Abstract
The POTE gene family is composed of 13 highly homologous paralogs preferentially expressed in prostate, ovary, testis and placenta. We produced 10 monoclonal antibodies (MAbs) against 3 representative POTE paralogs: POTE-21, POTE-2γC and POTE-22. One reacted with all 3 paralogs, 6 MAbs reacted with POTE-2γC and POTE-22, and 3 MAbs were specific to POTE-21. Epitopes of all 10 MAbs were located in the cysteine-rich repeats (CRRs) motifs located at the N-terminus of each POTE paralog. Testing the reactivity of each MAb with 12 different CRRs revealed slight differences among the antigenic determinants, which accounts for differences in cross-reactivity. Using MAbs HP8 and PG5 we were able to detect a POTE-actin fusion protein in human testis by immunoprecipitation followed by Western blotting. By immunohistochemistry we demonstrated that the POTE protein is expressed in primary spermatocytes, implying a role in spermatogenesis.
Keywords: POTE paralogs, primary spermatocytes, spermatogenesis
Introduction
The POTE gene family is primate-specific and is expressed in prostate, ovary, testis, placenta and in many cancers [1–3]. The POTE family consists of 13 highly homologous variants dispersed among 8 different chromosomes: 2, 8, 13, 14, 15, 18, 21 and 22. The POTE proteins are made up of amino terminal cysteine-rich repeats (CRRs) of 37 amino acids each, ankyrin repeat motifs of 33 amino acids, and an α helical region similar to spectrins. Each paralog codes for a different number of CRRs and ankyrin repeats. The length of α helical region varies among paralogs and some paralogs do not contain this region. We have recently reported that several members of the POTE gene family contain an actin retroposon inserted at the carboxyl terminus of an ancestral POTE paralog in the process of gene evolution [4]. The POTE-2α and POTE-2γ actin fusion genes are expressed in embryonic stem cells and breast cancer cell lines [4, 5]. However the function of the POTE genes is not yet known. Examination of the expression pattern of the POTE proteins is an important step in order to understand the biological function of the POTE family. To investigate expression of POTE protein, versatile antibodies that are usable for different kinds of experiments are required.
POTE was originally discovered as a gene preferentially expressed in prostate, ovary, testis and placenta by a computer-based screening strategy using EST database [1–2]. Subsequent RT-PCR and in situ hybridization studies confirmed these findings. In a survey of mRNA expression we found POTE paralog expression varied among different tissues and POTE-2γC and POTE-22 were the major transcripts in many cancer cell lines and tissues [3]. For the purpose of detection of POTE proteins, we selected these two major paralog proteins as well as the prototype POTE, POTE-21, as antigens for producing monoclonal antibodies (MAbs).
The first POTE gene discovered is POTE-21, and it is located in chromosome 21 and encodes a protein of 66 kDa, which consists of 3 CRRs and 5 ankyrin repeat motifs followed by spectrin-like α helical region [2]. Both POTE-2γC and POTE-22 have a similar structure to POTE-21 except that they do not contain the α helical region. The POTE-2γC gene is located on chromosome 2 and encodes a protein of 39 kDa, which consists of 3 CRRs and 5 ankyrin repeat motifs. These POTE proteins are associated with the inner aspect of plasma membrane through the CRRs [6]. POTE-22 is located on chromosome 22 and encodes a protein of 34 kDa, which consists of 4 CRRs, and 2 ankyrin repeat motifs. When amino acids 1–130 of these three paralogs are aligned, 95 of 130 (73%) are identical. Because of the high homology, cross-reactivity of MAbs to other paralogs is to be expected. Both cross-reactive and paralog-specific MAbs should be useful, because we will be able to detect general expression with cross-reactive MAbs and paralog-specific expression with others.
Here we describe the production and characterization of 10 MAbs against POTE-21, POTE-2γC and POTE-22. All 10 MAbs worked in both Western blotting and immunofluorescence. The cross-reactivity to other paralogs was examined by ELISA, Western blotting, and immunofluorescence.
