Abstract
The protein product of the fra-2 gene (Fra-2), a fos-family member, can compete with Fos protein for participation in AP-1 transcription factor complexes and each protein can contribute different transactivational consequences to an AP-1 complex. To date, there is limited characterization of fra-2 mRNA expression in the rat forebrain. We examined basal and restraint-induced mRNA expression (in situ hybridization) of fra-2 in the rat forebrain and compared its temporal-spatial pattern to c-fos. In contrast to the very low basal expression of c-fos, fra-2 basal expression was moderately high throughout cortex and some subcortical structures, including prominent basal expression in the hypothalamic paraventricular nucleus (PVN). Restraint-induced fra-2 expression was quantified in the prefrontal cortex (PFC), lateral septum (LS) and PVN. Maximal fra-2 gene induction in the PFC and LS was delayed (60 min) after restraint onset with respect to c-fos (15 min), whereas in the PVN, fra-2 mRNA increased within 15 min of restraint. Additionally we compared c-fos and fra-2 gene expression in rats given shorter or longer restraint durations, but equal total time from stress onset to sample collection, to determine the extent to which the kinetics of gene induction matched that of a hypothalamic-pituitary-adrenal axis hormone response. Rats given 45 min recovery after 15 min restraint showed less c-fos expression in the PVN, less fra-2 expression in the prelimbic and infralimbic PFC, and no difference in the LS compared with rats restrained for 60 min. Thus, the expression of both genes was sensitive to stressor duration, but this sensitivity varied with brain region. Differential basal and stress-induced expression patterns of the fra-2 and c-fos genes are likely to have important functional consequences for AP-1 transcription factor dependent regulation of neural plasticity.
Keywords: ACTH, CORT, hypothalamus-pituitary-adrenal axis, IEG
Introduction
Inducible immediate-early genes (IEGs), or genes which respond rapidly and transiently to a variety of stimuli (Robertson, 1992), may serve as markers of neuronal activity and thereby aid in our understanding of the neural basis of behavior. Of these IEGs, neuroscientists have paid particular attention to c-fos gene expression (Sheng and Greenberg, 1990). Another member of the fos-family, fra-2, was isolated in 1990 (Nishina et al., 1990) and subsequently characterized in terms of promoter elements and pharmacological expression profile in the chicken embryo (Yoshida et al., 1993) and mouse (Foletta et al., 1994). The general structural organization of the fra-2 gene is very similar to the c-fos gene (Nishina et al., 1990, Yoshida et al., 1993, Foletta et al., 1994). There are some indications, however, that fra-2 gene expression patterns and protein function differ from c-fos, and that fra-2 expression may be especially important for long-term neural and endocrine adaptation to repeated stress (Sabban et al., 2004, Hebert et al., 2005), drugs of abuse (Liu et al., 2005), brain injury (Pennypacker et al., 2000, Butler and Pennypacker, 2004), photoperiod phase shift (Engel et al., 2005), and recovery from sleep deprivation (Terao et al., 2003).
Both physical and psychological stressors produce rapid and transient induction of c-fos gene expression in a variety of rodent brain regions (Kovacs, 1998). These expression patterns have been used to assist with delineating stress-responsive neural circuits (Pacak and Palkovits, 2001). Moreover, the induction of c-fos is believed to be important for coordinating adaptive cellular changes within these structures in response to stress (Herman and Cullinan, 1997). To date, there is very limited characterization of the basal and stimulus-induced spatial-temporal expression pattern of fra-2 in the rat brain. Early northern blot studies revealed readily detectable basal levels of fra-2 in whole mouse brain homogenates, contrasting with the much lower basal expression of other members of the fos family (including c-fos, fra-1, fra-2, and fosB) (Foletta et al., 1994). fra-2 mRNA expression, as detected by in situ hybridization, has only been described for the hippocampus (Beer et al., 1998), and the suprachiasmatic (SCN) and paraventricular nuclei (PVN) of the hypothalamus (Honkaniemi et al., 1994, Schwartz et al., 2000).
In this study we have used in situ hybridizaton to directly compare the time-course of fra-2 and c-fos gene induction in rat forebrain in response to acute psychological stress (restraint). The only other study to examine stress-induced fra-2 mRNA in the brain used systemic injection of capsaicin as its stimulus, and the analysis was restricted to the PVN (Honkaniemi et al, 1994). Additionally the neural response to capsaicin, a putative systemic stressor, may differ substantially from that of processive stressors such as restraint, which require higher-order processing (Herman and Cullinan, 1997). For this study we have examined parallel fra-2 and c-fos gene expression at three rostral-caudal levels of rat forebrain that contain key structures believed to be part of a network of stress-reactive forebrain structures - prefrontal cortex, lateral septum and PVN (Herman et al., 2003). Because of potential differential mechanisms of transcriptional control in the fra-2 and c-fos gene, studying their spatial-temporal expression patterns on adjacent brain sections from the same animals may reveal new information regarding neural recruitment patterns of the brain undergoing stress. An additional component of this study is that we directly compared gene expression in rats at the same point in time after restraint onset, but with different durations of acute stress (continuous versus some period of restraint recovery). This comparison can be informative for both intrinsic gene expression kinetics as well as system-level differences in ongoing neural activity as a result of different durations of restraint.
A portion of this manuscript has appeared previously in abstract form (Weinberg et al., 2005).
Experimental Procedures
Animals
Male Sprague–Dawley rats (Harlan Laboratories, Indianapolis, IN, USA) were allowed a two-week acclimation period after arrival to the animal facilities at the University of Colorado before experimental use (10 weeks old, weight range 282–329 g at experimental onset). Rats were housed in pairs in polycarbonate tubs with wood shavings, and were given food (Purina Rat Chow; Ralston Purina, St. Louis, MO, USA) and tap water ad libitum. The colony room lights were regulated on a 12-h light/dark cycle, with lights on at 07:00 h. Procedures for ethical treatment of animals conformed to the guidelines found in the “Guide for the Care and Use of Laboratory Animals,” DHHS Publication No. (NIH) 80–23, revised 1996 ed. and were approved by the University of Colorado Institutional Animal Care and Use Committee. All efforts were made to minimize the number of animals used and their suffering.
