Abstract
Background and purpose:
Artemisinin and its derivatives exhibit potent immunosuppressive activity. The purpose of the current study was to examine the immunosuppressive activity of artemether directly on T lymphocytes and to explore its potential mode of action.
Experimental approach:
In vitro, T-cell proliferation was measured using [3H]-thymidine incorporation assay in cells stimulated with ConA, alloantigen and anti-CD3 antibody. CFSE-labeled cell division and cell cycle distribution were monitored by flow cytometry. In vivo, the effects of artemether were evaluated in delayed-type hypersensitivity (DTH) and purified T-cell responses to ovalbumin in ovalbumin-immunized mice. The activation of extracellular signal-regulated kinase1/2 (ERK1/2) and Raf1 were assessed by Western blot analysis and the activation of Ras was tested in pull-down assays.
Key results:
We show that, in vitro, artemether suppressed ConA- or alloantigen-induced splenocyte proliferation, influenced production of the cytokines IL-2 and IFN-γ and inhibited cell cycle progression through the G0/G1 transition. In vivo, administration of artemether attenuated CD4 T-cell-mediated DTH reaction, and suppressed antigen-specific T-cell response in immunized mice. Further experiments showed that, treatment with artemether impaired both antigen- and anti-CD3-induced phosphorylation of ERK. In primary T cells, artemether profoundly inhibited anti-CD3-induced phosphorylation of Raf1 and activation of Ras.
Conclusions and implications:
This study provided experimental evidence of the immunosuppressive effects of artemether directly on T cells both in vitro and in vivo. Its immunosuppressive mechanism involved inhibition of the activation of the Ras-Raf1-ERK1/2 protein kinase cascade in T cells.
Keywords: artemether, immunosuppression, T-cell activation, Ras–Raf1–ERK1/2 cascade, artemisinin derivative
Introduction
Artemisinin is derived from the herb Artemisia annua L., and artemether is a semi-synthetic derivative of artemisinin (Supplementary Figure 1). Artemisinin is a potent anti-malarial agent with low toxicity, and its derivative artemether has improved efficacy in malaria treatment relative to artemisinin itself (Balint, 2001). Besides their anti-malarial effects, the immunosuppressive effects of artemisinin derivatives were also reported in laboratory studies (Shen et al., 1984; Chen and Gao, 1990; Li and Wu, 2003; Noori et al., 2004). They had also been used for the treatment of systemic lupus erythematosus (SLE) and allergic contact dermatitis with promising results (Chen and Maibach, 1994; Yu and Gao, 1997; Gladman et al., 1998; Tam et al., 2000; Zhang et al., 2002). In our previous studies, we synthesized a series of new artemisinin derivatives and discovered several of these compounds with potent immunosuppressive activity (Yang et al., 2005, 2006; Zhang et al., 2006). The preliminary studies of a novel artemisinin derivative, SM735, showed its immunosuppressive activities both in vitro and in vivo (Zhou et al., 2005). All these studies indicated that artemisinin and its derivatives exert potent immunosuppressive effect. However, the underlying mechanisms mediating their immunosuppressive activities are still largely unknown. In the present study, we focused on T lymphocytes, investigated the immunosuppressive effect of artemether on T cells both in vitro and in vivo and explored its potential mode of action.
T cells play a pivotal role in immune response. Autoreactive T-cell proliferation has been implicated in the pathogenesis of a variety of autoimmune diseases, such as SLE and rheumatoid arthritis (RA) (Xiao and Link, 1999; VanderBorght et al., 2001). Activation of T cells occurs normally upon interaction with an antigen-presenting cell by the engagement of T-cell receptors (TCR) (Alberola-Ila et al., 1997). A defined program is thus initiated (van-Leeuwen and Samelson, 1999; Van-Oers, 1999) that leads to T-cell proliferation and the induction of cytokines. Once the cell is activated, G1 cyclins (D-type cyclins) and cyclin-dependent kinase 6/4 (CDK6/4) are induced and cyclin-dependent kinase inhibitor p27kip protein is reduced; these changes regulate quiescent cell entry into the cell cycle (Lea et al., 2003). Concomitant with the entry of cells into the cell cycle, DNA synthesis and cell division are initiated. In this study, we describe the influence of artemether on the cell cycle progression and illustrate the in vitro and in vivo effect of artemether on T-cell proliferation and cytokine production.
