Abstract
Background and purpose:
Next to its broad antimicrobial spectrum, the therapeutic advantages of the fluoroquinolone antimicrobial drug Danofloxacin-Mesylate (DM) are attributed to its rapid distribution to the major target tissues such as lungs, intestines and the mammary gland in animals. Previous analyses revealed that effective drug concentrations are achieved also in luminal compartments of these organs, suggesting that active transport proteins facilitate excretion into the luminal space. Members of the ATP-Binding Cassette (ABC) superfamily, including P-gp, BCRP and MRP2 are known to be expressed in many tissue barriers and in cell-membranes facing luminal compartments. Hence we hypothesized that DM is a substrate for one of these efflux-transporters.
Experimental approach:
Confluent monolayers of Caco-2 cells, grown on microporous membranes in two-chamber devices were used. DM concentrations were measured by fluorimetric assay after HPLC of the culture media.
Key results:
DM transport across Caco-2 cells was asymmetric, with a rate of secretion exceeding that of absorption. The P-gp inhibitors PSC833 and GF120918 and the MRP-inhibitor MK571 partially decreased the secretion of DM and increased its absorption rate. The BCRP inhibitor, Ko143, decreased secretion only at a concentration of 1 μM. When DM was applied together with ciprofloxacin, secretion as well as absorption of DM decreased.
Conclusions and Implications:
DM is a substrate for the efflux transporters P-gp and MRP2, whereas the specific role of BCRP in DM transport needs further evaluation. These findings provide a mechanistic basis for the understanding of the pharmacokinetics of DM in healthy and diseased individuals.
Keywords: fluoroquinolones, danofloxacin, mesylate, ABC, transport, ABCB1, ABCC2, ABCG2
Introduction
Fluoroquinolone antimicrobials are widely used in the therapy of infectious diseases in consideration of their broad antimicrobial activity as well as their beneficial pharmacokinetics, characterized by a large volume of distribution. Fluoroquinolones can be applied orally or by parenteral injection and initial data indicated that the major route of elimination is renal excretion as only a small fraction was found to be eliminated with biliary fluid. Despite this low rate of biliary elimination, drug concentrations in the gut lumen are high following parenteral application, as demonstrated for ciprofloxacin, the first widely marketed fluoroquinolone (Sorgel et al., 1989). These findings suggested a trans-intestinal elimination, and a few years later, Rabbaa et al. (1995) hypothesized that permeability-glycoprotein (P-gp) might be involved in the trans-epithelial secretion of ciprofloxacin as well as ofloxacin. P-gp is a member of the superfamily of ATP-binding cassette (ABC) transporters. These transporters use ATP to pump compounds out of the cellular cytoplasm, hence contributing to the function of biological barriers, such as the blood–brain barrier and the intestinal barrier. Moreover, it was considered that transporter-dependent secretion of antimicrobials from the basolateral site to the luminal surfaces of the alveolar space or the luminal space of the large intestines provided a therapeutic advantage against bacteria that colonize these luminal surfaces.
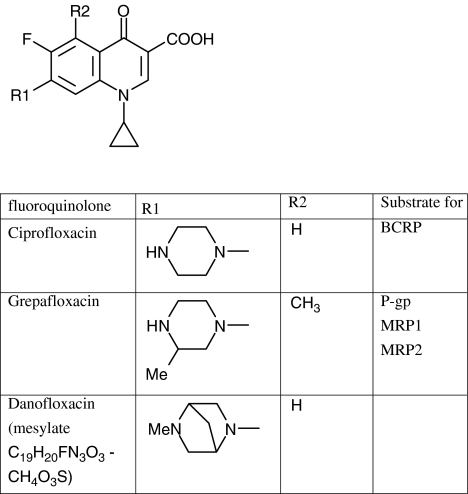
Danofloxacin–mesylate (DM, Figure 1) is a fluoroquinolone antibacterial drug for veterinary use. Its use is indicated in cases of Gram-negative infections of the respiratory tract and intestinal tract in various animal species and the mammary gland in cattle. The recommended dose is 6 mg kg−1 with subcutaneous injection on a single occasion, based on the concept of concentration-dependent killing that is applied to this group of fluoroquinolones. Danofloxacin itself was initially selected by QSAR analysis, but never entered clinical studies (Braish and Fox, 1990). In veterinary medicine, only danofloxacin–mesylate has been licensed. The oral bioavailability of DM varies among species, for example, 99% in fasted chickens (Knoll et al., 1999) and approximately 22% in horses (Fernandez-Varon et al., 2006), whereas parenteral injection was recommended for all animal species with the exception of poultry. Kinetic analyses of DM disposition following Intramuscular or Subcutaneous injection indicated that high drug concentrations exceeding those in plasma were detected in the luminal compartments of the lungs and the intestines as well as in the milk (Friis and Nielsen, 1997; McKellar et al., 1998; Shem-Tov et al., 1998; Lindecrona et al., 2000). As the previously published kinetic data did not provide an explanation for the high drug concentrations that were measured in the luminal space of the intestines after parenteral injection, we hypothesized that DM, like enrofloxacin and grepafloxacin, is a substrate for efflux transporters. Among these transporters, P-gp, breast cancer resistant protein (BCRP) and multi-drug resistance associated protein (MRP2) are known to be directed towards the luminal compartments in various organs, including the above-mentioned target organs for the clinical use of DM (de Lange et al., 2000; Nakajima et al., 2000; Naruhashi et al., 2001,; Yamaguchi et al., 2002; Sasabe et al., 2004; Merino et al., 2006). Hence, we investigated the effects of the lumen-directed transporters on DM absorption and secretion in Caco-2 cell monolayers, known to express P-gp, MRP2 and BCRP at the apical cell membranes (Taipalensuu et al., 2001; Prime-Chapman et al., 2004; Xia et al., 2005). Our results indicate that DM secretion is P-gp and MRP2-dependent and are suggestive of an additional role of BCRP in DM transmembrane transport. Extrapolation of these data to whole body disposition of DM suggests that the widespread transporter P-gp will mediate the secretion of this fluoroquinolone antimicrobial drug into luminal compartments of organs, whereas MRP2 will have a similar role in the main drug-eliminating organs.
