Abstract
Background and purpose:
Cyclosporine and FK506 are thought to act by targeting the Ca2+-dependent protein phosphatase, calcineurin. The aim of the present study was to determine whether cyclosporine and FK506 stabilize mast cells and basophils by interacting with calcineurin.
Experimental approach:
The effects of cyclosporine and FK506 on the IgE-mediated release of histamine from mast cells and basophils were evaluated. The presence of calcineurin in cells was determined by Western blotting. Ca2+-dependent protein phosphatase activities were assessed in cell extracts using a synthetic phosphorylated peptide that is known to serve as a substrate for calcineurin.
Key results:
FK506 was about 100-fold more potent than cyclosporine as an inhibitor of IgE-dependent histamine release from mast cells and basophils. Immunoblotting of solubilized preparations of purified cells demonstrated the presence of calcineurin in mast cells and basophils. In enzyme assays, mast cells expressed approximately 7-fold higher Ca2+-dependent protein phosphatase activity than basophils. Whereas cyclosporine effectively inhibited Ca2+-dependent protein phosphatase activity in cell extracts, FK506 was considerably less effective.
Conclusions and implications:
FK506 and cyclosporine inhibit the stimulated release of histamine from mast cells and basophils. However, the ability of cyclosporine, but not FK506, to inhibit Ca2+-dependent protein phosphatase activity questions whether FK506 stabilizes mast cells and basophils by interacting with calcineurin.
Keywords: protein phosphatases, calcineurin, cyclosporine, FK506, rapamycin, mast cells, basophils
Introduction
The immunosuppressants, cyclosporine and FK506 (also known as tacrolimus), are used in the treatment of autoimmune disorders and for preventing the rejection of transplanted tissues (Borel, 1989). In the therapeutic context, a particularly important action of these drugs is the inhibition of the activation of T-helper lymphocytes. T-helper cells are important orchestrators of immune responses and both cyclosporine and FK506 have been shown to inhibit the generation of cytokines, such as interleukin-2, from these cells (Kincaid, 1995). Stimulation of T-helper cells leads to increases in intracellular Ca2+ which can lead to the activation of the Ca2+- and calmodulin-dependent protein phosphatase, calcineurin (Klee et al., 1998; Rusnak and Mertz, 2000; Crabtree, 2001). Calcineurin dephosphorylates cytosolic nuclear factor of activated T cells (NFAT) and this permits NFAT to enter the nucleus and to interact with response elements to induce the expression of a variety of cytokines (Crabtree, 2001). Both cyclosporine and FK506 block this pathway by inhibiting the activity of calcineurin (Liu et al., 1991; Klee et al., 1998). These compounds do not bind directly to calcineurin but must first bind to proteins known as immunophilins (Liu et al., 1991). Both cyclosporine and FK506 have their own distinct cognate immunophilins referred to as cyclophilin and FK506 binding protein (FKBP), respectively (Schreiber and Crabtree, 1992; Liu, 1993).
Cyclosporine and FK506 may also be potentially useful compounds for the treatment of allergic disorders (Pacor et al., 2004). Attenuation by these drugs of the generation of cytokines such as interleukin-4 (Plath et al., 2003), which is involved in the generation of immunoglobulin (Ig)E (Del-Prete et al., 1988), could be beneficial. In addition to effects on cytokine expression, cyclosporine and FK506 have also been shown to inhibit exocytosis in a variety of cells that may be involved in the mediation of allergies (Kincaid, 1995). In mast cells and basophils, both cyclosporine and FK506 inhibit the antigen-induced release of histamine and the generation of products of arachidonate (Triggiani et al., 1989; Cirillo et al., 1990; Hultsch et al., 1990; De Paulis et al., 1991a, 1991b, 1992; Plath et al., 2003). Because these autacoids are proinflammatory, preventing mediator release from mast cells and basophils could be an additional potential therapeutic benefit of these drugs.
That cyclosporine and FK506 inhibit mast cell and basophil inflammatory responses suggests that calcineurin is important in regulating this activity in these cells. The aim of the present study was to determine whether cyclosporine and FK506 inhibit the IgE-mediated release of histamine from both human lung mast cells and basophils by interacting with calcineurin.
Methods
Buffers
Phosphate-buffered saline (PBS) was employed in these studies. PBS contained (mM): NaCl 137; Na2HPO4·12H2O 8; KCl 2.7; KH2PO4 1.5. PBS-bovine serum albumin (BSA) was PBS which additionally contained: CaCl2·2H2O 1 mM; MgCl2·6H2O 1 mM; glucose 5.6 mM; BSA 1 mg ml−1; DNase 15 μg ml−1. PBS-human serum albumin (HSA) was PBS additionally supplemented with: CaCl2·2H2O 1 mM; MgCl2·6H2O 1 mM; glucose 5.6 mM; HSA 30 μg ml−1. The pH of all PBS buffers was titrated to 7.3.
Hypotonic lysis buffer contained: Tris 50 mM; ethylenediaminetetraacetic acid (EDTA) 1 mM; ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′,-tetraacetic acid (EGTA) 0.1 mM; dithiothreitol 0.5 mM; phenyl methyl sulphonyl fluoride 50 μg ml−1; soybean trypsin inhibitor 50 μg ml−1; leupeptin 5 μg ml−1; aprotinin 5 μg ml−1. The pH was titrated to 8.0.
Western lysis buffer contained: Tris 50 mM; NaCl 150 mM; phenyl methyl sulphonyl fluoride 50 μg ml−1; soybean trypsin inhibitor 50 μg ml−1; leupeptin 5 μg ml−1; aprotinin 5 μg ml−1; Triton X-100 0.5%. The pH was titrated to 8.0.
Preparation of inhibitors and stimuli
FK506 (5 mM), cyclosporine (5 mM) and rapamycin (1 mM) were prepared as stock solutions in dimethyl sulphoxide. Okadaic acid (0.5 mM stock) and calyculin (0.1 mM stock) were prepared in 10% dimethyl sulphoxide. Microcystin was prepared as a 0.1 mM stock solution in 10% methanol. All of these stock solutions were stored at −20°C. Lyophilized polyclonal goat anti-human IgE antibody, was reconstituted in distilled water and stored at 4°C. The drugs were diluted to the desired concentration in buffer just before use. Preliminary experiments indicated that the vehicles used to prepare the drugs had no effect on histamine release or protein phosphatase assays.
Isolation of mononuclear cells and human basophils
Mixed leucocyte preparations were obtained from whole blood by dextran sedimentation. Briefly 50 ml of venous blood was mixed with 12.5 ml of 6% dextran and 5 ml of 100 mM EDTA, then allowed to sediment for 90 min at room temperature. The upper buffy coat layer was removed, cells were recovered by centrifugation (400 × g, 8 min) and washed twice with PBS. These mixed cell preparations were used in the histamine release experiments.