Materials and methods
Plasmids
We used 4 vectors for expression of each paralog: pcDNA3 (Invitrogen, Carlsbad, CA) for full-length protein expression in mammalian cells, an Fc fusion vector derived from pSegTag2 (Invitrogen) to make POTE fragments (amino acid 1-130 of each paralog) as fusion proteins with rabbit IgG1 Fc portion in 293T cells [7], pGEX6P-3 (Amersham Biosciences, Piscataway, NJ) to make glutathione S-transferase (GST)-fusion proteins in E. coli, and pEGFP-N1 (Novagen, Madison, WI) to express each CRR as an EGFP-fusion protein. To make the cDNA inserts, the POTE paralog cDNAs were PCR-amplified using appropriate primers containing restriction enzyme sites from the original plasmid clones [2] (The GenBank accession numbers are: POTE-21, AY172978; POTE-2γC, AY462873; POTE-22, AY466021, POTE-2α, AY462871).
Recombinant protein expression in 293T cells
To harvest Fc-fusion proteins in the supernatants and to obtain POTE-expressing cells for Western blot, immunofluorescence or FACS analysis, 293T cells were transfected with each plasmid using Lipofectamine and Plus reagent (Invitrogen) as described previously [7].
Preparation of GST-fusion proteins
GST-fusion expression plasmids were transformed into E. coli GC5 (GeneChoice, Frederick, MD) and the fusion proteins were expressed by inducing with 0.1 mM IsoPropyl β-D-ThioGalactoside for 6 h. All the GST-fusion proteins were expressed as inclusion bodies and washed as previously described [8].
Production of Mabs
Balb/C mice were immunized 3–5 times with 20 μg of proteins or DNA. For POTE-2γC or POTE-22, GST-fusion proteins were i.p. injected after solubilization in 0.5% SDS at 80°C for 10 min and 1:10 dilution with PBS. For POTE-21, POTE-21-DNA in pcDNA3 was i.d. injected and POTE-21-Fc protein was i.p. injected for the final boost immunization. Three days after final boost, the spleen cells were fused with SP2/0-neo myeloma cells as described previously [9]. The hybridomas were screened for secretion of specific MAbs in an ELISA using POTE-21-Fc, GST-POTE-2γC or GST-POTE-22 as the coated antigens. MAbs to the Fc portion or to GST were subtracted by the reactivity with rabbit Fc or GST-PRAC2 in a similar ELISA. The isotype of the MAbs was determined by mouse MAb isotyping reagents (ISO2; Sigma-Aldrich, St. Louis, MO). Ig concentrations in the culture supernatants were determined by a sandwich ELISA. All procedures were conducted in accordance with National Institutes of Health guidelines as approved by the Animal Care and Use Committee of the National Cancer Institute.
ELISA
Two mg/ml of GST-POTE fusion proteins were solubilized in 0.5% SDS at 80°C for 10 min and 1000-fold diluted in PBS just before the coating. For Fc-fusion proteins antigen, goat anti-rabbit IgG was firstly coated then the rabbit Fc-fusion proteins were captured. Incubation with MAbs followed by secondary antibody and substrate was carried out as described previously [9].
Western blotting
Twenty ng of GST-fusion proteins or 20 μg of cell lysates were separated on 4–20% SDS polyacrylamide gels (Bio-Rad, Hercules, CA) under reducing condition. Transfer of proteins to a polyvinylidene diflouride (PVDF) membrane (0.2 μm; Immuno-Blot; Bio-Rad) and the immunostaining by the MAbs were carried out as previously described [9].
Immunofluorescence antibody
293T cells were transfected with a POTE-21, a POTE-2γC or a POTE-22 plasmid. After 24 h, the cells were detached and seeded into 4-well chamber slide (Nalge Nunc) and incubated another 24 h. Fixation and staining using Alexa 594-conjugated goat-anti-mouse IgG (1:1000 dilution; Invitrogen) were performed as previously described [10].
FACS analysis
293T cells were transfected with EGFP fusion expression plasmid for each CRR or 2 or 3 sequential CRR of POTE-21, POTE-22, POTE-2γC, POTE-2α or EGFP vector alone. Forty-eight h after transfection, the cells were harvested, fixed and permeabilized with Fixation/Permeabilization buffer and Permeabilization buffer (eBioscience) according to manufacturer’s instruction. The cells were incubated with each MAb followed by allophycocyanin (APC)–labeled goat anti-mouse IgG F(ab’)2 (Jackson). After washing, the APC-fluorescence associated with transfected cells (EGFP-positive) was measured using a FACSCalibur Flow Cytometer (Becton Dickinson, Franklin Lakes, NJ).
