA or by VEGF. Because L-mevalonate prevented pitavastatin's ability to increase S1P1 receptor protein and mRNA expression (Figure 4a and b), inhibition of HMG-CoA reductase activity in BAEC was responsible for this action of pitavastatin. Inhibition of HMG-CoA reductase leads not only to decreased production of cholesterol, but also decreases isoprenoids. Decreased production of cell isoprenoids ultimately leads to attenuated protein isoprenylation, which plays pivotal roles in regulating subcellular localization and functions of several important signaling proteins, including small G-proteins (Graaf et al., 2004). It has been postulated that statins modulate gene expression of cardiovascular cells by inhibiting protein isoprenylation (Liao, 2002). In the current studies, we found that GGTI-286, an inhibitor of geranylgeranyl transferase I, but not FTI-277, an inhibitor of farnesyltransferase, mimicked the ability of statins to increase S1P1 receptor protein (Figure 4c). Squalene, an intermediate metabolite that occurs immediately proximal to cholesterol and is no longer able to be metabolized to isoprenoids (Liao, 2002), did not reverse pitavastatin-induced upregulation of S1P1 receptor protein and mRNA (Figure 4b and data not shown). Thus, inhibition of protein geranylgeranylation, possibly including that of small G-proteins (Liao, 2002), may participate in statin-induced upregulation of S1P1 receptor expression and S1P signaling.
Our experiments indicated that the phosphorylation of eNOS evoked by S1P was markedly augmented in cells that were pretreated with pitavastatin (Figure 5). Induction of S1P1 receptors is therefore functionally coupled to the enhancement of cellular responses to subsequent stimulation with S1P. Because stimulation of S1P1 receptors could exert protective actions in endothelial cells, via the activation of eNOS (Kimura et al., 2001; Kwon et al., 2001; Rikitake et al., 2002; Dantas et al., 2003; Hla, 2003), we speculate that enhancement of endothelial responses to S1P may represent another molecular mechanism by which statins could improve endothelial functions (Davignon, 2004).
Implications of S1P1 receptor induction by statins were further examined by using normal human HDL in our cell culture model. HDL induced patterns of phosphorylation and activation responses of eNOS similar to those following purified S1P (Figure 6, see also Kimura et al., 2001; Nofer et al., 2004). S1P, which was found to be specifically enriched in HDL fractions in normal human sera, has been proposed to play key roles in transducing endothelial responses to HDL stimulation (Okajima, 2002). Our studies demonstrated that pitavastatin-treated BAEC, which expressed elevated levels of S1P1 receptors, exhibited greater responses to subsequent treatment with HDL, both in protein phosphorylation and in NO production (Figure 6). Importantly, when induction of S1P1 receptors by pitavastatin had been blocked by co-treatment of BAEC with L-mevalonate, enhancement of the phosphorylation of eNOS was completely abolished (Figure 7).
Earlier studies had implicated S1P3 or SR-BI receptor subtypes in the endothelial responses to HDL in different experimental settings (Yuhanna et al., 2001; Nofer et al., 2004). Our data using BAEC indicated that induction of S1P1 receptor played a major role in mediating the enhanced response to HDL induced by the statins. Neither the S1P3 nor the SR-BI receptors appear to be involved in this effect of the statins, because expression of these two receptors was not altered by statins, under these conditions (Figure 3). Moreover, siRNA specific to S1P1 attenuated eNOS responses to HDL without altering expression of other two related receptor subtypes (Figure 8). The apparent discrepancy of our findings with those of Nofer et al. (2004) who found that S1P3 receptor null mice have impaired eNOS responses to HDL, may derive from the differences of species (cows vs mice) or those of experimental settings (cell culture vs isolated blood vessels). Taken together, however, our present results identify the S1P1 receptor subtype in BAEC, whose expression can be modulated by statins, as a novel point of control at which HDL modulates endothelial functions. Using HUVEC, we also confirmed that pitavastatin increased S1P1 receptor protein (Figure 2c) and the phosphorylation of eNOS in responses to S1P (data not shown). Thus, major observations of the current experiments in BAEC can be reproduced in human endothelial cells as well. However, it remains to be determined whether or not statins modulate S1P receptor expression levels in vivo or in clinical settings.