Mononuclear cells were isolated from blood by Percoll density centrifugation according to methods described in detail elsewhere (Weston et al., 1997). Flotation of blood over Percoll density gradients also generated a basophil-enriched (5–15% purity) fraction which was purified further by immunomagnetic bead separations according to the method described (Weston et al., 1997). In brief, the basophil-enriched fraction was incubated (1 h) over ice with monoclonal (IgG2A) mouse anti-human IgE and then incubated (30 min) with Dynal magnetic beads coated with a rat anti-mouse IgG2A antibody at a ratio of beads to cells of 4:1. The magnetic fraction was harvested, using a Dynal MPC-1 magnet and the magnetically adherent cells counted with alcian blue to determine basophil purities (Gilbert and Ornstein, 1975). This fraction typically contained 1–3 × 106 basophils at purities of 91–99%. These purified cells were used to prepare extracts for use in protein phosphatase assays and for immunoblotting.
Isolation and purification of human lung mast cells
Mast cells were isolated from human lung tissue by a modification of the method described by Ali and Pearce (1985). Macroscopically normal tissue from lung resections of patients was obtained with the approval of the Local Research Ethics Committee. The tissue was chopped vigorously for 10 min with scissors in a small volume of PBS buffer and then washed over a nylon mesh (100 μm pore size; Incamesh, Warrington, UK) with 0.5–1 l of PBS buffer to remove lung macrophages. The tissue was reconstituted in PBS-BSA (10 ml per gram of tissue) containing collagenase Ia (0.1 mg ml−1 of PBS-BSA) and agitated by using a water-driven magnetic stirrer immersed in a water bath set at 37°C. The supernatant (containing some mast cells) was separated from the tissue by filtration over nylon mesh. The collagenase-treated tissue was then reconstituted in a small volume of PBS-BSA buffer and disrupted mechanically with a syringe. The disrupted tissue was then washed over nylon gauze with PBS-BSA (300–600 ml). The pooled filtrates were sedimented (400 × g, room temperature, 8 min), the supernatant discarded and the pellets reconstituted in PBS-BSA (100 ml). The pellet was washed twice more. Mast cells were visualized by microscopy using an alcian blue stain (Gilbert and Ornstein, 1975). Of the total cells, 3–13% were mast cells. This method generated 2–9 × 105 mast cells per gram of tissue. Mast cells prepared in this manner were used in mediator release experiments.
For protein phosphatase assays and for immunoblotting, mast cells were purified further by Percoll density centrifugation followed by immunomagnetic bead separations according to methods described elsewhere (Peirce et al., 1997). The protocol (i.e. incubation times, buffers, cell numbers) for immunomagnetic bead separation was essentially the same as that described for basophils except that a monoclonal (IgG1) anti-c-kit antibody and Dynal beads coated with rat anti-mouse IgG1 were employed. These methods generated mast cell purities of 92–99% with cell yields of between 1 and 3 × 106 mast cells.
Mediator release
Mediator release experiments were performed in PBS-HSA buffer. Mast cells and basophils were incubated with or without an immunosuppressant for 5 min, unless otherwise indicated in the text, before challenge with stimulus. Preliminary experiments indicated that extending the incubation time from 5 up to 60 min did not alter the inhibitory effects of drugs. Mediator release was initiated immunologically with anti-IgE. Concentrations of anti-IgE were chosen that elicited about 30% of the total histamine content from cell preparations. In human lung mast cells, this was a fixed concentration (1:300) of anti-IgE but in basophils, which show greater variability in their responses to anti-IgE, the concentration (1:30 000 to 1:3000) varied from donor to donor. Alternatively, cells were activated non-immunologically with ionophore A23187 (0.2 μM). Stimulus-induced secretion was allowed to proceed for 25 (mast cells) or 45 (basophils) min at 37°C after which time the cells were pelleted by centrifugation (400 × g, room temperature, 4 min). Histamine released into the supernatant was determined by the modified (Ennis, 1991) automated fluorometric method of Siraganian (1974). Total histamine content was determined by lysing aliquots of the cells with perchloric acid at a final concentration of 1.6%. Cells incubated in buffer alone served as a measure of spontaneous histamine release, which ranged from 2 to 8% of the total histamine content. None of the drugs used in this study influenced spontaneous histamine release. Histamine release was thus expressed as a percentage of the total histamine content after subtracting the spontaneous histamine release. All assays were performed in duplicate.
Immunoblotting
Cell extracts were prepared for use in immunoblotting according to methods described elsewhere (Fruman et al., 1992). Purified cells were incubated (10 min on ice) with Western lysis buffer (10 μl per 1 × 106 cells) and then the lysates clarified by centrifugation (13 000 × g, 3 min). The lysates were subjected to polyacrylamide gel electrophoresis in the presence of sodium dodecyl sulphate using 15% gels and then the separated proteins transferred (14 h) electrophoretically to nitrocellulose membranes. Membranes were probed with a monoclonal antibody (IgG1 from clone VA1) to calcineurin B followed by incubation with secondary antibody. Protein bands were visualized by the addition of enhanced chemiluminescence (ECL) reagents and signals detected on ECL film. Relevant bands were subjected to densitometry (Shimadzu CS-9001 Densitometer).
Protein phosphatase assays
Lysates of purified cell preparations, for use in protein phosphatase assays, were prepared as described elsewhere (Fruman et al., 1992). Purified cells were resuspended in hypotonic lysis buffer (1 × 106 cells in 10 μl) and disrupted by three cycles of freeze–thawing. Following centrifugation (13 000 × g, 10 min), supernatants were snap frozen in liquid nitrogen and stored at −80°C for use at a later date.
A peptide of 19 amino acids (DLDVPIPGRFDRRVSVAAE) corresponding to the sequence in the RII regulatory subunit of cAMP-dependent protein kinase (PKA) that contains the autophosphorylation site was used as the substrate for calcineurin (Fruman et al., 1992). The RII peptide was phosphorylated by incubating the peptide (400 μM) at 37°C for 100 min in the presence of 100 mM Tris-HCl (pH 7.4), 0.8 mM [γ32P]ATP (specific activity, 8 × 105 c.p.m. nmol−1), 16 mM MgCl2 and 100 U ml−1 catalytic subunit of PKA. The phosphopeptide was isolated from the reaction mixture by absorption on Dowex AG1-X8 anion exchange resin and elution with 30% acetic acid.
Assays were performed in a total reaction volume of 60 μl containing cell extract (0.5 × 106 cell equivalents), assay buffer (50 mM Tris-HCl at pH 7.4, 0.1 M NaCl, 1 mM dithiothreitol and 0.1 mg ml−1 BSA), phosphopeptide (5 μM) and Ca2+. Inhibitors such as EGTA, cyclosporine, FK506, microcystin, okadaic acid and calyculin were also added to the reaction mixture as appropriate. The reaction was initiated by the addition of cell extract (20 μl) and at times 2.5 and 5 min, aliquots (25 μl) of the reaction mixture were removed and added to 175 μl of ice-cold potassium phosphate (100 mM) in 12.5% trichloroacetic acid and kept on ice for 10 min. The samples were then centrifuged (13 000 × g, 3 min) and an aliquot (180 μl) of the supernatant was loaded on to a Dowex AG-50W cation-exchange column (1 ml). Free 32P, liberated from the peptide by protein phosphatase, did not bind to the column and was eluted with 1 ml (2 × 0.5 ml) of water. The eluted radiolabel was dissolved in scintillant (Optiphase, Fisons, Loughborough, UK) and counted (Beckman LS 5000 SE scintillation counter). Liberated 32P was corrected for 32P in blank incubations carried out in the absence of protein phosphatase. Linear rates of 32P release were observed over 5 min. In addition to the methods described above, protein phosphatase activity was also assessed using a commercially available QuantiZyme assay system (Biomol Research Laboratories Inc., Plymouth Meeting, PA, USA). The kit was used according to the manufacturer's instructions.