Figure 1.
Chemical structure of danofloxacin, ciprofloxacin and grepafloxacin. The latter two fluoroquinolones had been previously found to be a substrate for ATP-dependent drug transporters.
Materials and methods
Caco-2 cell cultures
Caco-2 cells (American Type Culture Collection, Rockville, MO, USA, ATCC HTB-37), passage 95–105, were cultured in Dulbecco's modified Eagle's medium (DMEM, Gibco, NY, USA) with 4.5 g l−1 glucose, supplemented with 10% (v/v) fetal bovine serum (FBS), 0.1 mM nonessential amino acids, 2 mM L-glutamine, 100 U ml−1 penicillin and 100 μg ml−1 streptomycin. Cells were cultured in 75cm2 tissue culture T-flasks at 37°C in a humidified atmosphere of 5% CO2 in air for subsequent plating onto the cell culture inserts (0.4 μm pore size, high-pore density, polyethylene terephthalate (PET) micro-porous membranes, surface area 0.3 cm2, BD, Falcon, NJ, USA). Caco-2 cells were seeded at a density of 30 000 cells per insert. Transport experiments were conducted after 20–21 days, using only those cell monolayers on inserts with a transepithelial electrical resistance (TEER) value above 300 Ω cm2, as measured by an epithelial volt-ohm meter (Millicell-ERS, Millipore Corporation, Bedford, MA, USA).
Transport studies
Bidirectional transport studies were performed in DMEM without FBS and phenol red, supplemented with 1% (v/v) non-essential amino acids and 2 mM L-glutamine, pH 7.4. The experiments were initiated by adding medium containing DM, to either the apical compartment at a volume of 300 μl, or to the basolateral compartment at a volume of 700 μl, for measuring absorption and secretion, respectively. Aliquots from both compartments were taken after 1 h, and subjected to high-performance liquid chromatography (HPLC) analysis. In the subsequent experiments, stocks of the inhibitors PSC833, GF120918, MK571 and Ko143 were dissolved in dimethyl sulfoxide (DMSO), and were added to the medium in the given concentrations. The final concentration of the solvent DMSO was set to 0.1% in all experiments. The inhibitors, as well as ciprofloxacin, were added to both compartments of the two-chamber system. After 1 h of incubation at 37°C in a humidified atmosphere of 5% CO2 in air, samples from both compartments were collected separately for HPLC analysis.
HPLC analysis
The HPLC analyses were conducted according to the method previously described by Garcia et al. (2000), with minor modification. Briefly, samples of 20 μl were directly injected into the HPLC system, consisting of a high-pressure pump, an autoinjector (Gyna 50) and a fluorescence detector (Detector Jasco, IJsselstein, The Netherlands Model FP 920). A spherisorb-ODS2 column was used for the separation of the samples, using a mobile phase of acetonitrile in an aqueous phase (16:84, v/v) set to pH 3.0 and a flow rate of 1.0 ml min−1. The aqueous phase consisted of tetrabutylammonium hydrogen phosphate (TBAP), 0.02 M and potassium dihydrogen phosphate, 0.02 M dissolved in demineralized water. Excitation and emission wavelengths of 280 and 440 nm, respectively, were used for the detection of DM. In order to allow a parallel measurement of DM and ciprofloxacin, in the corresponding experiments the percentage of acetonitrile in the mobile phase was decreased to 10% (v/v). The area under curve was integrated by Chromeleon software (Separations, HI Ambacht, The Netherlands) and compared with standard curves prepared with cell culture medium for the quantification of DM concentrations.
Data analysis
The apparent permeability (Papp) was calculated according to the following equation:
where V is the volume of receiver compartment (cm3), dC/dt the rate of time-dependent increase in the concentration in the receiver compartment (mol dm−3 s−1), A the surface area of microporous membrane of the inserts (cm2) and C0, is the initial concentration of DM in the donor compartment (mol dm−3).
One-way analysis of variance (ANOVA), followed by Dunnett's multiple comparison test (Graph Pad Prism software, version 2.01; Graph Pad software Inc., San Diego, CA, USA) was used to assess the statistical significance of observed differences. Differences were considered to be statistically significant when P<0.05.
Chemicals
Danofloxacin-mesylate was kindly supplied by Pfizer (Sandwich, UK). Ciprofloxacin was obtained from Fluka (Seelze, Germany). MK-571 sodium salt was obtained from Alexis Biochemicals (Grünberg, Germany). PSC833 was a generous gift from Novartis Pharma AG (Basel, Switzerland), GF120918 was donated by GlaxoSmithKline (Stevenage, Herts, UK) and Ko143 was a generous gift from Professor GJ Koomen (University of Amsterdam, The Netherlands).