Grouping and Testing
Rats were divided into six groups designated according to their duration of restraint and post-restraint recovery time, respectively: 0–0, 15–0, 15–45, 60–0, 60–120, and 180–0. Group 0–0 rats were taken from their home cages and killed without any restraint exposure. Groups 15–0, 60–0, and 180–0 were taken from their home cages and restrained for 15, 60, or 180 min and then immediately killed. Rats in groups 15–45 and 60–120 were restrained for 15 or 60 min, replaced in their home cages for 45 or 120 min, after which time they were killed. The 15–45 and 60–120 groups were established for comparison to the 60–0 and 180–0 groups, respectively.
Restraint involved taking rats from the home cage and placing them in adjustable length (15.5 +/− 2.5 cm long and 6.3 cm diameter) Plexiglas tubes with air holes in the front, top and back. This stressor is considered to be primarily psychological in nature because it does not produce pain or direct physical insult (Herman and Cullinan, 1997). Restraint took place in a separate room adjacent to the home cage room. The 180–0 group consisted of four rats. Otherwise, all groups consisted of six rats. Cage-mates were restrained simultaneously.
Upon rapid decapitation, brains were collected, flash-frozen in an isopentane bath maintained between −40°C and −30°C and stored at −80°C. Trunk blood was taken in EDTA coated tubes, kept on wet ice and centrifuged at 5000g for ten minutes within forty-five minutes of sacrifice. Plasma was then rapidly frozen and stored at −80°C until hormone assay.
Corticosterone and ACTH radioimmunoassay
Corticosterone (CORT) and ACTH plasma concentrations were measured by radioimmunoassay, as described previously (Girotti et al., 2006). Assay sensitivity for CORT was approximately 0.5 μg/100 ml for a 20 μl sample. The detection limit forACTH was approximately 15 pg/ml for a 50 μl sample.
In situ hybridization histochemistry
Ten micrometer brain sections were cut on a cryostat (Leica model 1850) through the extent of the prefrontal cortex (approximately 3.20 mm anterior to bregma (Paxinos G, 1998)), rostral septum (approximately 0.20 mm anterior to bregma) PVN (approximately 1.80 mm posterior to bregma), thaw-mounted onto poly-L-lysine-coated slides and stored at −80°C. In situ hybridization for c-fos or fra-2 mRNA was performed as described previously (Girotti et al., 2006). For generation of the c-fos probe, plasmids containing a fragment of c-fos cDNA (courtesy of Dr. T. Curran, St. Jude Children’s Research Hospital, Memphis) were used. For the generation of fra-2 ribonucleotide probe, a 450 bp fragment (Exon 4) was amplified from GenBank accession number NM_012954 using the primer set: 5’-gcagaaggagaaggagaag-3’ (forward) and 5’-agagtgggggagttcaag-3’ (reverse). Primer products were cloned into pCR-II TOPO vectors (Invitrogen) to generate RNA probes. Direct sequencing revealed the correct sequence and orientation of the cloned fragment. [35S]-labeled complementary RNA probes were generated using standard in vitro transcription reagents (Promega, Madison, WI, USA). After the hybridization assay procedure, sections were dehydrated and exposed to X-ray film for 1–4 weeks.
Image Analysis
Semi-quantitative analyses of autoradiographs were performed on digitized images from X-ray films (NIH Image) as described (Campeau and Watson, 1997). For the prefrontal cortex, a 25 x 25 pixel square was placed within the prelimbic (PL), infralimbic (IL) or ventral orbital (VO) prefrontal cortex, as determined by using the Paxinos and Watson (Paxinos G, 1998) atlas for guidance. For the lateral septum (LS) and paraventricular nucleus of the hypothalamus (PVN), regions of interest (ROIs) were drawn around the brain structure using the Paxinos and Watson atlas for guidance. The ROIs were first drawn on images with positive signals then saved and positioned on control brain images according to identifiable landmarks. Background areas were chosen in the white matter or in a cell-poor area close to the ROIs. Signal pixels of the brain area of interest were defined as being 3.5 standard deviations above the mean of the set background. The number of pixels and the average pixel values above the set background were then computed and multiplied, giving an integrated densiometric measure of arbitrary units (integrated gray level).
Statistical Analyses
Time course data (groups 0–0, 15–0, 60–0, and 180–0) were analyzed with separate one-way analyses of variance (ANOVA) tests using a Statistical Analysis System (SAS) package for UNIX. When testing group differences in ACTH, CORT, and gene expression (in situ hybridization data), post-hoc tests (Fisher's least significant difference test) were performed to determine points of significant difference between treatment groups and the results are indicated on the data figures. When comparing groups of rats of different stressor durations but same total time from stimulus onset to death (i.e. 15–45 versus 60–0 and 60–120 versus 180–0) groups were compared by two-tailed independent sample t-tests (Microsoft Excel). In all statistical comparisons, an α level of P<0.05 was used to determine statistical significance. All data were log(10) transformed prior to statistical analysis due to heterogeneity of variance. Data presented represent group mean +/− S.E.M.