Data analysis
Maximal responses (Emax) and potencies (pEC50) were determined by nonlinear regression analysis (GraphPad Prism, version 3.0a). To determine whether there was any difference in the responses after treatments with drugs, repeated measures analysis of variance was performed.
Materials
The following were purchased from the sources indicated; anti-human IgE, aprotinin, BSA, collagenase, dimethyl sulphoxide, DNase, Dowex AG-50W, Dowex AG1-X8, dithiothreitol, HSA, leupeptin, Percoll, phenyl methyl sulphonyl fluoride, soybean trypsin inhibitor, Tween 20, Triton X-100 (all Sigma, Poole, UK); EDTA, calcium chloride and magnesium chloride (BDH, Poole, UK); rapamycin (Calbiochem, Nottingham, UK); microcystin (Gibco BRL, Dundee, UK); okadaic acid and calyculin (Alexis Corporation, Nottingham, UK); acrylamide, Tris, sodium dodecyl sulphate (Bio-Rad, Hemel Hempstead, UK); monoclonal (IgG1) mouse anti-human c-kit and monoclonal (IgG2A) mouse anti-human IgE (Immunotech, Marseilles, France); magnetic beads coated with rat anti-mouse IgG1 or IgG2A antibody (Dynal, Wirral, UK); ECL reagents, ECL film, nitrocellulose membranes (Amersham, Little Chalfont, UK); monoclonal (IgG1) anti-human calcineurin B antibody (Upstate Biotechnology Inc., NY, USA); anti-mouse IgG1 (Serotec, Oxford, UK).
The RII peptide was a kind gift from Dr C Hollis, AstraZeneca, Loughborough, UK. FK506 was a kind gift from Dr K Murato, Fujisawa GmbH, Munich, Germany. Cyclosporine was a gift from Dr J Maddox, Sheffield University, UK.
Results
Functional studies
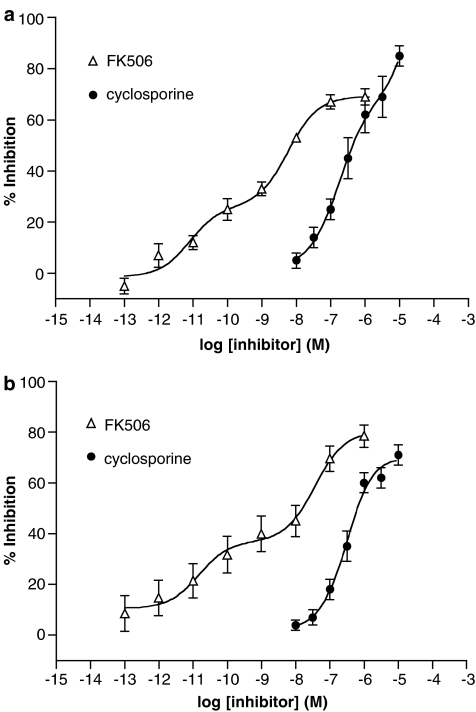
The effects of cyclosporine and FK506 on histamine release induced by anti-IgE from mast cells and basophils were assessed (Figure 1). Both cyclosporine and FK506 inhibited histamine release in a concentration-dependent manner and FK506 was 80- to 120-fold more potent than cyclosporine in basophils and mast cells, respectively (Table 1). The inhibitory effects of FK506, unlike those of cyclosporine, appeared to be biphasic.
Figure 1.
Effect of FK506 and cyclosporine on histamine release from (a) mast cells and (b) basophils. Cells were incubated (5 min) with either inhibitor before activation with anti-IgE. Results are expressed as the % inhibition of the control histamine releases which were 27±3% (mast cells) and 28±3% (basophils). Statistically significant (P<0.01) levels of inhibition were obtained for FK506 (⩾0.01 nM) and cyclosporine (⩾0.1 μM). Values are means±s.e.m. for eight (mast cells) and 10 (basophils) experiments.
Table 1.
Potency (pEC50) and efficacy (Emax) of FK506 and cyclosporine as inhibitors of IgE-mediated histamine release from mast cells and basophils
|
FK506 |
Cyclosporine |
|||
|---|---|---|---|---|
| Emax (%) | pEC50 | Emax (%) | pEC50 | |
| Basophils | 76±3 | 8.5±0.2 | 70±4 | 6.6±0.1 |
| Mast cells | 69±4 | 8.7±0.3 | 80±4 | 6.6±0.2 |
Abbreviation: Ig, immunoglobulin.
Values shown are for the inhibition of IgE-mediated histamine release from mast cells and basophils by FK506 (10−13 to 10−6 M) and cyclosporine (10−8 to 10−5 M). Cells were incubated for 5 min with or without an inhibitor before challenge with anti-IgE. Control histamine releases ranged from 23±3 to 30±4%. Data are means±s.e.m. and are based on 17 (mast cells) to 25 (basophils) experiments for FK506 and 13 (mast cells) to 14 (basophils) experiments for cyclosporine.
In addition to effects on IgE-mediated histamine release, the effects of FK506 and cyclosporine on histamine release from mast cells and basophils induced by the Ca2+-ionophore, A23187, were also studied. Both compounds inhibited histamine release induced by ionophore A23187 in a concentration-dependent manner. FK506 was roughly equipotent as an inhibitor of ionophore A23187-induced and IgE-dependent histamine release whereas cyclosporine was about 10-fold more potent as an inhibitor of ionophore A23187-induced release than that mediated by anti-IgE (Tables 1 and 2).
Table 2.
Potency (pEC50) and efficacy (Emax) of FK506 and cyclosporine as inhibitors of ionophore A23187-induced histamine release from mast cells and basophils
|
FK506 |
Cyclosporine |
|||
|---|---|---|---|---|
| Emax (%) | pEC50 | Emax (%) | pEC50 | |
| Basophils | 97±3 | 8.3±0.1 | 98±2 | 7.5±0.3 |
| Mast cells | 56±5 | 8.7±0.3 | 69±6 | 7.3±0.3 |
Values shown are for the inhibition of ionophore A23187-mediated histamine release from mast cells and basophils by FK506 (10−10 to 10−6 M) and cyclosporine (10−9 to 10−5 M). Mast cells and basophils were incubated for 5 min with or without an inhibitor before challenge with ionophore A23187 (0.2 μM). Control histamine releases ranged from 27±5 to 40±5%. Data are means±s.e.m. and are based on four (basophils) to 10 (mast cells) experiments for FK506 and on eight (basophils) to 14 (mast cells) experiments for cyclosporine.