Results
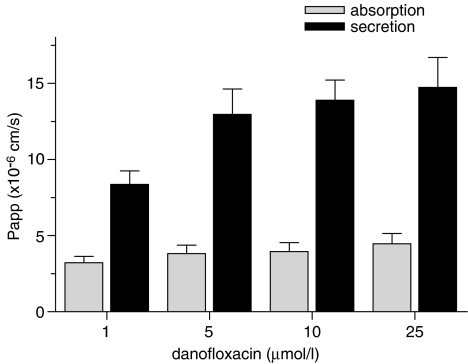
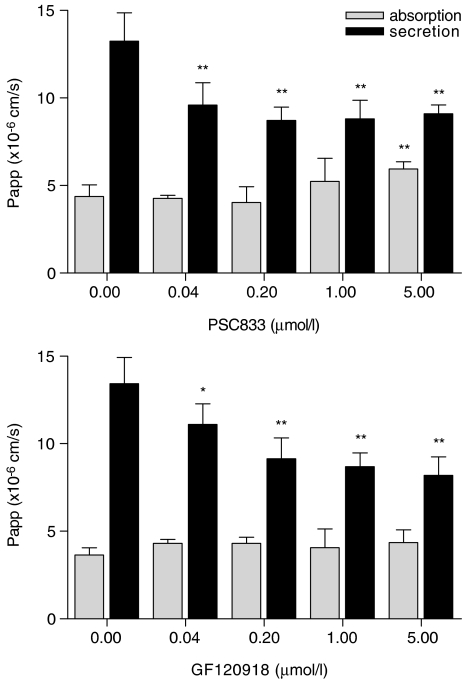
Experiments with a concentration range between one and 25 μM DM indicated an asymmetric transport of DM at all concentrations tested. Secretion, expressed as apparent permeability (Papp), increased with increasing concentrations, whereas absorption remained constant (Figure 2). The rate of secretion exceeded that of absorption 2.5 to 3.5 times. These findings suggest that the efflux of DM to the apical compartment is indeed a carrier-mediated process in Caco-2 cells. For the following experiments, a series of inhibitors for ATP-dependent transporters was selected to assess the contribution of individual transporters to DM transport. In these experiments, the inhibitors were added at different concentrations to both compartments, whereas a constant concentration (10 μM) of DM was used. As potent inhibitor for P-gp, PSC833 (Aouali et al., 2005) was selected. PSC833 decreased the secretion of DM, and subsequently increased its absorption (Figure 3, upper panel), demonstrating that P-gp is involved in the secretion of DM.
Figure 2.
Concentration-dependent transport of danofloxacin: Caco-2 cell monolayers were incubated with increasing concentrations of danofloxacin added to the basolateral or apical compartment. Data represent means±s.d. of six or more replicates.
Figure 3.
The effect of PSC833 and GF120918 on absorptive and secretory transport of danofloxacin. Caco-2 cell monolayers were incubated with 10 μM danofloxacin in the absence or presence of increasing concentrations of PSC833 or GF120918 added to the apical and basolateral compartment. Data represent means±s.d. of six replicates. Significant differences are marked; *P<0.05, **P<0.01.
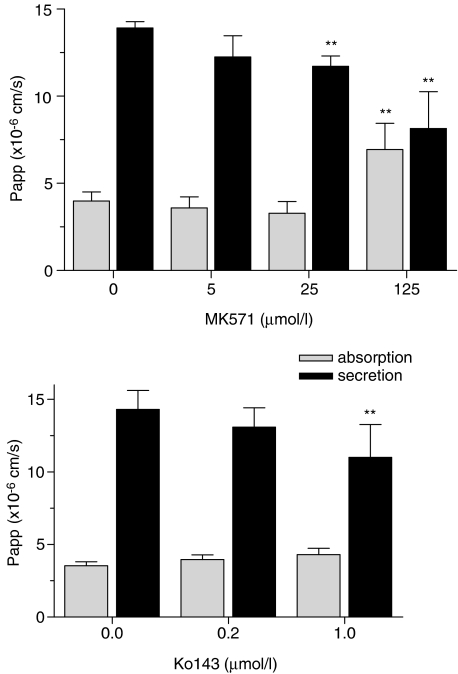
However, as secretion was not completely blocked by PSC833, it was concluded that P-gp is not the only carrier responsible for the secretion of DM in the Caco-2 cell model. Therefore, we measured the effect of GF120918, a dual inhibitor for P-gp and BCRP (de Bruin et al., 1999). Secretion was again decreased (Figure 3, lower panel), whereas absorption remained apparently unaffected. With the aim to further analyze the role of BCRP, transport of DM was measured in the presence of Ko143 (Figure 4, upper panel). Ko143, considered to be a potent inhibitor of BCRP-mediated transports (Allen et al., 2002), decreased the secretion of DM, without significantly affecting its absorption. Finally, an inhibitor for MRPs, MK571 (Gekeler et al., 1995), was found to decrease the secretion and increase absorption of DM (Figure 4, lower panel) suggesting that MRP2, thus far the only MRP-transporter recognized at the apical membrane of Caco-2 cells, is also involved in DM secretion.
Figure 4.
The effect of MK571and Ko143 on absorptive and secretory transport of danofloxacin. Caco-2 cell monolayers were incubated with 10 μM danofloxacin in the absence or presence of increasing concentrations of MK571 or Ko143 added to the apical and basolateral compartment. Data represent means±s.d. of six replicates. Significant differences are marked, **P<0.01.