Within minutes of TCR stimulation, several signal transduction pathways are thought to come into play. One of these signaling pathways involves the small GTPase Ras. In resting cells, Ras is maintained in inactivated Ras-GDP form. After activation, Ras is converted into active Ras-GTP followed by downstream signaling resulting in the activation of extracellular signal-regulated kinase (ERK) (Izquierdo et al., 1993; Moodie et al., 1993). During TCR signaling, ERK/mitogen-activated protein kinase plays a critical role, which acts downstream of the early signaling events and, ultimately, results in the efficient transcription of genes encoding cytokines and cytokine receptors, which promote further activation and proliferation of mature T lymphocytes (Crabtree, 1989; Su and Karin, 1996; Whitmarsh and Davis, 1996). Previous studies also indicated the Ras–ERK pathway has been implicated in the regulation of the molecular events that drive cell cycle progression. The Ras–ERK pathway acts transcriptionally to induce the cyclin D1 gene (Aktas et al., 1997; Cheng et al., 1998). The selective inhibition of ERK by the mitogen-activated protein kinase kinase (MEK) inhibitor PD98059 completely prevents entry into S phase (Geginat et al., 1999).
In this study, we provide evidence, for the first time, to demonstrate the effect of artemether on T-cell-mediated immune responses. Our results also reveal possible mechanisms of artemether on T-cell activation and proliferation and elucidate the action of artemether on the Ras–Raf1–ERK1/2 cascade.
Materials and methods
Experimental animals
Female BALB/c and C57BL/6 mice (6–8 weeks old) were obtained from Shanghai Laboratory Animal Center of the Chinese Academy of Sciences (Certificate no. 99-003). Mice were housed in specific pathogen-free conditions. All experiments were carried out according to the NIH Guidelines for Care and Use of Laboratory Animals and were approved by Bioethics Committee of the Shanghai Institute of Materia Medica.
Cell preparation
Mice were killed by cervical dislocation, and the spleens or lymph nodes were removed aseptically. Mononuclear cell suspensions were prepared and resuspended in RPMI 1640 medium (containing 10% fetal bovine serum (FBS)) supplemented with penicillin (100 U ml−1) and streptomycin (100 μg ml−1). Purified T cells were prepared by using immunomagnetic negative selection to deplete B cells and I-A+ antigen presenting cell (APC) as described by Zhu et al. (2006). Purity of the resulting T-cell populations was examined by flow cytometry and was consistently >95%. Splenic APC-enriched populations were separated by using immunomagnetic-negative selection to deplete the surface Ig+ cells (B cells) and T cells as described by Zhu et al. (2006). Purity of the resulting APC-enriched populations was examined by flow cytometry. The resulting purified splenic APC-enriched populations contained less than 1% of T and B cells.
MTT assay
Cytotoxicity was assessed with 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay, as described previously (Wu et al., 2005). Briefly, splenocytes (4 × 105 cells well−1) were cultured in triplicates in a 96-well flat-bottomed plate (Costar, Corning Incorporated, Corning, NY, USA) for 48 or 96 h. MTT (5 mg ml−1) was pulsed 4 h before the end of the culture, and then solvent (10% sodium dodecyl sulfate (SDS), 50% N,N-dimethy formamide, pH 7.2) was added to dissolve the precipitate. After incubating the solution for 7 h, the OD570 readings were taken with a microplate reader (Model 550, Bio-Rad, Hercules, CA, USA).
Proliferation assays
The proliferation of splenocytes in response to concanavalin A (ConA) or alloantigen was determined by [3H]thymidine uptake, as described previously (Wu et al., 2005). Briefly, BALB/c splenocytes suspension (4 × 105 cells well−1) was cultured with ConA (5 μg ml−1) or 30 Gy (Gammacell 3000, Canada) irradiated C57BL/6 splenocyte (4 × 105 cells well−1) as stimulator in a 96-well flat-bottomed plate (Costar). The cultures were incubated for 48 and 96 h for ConA-and alloantigen-induced proliferation, respectively. The cultures were then pulsed with 0.5 μCi [3H]thymidine for 8 h (ConA-induced proliferation) or 24 h (alloantigen-induced proliferation) before the termination of cultures. The cultured cells were harvested onto glass fiber filters. The radioactivity incorporated was determined with a Beta Scintillation Counter (MicroBeta Trilux, Wellesley, MA, USA). To determine cytokine levels, splenocytes (4 × 106 cells well−1) were cultured with ConA (2 μg ml−1) in 24-well plates (Costar). The cultures were incubated at 37°C, 5% CO2 for 16, 24 and 36 h. Interleukin-2 (IL-2) and interferon (IFN)-γ levels in culture supernatants were measured by enzyme-linked immunosorbent assay (ELISA) (BD Biosciences and Pharmingen, San Diego, CA, USA) following the manufacturer's instruction.