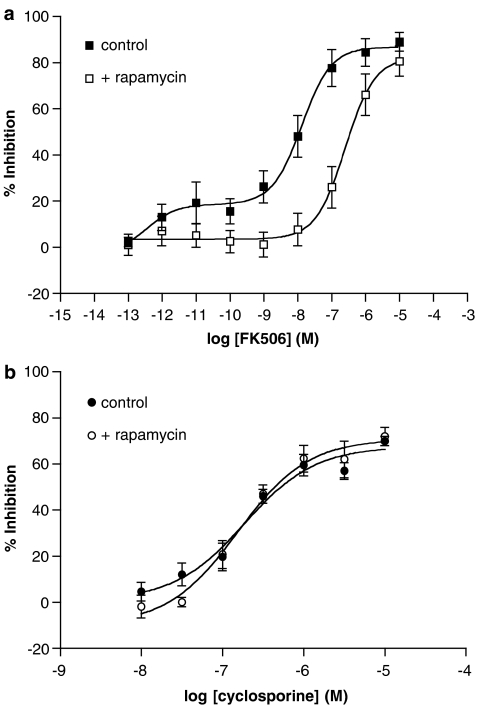
The immunosuppressant, rapamycin, has been shown to act as an antagonist of the actions of FK506 at calcineurin by interacting with FKBP (Dumont et al., 1994). The epitopes created by the FK506–FKBP complex are distinct from those of the rapamycin–FKBP complex, and hence interact with different effector proteins (Bierer et al., 1990). Preliminary studies demonstrated that rapamycin, at lower concentrations (10−9 to 10−6 M), had no significant (P>0.05) effect on the IgE-mediated release of histamine from mast cells and basophils although rapamycin, at a higher concentration (10−5 M), did inhibit release of histamine from mast cells and basophils (22±3 and 44±15% inhibition, respectively) to a significant (P<0.05) degree (n=7–9, data not shown). The effects of rapamycin (3 × 10−7 M) on the inhibitory effects of FK506 (10−13 to 10−5 M) and cyclosporine (10−8 to 10−5 M) were then investigated. Rapamycin antagonized the inhibitory effects of FK506, but not those of cyclosporine, in basophils (Figure 2). Similar results were observed in mast cells wherein rapamycin antagonized the inhibitory effects of FK506 but not those of cyclosporine (data not shown, n=4). In both cell types, FK506 was about 50-fold less potent in the presence of rapamycin.
Figure 2.
Effect of rapamycin on the (a) FK506 and (b) cyclosporine inhibition in basophils. Cells were incubated (15 min) with or without rapamycin (300 nM) and then together with or without either FK506 or cyclosporine before activation with anti-IgE. Values are expressed as the % inhibition of the control histamine releases that were 29±3 and 23±3% in the absence and presence of rapamycin, respectively. Values are means±s.e.m. for four experiments.
Immunoblotting
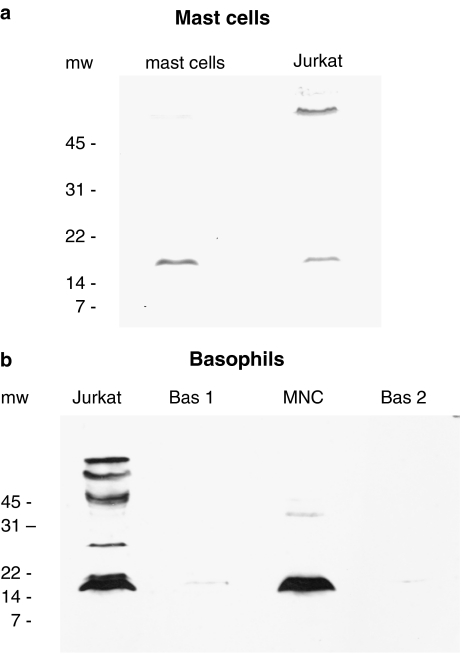
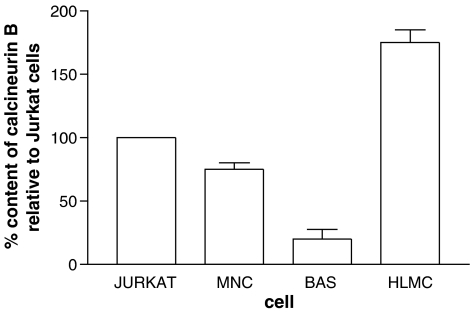
Immunoblotting experiments were performed in order to establish whether calcineurin was present in basophils and mast cells. Calcineurin exists as a heterodimer of calcineurin A (catalytic subunit) and calcineurin B (regulatory subunit). Studies by others using Jurkat cells (a clone of human T lymphocytes) have shown that calcineurin B has a molecular weight of ∼17 kDa (Fruman et al., 1992). For this reason, Jurkat cells were used as internal positive controls in these experiments. Immunoblotting with a monoclonal antibody to calcineurin B identified a protein migrating at about 18 kDa in both mast cells and in Jurkat cell extracts (Figure 3a). Mast cells contained more of this protein. Similar experiments with basophils suggested that these cells have substantially less calcineurin B, on a per cell basis, than either Jurkat cells or mononuclear cells (Figure 3b). Densitometric analysis of these blots indicated that mast cells possessed 1.8-, 2.3- and 9.6-fold more calcineurin B than Jurkat cells, mononuclear cells and basophils, respectively (Figure 4).
Figure 3.
Immunoblot for calcineurin B in (a) mast cells and (b) basophils. (a) Mast cells (99% purity) and Jurkat cells, both at 0.5 × 106 cell equivalents, were probed for calcineurin B. The exposure time was 1 min. The blot is representative of a total of three experiments of identical design. (b) The lanes marked Bas 1 and Bas 2 represent two basophil preparations (both at a purity of 99%) generated from two different donors. The lane marked MNC represents mononuclear cells derived from donor, Bas 1. Jurkat cells were also included. All preparations were used at 0.3 × 106 cell equivalents. The exposure time (10 min) was necessarily high in order to permit visualization of the basophil lanes. The blot is representative of a total of four experiments of similar design. Molecular weight (mw; kDa) markers are indicated.
Figure 4.
Densitometry of immunoblots for calcineurin B. Jurkat cells were included as internal positive controls in all immunoblots performed and the figure shows the quantities of calcineurin B in mast cells (HLMC), mononuclear cells (MNC) and basophils (BAS) relative to those in Jurkat cells. Purities were ⩾95% for both mast cells and basophils. Values are means and vertical lines, s.e.m., n=4 (mononuclear cells and basophils) and n=3 (mast cells).
Protein phosphatase activity
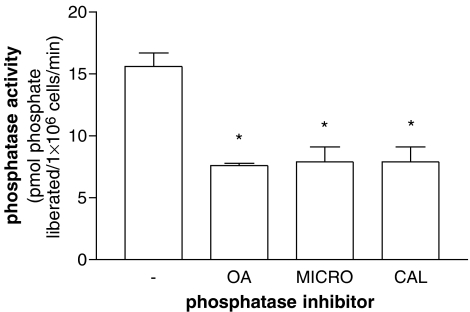
Calcineurin is a Ca2+-dependent protein phosphatase. For this reason, extracts derived from purified cells were assessed for Ca2+-dependent phosphatase activity employing a phosphopeptide (RII peptide) that is a substrate for calcineurin. However, this peptide is also recognized by protein phosphatases that do not require Ca2+ for activity (Fruman et al., 1992). Experiments utilizing okadaic acid, microcystin and calyculin (all at 50 nM) indicated that RII peptide phosphatase activity in mononuclear cell extracts was inhibited by about 50% by all three of these compounds (Figure 5). These compounds are known to be effective inhibitors of both protein serine/threonine phosphatase (PP) 1 and PP2A (Cohen et al., 1990; MacKintosh, 1993; MacKintosh and MacKintosh, 1994) suggesting that either PP1 and/or PP2A might be active against the RII phosphopeptide.