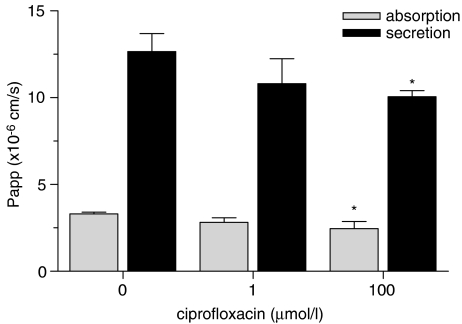
In a third experimental setting, we evaluated the potential for common pathways in the transport of DM and ciprofloxacin, a related fluoroquinolone (Figure 5). Coincubation of DM with ciprofloxacin resulted in a decreased secretion of DM as well as a decreased absorption of DM, indicating drug–drug interactions at the level of membrane transport.
Figure 5.
The effect of ciprofloxacin on absorptive and secretory transport of danofloxacin. Caco-2 cell monolayers were incubated with 10 μM danofloxacin in the absence or presence of ciprofloxacin added to the apical and basolateral compartment at increasing concentrations. Data represent means±s.d. of three replicates. Significant differences are marked, *P<0.05.
Discussion
The involvement of ATP-dependent efflux transporters in the disposition and the excretion of DM had not been investigated before, in spite of finding high drug concentrations at the luminal side of the intestines, the lung and the mammary gland following subcutaneous injection of DM. It was the aim of the present study to assess whether or not DM, like grepafloxacin, is a substrate for ATP-dependent efflux transporters and evaluate the potential role of individual transporters in danofloxacin secretion into luminal compartments. The Caco-2 model used here has been validated before and applied to elucidate the transmembrane transport of various drugs and toxins. In the present experiments, Caco-2 cells were grown in two chamber systems and DM was added to the apical or basolateral compartment in the absence or presence of known inhibitors of ATP-dependent efflux transporters. Measurement of the membrane transconductance at the beginning and the end of the experiments indicated that the given treatments did not affect the integrity of the cell monolayer. With this model, we demonstrated that DM is a substrate for more than one transporter. The inhibitory effects of PSC833 and GF120918 clearly show that secretion is P-gp dependent, whereas the other inhibitors, MK571 and Ko143, predict a role for MRP2 and BCRP in the organ-specific secretion. The limited effect of Ko143, used as inhibitor for BCRP, on DM secretion might be due to a compensatory increase in the transport by P-gp or to nonspecified effects on other membrane transporters. Ko143 has been described to be a potent inhibitor of BCRP, but a minimal effect on P-gp and MRP1 function has been observed at a concentration of 0.50 μM, which falls within the concentration range tested here (see Figure 4, upper panel), whereas it lacks any effect on MRP2 function in this concentration range (Allen et al., 2002). In the light of these previous findings, it can be argued that the inhibitory effect of Ko143 on DM secretion that was only seen at the highest concentration used (1 μmol l−1), results from a nonspecific inhibition of P-gp. However, when PSC833 and Ko143 were used together (data not shown), DM secretion decreased further.
Ciprofloxacin, a substrate for BCRP, but not for P-gp or MRP2 (Lowes and Simmons, 2002; Merino et al., 2006), decreased the secretion and absorption of danofloxacin. A common pathway in the transport of danofloxacin and ciprofloxacin seems likely. A basolateral carrier affecting the transepithelial transport of ciprofloxacin has already been suggested (Griffiths et al., 1994). Basolateral carriers may belong to the MRP-family of active membrane carriers or to the family of solute (facilitated) carriers (SLC) (Griffiths et al., 1994; Zakelj et al., 2006). The latter are mainly responsible for cellular uptake of substrates, but depending on the cosubstrates and their concentrations they may serve as efflux carriers as well. Thus, whether inhibition of DM transport by ciprofloxacin has resulted from competitive inhibition for a basolateral and/or apical transporter (BCRP) needs further evaluation. However, the involvement of a basolateral active efflux transporter in the absorption of DM is suggested by the increased secretion of DM that was found when increasing concentrations of DM were applied, leading to the saturation of this basolateral efflux transporter that counteracted partly the secretion. This would also explain the observed tendency for a decreased absorption at lower concentrations of MK571 considering that MRPs other than MRP2 are likely to be situated in the basolateral membrane of intestinal cells.
Our finding that DM is a substrate for multiple transporters is not surprising, because various drugs are substrates for more than one transporter, including the fluoroquinolone grepafloxacin (Lowes and Simmons, 2002; Naruhashi et al., 2002; Sasabe et al., 2004) and likely sparfloxacin (Cormet-Boyaka et al., 1998). P-gp is expressed in alveolar epithelial type I epithelium within the human and rat lung tissue (Campbell et al., 2003), although BCRP is expressed in the bronchial and bronchiolar epithelium, similar to P-gp, and in the endothelial cells (Scheffer et al., 2002), but luminal staining of alveolar cells has not been demonstrated yet. High concentrations of grepafloxacin, compared to plasma concentrations, were previously measured in the epithelial lining fluid (ELF) of the rat lung (Lowes and Simmons, 2002; Zhao et al., 2002), whereas this was not observed for ciprofloxacin (Deguchi et al., 2003). Similarly, concentrations of levofloxacin and sparfloxacin, both described as substrates for P-gp (Ito et al., 1997; Cormet-Boyaka et al., 1998; de Lange et al., 2000), in ELF exceeded those in plasma in humans (Wise and Honeybourne, 1996; Andrews et al., 1997). It thus seems that P-gp determines the vectorial transport into the bronchiolar and alveolar space. Previous in vivo investigations had indicated that DM rapidly penetrates respiratory tract tissues and secretions of calves (Friis, 1993a; McKellar et al., 1999), suggesting an active secretion of danofloxacin into these secretions, that may be mediated by P-gp (Friis, 1993b). Similar results were obtained in pigs (Friis and Nielsen, 1997). Other mechanisms involved in tissue distribution or accumulation are tissue-specific uptake and binding, and high tissue concentrations may thus result from binding of fluoroquinolones to cellular components as exemplified for grepafloxacin, which binds to phophatidylserine (Suzuki et al., 2002).