Cell cycle progression assays
To examine the effect of artemether on cell division, splenocytes of BALB/c mice were stained with the 5-carboxyfluorescein diacetate succinimide ester (CFSE) at a concentration of 2.5 μM, and then incubated for 20 min in a 37°C water bath, in RPMI 1640. The staining was stopped by the addition of FBS to reach a concentration of 20% of the total volume. Labeled cells were washed twice in phosphate-buffered saline (PBS), counted, then co-cultured with ConA (5 μg ml−1) in the absence or presence of artemether for 72 h. Cells were collected and incubated with 2.4G2 anti-Fc receptor monoclonal antibody, and then stained with the following monoclonal antibodies: PE-conjugated anti-CD4 or anti-CD8 monoclonal antibody. The data were acquired by gating on CD4+ or CD8+ cell population on a FACSCalibur (Becton Dickinson, San Jose, CA, USA). The sequential loss of CFSE fluorescence was used to measure cell division. Division numbers were obtained from an interval analysis of the CFSE histogram by using CellQuest software (Becton Dickinson, San Jose, CA, USA).
To examine the effect of artemether on cell cycle distribution, T cells from lymph nodes of BALB/c mice were stimulated with ConA for 16 h, and then collected and washed with cold PBS and fixed with 70% ethanol for 30 min at 4°C. Then, the fixed cells were washed with PBS and stained with 20 μg ml−1 of propidium iodide (PI) containing 10 μg ml−1 RNase A in the dark at room temperature for 20 min. The DNA contents of the cells (1 × 104 cells group−1) were analyzed by using Modfit software (Becton Dickinson, San Jose, CA, USA).
For analyzing the cell cycle regulation proteins, T cells were activated with anti-CD3 monoclonal antibody and harvested at 24 h post activation for Western blotting. Whole-cell extracts were obtained as described previously (Zhu et al., 2006).
DNFB-induced delayed type hypersensitivity reaction
The experiments for studying 2,4-dinitrofluorobenzene (DNFB)-induced delayed-type hypersensitivity (DTH) reaction were performed as described previously (Zhou et al., 2005). The sensitization was induced by topical application of 20 μl of 0.5% (v/v) DNFB in acetone:olive oil (4:1) onto each hind foot of mice on days 0 and 1. The mice were challenged by application of 10 μl of 0.5% (v/v) DNFB on the inner and outer surfaces of the right ear on day 9. The increase in the ear patch weight (8-mm punches) and ear thickness between the left and right ear was measured 72 h after challenge. Vehicle and artemether (5, 50 and 100 mg kg−1) were given by the oral route and CsA (2 mg kg−1) was given intraperitoneally 1 day before challenge and then once a day for 3 days (days 8–10).
Anti-ovalbumin T-cell immune responses
Ovalbumin at 2 mg ml−1 in PBS was emulsified in an equal volume of complete Freund's adjuvant (CFA). The emulsion (100 μl containing 100 μg ovalbumin) was injected subcutaneously into the shaved backs of the BALB/c mice on day 1. Ovalbumin-immunized mice were treated orally with vehicle or artemether, once a day for seven consecutive days. On day 7, purified T cells (4 × 105 cells well−1) from draining lymph nodes of ovalbumin-immunized mice with or without artemether treatment were co-cultured with 30 Gy γ-irradiated APC-enriched cells (1 × 105 cells well−1) from splenocytes of naïve mice in 96-well flat-bottomed plates in the presence of ovalbumin (100 μg ml−1). Cells were pulsed with 0.5 μCi [3H]thymidine per well for 8 h before harvesting and assessed for [3H]thymidine incorporation at 72 h. To determine cytokines, the supernatants were harvested at 48 h and the levels of IL-2 and IFN-γ were measured by ELISA.