Figure 5.
Effects of phosphatase inhibitors (50 nM) on protein phosphatase activity in mononuclear cell extracts. Dephosphorylation of radiolabelled RII phosphopeptide was followed over 5 min in the presence of Ca2+ (1 mM) and in the absence (−) or presence of okadaic acid (OA), microcystin (MICRO) or calyculin (CAL). Protein phosphatase activity is expressed as the liberation of phosphate from RII peptide per minute per 106 cells. All three phosphatase inhibitors reduced the control protein phosphatase activity to a statistically significant (*P<0.05) degree. Results are means and vertical lines, s.e.m., n=4.
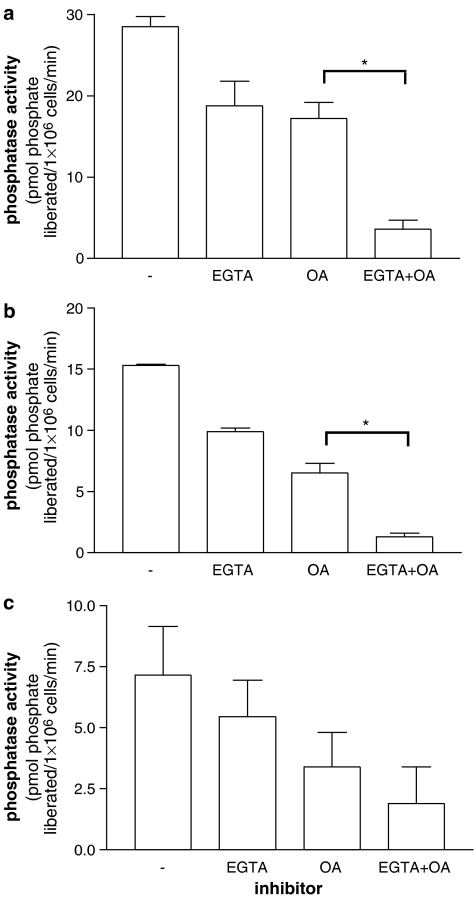
In extracts of mast cells, mononuclear cells and basophils, a Ca2+-dependent protein phosphatase activity was demonstrated by the inhibition of dephosphorylation of the RII peptide by EGTA (5 mM) (Figure 6). Moreover, this inhibition by EGTA was still evident in the presence of okadaic acid (50 nM), which inhibits dephosphorylation of the RII peptide by PP1/PP2A (Figure 6). However, of the three cell types, mast cells possessed the highest levels of Ca2+-dependent protein phosphatase activity and basophils the least. Respective Ca2+-dependent protein phosphatase activities were 12.8±3, 5.3±0.7 and 1.8±0.7 pmol of phosphate liberated per minute by extracts derived from 1 × 106 mast cells, mononuclear cells and basophils. Thus, mast cells possess 2.4- and 7.1-fold more Ca2+-dependent protein phosphatase activity than mononuclear cells and basophils, respectively.
Figure 6.
Ca2+-dependent protein phosphatase activities in (a) mast cells, (b) mononuclear cells and (c) basophils. Dephosphorylation of radiolabelled RII phosphopeptide was followed over 5 min in the presence of Ca2+ (1 mM) and in the absence (−) or presence of EGTA (5 mM) and/or okadaic acid (OA, 50 nM). Protein phosphatase activity is expressed as the liberation of phosphate from RII peptide per minute per 106 cells. Purities were 95±1% (range 92–99%) for mast cells and 97±2% (range 91–99%) for basophils. EGTA reduced the protein phosphatase activity to a statistically significant (*P<0.05) degree in mast cells and mononuclear cells but not in basophils (comparison between protein phosphatase activities in the presence of OA and EGTA+OA). Results are means and vertical lines, s.e.m., n=4. Note that the y axis range of values differs for each panel.
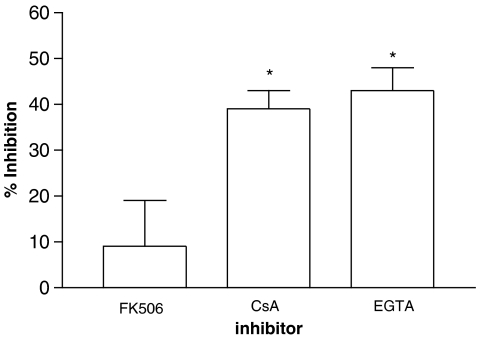
In further experiments, the effects of cyclosporine and FK506 on protein phosphatase activity in cell extracts were investigated. The effects of cyclosporine (10−6 M) and FK506 (10−8 M) on protein phosphatase activity in mononuclear cell extracts were assessed initially. Whereas cyclosporine was almost as effective as EGTA (5 mM) as an inhibitor of the protein phosphatase activity, FK506 was, by comparison, relatively ineffective (Figure 7). In order to determine whether the modest inhibitory effect of FK506 was related to the concentration used, higher concentrations (10−8, 10−7 and 10−6 M) of FK506 were studied. However, both low and higher concentrations of FK506 inhibited phosphatase activity (∼10% inhibition) to the same extent (n=2, data not shown).
Figure 7.
Inhibition of the protein phosphatase activity by immunosuppressants. Mononuclear cell extracts were prepared and dephosphorylation of the RII phosphopeptide was followed in the absence or presence of either FK506 (10 nM), cysclosporine, (CsA, 1 μM) or EGTA (5 mM). EGTA and cyclosporine, but not FK506, inhibited the protein phosphatase activity to a statistically significant (*P<0.05) degree. Results are expressed as the percent inhibition of phosphatase activity. Results are means and vertical lines, s.e.m., n=5.
In these experiments, extracts from lysed cells were prepared and the effects of cyclosporine and FK506 on activity in these extracts determined. In an alternative approach, the mononuclear cells were first pretreated (15 min) with either cyclosporine (10−6 M) or FK506 (10−8 M) before disruption of the cells and assessment of protein phosphatase activities. FK506 was still a relatively ineffective inhibitor (9±7% inhibition; P>0.05), compared to cyclosporine (43±8% inhibition; P<0.05), of the protein phosphatase activity when adopting this experimental protocol (n=5).
The effects of cyclosporine and FK506 on phosphatase activity in mononuclear cell preparations were mimicked in mast cell and basophil preparations. Whereas cyclosporine (10−6 M) inhibited phosphatase activity in extracts prepared from purified basophils (n=4) and mast cells (n=2) by 30–42%, FK506 (10−8 M) inhibited the phosphatase activity by ⩽10%.