Expression of BCRP is highly upregulated in the lactating mammary gland of cows, human and mice (Jonker et al., 2005). High concentrations of danofloxacin in the milk of cows exceeding those of serum were previously reported (Shem-Tov et al., 1998), and this phenomenon was explained by ion trapping. However, it is highly likely that BCRP plays an important role in the secretion of danofloxacin into the milk, as was shown for ciprofloxacin in rats (Merino et al., 2006) and enrofloxacin in ewes (Pulido et al., 2006), and hence this mechanism should be further elucidated.
Expression of all three transporters is high in the small intestines. Although the expression of MRP2 decreases along the human intestinal tract, P-gp and BCRP are highly expressed in the large intestines as well (Maliepaard et al., 2001; Langmann et al., 2003). Active intestinal secretion, presumably mediated by one or more of these transporters, has been reported for grepafloxacin (Naruhashi et al., 2001, 2002; Yamaguchi et al., 2002; Fernandez-Teruel et al., 2005), sarafloxacin (Fernandez-Teruel et al., 2005) and ofloxacin (Rabbaa et al., 1996), and is highly suggested for other fluoroquinolones, as an asymmetric transport directed to the luminal side could be demonstrated in Caco-2 cells (Griffiths et al., 1994; Cormet-Boyaka et al., 1998; Yamaguchi et al., 2000; Naruhashi et al., 2001; Ruiz-García et al., 2002; Volpe, 2004). In in vivo studies, high concentrations of DM were found in the intestinal contents in healthy pigs (Lindecrona et al., 2000), cattle (Von Traeder and Kleinhaus, 2002) and sheep (McKellar et al., 1998), and it has to be assumed that the underlying mechanism is an active secretion of danofloxacin by MRP2 and P-gp localized in the brush border membranes of epithelial cells.
It is worthwhile to recall that numerous factors affect the function of transporters, including genetic variation, gender, feed components, comedication with substrate drugs, infection and inflammation. Expression of these transporters is partly coregulated with phase I and II metabolizing enzymes and typically depends on the activation of nuclear transcription factors, such as constitutive androstane receptor (CAR) and pregnane X receptor (PXR) (Eloranta et al., 2005). Activation of these nuclear receptors by physiological ligands, such as hormones (e.g. cortisol, estradiol, progesteron and thyroid hormone) or xenobiotics leads to changes in the rate of transcription of the drug -transporters. In turn, inflammatory mediators (including IL-6) are known to decrease the expression and function of P-gp and MRP2, similar to the decrease in the activity of various CYP450 isozymes in the liver and the gastro-intestinal tract (Fernandez et al., 2004; Kalitsky-Szirtes et al., 2004). These mechanisms are likely to explain the previously observed decreases in the systemic clearance and secretion of DM into the intestinal lumen in the diseased animals (Lindecrona et al., 2000). Thus, the disposition of fluoroquinolones into the target tissues may highly vary among individuals, with possible consequences for efficacy and resistance development in microbes.
In addition, certain plant-derived polyphenolic compounds, structurally related to the quinolone antimicrobials, are substrates for P-gp, MRP2 and BCRP. High levels of these compounds, including the flavenoids genestin, quercetin, naringenin, hesperetin (for a review see Morris and Zhang, 2006), may occur in food and feed materials, especially in soybean products and (citrus) fruits and hence may decrease the secretion of fluoroquinolones, including DM into the intestinal lumen.
In conclusion, the present results indicate that DM is a substrate for multiple transporters, including P-gp and MRP2, whereas the role of BCRP in DM secretion remains elusive. The finding that DM is a substrate for efflux transporters explains the previously observed secretion of DM into the intestinal lumen after parenteral application and the high concentrations in bronchial secretions, which may contribute to the therapeutic efficacy of this fluoroquinolone.
Acknowledgments
We greatly appreciate the gift of Professor/Dr GJ Koomen and the Van ‘t Hoff Institute for Molecular Sciences, University of Amsterdam, providing us with Ko 143 for the inhibition studies. We thank Lilian de Nijs-Tjon and Marjolein van der Doelen for their technical assistance.
Abbreviations
- ABC
ATP-binding cassette
- ATP
adenosine tri-phospate
- BCRP
breast cancer resistance protein
- bw
body weight
- CAR
constitutive androstane receptor
- CYP
cytochrome P
- DM
danofloxacin-mesylate
- DMEM
Dulbecco's modified Eagle's medium
- DMSO
dimethyl sulfoxide
- ELF
epithelial lining fluid
- FBS
fetal bovine serum
- HPLC
high-performance liquid chromatography
- IL
interleukin
- IM
intramuscular
- MRP
multi-drug resistance associated protein
- Papp
apparent permeability
- P-gp
permeability-glycoprotein
- PXR
pregnane X receptor
- SC
subcutaneous
- SLC
solute carrier
- TEER
trans-epithelial electrical resistance
- TBAP
tetrabutylammonium hydrogen phosphate
Conflict of interest
The authors state no conflict of interest.