Immunoprecipitation Western blotting
Human testis and liver were obtained from the Cooperative Human Tissue Network (Philadelphia, PA). The frozen tissue was ground in a mortar set and lysed with RIPA buffer (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 5 mM EDTA, 1% Nonidet P-40, 1% deoxycholate, 0.1% SDS, 1 mM PMSF, 1 mg/ml leupeptin, 1 mg/ml aprotinin). After sonication and centrifugation, 4 mg protein was immunoprecipitated by HP8 antibody and detected in Western blotting using PG5 MAb as previously described [4].
Immunohistochemistry
Formalin-fixed, paraffin-embedded human tissues (Cooperative Human Tissue Network) were cut into 5-micron sections and pretreated with microwave antigen retrieval in 10 mM citrate buffer pH6.0 under pressure. Sections were incubated with PG5 antibody at 60 ng/ml overnight at 4°C. The primary antibody was detected by a second reagent “Catalyzed Signal Amplification” for use with mouse primary antibodies (Kit K1500; DakoCytomation, Carpentaria, CA). Daminobenzidine was used as chromogen. 293T cells transfected with POTE expression construct (POTE-2 alpha actin cDNA) were used as a positive control; untransfected 293T cells were used as a negative control. Slides were counterstained with hematoxylin.
In situ hybridization
Sense and anti-sense riboprobes for POTE-21 (GenBank accession no. NM-174981, bps 578-1413) were transcribed and purified with Megascript high yield in vitro transcription kit and MEGA Clear purification kit (Ambien, Austin TX), respectively. Sections were treated with 10 mg/ml proteinase K (Roche) in PBS for permeabilization, fixed in 4% paraformaldehyde, acetylated in 0.1M triethanolamine (Sigma) containing 0.25% acetic anhydride (Sigma). Pre-hybridization were performed in hybridization buffer (50% distilled formamide, 5x SSC; 1% SDS, 50 mg/ml yeast tRNA, 50 mg/ml heparin sodium salt) at 65°C for 1 h, then hybridized in hybridization buffer containing 0.5 ng/ml of probe at 65°C for 18 h. After washing, the bound probe was visualized by alkaline phosphatase conjugated sheep anti-Digoxigenin antibody (Roche) followed by BM purple AP substrate (Roche).
Results
Production of MAbs and their cross-reactivity
We performed 10 fusion experiments and obtained 10 stable hybridomas. The characteristics of the MAbs are summarized in Table 1. All the MAbs reacted with the paralog used for immunization and some showed cross-reactivity with the other 2 paralogs as expected from the high homology.
Table 1.
Characteristics of anti-POTEs MAbs
| Immunogen* | Mab | Subclass† | Reactivity | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ELISA
|
Western blotting
|
IFA‡ |
|||||||||||||||
| GST-POTEs § |
POTEs-Fc# |
GST-POTEs
|
POTEs/293T¶ |
POTEs/293T
|
|||||||||||||
| 21 | 2γC | 22 | 21 | 2γC | 22 | 21 | 2γC | 22 | 21 | 2γC | 22 | 21 | 2γC | 22 | |||
| POTE-2γC | PG5 | 2b | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| PG4 | 2a | − | + | + | − | + | − | − | + | + | − | + | + | − | + | + | |
| PG6 | 1 | − | + | + | − | + | − | − | + | + | − | + | + | − | + | + | |
| PG7 | 2a | − | + | + | − | + | − | − | + | + | − | + | + | − | + | + | |
| PG31 | 2b | − | + | + | − | + | + | − | + | + | − | + | + | − | + | + | |
| POTE-22 | HP8 | 1 | − | + | + | − | + | + | − | + | + | − | + | + | − | + | + |
| HP18 | 1 | − | + | + | − | + | + | − | + | + | − | + | + | − | + | + | |
| POTE-21 | K24 | 2b | + | − | − | + | − | − | + | − | − | + | − | − | + | − | − |
| PA1 | 2b | + | − | − | + | − | − | ND | ND | ND | + | − | − | + | − | − | |
| PA8 | 2a | + | − | − | + | − | − | + | − | − | + | − | − | + | − | − | |
For POTE-2γC and POTE-22, GST-POTE-2γC full-length or GST-POTE-22 full-length fusion proteins were used as immunogens. For POTE-21, DNA plasmid that encode full-length POTE-21 or POTE-21(1-130 amino acids) fragment-rabbit Fc fusion protein were used.