Discussion
The results of the present study indicate that cyclosporine and FK506 inhibit the IgE- and non-IgE-dependent release of histamine from mast cells and basophils suggesting that this property may contribute, at least in part, to some of the anti-allergic effects of these immunosuppressive agents. Our findings are in broad agreement with published data regarding the effects of cyclosporine and FK506 on basophils and mast cells (Triggiani et al., 1989; Cirillo et al., 1990; Hultsch et al., 1990; De Paulis et al., 1991a, 1991b, 1992; Plath et al., 2003). FK506 was at least an order of magnitude more potent than cyclosporine at inhibiting the stimulated release of histamine from these cell types. This rank order of activity for the inhibition of histamine release could suggest that these drugs act by attenuating the Ca2+-dependent protein phosphatase, calcineurin. This is because FK506 is more potent than cyclosporine as an inhibitor of isolated calcineurin (Schreiber and Crabtree, 1992; Liu, 1993).
Cyclosporine and FK506 are not thought to interact with calcineurin directly to inhibit phosphatase activity. Rather, cyclosporine and FK506 form complexes with intracellular immunophilin receptors such as cyclophilin and FKBP, respectively, and it is these complexes, rather than the drugs by themselves, that inhibit calcineurin (Liu et al., 1991; Clipstone and Crabtree, 1992). Rapamycin, an immunosuppressant with a different mechanism of action (Jayaraman and Marks, 1993; Morice et al., 1993), may act as a pharmacological antagonist to FK506 as it can bind to the same immunophilin (FKBP12) and block the actions of FK506 (Dumont et al., 1994). Studies by others have shown that rapamycin can antagonize the inhibitory effects of FK506 on IgE-mediated histamine release from basophils and mast cells (De Paulis et al., 1991a, 1991b, 1992). These findings were replicated in the present study as rapamycin antagonized the effects of FK506 in both mast cells and basophils suggesting an involvement of FKBP in the mediation of the effects of FK506.
In further studies, we sought to establish whether calcineurin could be detected in mast cells and basophils. Immunoblotting indicated that the regulatory subunit, calcineurin B, was present in mast cells and basophils. Moreover, protein phosphatase assays using the RII phosphopeptide, a substrate that is recognized by calcineurin, indicated that extracts of mast cells and basophils dephosphorylate this peptide in a Ca2+-dependent manner. Overall, these data indicate that the purported target of cyclosporine and FK506, calcineurin, is present in these cells. However, whether these agents inhibit histamine release by the same mechanism, and whether this involves calcineurin, is open to question.
It seems likely that FK506 acts in a mechanistically similar manner in mast cells and basophils to prevent IgE-mediated histamine release because FK506 was equipotent in both cell types. For the same reasons, cyclosporine may act in a similar manner in both cell types. However, whether both agents act to prevent degranulation by identical mechanisms is open to question. For example, when the effects of these agents were assessed on histamine release induced by the Ca2+ ionophore, A23187, cyclosporine was about 10-fold more potent as an inhibitor of histamine release when compared to effects on IgE-dependent release. By contrast, FK506 was equipotent against release induced by either anti-IgE or ionophore A23187. Moreover, FK506, unlike cyclosporine, appears to inhibit histamine release in a biphasic manner (see Figure 1). Overall, these findings highlight differences between FK506 and cyclosporine as inhibitors of degranulation.
Although both mast cells and basophils appear to express calcineurin B it is of interest that mast cells appear to contain almost 10-fold higher levels of this subunit than basophils, as assessed by densitometry. Furthermore, mast cells contained twofold more of this subunit than either Jurkat cells (T-lymphocyte cell line) or mononuclear cells, about 70% of which would be expected to be lymphocytes and the remainder monocytes. These data indicate that mast cells have higher levels of calcineurin B than T lymphocytes, although caution should be employed when extrapolating results obtained with Jurkat cells to T lymphocytes in general. Although calcineurin, undoubtedly, plays an essential role in T-lymphocyte function (Kincaid, 1995), these cells express low levels of calcineurin in comparison with B lymphocytes (Kincaid et al., 1987). Indeed, it is noteworthy that overexpression of calcineurin in Jurkat cells renders them more resistant to the effects of cyclosporine and FK506 (Clipstone and Crabtree, 1992).
The immunoblotting data presented here are in good agreement with measurements of the Ca2+-dependent protein phosphatase activities as the activity in mast cells is about seven- and twofold higher than that detected in basophils and mononuclear cells, respectively. These data indicate that there is a good correlation between the levels of calcineurin B assessed by immunoblotting and the measured Ca2+-dependent protein phosphatase activity. These findings provide evidence that mast cells contain significant quantities of calcineurin whereas basophils do not express the phosphatase anywhere near as prominently. Despite this large disparity in calcineurin content, it is of interest that the potency of FK506 as an inhibitor of histamine release is similar in both mast cells and basophils and the same holds true for cyclosporine.
In further studies, the effects of cyclosporine and FK506 on protein phosphatase activity in cell extracts were determined. Cyclosporine (1 μM) was substantially more efficient than FK506 (10 nM) at inhibiting the dephosphorylation of RII phosphopeptide by cell extracts. It is interesting, therefore, to note that whereas cyclosporine (1 μM) inhibits histamine release and protein phosphatase activity, FK506 (10 nM) inhibits histamine release but fails to attenuate protein phosphatase activity. These data question whether FK506 inhibits histamine release by interacting with calcineurin.
Studies by others have shown that FK506 can have modest effects on the activity of calcineurin in cell extracts (Kaye et al., 1992; Kung and Halloran, 2000; Kung et al., 2001; Perrino et al., 2002). One possible reason for the greater activity of cyclosporine, compared to FK506, might be related to the content of cognate immunophilins in the cells studied (Kaye et al., 1992; Kung and Halloran, 2000). Therefore, the difference in inhibitory effects of cyclosporine and FK506 on phosphatase activity could be related to limiting levels of FKBP compared to cyclophilins.
Alternative explanations that could account for the relative effectiveness of cyclosporine and FK506 to inhibit dephosphorylation of the RII phosphopeptide may relate to the content of calcineurin A isoforms expressed by cells. Calcineurin is made up of two subunits, calcineurin A (catalytic) and calcineurin B (regulatory) and the existence of different isoforms of calcineurin A (α, β and γ) has been reported (Guerini et al., 1990). The distribution of these isoforms differs among tissues and higher levels of the α than the β isoform of calcineurin A are found in mammalian brain (Kuno et al., 1992) whereas in lymphocytes the converse is true (Jiang et al., 1997). Furthermore, it has been demonstrated that the α isoform is more sensitive to inhibition by FK506-FKBP12 than the β isoform whereas the cyclosporine–cyclophilin complex inhibits both isoforms to a similar degree (Perrino et al., 2002).
Although a preponderance of data argues against a role for calcineurin in the regulation of mast cells and basophils by FK506, the possibility cannot be excluded that, in intact cells, FK506 targets calcineurin. Discrete compartments that are linked to degranulation and that may favourably express calcineurin and cognate immunophilins may exist in the intact cell. FK506 may selectively inhibit calcineurin in these compartments but this activity may not be reflected in a broken cell assay assessing total phosphatase activity.