References
- Allen JD, Van Loevezijn A, Lakhai JM, Van Der Valk M, Van Tellingen O, Reid G, et al. Potent and specific inhibition of the breast cancer resistance protein multidrug transporter in vitro and in mouse intestine by a novel analogue of fumitremorgin C. Mol Cancer Ther. 2002;1:417–425. [PubMed] [Google Scholar]
- Andrews J, Honeybourne D, Jevons G, Brenwald N, Cunningham B, Wise R. Concentrations of levofloxacin (HR 355) in the respiratory tract following a single oral dose in patients undergoing fibre-optic bronchoscopy. J Antimicrob Chemother. 1997;40:573–577. doi: 10.1093/jac/40.4.573. [DOI] [PubMed] [Google Scholar]
- Aouali N, Eddabra L, Macadre J, Morjani H. Immunosuppressors and reversion of multidrug-resistance. Crit Rev Oncol/Hematol Immunosuppressive Treatment Induction Cancer. 2005;56:61–70. doi: 10.1016/j.critrevonc.2004.12.010. [DOI] [PubMed] [Google Scholar]
- Braish TF, Fox DE. Synthesis of (S,S)- and (R,R)-2-alkyl-2,5-diazabicyclo[2.2.1]heptanes. J Org Chem. 1990;55:1684–1687. [Google Scholar]
- Campbell L, Abulrob a-NG, Kandalaft LE, Plummer S, Hollins aJ, Gibbs A, et al. Constitutive expression of P-glycoprotein in normal lung alveolar epithelium and functionality in primary alveolar epithelial cultures. J Pharmacol Exp Ther. 2003;304:441–452. doi: 10.1124/jpet.102.042994. [DOI] [PubMed] [Google Scholar]
- Cormet-Boyaka E, Huneau JF, Mordrelle A, Boyaka PN, Carbon C, Rubinstein E, et al. Secretion of sparfloxacin from the human intestinal Caco-2 cell line is altered by P-glycoprotein inhibitors. Antimicrob Agents Chemother. 1998;42:2607–2611. doi: 10.1128/aac.42.10.2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bruin M, Miyake K, Litman T, Robey R, Bates SE. Reversal of resistance by GF120918 in cell lines expressing the ABC half-transporter, MXR. Cancer Lett. 1999;146:117–126. doi: 10.1016/s0304-3835(99)00182-2. [DOI] [PubMed] [Google Scholar]
- De Lange EC, Marchand S, Van Den Berg D, Van Der Sandt IC, De Boer aG, Delon A, et al. In vitro and in vivo investigations on fluoroquinolones; effects of the P-glycoprotein efflux transporter on brain distribution of sparfloxacin. Eur J Pharm Sci. 2000;12:85–93. doi: 10.1016/s0928-0987(00)00149-4. [DOI] [PubMed] [Google Scholar]
- Deguchi Y, Sun J, Tauchi Y, Sakai S, Morimoto K. Distribution characteristics of grepafloxacin, a fluoroquinolone antibiotic, in lung epithelial lining fluid and alveolar macrophage. Drug Metab Pharmacokinet. 2003;18:319–326. doi: 10.2133/dmpk.18.319. [DOI] [PubMed] [Google Scholar]
- Eloranta JJ, Meier PJ, Kullak U, Gerd A.Coordinate transcriptional regulation of transport and metabolism. Methods in enzymology Phase II Conjugation Enzymes and Transport Systems 2005Academic Pressx: New York; 511–530.In: Helmut Sies aLP (ed.) [DOI] [PubMed] [Google Scholar]
- Fernandez C, Buyse M, German-Fattal M, Gimenez F. Influence of the pro-inflammatory cytokines on P-glycoprotein expression and functionality. J Pharm Pharm Sci. 2004;7:359–371. [PubMed] [Google Scholar]
- Fernandez-Teruel C, Gonzalez-Alvarez I, Casabo VG, Ruiz-Garcia A, Bermejo M. Kinetic modelling of the intestinal transport of sarafloxacin. Studies in situ in rat and in vitro in Caco-2 cells. J Drug Target. 2005;13:199–212. doi: 10.1080/10611860500087835. [DOI] [PubMed] [Google Scholar]
- Fernandez-Varon E, Ayala I, Marin P, Carrion A, Martos N, Escudero E, et al. Pharmacokinetics of danofloxacin in horses after intravenous, intramuscular and intragastric administration. Equine Vet J. 2006;38:342–346. doi: 10.2746/042516406777749245. [DOI] [PubMed] [Google Scholar]
- Friis C. Penetration of danofloxacin into the respiratory tract tissues and secretions in calves. Am J Vet Res. 1993a;54:1122–1127. [PubMed] [Google Scholar]
- Friis C. Penetration of danofloxacin into the respiratory tract tissues and secretions in calves. Am J Vet Res. 1993b;54:1122–1127. [PubMed] [Google Scholar]
- Friis C, Nielsen JP. Penetration of danofloxacin into the respiratory tract tissues and secretions in healthy and Actinobacillus pleuropneumoniae infected pigs. J Vet Pharmacol Ther. 1997;20:87–109. [Google Scholar]
- Garcia MA, Solans C, Aramayona JJ, Rueda S, Bregante MA. Development of a method for the determination of danofloxacin in plasma by HPLC with fluorescence detection. Biomed Chromatogr. 2000;14:89–92. doi: 10.1002/(SICI)1099-0801(200004)14:2<89::AID-BMC931>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Gekeler V, Ise W, Sanders KH, Ulrich WR, Beck J. The leukotriene LTD4 receptor antagonist MK571 specifically modulates MRP associated multidrug resistance. Biochem Biophys Res Commun. 1995;208:345–352. doi: 10.1006/bbrc.1995.1344. [DOI] [PubMed] [Google Scholar]
