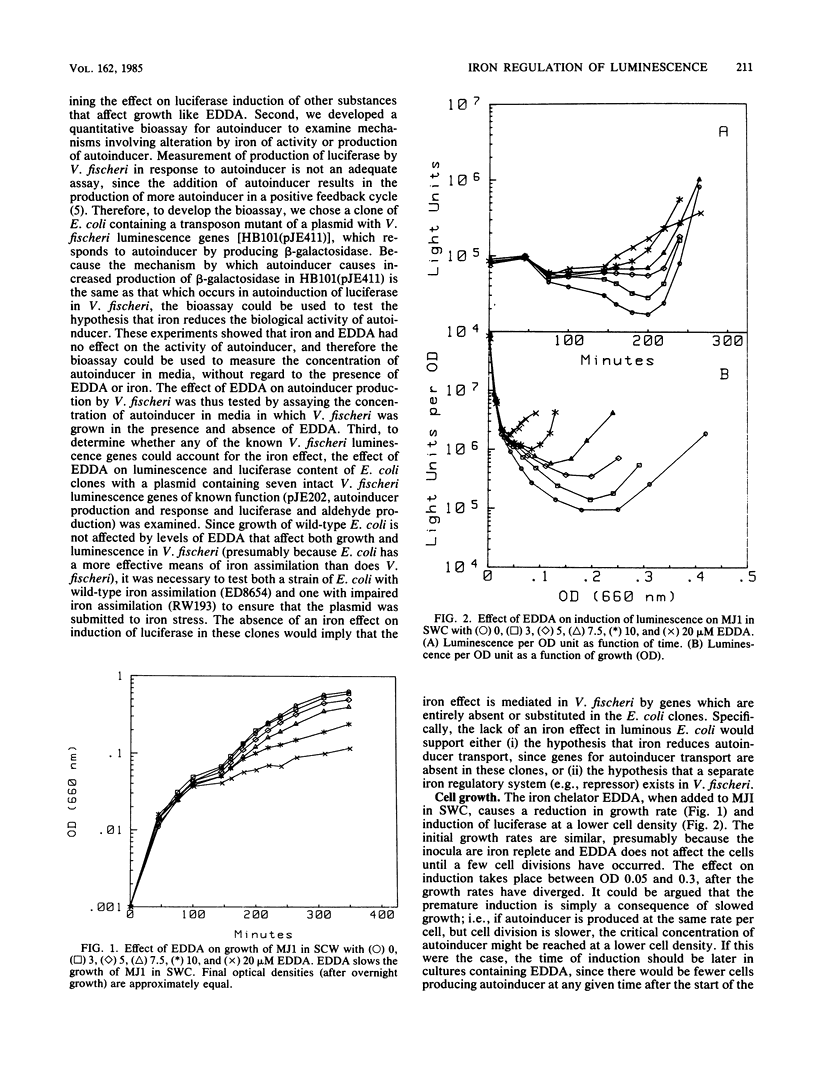
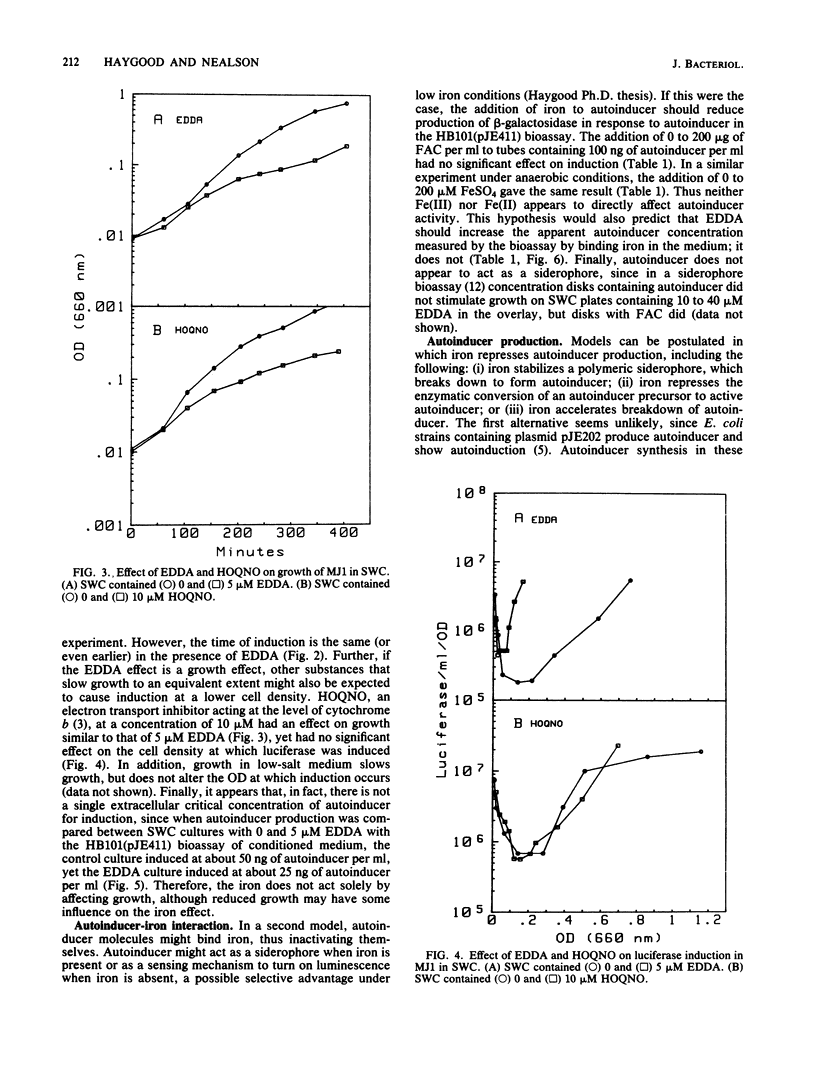
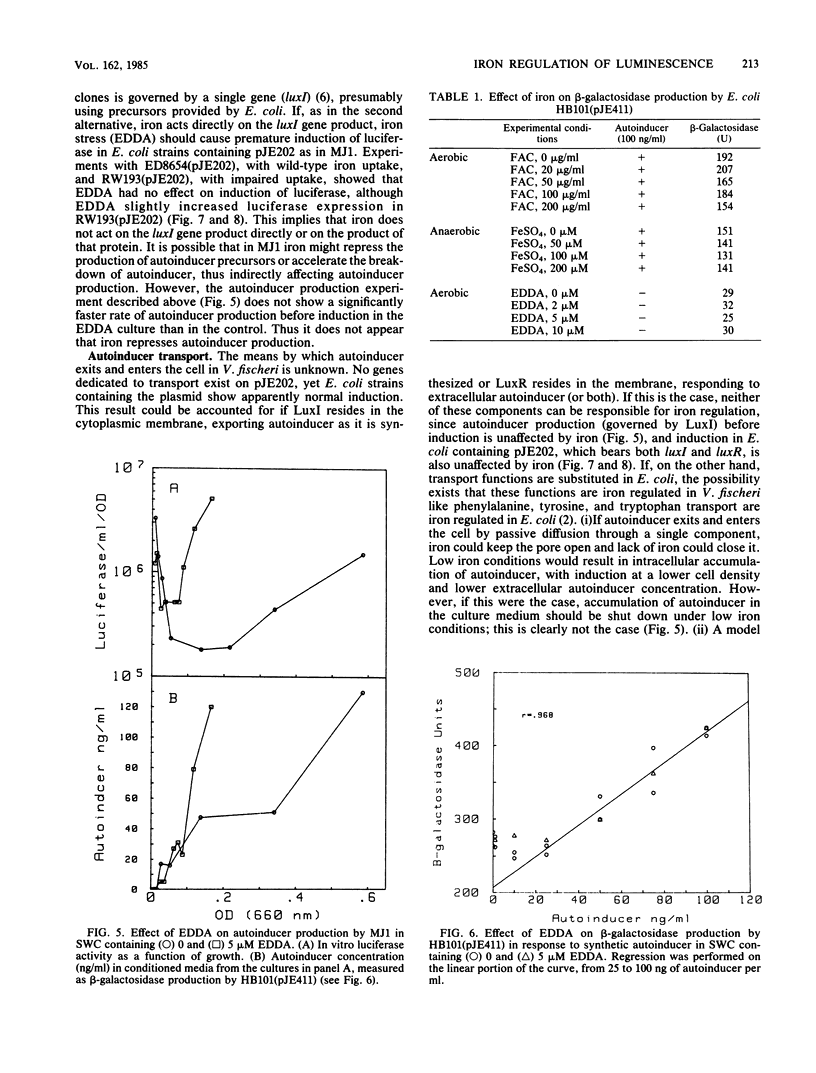
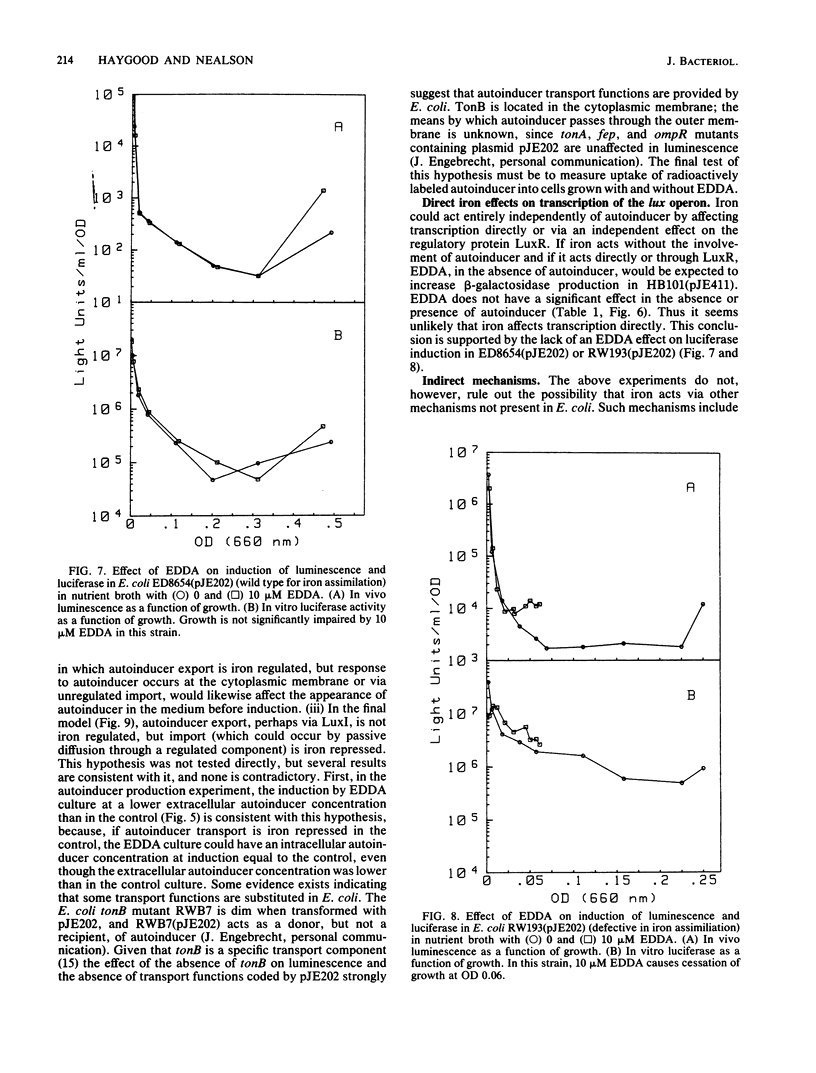
Abstract
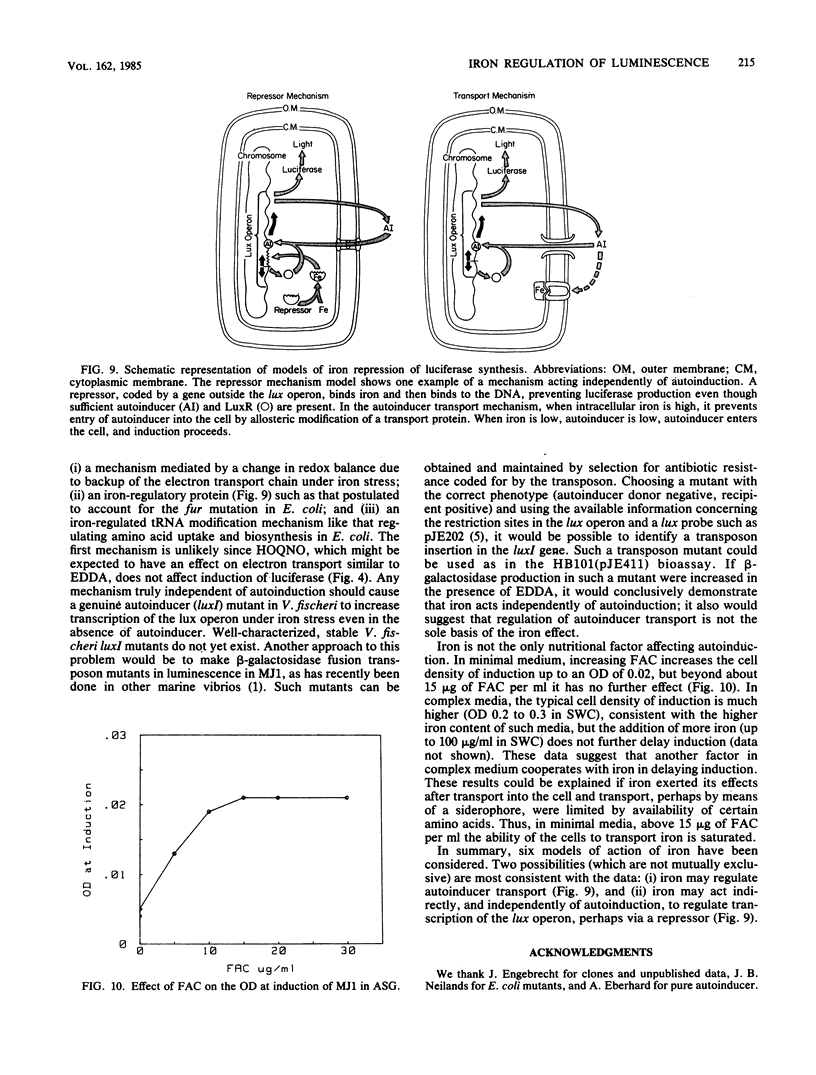
Synthesis of luciferase (an autoinducible enzyme) is repressed by iron in the symbiotic bioluminescent bacterium Vibrio fischeri. Possible mechanisms of iron regulation of luciferase synthesis were tested with V. fischeri and with Escherichia coli clones containing plasmids carrying V. fischeri luminescence genes. Experiments were conducted in complete medium with and without the synthetic iron chelator ethylenediamine-di(o-hydroxyphenyl acetic acid). Comparison of the effect of ethylenediamine-di(o-hydroxyphenyl acetic acid) and another growth inhibitor, (2-n-heptyl-4-hydroxyquinoline-N-oxide), showed that iron repression is not due to inhibition of growth. A quantitative bioassay for autoinducer was developed with E. coli HB101 containing pJE411, a plasmid carrying V. fischeri luminescence genes with a transcriptional fusion between luxI