Results
Effect of restraint on HPA axis hormone secretion
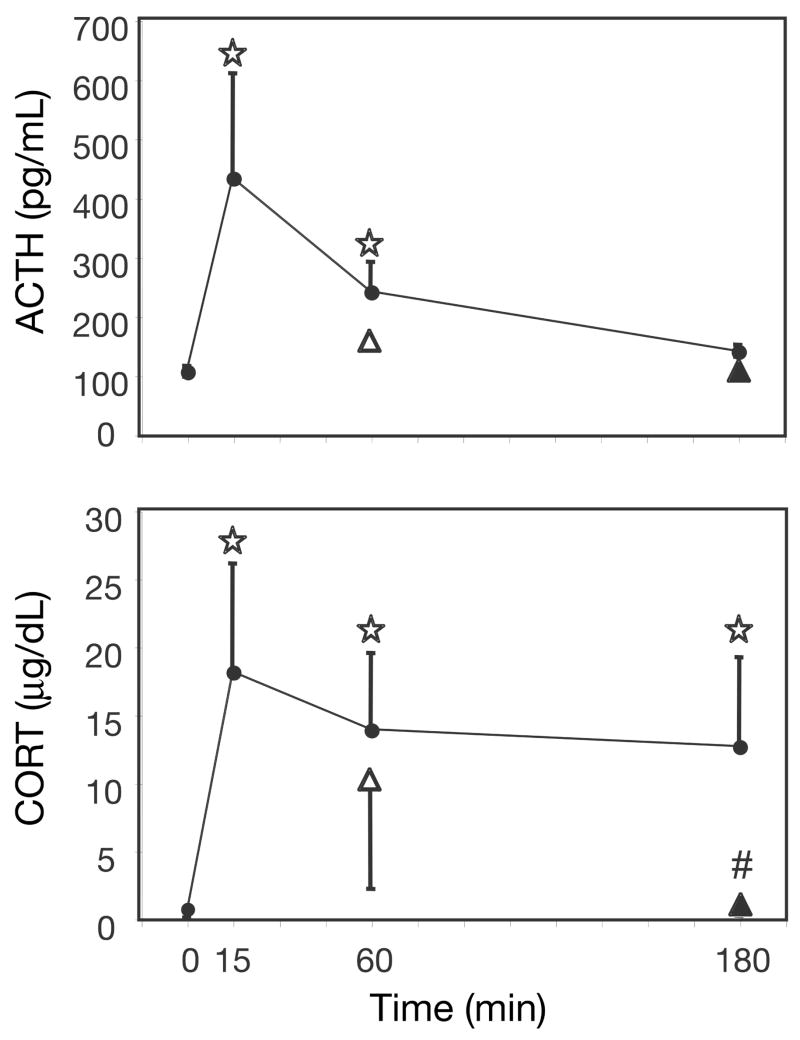
One-way ANOVAs indicate a significant main effect of time on plasma ACTH [F(3,18) = 6.23, p < 0.005], and a significant main effect of time on CORT levels [F(3,18) = 9.79, p < 0.0001]. Post-hoc tests indicate that circulating plasma levels of ACTH and CORT (Fig 1) were significantly higher than control after both 15 and 60 min of restraint. CORT, but not ACTH, was also elevated after 180 min restraint. Rats given a 45 min recovery period (15–45) did not have significantly different plasma levels of ACTH or CORT than rats restrained for the full duration of time (60–0), however rats given 180 min of restraint (180–0) had higher plasma CORT than rats given 60 min of restraint followed by 120 min of recovery (60–120).
Figure 1.
ACTH and CORT plasma levels after different periods of restraint. ACTH (top) and CORT (bottom) were measured in the plasma of rats at 0, 15, 60, or 180 min of restraint stress (●). Two additional data points represent plasma hormone levels of rats given a recovery period post-restraint but equal total time from restraint onset to death. One group was given 15 min of restraint followed by 45 min of recovery (△), and another group was given 60 min of restraint followed by 120 min of recovery (▲).⋆, significantly different from home-cage control (0 min); Fisher’s LSD, P<0.05. #, significantly different from the group given equal total time from restraint onset to death; independent samples t-test, P<0.05.
Basal c-fos and fra-2 expression
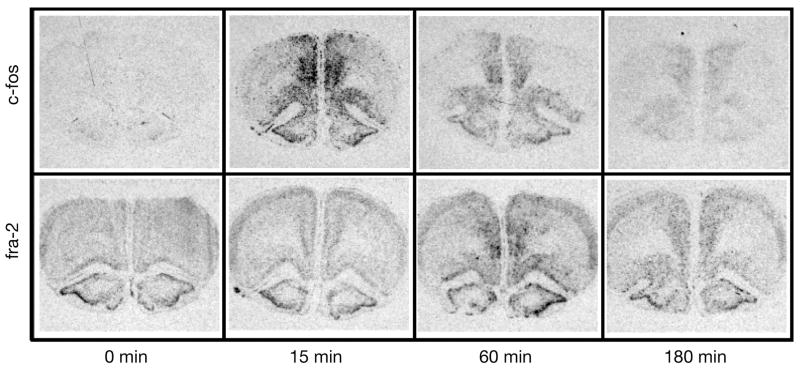
Under no-stress conditions (0–0) there was very little discernable c-fos mRNA expression on coronal sections at the level of the prefrontal cortex, lateral septum (with the exception of some signal within the doral endopiriform nucleus) and the PVN. In contrast, at each rostral-caudal level examined there was clearly visible fra-2 mRNA expression throughout cortex with especially prominent expression in the outer layers of neocortex, in the innermost layer of the prelimbic and infralimbic area and layer two of the piriform cortex (Fig 2). At the level of the lateral septum there was also prominent fra-2 mRNA expression in the shell of the nucleus accumbens and the doral endopirifom nucleus. At the level of the PVN there was also pronounced fra-2 mRNA expression within the parvo and magnocellular regions of the PVN, the supraoptic nucleus and the principal cell body layer of the hippocampus and dentate gyrus. Relative c-fos and fra-2 mRNA levels were quantified for each restraint condition within the prelimbic, infralimbic and ventral orbital regions of prefrontal cortex, the lateral septum and the PVN.
Figure 2.
Basal c-fos and fra-2 mRNA expression in the rat forebrain. Top row illustrates neuroanatomical subregions of coronal sections collected at the rostral-caudal level of the prefrontal cortex (PFC), lateral septum (LS), and hypothalamic paraventricular nucleus (PVN) (adapted from Paxinos and Watson (Paxinos G, 1998)). Numbers indicate regions of interest for densitometry analysis: 1 = prelimbic PFC, 2 = infralimbic PFC, 3 = ventral orbital PFC, 4 = LS, 5 = PVN. Letters indicate additional brain regions with relatively high basal fra-2 mRNA: a = piriform cortex, b = nucleus accumbens shell, c = endopiriform nucleus, d = supraoptic nucleus, e = hippocampus, f = dentate gyrus. The bottom row presents representative autoradiogram images of c-fos expression (left half of each coronal section) and fra-2 expression (right side of each coronal section) for comparison purposes.