- Griffiths NM, Hirst BH, Simmons NL. Active intestinal secretion of the fluoroquinolone antibacterials ciprofloxacin, norfloxacin and pefloxacin; a common secretory pathway. J Pharmacol Exp Ther. 1994;269:496–502. [PubMed] [Google Scholar]
- Ito T, Yano I, Tanaka K, Inui K-I. Transport of quinolone antibacterial drugs by human P-glycoprotein expressed in a kidney epithelial cell line, LLC-PK1. J Pharmacol Exp Ther. 1997;282:955–960. [PubMed] [Google Scholar]
- Jonker JW, Merino G, Musters S, Van Herwaarden aE, Bolscher E, Wagenaar E, et al. The breast cancer resistance protein BCRP (ABCG2) concentrates drugs and carcinogenic xenotoxins into milk. Nat Med. 2005;11:127–129. doi: 10.1038/nm1186. [DOI] [PubMed] [Google Scholar]
- Kalitsky-Szirtes J, Shayeganpour A, Brocks DR, Piquette-Miller M. Suppression of drug-metabolizing enzymes and efflux transporters in the intestine of endooxin-treated rats. Drug Metab Dispos. 2004;32:20–27. doi: 10.1124/dmd.32.1.20. [DOI] [PubMed] [Google Scholar]
- Knoll U, Glunder G, Kietzmann M. Comparative study of the plasma pharmacokinetics and tissue concentrations of danofloxacin and enrofloxacin in broiler chickens. J Vet Pharmacol Ther. 1999;22:239–246. doi: 10.1046/j.1365-2885.1999.00217.x. [DOI] [PubMed] [Google Scholar]
- Langmann T, Mauerer R, Zahn A, Moehle C, Probst M, Stremmel W, et al. Real-time reverse transcription-PCR expression profiling of the complete human ATP-binding cassette transporter superfamily in various tissues. Clin Chem. 2003;49:230–238. doi: 10.1373/49.2.230. [DOI] [PubMed] [Google Scholar]
- Lindecrona RH, Friis C, Nielsen JP. Pharmacokinetics and penetration of danofloxacin into the gastrointestinal tract in healthy and in Salmonella typhimurium infected pigs. Res Vet Sci. 2000;68:211–216. doi: 10.1053/rvsc.1999.0361. [DOI] [PubMed] [Google Scholar]
- Lowes S, Simmons NL. Multiple pathways for fluoroquinolone secretion by human intestinal epithelial (Caco-2) cells. Br J Pharmacol. 2002;135:1263–1275. doi: 10.1038/sj.bjp.0704560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maliepaard M, Scheffer GL, Faneyte IF, Van Gastelen MA, Pijnenborg aCLM, Schinkel aH, et al. Subcellular localization and distribution of the breast cancer resistance protein transporter in normal human tissues. Cancer Res. 2001;61:3458–3464. [PubMed] [Google Scholar]
- Mckellar Q, Gibson I, Monteiro A, Bregante M. Pharmacokinetics of enrofloxacin and danofloxacin in plasma, inflammatory exudate, and bronchial secretions of calves following subcutaneous administration. Antimicrob Agents Chemother. 1999;43:1988–1992. doi: 10.1128/aac.43.8.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mckellar QA, Gibson IF, Mccormack RZ. Pharmacokinetics and tissue disposition of danofloxacin in sheep. Biopharm Drug Dispos. 1998;19:123–129. doi: 10.1002/(sici)1099-081x(199803)19:2<123::aid-bdd89>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Merino G, Alvarez aI, Pulido MM, Molina aJ, Schinkel aH, Prieto JG. Breast cancer resistance protein (BCRP/ABCG2) transports fluoroquinolone antibiotics and affects their oral availability, pharmacokinetics and milk secretion. Drug Metab Dispos. 2006;34:690–695. doi: 10.1124/dmd.105.008219. [DOI] [PubMed] [Google Scholar]
- Morris ME, Zhang S. Flavonoid–drug interactions: effects of flavonoids on ABC transporters. Life Sci. 2006;78:2116–2130. doi: 10.1016/j.lfs.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Nakajima Y, Hattori K, Shinsei M, Matsunaga N, Iizasa H, Sasabe H, et al. Physiologically-based pharmacokinetic analysis of grepafloxacin. Biol Pharm Bull. 2000;23:1077–1083. doi: 10.1248/bpb.23.1077. [DOI] [PubMed] [Google Scholar]
- Naruhashi K, Tamai I, Inoue N, Muraoka H, Sai Y, Suzuki N, et al. Active intestinal secretion of new quinolone antimicrobials and the partial contribution of P-glycoprotein. J Pharm Pharmacol. 2001;53:699–709. doi: 10.1211/0022357011775820. [DOI] [PubMed] [Google Scholar]
- Naruhashi K, Tamai I, Inoue N, Muraoka H, Sai Y, Suzuki N, et al. Involvement of multidrug resistance-associated protein 2 in intestinal secretion of grepafloxacin in rats. Antimicrob Agents Chemother. 2002;46:344–349. doi: 10.1128/AAC.46.2.344-349.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prime-Chapman HM, Fearn RA, Cooper aE, Moore V, Hirst BH. Differential MRP1-6 isoform expression and function in human intestinal epithelial Caco-2 cells. J Pharmacol Exp Ther. 2004;311:476–484. doi: 10.1124/jpet.104.068775. [DOI] [PubMed] [Google Scholar]