All the MAbs possess κ light chain.
Immunofluorescence antibody.
GST-POTEs full-length fusion proteins were used as antigens.
POTEs (1-130 amino acids) fragment-rabbit Fc fusion protein were used as antigens.
293T cell lysates transfected with full-length POTEs expression vectors.
ND, not determined.
In an ELISA using GST-POTE fusion proteins as the antigen, MAb PG5 reacted with all the three paralogs and the other 4 anti-POTE-2γC MAbs reacted with both GST-POTE-2γC and GST-POTE-22 but not with GST-POTE-21. Two anti-POTE-22 MAbs showed the same pattern. The 3 anti-POTE-21 MAbs were specific to POTE-21. When POTE fragments containing 3 CRR-Fc fusion proteins were used in an ELISA. The patterns of reactivity were the same except that PG4, PG6 and PG7 did not react with the POTE-22 fragment-Fc, indicating that these three MAbs recognize the fourth CRR of POTE-22 and reactivity was confirmed in FACS analysis.
Fig. 1A shows representative Western blots. PG5 detected POTE-2γC, POTE-22 and POTE-21 as major bands around 65 kDa, 60 kDa and 92 kDa, respectively, in GST-POTEs fusion proteins, but not a control fusion protein GST-PRAC2. In a Western blot using 293T cells transfected with POTEs-expression vectors, PG5 bound to POTE-2γC, POTE-22 and POTE-21 at around 39 kDa, 34 kDa and 66 kDa, but not to CD30 (data not shown). Each MAb showed the same specificity as ELISA using GST-fusion proteins with both GST-fusion proteins and POTE proteins expressed in 293T cells (Fig. 1A and Table 1).
Fig. 1.

Reactivity of anti-POTE MAbs to different POTE paralogs in Western blotting and immunofluorescence antibody. (A) Twenty ng of GST-POTE paralogs (lane 1, GST-POTE-2γC; lane 2, GST-POTE-22; lane 3, GST-POTE-21) or GST-PRAC2 (lane 4) fusion proteins were blotted on a PVDF membrane after separation on a 4–20% SDS-PAGE gel under reducing conditions. The proteins were detected with 1.25–2.5 μg/ml of each MAb, followed by alkaline phosphatase-conjugated anti-mouse IgG and BCIP/NBT substrate. (B) 293T cells were transfected with POTE-21, POTE-2γC or POTE-22 plasmid. Twenty-four h after transfection, the cells were detached and seeded into chamber slides. The next day, the cells were fixed in 3.7% formaldehyde and incubated with anti-POTEs MAbs at 5 μg/ml each. Alexa 594-conjugated goat-anti-mouse IgG was used as the secondary reagent. Anti-POTEs staining is seen in red. Nuclei were stained with DAPI in blue.
Fig. 1B shows POTE staining using representative anti-POTEs MAbs in 293T cells transfected with POTE-2γC or POTE-22. All the MAbs bound to POTE proteins with the same cross-reactivity pattern as observed in Western blotting (Table 1). Thus all the MAbs can react with formalin-fixed antigen with the same specificity as in Western blotting. Our immunofluorescence data also showed clear plasma membrane localization of both paralogs is consistent with our previous finding that hemagglutinin-tagged POTE-21 was associated in the plasma membrane in PC-3 cells [10].