Improvement of NO bioavailability has been identified as a key point at which statins exhibit favorable cardiovascular actions (Davignon, 2004). For example, statins have been shown to counteract down-regulation of eNOS expression by hypoxia (Laufs et al., 1997). Although statins per se under the current conditions did not increase expression levels of eNOS protein (Figure 1), in agreement with an earlier report by Lamas and colleagues (Hernandez-Perera et al., 1998), our experiments indicated that pitavastatin increased eNOS responses elicited by S1P as well as by HDL (Figures 5 and 6). It is therefore likely that statins not only modulate expression of eNOS protein, but also facilitate receptor-regulated activation of eNOS. Because eNOS activity is predominantly regulated by various receptor pathways of endothelial cells (Loscalzo and Welch, 1995), including those for S1P (Igarashi et al., 2001a; Dantas et al., 2003), our studies may provide an additional point of control whereby statins increase NO bioavailability in vivo. Notably, statins directly activate eNOS in a shorter time window via PI3-K-Akt pathways (Kureishi et al., 2000). Thus, induction of S1P1 receptor and enhancement of S1P-induced eNOS activation may represent relatively longer term endothelial responses elicited by statins. Treatment with statins influences serum HDL cholesterol concentrations in patients (reviewed in von Eckardstein et al., 2000). Because S1P is produced by sphingosine kinases and degraded by S1P-lyases in mammals (Saba and Hla, 2004), it will be interesting to explore how statins may regulate activities of these S1P-related enzymes and ultimately the S1P content of serum, especially that of HDL fractions.
In conclusion, our present study documents that statins (HMG-CoA reductase inhibitors) increase expression of S1P1 receptors in cultured vascular endothelial cells. Pharmacological experiments showed that inhibition of HMG-CoA reductase and subsequent decreases in protein geranylgeranylation were involved in statin-induced S1P1receptor up-regulation. Induction of S1P1 receptors was associated with statin-promoted enhancement of eNOS responses to subsequent stimulation with S1P or with HDL; conversely, knockdown of S1P1 receptors by siRNA attenuated responses of eNOS in endothelial cells. Thus, induction of S1P1 receptors may represent a novel feature of the pleiotropic effects of statins by which they mediate increased activity of endothelial NOS in response to sphingolipid compounds, associated with HDL.
External data objects
Acknowledgments
We thank Dr Roger A Sabbadini for providing anti-EDG-1 (S1P1) antibody. This work was in part supported by Grants-in-Aid to JI (15790119) and to HK (15590186) from the Ministry of Education, Culture, Sports, Science and Technology of Japan, as well as by a Grant-in-Aid to JI. by Nankai Ikueikai (Kagawa, Japan).
Abbreviations
- BAEC
bovine aortic endothelial cells
- eNOS
endothelial isoform of nitric oxide synthase
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- HDL
high-density lipoproteins
- HMG-CoA
3-hydroxy-3-methylglutaryl coenzyme A
- HRP
horseradish peroxidase
- HUVEC
human umbilical vein endothelial cells
- PI3-K
phosphoinositide 3′-OH kinase
- RT-PCR
reverse transcription-PCR
- S1P
sphingosine 1-phosphate
- siRNA
small interfering RNA
- VEGF
vascular endothelial growth factor
Conflict of interest
The author states no conflict of interest.
Supplementary Information accompanies the paper on British Journal of Pharmacology website (http://www.nature.com/bjp).