and E. coli lacZ. Bioassay experiments showed no effect of iron on either autoinducer activity or production (before induction) or transcription of the lux operon. Ethylenediamine-di(o-hydroxyphenyl acetic acid) did not affect luciferase induction in E. coli strains with wild-type iron assimilation (ED8654) or impaired iron assimilation (RW193) bearing pJE202 (a plasmid with functional V. fischeri lux genes), suggesting that the genes responsible for the iron effect are missing or substituted in these clones. Two models are consistent with the data: (i) iron represses autoinducer transport, and (ii) iron acts through an autoinduction-independent regulatory system (e.g., an iron repressor).
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belas R., Mileham A., Simon M., Silverman M. Transposon mutagenesis of marine Vibrio spp. J Bacteriol. 1984 Jun;158(3):890–896. doi: 10.1128/jb.158.3.890-896.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck M., Griffiths E. Regulation of aromatic amino acid transport by tRNA: role of 2-methylthio-N6-(delta2-isopentenyl)-adenosine. Nucleic Acids Res. 1981 Jan 24;9(2):401–414. doi: 10.1093/nar/9.2.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engebrecht J., Nealson K., Silverman M. Bacterial bioluminescence: isolation and genetic analysis of functions from Vibrio fischeri. Cell. 1983 Mar;32(3):773–781. doi: 10.1016/0092-8674(83)90063-6. [DOI] [PubMed] [Google Scholar]
- Engebrecht J., Silverman M. Identification of genes and gene products necessary for bacterial bioluminescence. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4154–4158. doi: 10.1073/pnas.81.13.4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles A. A., Khimji P. L. Enterobacterial chelators of iron: their occurrence, detection, and relation to pathogenicity. J Med Microbiol. 1975 Nov;8(4):477–490. doi: 10.1099/00222615-8-4-477. [DOI] [PubMed] [Google Scholar]
- Nealson K. H. Autoinduction of bacterial luciferase. Occurrence, mechanism and significance. Arch Microbiol. 1977 Feb 4;112(1):73–79. doi: 10.1007/BF00446657. [DOI] [PubMed] [Google Scholar]
- Neilands J. B. Microbial envelope proteins related to iron. Annu Rev Microbiol. 1982;36:285–309. doi: 10.1146/annurev.mi.36.100182.001441. [DOI] [PubMed] [Google Scholar]
- Prody C. A., Neilands J. B. Genetic and biochemical characterization of the Escherichia coli K-12 fhuB mutation. J Bacteriol. 1984 Mar;157(3):874–880. doi: 10.1128/jb.157.3.874-880.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers H. J. Iron-Binding Catechols and Virulence in Escherichia coli. Infect Immun. 1973 Mar;7(3):445–456. doi: 10.1128/iai.7.3.445-456.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruby E. G., Nealson K. H. Symbiotic association of Photobacterium fischeri with the marine luminous fish Monocentris japonica; a model of symbiosis based on bacterial studies. Biol Bull. 1976 Dec;151(3):574–586. doi: 10.2307/1540507. [DOI] [PubMed] [Google Scholar]
- Wayne R., Frick K., Neilands J. B. Siderophore protection against colicins M, B, V, and Ia in Escherichia coli. J Bacteriol. 1976 Apr;126(1):7–12. doi: 10.1128/jb.126.1.7-12.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]