Epitope location
To elucidate the epitope location of the anti-POTE MAbs, we prepared GST-fusion proteins of CRR portion and ankyrin repeat domains for each paralog. In an ELISA using GST-POTE-2γC1-130(CRRs), GST-POTE-2γC172-336(Ankirin), GST-POTE-221-167(CRRS) and GST-POTE-22208-273(Ankirin), all the anti-POTE-2γC or POTE-22 MAbs reacted with GST-fusion proteins of CRRs indicating that the epitopes recognized are located in the CRRs of POTE-2γC or POTE-22 (data not shown). To further map the epitope, we tested the reactivity of each MAb with EGFP fusion proteins containing CRRs derived from each POTE paralog (Fig. 2). Representative data is shown in Fig. 2A. MAb PG5 bound to various CRRs in different POTE paralogs. HP8 and HP18 showed somewhat less cross-reactivity. The 3 POTE-21-specific MAbs, K24, PA1 and PA8 did not bind to POTE-21 CRR1. The 10 anti-POTE MAbs can be classified into 5 groups based on reactivity patterns (Fig. 2B, I-V). Since all the MAbs reacted in Western blot with SDS-denatured antigens, their epitopes are located in linear sequences. By the inspection of the sequences of the CRRs, we have indicated the most likely location of the epitopes as shown in Fig. 2C. To find the crucial residues forming each epitope, the following criteria were employed; 1) find common residues in the CRRs with which the group of MAbs reacted, 2) there must be more than three sequential residues, and 3) eliminate common sequential residues in the CRRs with which the group of MAbs did not react. According to the criteria, crucial residues for epitope I, II and IV were SNV, YDD and AMKT, respectively. Although POTE-21 CRR2 contains 4 unique residues, these data were not enough to indicate which one is important for epitope V. Epitope III appeared to crossover two CRRs because PG31 did not recognize any individual CRR tested but did recognize connected CRRs.
Fig. 2.
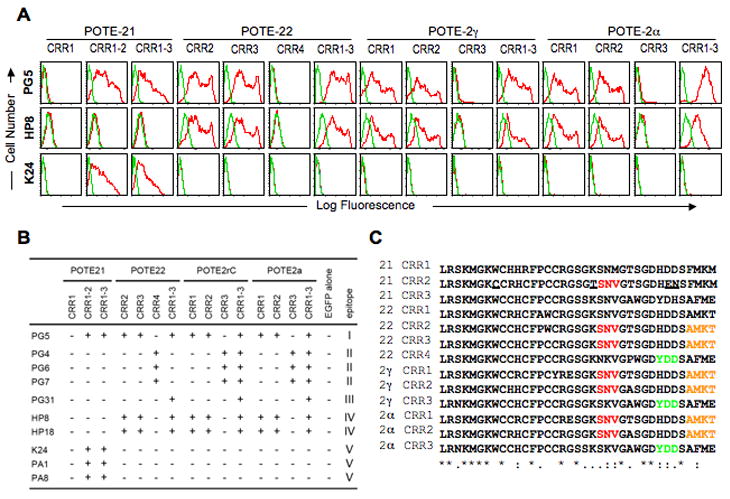
Epitope location of anti-POTE MAbs. (A) Representative FACS data of the anti-POTE MAbs using 293T cells transfected with EGFP fusion expression plasmid for each CRR or 2 or 3 sequential CRRs of POTE-21, POTE-22, POTE-2γC or POTE-2α. Two days after transfection, the cells were stained with 7-AAD, fixed, permeabilized, and incubated with the anti-POTE MAbs, followed by APC–labeled goat anti-mouse IgG F(ab′)2. Live and transfected cells were gated for non-staining with 7-AAD (FL3) and EGFP fluorescence (FL1), respectively. Gated cells were analyzed by APC staining (FL4). Histogram versus log fluorescence (red) with the negative control (green, staining of the cells transfected with EGFP vector alone). (B) Summary of the reactivity of anti-POTE MAbs with CRRs in various POTE paralogs. The patterns of reactivity fell into five groups (I–V). (C) Multiple sequence alignment of each CRR by ClustalW. The crucial residues for epitope I, II and IV, deduced from the criteria described in the text, are marked in red, green and orange, respectively. The unique residues in POTE-21 CRR2 are underlined. POTE-21 CRR3 and POTE-22 CRR1 were not considered because they were not tested.