References
- Dantas AP, Igarashi J, Michel T. Sphingosine 1-phosphate and control of vascular tone. Am J Physiol Heart Circ Physiol. 2003;284:H2045–H2052. doi: 10.1152/ajpheart.01089.2002. [DOI] [PubMed] [Google Scholar]
- Davignon J. Beneficial cardiovascular pleiotropic effects of statins. Circulation. 2004;109:III39–III43. doi: 10.1161/01.CIR.0000131517.20177.5a. [DOI] [PubMed] [Google Scholar]
- Durrington P. Dyslipidaemia. Lancet. 2003;362:717–731. doi: 10.1016/S0140-6736(03)14234-1. [DOI] [PubMed] [Google Scholar]
- Gonzalez E, Nagiel A, Lin AJ, Golan DE, Michel T. Small interfering RNA-mediated down-regulation of caveolin-1 differentially modulates signaling pathways in endothelial cells. J Biol Chem. 2004;279:40659–40669. doi: 10.1074/jbc.M407051200. [DOI] [PubMed] [Google Scholar]
- Graaf MR, Richel DJ, Van Noorden CJ, Guchelaar HJ. Effects of statins and farnesyltransferase inhibitors on the development and progression of cancer. Cancer Treat Rev. 2004;30:609–641. doi: 10.1016/j.ctrv.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Han J, Parsons M, Zhou X, Nicholson AC, Gotto AM, Jr, Hajjar DP. Functional interplay between the macrophage scavenger receptor class B type I and pitavastatin (NK-104) Circulation. 2004;110:3472–3479. doi: 10.1161/01.CIR.0000148368.79202.F1. [DOI] [PubMed] [Google Scholar]
- Hernandez-Perera O, Perez-Sala D, Navarro-Antolin J, Sanchez-Pascuala R, Hernandez G, Diaz C, et al. Effects of the 3-hydroxy-3-methylglutaryl-CoA reductase inhibitors, atorvastatin and simvastatin, on the expression of endothelin-1 and endothelial nitric oxide synthase in vascular endothelial cells. J Clin Invest. 1998;101:2711–2719. doi: 10.1172/JCI1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hla T. Sphingosine 1-phosphate receptors. Prostaglandins. 2001;64:135–142. doi: 10.1016/s0090-6980(01)00109-5. [DOI] [PubMed] [Google Scholar]
- Hla T. Signaling and biological actions of sphingosine 1-phosphate. Pharmacol Res. 2003;47:401–407. doi: 10.1016/s1043-6618(03)00046-x. [DOI] [PubMed] [Google Scholar]
- Hla T, Maciag T. An abundant transcript induced in differentiating human endothelial cells encodes a polypeptide with structural similarities to G-protein-coupled receptors. J Biol Chem. 1990;265:9308–9313. [PubMed] [Google Scholar]
- Igarashi J, Bernier SG, Michel T. Sphingosine 1-phosphate and activation of endothelial nitric oxide synthase: differential regulation of Akt and MAP Kinase pathways bu EDG and bradykinin receptors in vascular endothelial cells. J Biol Chem. 2001a;276:12420–12426. doi: 10.1074/jbc.M008375200. [DOI] [PubMed] [Google Scholar]
- Igarashi J, Erwin PA, Dantas AP, Chen H, Michel T. VEGF induces S1P1 receptors in endothelial cells: implications for cross-talk between sphingolipid and growth factor receptors. Proc Natl Acad Sci USA. 2003;100:10664–10669. doi: 10.1073/pnas.1934494100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igarashi J, Michel T. Sphingosine 1-phosphate and isoform-specific activation of phosphoinositide 3-kinase beta. Evidence for divergence and convergence of receptor-regulated endothelial nitric-oxide synthase signaling pathways. J Biol Chem. 2001b;276:36281–36288. doi: 10.1074/jbc.M105628200. [DOI] [PubMed] [Google Scholar]
- Kimura T, Sato K, Kuwabara A, Tomura H, Ishiwara M, Kobayashi I, et al. Sphingosine 1-phosphate may be a major component of plasma lipoproteins responsible for the cytoprotective actions in human umbilical vein endothelial cells. J Biol Chem. 2001;276:31780–31785. doi: 10.1074/jbc.M104353200. [DOI] [PubMed] [Google Scholar]
- Krieger M. Scavenger receptor class B type I is a multiligand HDL receptor that influences diverse physiologic systems. J Clin Invest. 2001;108:793–797. doi: 10.1172/JCI14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kureishi Y, Luo Z, Shiojima I, Bialik A, Fulton D, Lefer DJ, et al. The HMG-CoA reductase inhibitor simvastatin activates the protein kinase akt and promotes angiogenesis in normocholesterolemic animals. Nat Med. 2000;6:1004–1010. doi: 10.1038/79510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon YG, Min JK, Kim KM, Lee DJ, Billiar TR, Kim YM. Sphingosine 1-phosphate protects human umbilical vein endothelial cells from serum-deprived apoptosis by nitric oxide production. J Biol Chem. 2001;276:10627–10633. doi: 10.1074/jbc.M011449200. [DOI] [PubMed] [Google Scholar]