In summary, the present work has shown that FK506 is more effective than cyclosporine as an inhibitor of histamine release from mast cells and basophils. However, whether either of these agents act by interacting with calcineurin to inhibit histamine release is hard to determine although the evidence is stronger for cyclosporine than FK506 especially as cyclosporine, but not FK506, was an effective inhibitor of Ca2+-dependent protein phosphatase activity. It is noteworthy that a significant body of work is accumulating showing that cyclosporine and FK506 may act via calcineurin-independent pathways (Mori et al., 1997; Salerno et al., 1998; Matsuda and Koyasu, 2000; Price et al., 2003). Notably, it has been reported that cyclosporine–cyclophilin and FK506–FKBP complexes suppress the activation of mitogen-activated protein kinase pathways independently of their interaction with calcineurin in T lymphocytes (Matsuda and Koyasu, 2000). The findings presented here add weight to a growing appreciation that cyclosporine and FK506 act by different mechanisms and not necessarily exclusively through calcineurin.
Acknowledgments
We are grateful to Mr T Locke, Mr G Cooper, Mr D Hopkinson, Mr Zurek, Mr G Rocco, Mr N Vaughan and Mr A Thorpe (Cardiothoracic Surgery), Dr SK Suvarna, Dr P Kitsanta and Dr C Layton (Histopathology) at the Northern General Hospital, Sheffield for their invaluable help in providing lung tissue specimens. The authors also thank Dr Clare Hollis (Astra Charnwood, Loughborough, UK) for the kind gift of RII peptide, which was used in the phosphatase assays and Dr K Murato (Fujisawa GmbH, Munich, Germany) for the kind gift of FK506. This work was supported in part by Asthma UK.
Abbreviations
- ECL
enhanced chemiluminescence
- FK506BP
FK506 binding protein
- HSA
human serum albumin
- NFAT
nuclear factor of activated T cells
- PBS
phosphate-buffered saline
- PKA
cAMP-dependent protein kinase
- PP
protein serine/threonine phosphatase
Conflict of interest
The authors state no conflict of interest.
References
- Ali H, Pearce FL. Isolation and properties of cardiac and other mast cells from the rat and guinea-pig. Agents Actions. 1985;16:138–140. doi: 10.1007/BF01983121. [DOI] [PubMed] [Google Scholar]
- Bierer BE, Matilla PS, Standaert RF, Herzenberg LA, Burakoff SJ, Crabtree G, et al. Two distinct signal transmission pathways in T lymphocytes are inhibited by complexes formed between an immunophilin and either FK506 or rapamycin. Proc Natl Acad Sci USA. 1990;87:9231–9235. doi: 10.1073/pnas.87.23.9231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borel JF. Pharmacology of cyclosporine (Sandimmune) IV. Pharmacological properties in vivo. Pharmacol Rev. 1989;41:259–371. [PubMed] [Google Scholar]
- Cirillo R, Triggiani M, Siri L, Ciccarelli A, Pettit GR, Condorelli M, et al. Cyclosporin A rapidly inhibits mediator release from human basophils presumably by interacting with cyclophilin. J Immunol. 1990;144:3891–3897. [PubMed] [Google Scholar]
- Clipstone NA, Crabtree GR. Identification of calcineurin as a key signalling enzyme in T-lymphocyte activation. Nature. 1992;357:695–697. doi: 10.1038/357695a0. [DOI] [PubMed] [Google Scholar]
- Cohen P, Holmes CF, Tsukitani Y. Okadaic acid: a new probe for the study of cellular regulation. Trends Biochem Sci. 1990;15:98–102. doi: 10.1016/0968-0004(90)90192-e. [DOI] [PubMed] [Google Scholar]
- Crabtree GR. Calcium, calcineurin and the control of transcription. J Biol Chem. 2001;276:2313–2316. doi: 10.1074/jbc.R000024200. [DOI] [PubMed] [Google Scholar]
- De Paulis A, Cirillo R, Ciccarelli A, Condorelli M, Marone G. FK506, a potent novel inhibitor of the release of proinflammatory mediators from human FcɛRI+ cells. J Immunol. 1991a;146:2374–2381. [PubMed] [Google Scholar]
- De Paulis A, Cirillo R, Ciccarelli A, De Crescenzo G, Oriente A, Marone G. Characterisation of the anti-inflammatory effect of FK-506 on human mast cells. J Immunol. 1991b;14:4278–4285. [PubMed] [Google Scholar]
- De Paulis A, Stellato C, Cirillo R, Ciccarelli A, Oriente A, Marone G. Anti-inflammatory effect of FK-506 on human skin mast cells. J Invest Dermatol. 1992;99:723–728. doi: 10.1111/1523-1747.ep12614216. [DOI] [PubMed] [Google Scholar]
- Del-Prete GF, Maggi E, Parronchi P, Chertien I, Tiri A, Macchia D, et al. IL-4 is an essential factor for IgE synthesis induced in vitro by human T cell clones and their supernatants. J Immunol. 1988;140:4193–4198. [PubMed] [Google Scholar]
- Dumont FJ, Kastner C, Iacovone F, Fischer PA. Quantitative and temporal analysis of the cellular interaction of FK-506 and rapamycin in T-lymphocytes. J Pharmacol Exp Ther. 1994;268:32–41. [PubMed] [Google Scholar]
- Ennis M. Current techniques of histamine determination: automated fluorometric assays. Handbook Exp Pharmacol. 1991;97:31–38. [Google Scholar]
- Fruman DA, Klee CB, Bierer BE, Burakoff SJ. Calcineurin phosphatase activity in T lymphocytes is inhibited by FK 506 and cyclosporin A. Proc Natl Acad Sci USA. 1992;89:3686–3690. doi: 10.1073/pnas.89.9.3686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert HS, Ornstein L. Basophil counting with a new staining method using alcian blue. Blood. 1975;46:279–282. [PubMed] [Google Scholar]
- Guerini D, Hubbard MJ, Krinks MH, Klee CB. Multiple forms of calcineurin, a brain isoenzyme of the calmodulin-stimulated protein phosphatase. Adv Second Messenger Phosphoprot Res. 1990;24:242–247. [PubMed] [Google Scholar]
- Hultsch T, Rodriguez JL, Kaliner MA, Hohman RJ. Cyclosporin A inhibits degranulation of rat basophilic leukemia cells and human basophils. J Immunol. 1990;144:2659–2664. [PubMed] [Google Scholar]
- Jayaraman T, Marks AR. Rapamycin-FKBP12 blocks proliferation, induces differentiation, and inhibits cdc2 kinase activity in a myogenic cell line. J Biol Chem. 1993;268:25385–25388. [PubMed] [Google Scholar]
- Jiang H, Xiong F, Kong S, Ogawa T, Kobayashi M, Liu JO. Distinct tissue and cellular distribution of two major isoforms of calcineurin. Mol Immunol. 1997;34:663–669. doi: 10.1016/s0161-5890(97)00054-0. [DOI] [PubMed] [Google Scholar]