Expression of POTE protein in human testis
To demonstrate the usefulness of the MAbs for the analysis of endogenous POTE proteins normal human tissue was analyzed. Because POTE-2α, POTE-2γ and POTE-22 are expressed more frequently than POTE-21 in most tissue and tumor samples [3], we chose the combination of HP8 and PG5 for the IP-Western. After immunoprecipitation with HP8 followed by Western blotting with PG5, a band of 140 kDa was detected in testis but not in liver (Fig. 3A). This band size is the same as POTE-2α-actin in POTE-2α-actin-transfected 293T lysates.
Fig. 3.
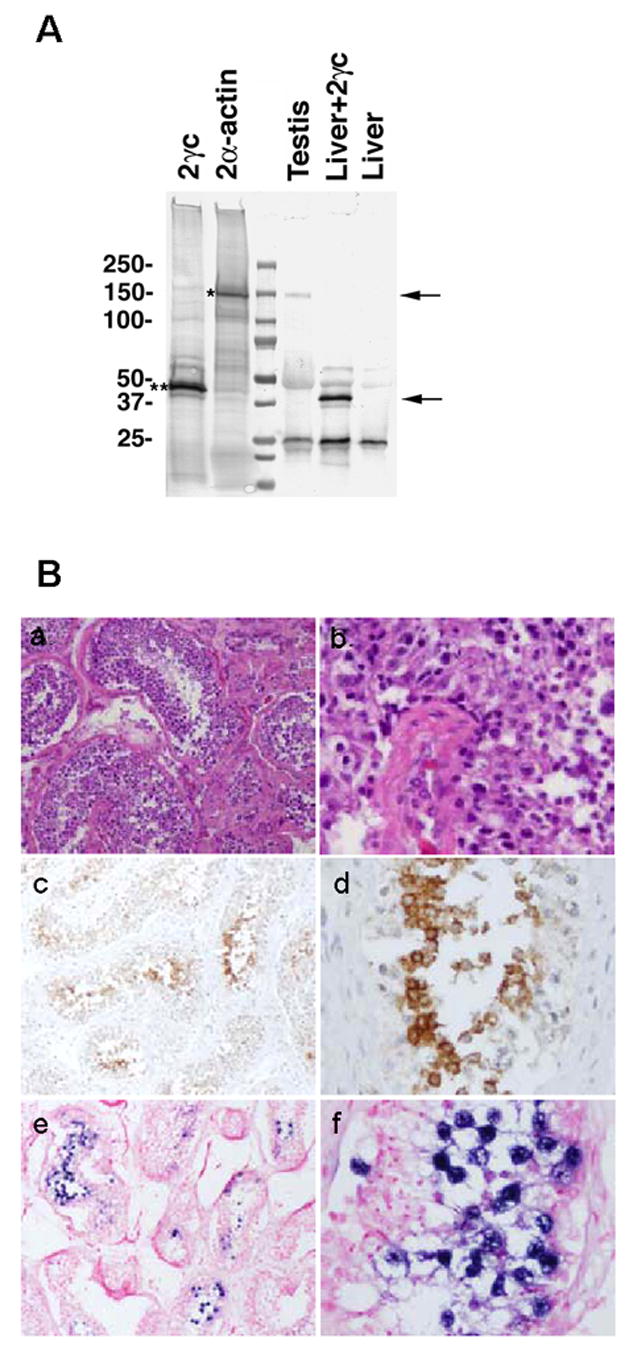
Expression of POTE-2α-actin protein in human testis. (A) Detection of POTE-2α-actin protein by immunoprecipitation. Human testis and liver tissue lysate was subjected to immunoprecipitation by using HP8 antibody. Immunoprecipitate was resolved in a 4–20% PAGE gel, transferred into a PVDF membrane and immunoblotted with PG5 antibody. A specific band of 140 kDa in size (upper arrow) was detected in testis and POTE-2α-actin-transfected 293T (positive control) lysates. (B) Localization of POTE in human testis tissue. H&E staining (a and b), POTE protein localization in immunohistochemistry using PG5 antibody (c and d) and POTE mRNA localization in in situ hybridization (e and f). The positive signal of POTE protein appeared as brown spots using diaminobenzidine. Positive signal of POTE mRNA by anti-sense probe is visualized by alkaline phosphatase conjugated sheep anti-Digoxigenin antibody followed by BM purple AP substrate. Sense probe did not show any signal. a, c and e, × 10; b, d and f, × 40.