Effect of restraint on c-fos and fra-2 expression in the prefrontal cortex
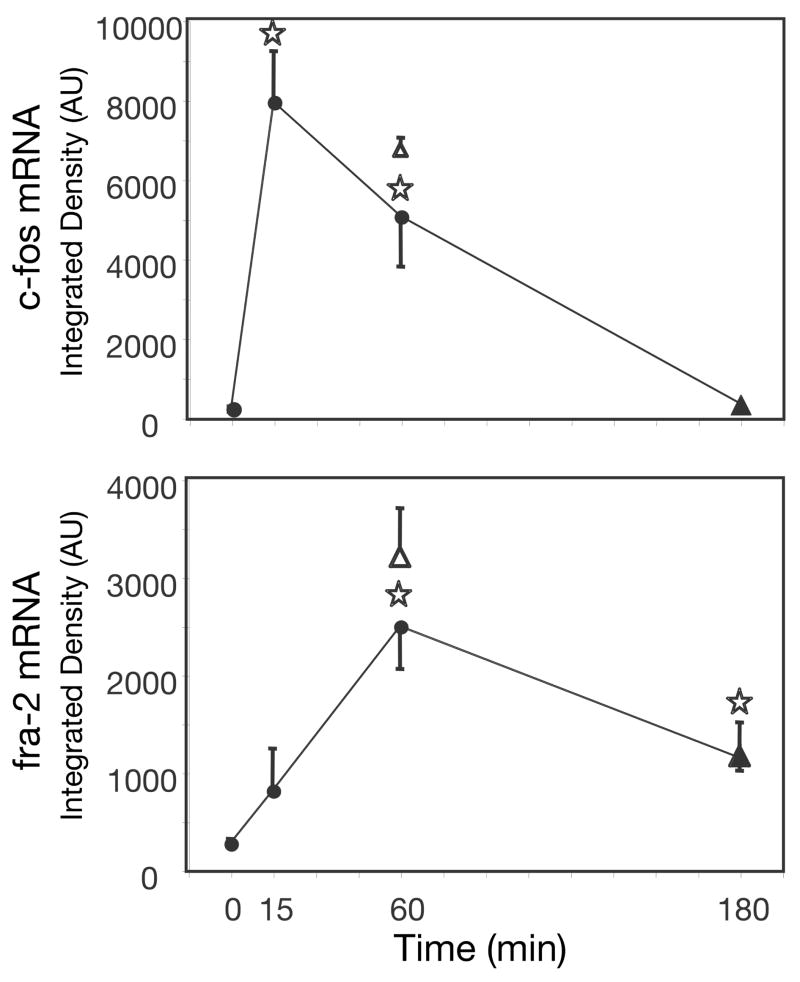
One-way ANOVAs of c-fos mRNA levels indicate a significant effect of time of restraint on c-fos gene expression in the prefrontal cortex: PL [F(3,17) = 63.98, p < 0.0001], IL [F(3,17) = 61.51, p < 0.0001], and VO [F(3,17) = 134.62, p < 0.0001]. Post-hoc tests indicate that c-fos gene expression in the PL, IL, and VO (Fig 3) was significantly greater in rats restrained for 15 min than in home-cage controls, and remained elevated after 60 min of restraint. Although this c-fos gene expression was still significantly higher than basal by 180 min of restraint it had greatly diminished from peak expression levels. Rats given a post-restraint recovery period (15–45 and 60–120) did not have significantly different levels of c-fos expression in the prefrontal cortex than rats restrained for the full duration of time (60–0 and 180–0, respectively).
Figure 3.
c-fos and fra-2 mRNA relative levels in the prefrontal cortex after different periods of restraint. c-fos (top) and fra-2 (bottom) expression were measured in the PL (left), IL (middle), and VO (right) of the prefrontal cortex of rats at 0, 15, 60, or 180 min of restraint stress (●). Two additional data points represent mRNA levels of rats given a recovery period post-restraint but equal total time from restraint onset to death. One group was given 15 min of restraint followed by 45 min of recovery (△), and another group was given 60 min of restraint followed by 120 min of recovery (▲).⋆, significantly different from home-cage control (0 min); Fisher’s LSD, P<0.05. #, significantly different from the group given equal total time from restraint onset to death; independent samples t-test, P<0.05.
One-way ANOVAs of fra-2 mRNA levels indicate a significant main effect of time on fra-2 gene expression in the PL [F(3,16) = 4.95, p < 0.05], a significant main effect in the IL [F(3,16) = 3.30, p < 0.05], and a trend in the VO [F(3,16) = 2.58, p = 0.09]. Post-hoc tests indicate that fra-2 gene expression in the PL, IL and VO (Fig 3) was significantly greater than control expression after 60 min of restraint. This expression declined by 180 min of restraint, however in the PL fra-2 expression at 180 min of restraint was still significantly greater than control. In both the PL and IL prefrontal cortex, rats restrained for 15 min and given a 45 min recovery period (15–45) expressed significantly less fra-2 than rats restrained for the full duration (60–0). Figure 4 shows sample autoradiograms of c-fos and fra-2 expression in the prefrontal cortex for rats given 0–0, 15–0, 60–0, or 180–0 min of restraint.
Figure 4.
Representative autoradiograms of stress-induced c-fos and fra-2 gene expression in the prefrontal cortex. Rats were exposed to 0, 15, 60, or 180 min of restraint. c-fos expression (top) is evident throughout the PL, IL, and VO by 15 min of restraint and has diminished by 180 min of restraint. fra-2 expression (bottom) significantly increases above control levels in the PL, IL, and VO after 60 min of restraint, and diminishes by 180 min of restraint.
Effect of restraint on c-fos and fra-2 expression in the lateral septum
A one-way ANOVA of c-fos mRNA expression indicates a significant effect of time on c-fos gene expression in the lateral septum: LS [F(3,18) = 14.57, p < 0.0001]. Post-hoc tests reveal that c-fos gene expression in the lateral septum (Fig 5) was significantly greater in rats restrained for 15 min than in home-cage controls, and remained elevated after 60 min of restraint. By 180 min of restraint c-fos expression was no longer significantly different than home-cage control expression. Rats given a post-restraint recovery period (15–45 and 60–120) did not have significantly different levels of c-fos expression in the lateral septum than rats restrained for the full duration of time (60–0 and 180–0 respectively). The LS showed a highly significant main effect of time on fra-2 expression: LS [F(3,18) = 14.23, p < 0.0001]. Post-hoc tests reveal that fra-2 gene expression in the lateral septum (Fig 5) was significantly greater than control expression after 60 min of restraint and remained significantly elevated relative to control at 180 min of restraint. Rats given a post-restraint recovery period (15–45 and 60–120) did not have significantly different levels of fra-2 expression in the lateral septum than rats restrained for the full duration of time (60–0 and 180–0, respectively). Figure 6 shows sample autoradiograms of c-fos and fra-2 expression in the lateral septum for rats given 0–0, 15–0, 60–0, or 180–0 min of restraint.