T-cell activation and Western blotting assay
Purified T cells (1 × 107 ml−1) from lymph nodes of OVA-immunized mice with or without artemether treatment were stimulated with ovalbumin (100 μg ml−1) for 10 min; purified primary T cells from lymph nodes of normal BALB/c mice were pretreated with artemether or the MEK inhibitor PD98059 2 h before stimulation with plate-coated anti-CD3 (5 μg ml−1) for 10 min. Then, the cells were collected and lysed in SDS sample buffer (62.5 mM Tris-HCl, pH 6.8, 2% SDS, 10% glycerol, 5% 2-mercaptoethanol and 0.02% bromphenol blue) and boiled for 5 min at 100°C. Proteins were resolved by 12% SDS-polyacrylamide gel electrophoresis (PAGE), transferred to the nitrocellulose membrane (Amersham Pharmacia Biotech, Buckinghamshire, UK) and blocked with 5% non-fat milk powder-TBST buffer (TBS containing 0.1% Tween20) for 1 h at room temperature. The membranes were incubated overnight at 4°C with a 1:1000 dilution of one of the polyclonal antibodies against phospho-Raf1, Raf1, phospho-ERK and ERK (Cell Signal Technology, Danvers, MA, USA). The blots were rinsed three times with TBST buffer for 15 min each. The washed blots were incubated with a 1:5000 dilution of horseradish peroxidase-conjugated secondary antibody (Biotechnology Company, Shanghai, China) for 1 h and then washed three times with the TBST buffer. The transferred proteins were visualized with an enhanced chemiluminescence detection kit (Amersham Pharmacia Biotech, Buckinghamshire, UK).
Detection of Ras activation
To study activation of Ras, the GTP-bound form of Ras was affinity-purified by the use of Raf1-Ras-binding domain (RBD)-GST complexed with glutathione beads following the manufacturer's instruction (Cytoskeleton, Denver, CO, USA). Complexes were analyzed by SDS-PAGE and immunoblot with Ras-specific antibody.
Statistical analysis
The cytotoxic concentration of the compound that reduced cell viability by 50% (CC50) and the inhibitory concentration of the compound that reduced cell proliferation by 50% (IC50) values were determined by using the Origin software package (Microcal Software), as described previously (Zhou et al., 2005). Student's t-test and one-way analysis of variance with Newman–Keuls multiple comparisons on post-tests were used to analyze data and compare groups. P<0.05 was considered to be statistically significant.
Reagents
Artemether were prepared at Shanghai Institute of Materia Medica using a previously published method (Liang and Li, 1996). Artemether was dissolved in peanut oil (100 g l−1) (Sigma-Aldrich, St Louis, MO, USA) as a stock solution and stored at 4°C. The stock solution was diluted to the required concentrations with RPMI 1640 supplemented with Tween80 (less than 0.3%). ConA, MTT, ovalbumin (grade V) and CFSE were purchased from Sigma-Aldrich (St Louis, MO, USA). [3H]-thymidine (1 mCi ml−1) was purchased from Shanghai Institute of Atomic Energy. 2,4-Dinitro-1-fluorobenzene (DNFB) and PD98059 were purchased from Merck (Whitehouse Station, NJ, USA). CFA was purchased from Difco Laboratory (Detroit, MI, USA). RPMI 1640 medium was purchased from Invitrogen (Carlsbad, CA, USA). FBS was purchased from Hyclone Laboratories (Logan, UT, USA). IL-2 and IFN-γ ELISA kits, anti-CD3 (145-2C11), and PE-anti-mouse-CD4 and PE-anti-mouse-CD8 antibodies and PI were BD Biosciences Pharmingen (San Diego, CA, USA) products. Polyclonal antibodies against Raf1, phospho-Raf1, ERK and phospho-ERK were purchased from Cell Signal Technology (Beverly, MA, USA). Polyclonal antibody against β-actin was from Sigma-Aldrich. Cyclin D3, CDK6 and p27kip were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). The Ras activation assay kit was purchased from Cytoskeleton (Denver, CO, USA).