To determine the location of POTE protein in normal testis, we performed immunohistochemistry using the PG5 antibody (Fig. 3B). Significant immunostaining was observed in cells with large nuclei within the seminiferous tubules (Fig. 3B–c and d). Based on the morphology and the location of these cells in H&E stained sections (Fig. 3B–a and b), these cells have the properties of primary spermatocytes. Eight different specimens analyzed showed a similar staining pattern (data not shown). In an in situ hybridization study, POTE mRNA was located in the same type of cells in the seminiferous tubules (Fig. 3B–e and f).
Discussion
We produced MAbs that can detect 3 POTE paralogs, POTE-2γC, POTE-22 and POTE-21 in Western blotting and immunofluorescence antibody. These anti-POTE paralog MAbs contain cross-reactive MAbs to other paralogs as well as paralog-specific MAbs. Using cross-reactive MAbs, we detected endogenous POTE protein in normal human testis by immunoprecipitation and immunohistochemistry. POTE protein was detected in primary spermatocytes, indicating that POTE may play a role in a specific stage of spermatogenesis.
Immunofluorescence studies in transfected cells revealed that POTE-2γC and POTE-22 are located in the plasma membrane as well as POTE-21. This result indicates that signals for plasma membrane localization are conserved in these three paralogs. These three paralogs might have some similar function. Besides the plasma membrane localization, the fact that the POTE family bears ankyrin repeats infers that they may be involved in signal transmission across the plasma membrane. Ankyrin repeat motives are protein recognition modules involved in various cellular functions [11]. We plan to use these MAbs to find proteins that binds to POTE family members.
Using GST-fusion proteins of deletion mutants of POTE-2γC or POTE-22, we performed epitope mapping of 7 anti-POTE-2γC or POTE-22 MAbs. Epitopes that all 7 anti-POTEs MAbs recognized were located in the amino terminal portion containing CRRs even though they were produced by immunization with full-length POTE-2γC or POTE-22. Although POTE family genes are primate-specific and POTE orthologous genes in non-primate mammalian species have not been found, we have recently identified a POTE homolog ANKRD26 (ankyrin repeat domain 26) in both mouse and human genomes [12]. Because ANKRD26 bears ankyrin repeat motifs but not CRRs, the mouse may not have self-tolerance to the CRR in POTE paralogs. Since all the MAbs reacted with SDS-denatured antigen in ELISA and Western blotting, these epitopes are not conformational but linear. FACS analysis using EGFP fusion protein for each CRR allowed us to predict some epitope location on amino acid sequences. Since more than 4 to 8 residues are considered to be required to form a linear epitope [13], these residues presumably form each epitope with several adjacent residues. An avidity effect can be expected in PG5, HP8 and HP18 by reacting with multiple CRR in each POTE paralog, which is shown in the FACS data. The combination of PG5 and HP8 was the best choice to screen expression of POTE in human tissue.
Immunoprecipitation followed by Western blot using this combination of MAbs revealed expression of POTE-2α-actin in human testis. The expression was not detected by direct Western blot (data not shown) probably because of the low ratio of expressing cells to total tissue, which was also indicated in an immunohistochemistry that showed the location of POTE-2α-actin in a specific cell type in spermatogenesis, primary spermatocyte. Our preliminary data suggest that POTE proteins are involved in apoptosis (unpublished data). This observation suggests that POTE protein might play a role in spermatogenesis. Up to 75% of normal adult testis germ cells die in the process of programmed cell death before reaching maturity [14].