Figure 5.
c-fos and fra-2 mRNA relative levels in the lateral septum (LS) after different periods of restraint. c-fos (top) and fra-2 (bottom) expression were measured in the LS of rats after 0, 15, 60, or 180 min of restraint stress (●). Two additional data points represent mRNA levels of rats given a recovery period post-restraint but equal total time from restraint onset to death. One group was given 15 min of restraint followed by 45 min of recovery (△), and another group was given 60 min of restraint followed by 120 min of recovery (▲).⋆, significantly different from home-cage control (0 min); Fisher’s LSD, P<0.05. #, significantly different from the group given equal total time from restraint onset to death; independent samples t-test, P<0.05.
Figure 6.
Representative autoradiograms of stress-induced c-fos and fra-2 gene expression in the lateral septum. Rats were exposed to 0, 15, 60, or 180 min of restraint. c-fos expression (top) is evident by 15 min of restraint and has diminished by 180 min of restraint. fra-2 expression (bottom) significantly increases above control levels after 60 min of restraint, and diminishes by 180 min of restraint.
Effect of restraint on c-fos and fra-2 expression in the PVN
A one-way ANOVA indicates a significant main effect of time on c-fos gene expression in the PVN: [F(3,17) = 63.03, p < 0.0001]. Post-hoc tests show that c-fos gene expression in the PVN (Fig 7) was significantly greater in rats restrained for 15 min than in home-cage controls, and remained elevated after 60 min of restraint. By 180 min of restraint c-fos expression was significantly reduced from expression at 60 min of restraint. Nonetheless, PVN c-fos expression at 180 min of restraint was greater than expression in control rats. Rats restrained for 15 min and given a 45 min recovery period (15–45) had significantly less c-fos expression in the PVN than rats restrained for the full duration of time (60–0). Rats restrained for 60 min and given a 120 min recovery time (60–120) had a trend (p = 0.07) for less c-fos expression in the PVN than rats restrained for the full duration (180–0). Within the PVN there was also a significant main of time on fra-2 expression: [F(3,17) = 5.16, p = 0.010]. Post-hoc tests reveal that fra-2 gene expression in the PVN (Fig 7) was significantly greater than control expression after 15 min of restraint and remained significantly greater than control at 60 min of restraint. This expression declined by 180 min of restraint. Rats restrained for 15 min and given a 45 min recovery period (15–45) did not have significantly different expression of fra-2 in the PVN than rats restrained for the full duration of time (60–0). Rats given a long recovery period (60–120) had significantly greater levels of fra-2 expression in the PVN than rats restrained for the full duration of time (180–0). Figure 8 shows sample autoradiograms of c-fos and fra-2 expression in the PVN for rats given 0–0, 15–0, 60–0, or 180–0 min of restraint.
Figure 7.
c-fos and fra-2 mRNA relative levels in the hypothalamic paraventricular nucleus (PVN) after different periods of restraint. c-fos (top) and fra-2 (bottom) expression were measured in the PVN of rats at 0, 15, 60, or 180 min of restraint stress (●). Two additional data points represent mRNA levels of rats given a recovery period post-restraint but equal total time from restraint onset to death. One group was given 15 min of restraint followed by 45 min of recovery (△), and another group was given 60 min of restraint followed by 120 min of recovery (▲).⋆, significantly different from home-cage control (0 min); Fisher’s LSD, P<0.05. #, significantly different from the group given equal total time from restraint onset to death; independent samples t-test, P<0.05.
Figure 8.

Representative autoradiograms of stress-induced c-fos and fra-2 gene expression in the hypothalamic paraventricular nucleus. Rats were exposed to 0, 15, 60, or 180 min of restraint. c-fos expression (top) is evident by 15 min of restraint and has diminished by 180 min of restraint. fra-2 expression (bottom) is evident even in home-cage control animals (0 min restraint). fra-2 expression significantly increases above control levels within 15 min of restraint, and diminishes by 180 min of restraint.
Discussion
This study investigated the temporal-spatial expression pattern of the fra-2 gene in rat forebrain in response to restraint. Expression of the fra-2 gene was directly compared to the more widely studied gene family member c-fos. Extensive characterization of restraint-induced c-fos expression in rat forebrain has previously been reported (Imaki et al., 1993, Cullinan et al., 1995, Girotti et al., 2006, Trneckova et al., 2006). We found that c-fos gene expression in the PFC, LS, and PVN peaked at 15 min of restraint, declined some by 60 min of restraint and had reduced to near no-stress levels by 180 min of continuous restraint. A notable difference between fra-2 and c-fos gene expression was the relatively high basal levels of fra-2 mRNA in cortical and some subcortical structures. Under AM basal conditions, fra-2 expression was moderate in the PFC and undetectable in the LS. In the PVN, however, there were high levels of basal fra-2 gene expression. This finding contrasts with the low basal fra-2 expression in the PVN described in a previous study (Honkaniemi et al., 1994). Both studies looked at unhandled adult male Sprague-Dawley rats during the A.M., so the discrepancy is not readily explainable. Because a number of immediate early genes (e.g. c-fos, zif268, NGFI-B) have very low basal expression in the PVN (Rivest and Rivier, 1994, Imaki et al., 1996, Umemoto et al., 1997), the relatively high basal fra-2 gene expression in the PVN may reflect phenotypic specific control of fra-2 basal expression rather than a downstream intracellular signaling response to the tonic presence of an extrinsic signal.