- Laufs U, Fata VL, Liao JK. Inhibition of 3-hydroxy-3-methylglutaryl (HMG)-CoA reductase blocks hypoxia-mediated down-regulation of endothelial nitric oxide synthase. J Biol Chem. 1997;272:31725–31729. doi: 10.1074/jbc.272.50.31725. [DOI] [PubMed] [Google Scholar]
- Lee H, Goetzl EJ, An S. Lysophosphatidic acid and sphingosine 1-phosphate stimulate endothelial cell wound healing. Am J Physiol. 2000;278:C612–C618. doi: 10.1152/ajpcell.2000.278.3.C612. [DOI] [PubMed] [Google Scholar]
- Lee MJ, Thangada S, Claffey KP, Ancellin N, Liu CH, Kluk M, et al. Vascular endothelial cell adherens junction assembly and morphogenesis induced by sphingosine-1-phosphate. Cell. 1999;99:301–312. doi: 10.1016/s0092-8674(00)81661-x. [DOI] [PubMed] [Google Scholar]
- Liao JK. Isoprenoids as mediators of the biological effects of statins. J Clin Invest. 2002;110:285–288. doi: 10.1172/JCI16421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loscalzo J, Welch G. Nitric oxide and its role in the cardiovascular system. Prog Cardiovasc Dis. 1995;38:87–104. doi: 10.1016/s0033-0620(05)80001-5. [DOI] [PubMed] [Google Scholar]
- Mandala S, Hajdu R, Bergstrom J, Quackenbush E, Xie J, Milligan J, et al. Alteration of lymphocyte trafficking by sphingosine-1-phosphate receptor agonists. Science. 2002;296:346–349. doi: 10.1126/science.1070238. [DOI] [PubMed] [Google Scholar]
- Morales-Ruiz M, Lee MJ, Zoellner S, Gratton JP, Scotland R, Shiojima I, et al. Sphingosine-1-phosphate activates Akt, nitric oxide production and chemotaxis through a Gi-protein/phosphoinositide 3-kinase pathway in endothelial cells. J Biol Chem. 2001;276:19672–19677. doi: 10.1074/jbc.M009993200. [DOI] [PubMed] [Google Scholar]
- Nofer JR, Van Der Giet M, Tolle M, Wolinska I, Von Wnuck Lipinski K, Baba HA, et al. HDL induces NO-dependent vasorelaxation via the lysophospholipid receptor S1P(3) J Clin Invest. 2004;113:569–581. doi: 10.1172/JCI18004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okajima F. Plasma lipoproteins behave as carriers of extracellular sphingosine 1-phosphate: is this an atherogenic mediator or an anti-atherogenic mediator. Biochim Biophys Acta. 2002;1582:132–137. doi: 10.1016/s1388-1981(02)00147-6. [DOI] [PubMed] [Google Scholar]
- Ridker PM. Rosuvastatin in the primary prevention of cardiovascular disease among patients with low levels of low-density lipoprotein cholesterol and elevated high-sensitivity C-reactive protein: rationale and design of the JUPITER trial. Circulation. 2003;108:2292–2297. doi: 10.1161/01.CIR.0000100688.17280.E6. [DOI] [PubMed] [Google Scholar]
- Rikitake Y, Hirata K, Kawashima S, Ozaki M, Takahashi T, Ogawa W, et al. Involvement of endothelial nitric oxide in sphingosine-1-phosphate-induced angiogenesis. Arterioscler Thromb Vasc Biol. 2002;22:108–114. doi: 10.1161/hq0102.101843. [DOI] [PubMed] [Google Scholar]
- Saba JD, Hla T. Point-counterpoint of sphingosine 1-phosphate metabolism. Circ Res. 2004;94:724–734. doi: 10.1161/01.RES.0000122383.60368.24. [DOI] [PubMed] [Google Scholar]
- Von Eckardstein A, Assmann G. Prevention of coronary heart disease by raising high-density lipoprotein cholesterol. Curr Opin Lipidol. 2000;11:627–637. doi: 10.1097/00041433-200012000-00010. [DOI] [PubMed] [Google Scholar]
- Yatomi Y, Igarashi Y, Yang L, Hisano N, Qi R, Asazuma N, et al. Sphingosine 1-phosphate, a bioactive sphingolipid abundantly stored in platelets, is a normal constituent of human plasma and serum. J Biochem (Tokyo) 1997;121:969–973. doi: 10.1093/oxfordjournals.jbchem.a021681. [DOI] [PubMed] [Google Scholar]
- Yuhanna IS, Zhu Y, Cox BE, Hahner LD, Osborne-Lawrence S, Lu P, et al. High-density lipoprotein binding to scavenger receptor-BI activates endothelial nitric oxide synthase. Nat Med. 2001;7:853–857. doi: 10.1038/89986. [DOI] [PubMed] [Google Scholar]
- Zamir LO, Devor KA, Sauriol F. Biosynthesis of the trichothecene 3-acetyldeoxynivalenol. Identification of the oxygenation steps after isotrichodermin. J Biol Chem. 1991;266:14992–15000. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.