- Kaye RE, Fruman DA, Bierer BE, Albers MW, Zydowsky LD, Ho SI, et al. Effects of cyclosporin A and FK506 on Fcɛ receptor type I-initiated increases in cytokine mRNA in mouse bone marrow-derived progenitor mast cells: Resistance to FK506 is associated with deficiency in FK506-binding protein FKBP12. Proc Natl Acad Sci USA. 1992;89:8542–8546. doi: 10.1073/pnas.89.18.8542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kincaid RL. The role of calcineurin in immune system responses. J Allergy Clin Immunol. 1995;96:1170–1177. doi: 10.1016/s0091-6749(95)70202-4. [DOI] [PubMed] [Google Scholar]
- Kincaid RL, Takayama H, Billingsley ML, Sitkovsky MV. Differential expression of calmodulin-binding proteins in B, T lymphocytes and thymocytes. Nature. 1987;330:176–178. doi: 10.1038/330176a0. [DOI] [PubMed] [Google Scholar]
- Klee CB, Ren H, Wang X. Regulation of the calmodulin-stimulated protein phosphatase, calcineurin. J Biol Chem. 1998;273:13367–13370. doi: 10.1074/jbc.273.22.13367. [DOI] [PubMed] [Google Scholar]
- Kung L, Batiuk TD, Palomo-Pinon S, Noujaim J, Helms LMH, Halloran PF. Tissue distribution of calcineurin and its sensitivity to inhibition by cyclosporine. Am J Transplant. 2001;1:325–333. doi: 10.1034/j.1600-6143.2001.10407.x. [DOI] [PubMed] [Google Scholar]
- Kung L, Halloran PF. Immunophilins may limit calcineurin inhibition by cyclosporine and tacrolimus at high drug concentrations. Transplant. 2000;70:327–335. doi: 10.1097/00007890-200007270-00017. [DOI] [PubMed] [Google Scholar]
- Kuno T, Mukai H, Ito A, Chang CD, Kishima K, Saito N, et al. Distinct cellular expression of calcineurin A alpha and beta in rat brain. J Neurochem. 1992;58:1643–1651. doi: 10.1111/j.1471-4159.1992.tb10036.x. [DOI] [PubMed] [Google Scholar]
- Liu J. FK506 and cyclosporin, molecular probes for studying intracellular signal transduction. Immunol Today. 1993;14:290–295. doi: 10.1016/0167-5699(93)90048-P. [DOI] [PubMed] [Google Scholar]
- Liu J, Farmer JD, Lane WS, Friedman J, Weissman I, Schreiber SL. Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP–FK506 complexes. Cell. 1991;66:807–815. doi: 10.1016/0092-8674(91)90124-h. [DOI] [PubMed] [Google Scholar]
- MacKintosh C.Assay and purification of protein (serine/threonine) phosphatases Protein Phosphorylation, A Practical Approach 1993IRL Press: Oxford; 197–230.In: Hardie DG (ed). [Google Scholar]
- MacKintosh C, MacKintosh RW. Inhibitors of protein kinases and phosphatases. Trends Biochem Sci. 1994;19:444–448. doi: 10.1016/0968-0004(94)90127-9. [DOI] [PubMed] [Google Scholar]
- Matsuda S, Koyasu S. Mechanisms of action of cyclosporine. Immunopharmacol. 2000;47:119–125. doi: 10.1016/s0162-3109(00)00192-2. [DOI] [PubMed] [Google Scholar]
- Mori A, Suko M, Kaminuma O, Inoue S, Ohmura T, Hoshino A, et al. IL-2-induced IL-5 synthesis, but not proliferation, of human CD4+ T cells is suppressed by FK506. J Immunol. 1997;158:3659–3665. [PubMed] [Google Scholar]
- Morice WG, Wiederrecht G, Brunn GJ, Siekierka JJ, Abraham RT. Rapamycin inhibition of interleukin-2-dependent p33cdk2 and p34cdc2 kinase activation in T lymphocytes. J Biol Chem. 1993;268:22737–22745. [PubMed] [Google Scholar]
- Pacor ML, Di Lorenzo G, Martinelli N, Mansueto P, Rini GB, Corrocher R. Comparing tacrolimus ointment and oral cyclosporine in adult patients affected by atopic dermatitis: a randomized study. Clin Exp Allergy. 2004;34:639–645. doi: 10.1111/j.1365-2222.2004.1907.x. [DOI] [PubMed] [Google Scholar]
- Peirce MJ, Cox SE, Munday MR, Peachell PT. Preliminary characterization of the role of protein serine/threonine phosphatases in the regulation of human lung mast cell function. Br J Pharmacol. 1997;120:239–246. doi: 10.1038/sj.bjp.0700915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrino BA, Wilson AJ, Ellison P, Clapp LH. Substrate selectivity and sensitivity to inhibition by FK506 and cyclosporin A of calcineurin heterodimers composed of the α or β catalytic subunit. Eur J Biochem. 2002;269:3540–3548. doi: 10.1046/j.1432-1033.2002.03040.x. [DOI] [PubMed] [Google Scholar]
- Plath KES, Grabbe J, Gibbs BF. Calcineurin antagonists differentially affect mediator secretion, p38 mitogen-activated protein kinase and extracellular signal-regulated kinases from immunologically activated human basophils. Clin Exp Allergy. 2003;33:342–350. doi: 10.1046/j.1365-2222.2003.01610.x. [DOI] [PubMed] [Google Scholar]
- Price RD, Yamaji T, Matsuoka N. FK506 potentiates NGF-induced neurite outgrowth via the Ras/Raf/MAP kinase pathway. Br J Pharmacol. 2003;140:825–829. doi: 10.1038/sj.bjp.0705522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusnak F, Mertz P. Calcineurin: form and function. Phys Rev. 2000;80:1483–1521. doi: 10.1152/physrev.2000.80.4.1483. [DOI] [PubMed] [Google Scholar]
- Salerno A, Bonanno CT, Caccamo N, Cigna D, Dominici R, Ferro C, et al. The effect of cyclosporin A, FK506 and rapamycin on the murine contact sensitivity reaction. Clin Exp Immunol. 1998;112:112–119. doi: 10.1046/j.1365-2249.1998.00537.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber SL, Crabtree GR. The mechanism of action of cyclosporin A and FK506. Immunol Today. 1992;13:136–142. doi: 10.1016/0167-5699(92)90111-J. [DOI] [PubMed] [Google Scholar]
- Siraganian RP. An automated continuous-flow system for the extraction and fluorometric analysis of histamine. Anal Biochem. 1974;57:383–394. doi: 10.1016/0003-2697(74)90093-1. [DOI] [PubMed] [Google Scholar]
- Triggiani M, Cirillo R, Lichtenstein LM, Marone G. Inhibition of histamine and prostaglandin D2 release from human lung mast cells by cyclosporin-A. Int Arch Allergy Appl Immunol. 1989;88:253–255. doi: 10.1159/000234801. [DOI] [PubMed] [Google Scholar]
- Weston MC, Anderson N, Peachell PT. Effects of phosphodiesterase inhibitors on human lung mast cell and basophil function. Br J Pharmacol. 1997;121:287–295. doi: 10.1038/sj.bjp.0701115. [DOI] [PMC free article] [PubMed] [Google Scholar]