Restraint (60 min) produced a significant increase in fra-2 mRNA in the PFC, septum and PVN. A trend for an increase was apparent in each of these brain regions within 15 min after restraint onset, but this increase was only statistically significant within the PVN. By 180 min of restraint, fra-2 expression returned to near-basal levels in all brain regions examined, although it was still significantly elevated in prelimbic PFC and LS compared to no-stress. Thus, fra-2 gene expression in rat brain appears to have immediate early gene properties, as described in vitro (Nishina et al., 1990), but the delay to reach peak expression may reflect an additional increase secondary to other IEG induction.
Although fra-2 and c-fos are members of the same gene family (fos-family), as described above, basal and restraint-induced temporal induction patterns between the two genes were different. There is considerable structural similarity between promoters of the two genes. Both genes share a number of sequentially ordered putative enhancer elements in the 5’ regulatory region (Yoshida et al., 1993, Foletta et al., 1994, Beer et al., 1998), allowing for the possibility of some overlap in trans-activation profiles. However, there are several key differences in the promoters of the two genes as well. The c-fos promoter contains two cyclic adenosine monophosphate (cAMP) binding protein response (CRE)-like elements upstream of the transcription start site (TATA box) that confers enhancement of c-fos expression via cAMP (Hartig et al., 1991). The fra-2 promoter lacks a TATA box and has only one CRE (Yoshida et al., 1993). The absence of this additional CRE may explain our observed relative lack of immediate (within 15 min of restraint) fra-2 expression compared to c-fos. Interestingly, the fra-2 promoter contains an AP-1 site next to the principal transcription start site (Yoshida et al., 1993) that may allow for trans-activation by other fos-family members participating in an AP-1 transcription factor complex. This may explain the late induction of fra-2 after restraint onset: Fos, the protein product of c-fos mRNA, may confer (via an AP-1 element in the fra-2 promoter) late induction of fra-2.
Differences in fra-2 and c-fos basal and stress-induced gene expression patterns are likely to have important functional consequences. The protein products of both c-fos and fra-2 share five key functional domains, although other protein subregions are much less similar (Nishina et al., 1990). These two proteins compete with one another to form one half of the heterodimeric AP-1 transcription factor complex, leading to changes in downstream expression of genes containing AP-1 elements in their promoter regions (Karin, 1995). Different AP-1 dimer combinations vary in their response element utilization and transactivation potency (Cuevas et al., 2005). For example, an AP-1 complex containing Fra-2 may have much less transactivational efficacy than one containing Fos, depending on the phosphorylation state of Fra-2 (Murakami et al., 1999). Consequently, the presence of Fra-2 in an AP-1 complex may antagonize Fos actions (Herdegen and Leah, 1998).
An additional feature of our study was the direct comparison of restraint duration on HPA axis hormone and gene expression levels at a given point in time after restraint onset. It has been observed before that HPA axis hormone secretion declines over the course of a single session of restraint, with some decrease evident within 60 min of restraint onset and substantial decreases after two hours of continuous restraint (Pecoraro et al., 2006). Although this within restraint-session habituation could be due to an exhaustion of CRH and/or ACTH peptide hormone reserves, one study found that after four hours of continuous restraint the exposure of rats to a novel stressor restored ACTH and corticosterone secretion to initial peak levels, indicating that some neural adaptation had occurred over the four hours of restraint (Dhabhar et al., 1997). It has similarly been observed that c-fos mRNA levels peak relatively soon (15–30 min) after the onset of restraint or immobilization regardless of stressor duration (Umemoto et al., 1997, Trneckova et al., 2007), as was also evident in this study. There is some evidence for c-fos expression to be inhibited through an autoregulatory negative feedback effect (Sassone-Corsi et al., 1988). Thus, the temporal pattern of stress-induced c-fos expression may be limited in the extent to which it reflects sustained neuronal activity. In this study we found that rats that had only 15 min of restraint had somewhat lower ACTH and CORT levels 60 min after restraint onset than rats that were restrained for the entire 60 min. Additionally, rats restrained continuously for 180 min had greater CORT levels than rats restrained for 60 min followed by 120 min of recovery. Rats restrained for only 15 min also had lower c-fos mRNA levels in the PVN and fra-2 mRNA levels in the PFC than rats restrained continuously for 60 min, whereas in the lateral septum restraint duration did not affect gene expression. Because gene expression was greater in rats that were restrained continuously for 60 min than for 15 min, the expression time-course of both these genes must be able to somewhat reflect more than just the first 15 min of neural activation after stress onset. Crane et al (Crane et al., 2005) also observed a greater number of Fos positive CRF cells in the PVN of rats given 60 min of continuous restraint compared to rats given 15 min of restraint. Interestingly, our data suggests that the sensitivity of c-fos and fra-2 expression to stress duration varies with brain region, and not necessarily in a parallel manner.
By 180 min after restraint onset there appears to be substantial within-session neural response habituation, such that in general there were limited differences in hormone or gene expression responses in rats restrained continuously compared to those restrained for only 60 min. This eventual neural habituation to prolonged stressor exposure may account for the failure of another study to see differences in PVN c-fos mRNA levels of rats immobilized continuously for 120 min compared to rats immobilized for just the first 60 min of the 120 min period (Trneckova et al., 2007).
In summary, fra-2, compared to c-fos, has greater basal gene expression in select cortical and subcortical forebrain regions, with notably high basal expression in the PVN. Expression of fra-2 gene expression is further increased by restraint in forebrain cortical and subcortical structures including the PFC, LS, and PVN. The relatively delayed induction pattern of fra-2 compared with c-fos in these brain regions may suggest some divergent dependence of these two genes on cellular factors for trans-activation. The effect of stressor duration on fra-2 and c-fos gene expression also differed across brain regions examined. Given the different transcriptional regulatory effects of Fra-2 or Fos protein participation in an AP-1 complex, these different basal and stress-dependent fra-2 and c-fos gene expression patterns are likely to have important functional consequences for transcriptionally-dependent aspects of neuroplasticity.
Acknowledgments
We would like to thank Dr. Gregory Carey for excellent statistical advice. This work was supported by United States Public Health Service Grants MH075968 and MH065977.