- Pulido MM, Molina aJ, Merino G, Mendoza G, Prieto JG, Alvarez aI. Interaction of enrofloxacin with breast cancer resistance protein (BCRP/ABCG2): influence of flavonoids and role in milk secretion in sheep. J Vet Pharmacol Ther. 2006;29:279–287. doi: 10.1111/j.1365-2885.2006.00744.x. [DOI] [PubMed] [Google Scholar]
- Rabbaa L, Dautrey S, Colas-Linhart N, Carbon C, Farinotti R. Stereoselectivity of ofloxacin intestinal transport in the rat. Drugs. 1995;49 Suppl 2:333–334. doi: 10.2165/00003495-199500492-00089. [DOI] [PubMed] [Google Scholar]
- Rabbaa L, Dautrey S, Colas-Linhart N, Carbon C, Farinotti R. Intestinal elimination of ofloxacin enantiomers in the rat: evidence of a carrier-mediated process. Antimicrob Agents Chemother. 1996;40:2126–2130. doi: 10.1128/aac.40.9.2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-García A, Huimin L, Plá-Delfina JM, Ming Hu Kinetic characterization of secretory transport of a new ciprofloxacin derivative ( CNV97100) across Caco-2 cell monolayers. J Pharm Sci. 2002;91:2511–2519. doi: 10.1002/jps.10244. [DOI] [PubMed] [Google Scholar]
- Sasabe H, Kato Y, Suzuki T, Itose M, Miyamoto G, Sugiyama Y. Differential involvement of multidrug resistance-associated protein 1 and P-glycoprotein in tissue distribution and excretion of grepafloxacin in mice. J Pharmacol Exp Ther. 2004;310:648–655. doi: 10.1124/jpet.104.065201. [DOI] [PubMed] [Google Scholar]
- Scheffer GL, Pijnenborg aCLM, Smit EF, Muller M, Postma DS, Timens W, et al. Multidrug resistance related molecules in human and murine lung. J Clin Pathol. 2002;55:332–339. doi: 10.1136/jcp.55.5.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shem-Tov M, Rav-Hon O, Ziv G, Lavi E, Glickman A, Saran A. Pharmacokinetics and penetration of danofloxacin from the blood into the milk of cows. J Vet Pharmacol Ther. 1998;21:209–213. doi: 10.1046/j.1365-2885.1998.00137.x. [DOI] [PubMed] [Google Scholar]
- Sorgel F, Naber KG, Jaehde U, Reiter A, Seelmann R, Sigl G. Gastrointestinal secretion of ciprofloxacin. Evaluation of the charcoal model for investigations in healthy volunteers. Am J Med. 1989;87:62S–65S. doi: 10.1016/0002-9343(89)90025-9. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Kato Y, Sasabe H, Itose M, Miyamoto G, Sugiyama Y. Mechanism for the tissue distribution of grepafloxacin, a fluoroquinolone antibiotic, in rats 10.1124/dmd.30.12.1393. Drug Metab Dispos. 2002;30:1393–1399. doi: 10.1124/dmd.30.12.1393. [DOI] [PubMed] [Google Scholar]
- Taipalensuu J, Tornblom H, Lindberg G, Einarsson C, Sjoqvist F, Melhus H, et al. Correlation of gene expression of ten drug efflux proteins of the ATP-binding cassette transporter family in normal human jejunum and in human intestinal epithelial caco-2 cell monolayers. J Pharmacol Exp Ther. 2001;299:164–170. [PubMed] [Google Scholar]
- Volpe DA. Permeability classification of representative fluoroquinolones by a cell culture method. AAPS PharmSci. 2004;6:e13. doi: 10.1208/ps060213. [DOI] [PubMed] [Google Scholar]
- Von Traeder W, Kleinhaus S. Konzentrationsabhängige dosierung von danofloxacin - ein beitrag zur optimierung der klinischen wirksamkeit und vermeidung der selektion resistenter kieme. Tierärztliche Umschau. 2002;57:102–107. [Google Scholar]
- Wise R, Honeybourne D. A review of the penetration of sparfloxacin into the lower respiratory tract and sinuses. J Antimicrob Chemother. 1996;37 Suppl A:57–63. doi: 10.1093/jac/37.suppl_a.57. [DOI] [PubMed] [Google Scholar]
- Xia CQ, Liu N, Yang D, Miwa G, Gan LS. Expression, localization, and functional characteristics of breast cancer resistance protein in Caco-2 cells. Drug Metab Dispos. 2005;33:637–643. doi: 10.1124/dmd.104.003442. [DOI] [PubMed] [Google Scholar]
- Yamaguchi H, Yano I, Hashimoto Y, Inui KI. Secretory mechanisms of grepafloxacin and levofloxacin in the human intestinal cell line Caco-2. J Pharmacol Exp Ther. 2000;295:360–366. [PubMed] [Google Scholar]
- Yamaguchi H, Yano I, Saito H, Inui K-I. Pharmacokinetic role of P-glycoprotein in oral bioavailability and intestinal secretion of grepafloxacin in vivo. J Pharmacol Exp Ther. 2002;300:1063–1069. doi: 10.1124/jpet.300.3.1063. [DOI] [PubMed] [Google Scholar]
- Zakelj S, Sturm K, Kristl A. Ciprofloxacin permeability and its active secretion through rat small intestine in vitro. Int J Pharm. 2006;313:175–180. doi: 10.1016/j.ijpharm.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Zhao YL, Cai SH, Wang L, Kitaichi K, Tatsumi Y, Nadai M, et al. Possible involvement of P-glycoprotein in the biliary excretion of grepafloxacin. Clin Exp Pharmacol Physiol. 2002;29:167–172. doi: 10.1046/j.1440-1681.2002.03627.x. [DOI] [PubMed] [Google Scholar]