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research and in part with federal funds from the National Cancer Institute, National Institutes of Health, under contract N01-CO-12400. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. We thank Lawrence Sternberg and Andrew Warner, Molecular Pathology Laboratory, Donna Butcher, Histotechnology Laboratory, SAIC-Frederick, NCI, Susan Garfield and Stephen Wincovitch, Confocal Microscopy Core Facility, NCI, NIH, for technical assistance in confocal microscopy, Barbara J. Taylor, FACS Core Facility, NCI, NIH for technical assistance in flow cytometry and Anna Mazzuca for editorial assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bera TK, Zimonjic DB, Popescu NC, Sathyanarayana BK, Kumar V, Lee B, Pastan I. POTE, a highly homologous gene family located on numerous chromosomes and expressed in prostate, ovary, testis, placenta, and prostate cancer. Proc Natl Acad Sci USA. 2002;99:16975–16980. doi: 10.1073/pnas.262655399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bera TK, Huynh N, Maeda H, Sathyanarayana BK, Lee B, Pastan I. Five POTE paralogs and their splice variants are expressed in human prostate and encode proteins of different lengths. Gene. 2004;337:45–53. doi: 10.1016/j.gene.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 3.Bera TK, Saint Fleur A, Lee Y, Kydd A, Hahn Y, Popescu NC, Zimonjic DB, Lee B, Pastan I. POTE paralogs are induced and differentially expressed in many cancers. Cancer Res. 2006;66:52–56. doi: 10.1158/0008-5472.CAN-05-3014. [DOI] [PubMed] [Google Scholar]
- 4.Lee Y, Ise T, Duc H, Fluer AS, Hahn Y, Liu X, Nagata S, Lee B, Bera TK, Pastan I. Evolution and expression of chimeric POTE actin genes in human genome. Proc Natl Acad Sci USA. 2006;103:17885–17890. doi: 10.1073/pnas.0608344103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bera TK, Saint-Fleur A, Ha D, Yamada M, Lee Y, Lee BK, Hahn Y, Kaufman DS, Pera M, Pastan I. Selective POTE paralogs on chromosome 2 are expressed in human embryonic stem cells. Stem Cells Dev. 2007 doi: 10.1089/scd.2007.0079. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Das S, Ise T, Nagata S, Maeda H, Bera TK, Pastan I. Palmitoylation of POTE family proteins for plasma membrane targeting. Biochem Biophys Res Commun. 2007;363:751–756. doi: 10.1016/j.bbrc.2007.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Onda M, Willingham M, Nagata S, Bera TK, Beers R, Ho M, Hassan R, Kreitman RJ, Pastan I. New monoclonal antibodies to mesothelin useful for immunohistochemistry, fluorescence-activated cell sorting, Western blotting, and ELISA. Clin Cancer Res. 2005;11:5840–5846. doi: 10.1158/1078-0432.CCR-05-0578. [DOI] [PubMed] [Google Scholar]
- 8.Pastan I, Beers R, Bera TK. Recombinant immunotoxins in the treatment of cancer. Methods Mol Biol. 2004;248:503–518. doi: 10.1385/1-59259-666-5:503. [DOI] [PubMed] [Google Scholar]
- 9.Nagata S, Salvatore G, Pastan I. DNA immunization followed by a single boost with cells: a protein-free immunization protocol for production of monoclonal antibodies against the native form of membrane proteins. J Immunol Methods. 2003;280:59–72. doi: 10.1016/s0022-1759(03)00192-3. [DOI] [PubMed] [Google Scholar]
- 10.Maeda H, Nagata S, Wolfgang CD, Bratthauer GL, Bera TK, Pastan I. TARP, a prostate-specific protein localizing in mitochondria. J Biol Chem. 2004;279:24561–24568. doi: 10.1074/jbc.M402492200. [DOI] [PubMed] [Google Scholar]
- 11.Mosavi LK, Cammett TJ, Desrosiers DC, Peng Z. The ankyrin repeat as molecular architecture for protein recognition. Protein Sci. 2004;13:1435–1448. doi: 10.1110/ps.03554604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hahn Y, Bera TK, Pastan IH, Lee B. Duplication and extensive remodeling shaped POTE family genes encoding proteins containing ankyrin repeat and coiled coil domains. Gene. 2006;366:238–245. doi: 10.1016/j.gene.2005.07.045. [DOI] [PubMed] [Google Scholar]
- 13.Berzofsky JA, Berkower IJ. Immunogenicity and antigen structure. In: Paul WE, editor. Fundamental Immunology. Vol. 5. Lippencott, Williams and Wilkins; Philadelphia: 2003. pp. 631–683. [Google Scholar]
- 14.Milligan CE, Schwartz LM. Programmed cell death during animal development. Br Med Bull. 1997;53:570–590. doi: 10.1093/oxfordjournals.bmb.a011631. [DOI] [PubMed] [Google Scholar]