Abbreviations
- ACTH
adrenal corticotropin hormone
- CORT
corticosterone
- CRE
cyclic adenosine monophosphate binding protein response element, HPA axis, hypothalamus-pituitary-adrenal axis
- IEG
immediate-early gene
- IL
infralimbic prefrontal cortex
- LS
lateral septum
- PFC
prefrontal cortex
- PL
prelimbic prefrontal cortex
- PVN
paraventricular nucleus of the hypothalamus
- SCM
sis-conditioned medium
- SCN
suprachiasmatic nucleus
- SRE
serum response element
- VO
ventral-orbital prefrontal cortex
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Beer J, Mielke K, Zipp M, Zimmermann M, Herdegen T. Expression of c-jun, junB, c-fos, fra-1 and fra-2 mRNA in the rat brain following seizure activity and axotomy. Brain Res. 1998;794:255–266. doi: 10.1016/s0006-8993(98)00233-9. [DOI] [PubMed] [Google Scholar]
- Butler TL, Pennypacker KR. Temporal and regional expression of Fos-related proteins in response to ischemic injury. Brain Res Bull. 2004;63:65–73. doi: 10.1016/j.brainresbull.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Campeau S, Watson SJ. Neuroendocrine and behavioral responses and brain pattern of c-fos induction associated with audiogenic stress. J Neuroendocrinol. 1997;9:577–588. doi: 10.1046/j.1365-2826.1997.00593.x. [DOI] [PubMed] [Google Scholar]
- Crane JW, French KR, Buller KM. Patterns of neuronal activation in the rat brain and spinal cord in response to increasing durations of restraint stress. Stress. 2005;8:199–211. doi: 10.1080/10253890500333817. [DOI] [PubMed] [Google Scholar]
- Cuevas BD, Uhlik MT, Garrington TP, Johnson GL. MEKK1 regulates the AP-1 dimer repertoire via control of JunB transcription and Fra-2 protein stability. Oncogene. 2005;24:801–809. doi: 10.1038/sj.onc.1208239. [DOI] [PubMed] [Google Scholar]
- Cullinan WE, Herman JP, Battaglia DF, Akil H, Watson SJ. Pattern and time course of immediate early gene expression in rat brain following acute stress. Neuroscience. 1995;64:477–505. doi: 10.1016/0306-4522(94)00355-9. [DOI] [PubMed] [Google Scholar]
- Dhabhar FS, McEwen BS, Spencer RL. Adaptation to prolonged or repeated stress--comparison between rat strains showing intrinsic differences in reactivity to acute stress. Neuroendocrinology. 1997;65:360–368. doi: 10.1159/000127196. [DOI] [PubMed] [Google Scholar]
- Engel L, Gupta BB, Lorenzkowski V, Heinrich B, Schwerdtle I, Gerhold S, Holthues H, Vollrath L, Spessert R. Fos-related antigen 2 (Fra-2) memorizes photoperiod in the rat pineal gland. Neuroscience. 2005;132:511–518. doi: 10.1016/j.neuroscience.2004.12.014. [DOI] [PubMed] [Google Scholar]
- Foletta VC, Sonobe MH, Suzuki T, Endo T, Iba H, Cohen DR. Cloning and characterisation of the mouse fra-2 gene. Oncogene. 1994;9:3305–3311. [PubMed] [Google Scholar]
- Girotti M, Pace TW, Gaylord RI, Rubin BA, Herman JP, Spencer RL. Habituation to repeated restraint stress is associated with lack of stress-induced c-fos expression in primary sensory processing areas of the rat brain. Neuroscience. 2006;138:1067–1081. doi: 10.1016/j.neuroscience.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Hartig E, Loncarevic IF, Buscher M, Herrlich P, Rahmsdorf HJ. A new cAMP response element in the transcribed region of the human c-fos gene. Nucleic Acids Res. 1991;19:4153–4159. doi: 10.1093/nar/19.15.4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert MA, Serova LI, Sabban EL. Single and repeated immobilization stress differentially trigger induction and phosphorylation of several transcription factors and mitogen-activated protein kinases in the rat locus coeruleus. J Neurochem. 2005;95:484–498. doi: 10.1111/j.1471-4159.2005.03386.x. [DOI] [PubMed] [Google Scholar]
- Herdegen T, Leah JD. Inducible and constitutive transcription factors in the mammalian nervous system: control of gene expression by Jun, Fos and Krox, and CREB/ATF proteins. Brain Res Brain Res Rev. 1998;28:370–490. doi: 10.1016/s0165-0173(98)00018-6. [DOI] [PubMed] [Google Scholar]
- Herman JP, Cullinan WE. Neurocircuitry of stress: central control of the hypothalamo-pituitary-adrenocortical axis. Trends Neurosci. 1997;20:78–84. doi: 10.1016/s0166-2236(96)10069-2. [DOI] [PubMed] [Google Scholar]
- Herman JP, Figueiredo H, Mueller NK, Ulrich-Lai Y, Ostrander MM, Choi DC, Cullinan WE. Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Front Neuroendocrinol. 2003;24:151–180. doi: 10.1016/j.yfrne.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Honkaniemi J, Kononen J, Kainu T, Pyykonen I, Pelto-Huikko M. Induction of multiple immediate early genes in rat hypothalamic paraventricular nucleus after stress. Brain Res Mol Brain Res. 1994;25:234–241. doi: 10.1016/0169-328x(94)90158-9. [DOI] [PubMed] [Google Scholar]
- Imaki T, Shibasaki T, Chikada N, Harada S, Naruse M, Demura H. Different expression of immediate-early genes in the rat paraventricular nucleus induced by stress: relation to corticotropin-releasing factor gene transcription. Endocr J. 1996;43:629–638. doi: 10.1507/endocrj.43.629. [DOI] [PubMed] [Google Scholar]
- Imaki T, Shibasaki T, Hotta M, Demura H. Intracerebroventricular administration of corticotropin-releasing factor induces c-fos mRNA expression in brain regions related to stress responses: comparison with pattern of c-fos mRNA induction after stress. Brain Res. 1993;616:114–125. doi: 10.1016/0006-8993(93)90199-w. [DOI] [PubMed] [Google Scholar]
- Karin M. The regulation of AP-1 activity by mitogen-activated protein kinases. J Biol Chem. 1995;270:16483–16486. doi: 10.1074/jbc.270.28.16483. [DOI] [PubMed] [Google Scholar]
- Kovacs KJ. c-Fos as a transcription factor: a stressful (re)view from a functional map. Neurochem Int. 1998;33:287–297. doi: 10.1016/s0197-0186(98)00023-0. [DOI] [PubMed] [Google Scholar]
- Liu Y, Chen GD, Lerner MR, Brackett DJ, Matsumoto RR. Cocaine up-regulates Fra-2 and sigma-1 receptor gene and protein expression in brain regions involved in addiction and reward. J Pharmacol Exp Ther. 2005;314:770–779. doi: 10.1124/jpet.105.084525. [DOI] [PubMed] [Google Scholar]
- Murakami M, Ui M, Iba H. Fra-2-positive autoregulatory loop triggered by mitogen-activated protein kinase (MAPK) and Fra-2 phosphorylation sites by MAPK. Cell Growth Differ. 1999;10:333–342. [PubMed] [Google Scholar]
- Nishina H, Sato H, Suzuki T, Sato M, Iba H. Isolation and characterization of fra-2, an additional member of the fos gene family. Proc Natl Acad Sci U S A. 1990;87:3619–3623. doi: 10.1073/pnas.87.9.3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacak K, Palkovits M. Stressor specificity of central neuroendocrine responses: implications for stress-related disorders. Endocr Rev. 2001;22:502–548. doi: 10.1210/edrv.22.4.0436. [DOI] [PubMed] [Google Scholar]
- Paxinos GWC. The rat brain in stereotaxic coordinates. Academic Press; San Diego: 1998. [Google Scholar]
- Pecoraro N, Ginsberg AB, Warne JP, Gomez F, la Fleur SE, Dallman MF. Diverse basal and stress-related phenotypes of Sprague Dawley rats from three vendors. Physiol Behav. 2006;89:598–610. doi: 10.1016/j.physbeh.2006.07.019. [DOI] [PubMed] [Google Scholar]
- Pennypacker KR, Eidizadeh S, Kassed CA, O'Callaghan JP, Sanberg PR, Willing AE. Expression of fos-related antigen-2 in rat hippocampus after middle cerebral arterial occlusion. Neurosci Lett. 2000;289:1–4. doi: 10.1016/s0304-3940(00)01250-7. [DOI] [PubMed] [Google Scholar]
- Rivest S, Rivier C. Stress and interleukin-1 beta-induced activation of c-fos, NGFI-B and CRF gene expression in the hypothalamic PVN: comparison between Sprague-Dawley, Fisher-344 and Lewis rats. J Neuroendocrinol. 1994;6:101–117. doi: 10.1111/j.1365-2826.1994.tb00559.x. [DOI] [PubMed] [Google Scholar]
- Robertson HA. Immediate-early genes, neuronal plasticity, and memory. Biochem Cell Biol. 1992;70:729–737. doi: 10.1139/o92-112. [DOI] [PubMed] [Google Scholar]
- Sabban EL, Hebert MA, Liu X, Nankova B, Serova L. Differential effects of stress on gene transcription factors in catecholaminergic systems. Ann N Y Acad Sci. 2004;1032:130–140. doi: 10.1196/annals.1314.010. [DOI] [PubMed] [Google Scholar]
- Sassone-Corsi P, Ransone LJ, Lamph WW, Verma IM. Direct interaction between fos and jun nuclear oncoproteins: role of the 'leucine zipper' domain. Nature. 1988;336:692–695. doi: 10.1038/336692a0. [DOI] [PubMed] [Google Scholar]
- Schwartz WJ, Carpino A, Jr, de la Iglesia HO, Baler R, Klein DC, Nakabeppu Y, Aronin N. Differential regulation of fos family genes in the ventrolateral and dorsomedial subdivisions of the rat suprachiasmatic nucleus. Neuroscience. 2000;98:535–547. doi: 10.1016/s0306-4522(00)00140-8. [DOI] [PubMed] [Google Scholar]
- Sheng M, Greenberg ME. The regulation and function of c-fos and other immediate early genes in the nervous system. Neuron. 1990;4:477–485. doi: 10.1016/0896-6273(90)90106-p. [DOI] [PubMed] [Google Scholar]
- Terao A, Greco MA, Davis RW, Heller HC, Kilduff TS. Region-specific changes in immediate early gene expression in response to sleep deprivation and recovery sleep in the mouse brain. Neuroscience. 2003;120:1115–1124. doi: 10.1016/s0306-4522(03)00395-6. [DOI] [PubMed] [Google Scholar]
- Trneckova L, Armario A, Hynie S, Sida P, Klenerova V. Differences in the brain expression of c-fos mRNA after restraint stress in Lewis compared to Sprague-Dawley rats. Brain Res. 2006;1077:7–15. doi: 10.1016/j.brainres.2006.01.029. [DOI] [PubMed] [Google Scholar]
- Trneckova L, Rotllant D, Klenerova V, Hynie S, Armario A. Dynamics of immediate early gene and neuropeptide gene response to prolonged immobilization stress: evidence against a critical role of the termination of exposure to the stressor. J Neurochem. 2007 doi: 10.1111/j.1471-4159.2006.04278.x. [DOI] [PubMed] [Google Scholar]
- Umemoto S, Kawai Y, Ueyama T, Senba E. Chronic glucocorticoid administration as well as repeated stress affects the subsequent acute immobilization stress-induced expression of immediate early genes but not that of NGFI-A. Neuroscience. 1997;80:763–773. doi: 10.1016/s0306-4522(97)00050-x. [DOI] [PubMed] [Google Scholar]
- Weinberg M, Girotti M, Francis A, VanElzakker M, Spencer RL. Fra-1 in the prefrontal cortex: stress regulation of immediate early gene expression. Proceedings of the Society for Neuroscience; Washington, DC. 2005. [Google Scholar]
- Yoshida T, Suzuki T, Sato H, Nishina H, Iba H. Analysis of fra-2 gene expression. Nucleic Acids Res. 1993;21:2715–2721. doi: 10.1093/nar/21.11.2715. [DOI] [PMC free article] [PubMed] [Google Scholar]