Results
Artemether inhibited ConA-induced splenocyte proliferation and MLR
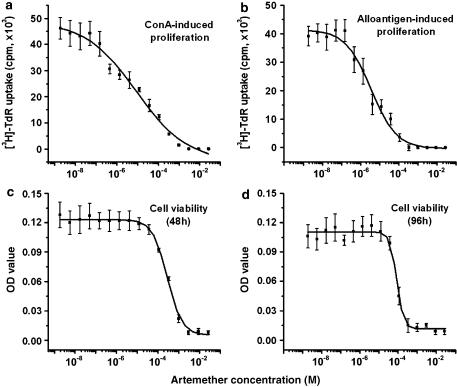
The activity of artemether was evaluated on splenocyte proliferation induced by ConA and mixed lymphocyte culture reaction (MLR) in vitro. ConA is a T-cell mitogen, and the MLR is a model of T-cell response to alloantigenic peptides complexed with major histocompatibility (MHC) proteins on APC. The desired concentration of artemether was incorporated into culture medium at the initiation of cultures. The results presented in Figure 1a and b illustrate the dose-dependent inhibitory effect of artemether on ConA- (Figure 1a) and alloantigen- (Figure 1b) induced proliferation of splenocytes, with IC50 values of 6.3±1.9 and 3.5±0.6 μM, respectively. The cytotoxicity of artemether was examined in the MTT assay and is presented in Figure 1c and d. The CC50 values for artemether were 350±39 and 80±0.4 μM at 48 and 96 h culture, respectively. The CC50 of artemether was 23–58 times higher than its IC50, which revealed the low toxicity of artemether in these cells. The results also indicated that the immunosuppressive activities of artemether observed here, at concentrations up to 50 μM, were not caused by its cytotoxicity.
Figure 1.
Artemether inhibited ConA- or alloantigen-induced proliferation and showed a low cytotoxicity profile in splenocytes. The in vitro cell viability and immunosuppressive activity were determined by MTT assay and [3H]-thymidine uptake assay, respectively. For proliferation assay, BALB/c splenocytes (4 × 105 well−1) were stimulated with ConA (5 μg ml−1) (a) or irradiated C57BL/6 splenocytes (1:1) (b) for 48 and 96 h, respectively, at increasing concentrations of artemether in a 96-well plate in triplicate. For cell viability measurement, the resting splenocytes were cultured with artemether at increasing concentrations for 48 h (c) or 96 h (d) in triplicate. Results presented are mean±s.e.m, n=3.
Effect of artemether on ConA-induced production of IL-2 and IFN-γ in splenocytes
IL-2 and IFN-γ are Th1-type cytokines produced and released upon T-cell activation and, we therefore examined the effect of artemether on their production in splenocytes. ConA strongly stimulated IL-2 and IFN-γ production and the levels of IFN-γ increased rapidly with the incubation time up to 36 h. However, levels of IL-2 decreased after 24 h. In preliminary assays, we observed a lower output of IL-2 following 5 μg ml−1 ConA than after 2 μg ml−1 in the 24 h culture (data not shown). We therefore used the lower concentration of ConA (2 μg ml−1) to stimulate the murine splenocytes and to assess the effects of artemether on IL-2 and IFN-γ production. The results from 16, 24 and 36 h cultures (Table 1) showed that artemether dose-dependently inhibited IL-2 production at 16 h (before the peak time). Afterwards, only high concentrations of artemether exhibited an inhibitory effect on IL-2 production. ConA-induced IFN-γ production was inhibited by artemether and the effect was more prominent at 36 h.
Table 1.
Effects of artemether on ConA-induced cytokine production in splenocytes
| Artemether (μM) | Time (h) | IL-2 (pg ml−1) | IFN-γ (pg ml−1) |
|---|---|---|---|
| 0 | 16 | 7668±59 | 2107±54 |
| 1 | 16 | 6311±68* | 2072±50 |
| 10 | 16 | 3998±24** | 1041±39* |
| 50 | 16 | 295±15** | 473±10** |
| 0 | 24 | 9339±40 | 3370±32 |
| 1 | 24 | 9104±57 | 2273±55* |
| 10 | 24 | 8519±70* | 1324±11* |
| 50 | 24 | 501±22** | 432±16** |
| 0 | 36 | 9162±62 | 6398±38 |
| 1 | 36 | 9178±48 | 4405±46** |
| 10 | 36 | 9086±38 | 1849±33** |
| 50 | 36 | 1423±24** | 478±25** |
Abbreviation: ConA, concanavalin A.
Splenocytes (4 × 106 cells well−1) were stimulated with ConA (2 μg ml−1) in the absence or presence of artemether in a 24-well plate for 16, 24 and 36 h at the concentrations as indicated, and the cytokine levels of IL-2 and IFN-γ in the culture supematants were determined by ELISA. IL-2 and IFN-γ levels in vehicle-treated splenocytes in the absence of ConA were 78±4 and 143±22 pg ml−1, respectively. Results were presented as mean±s.e.m., n=3.
P 0.05
P 0.01 versus vehicle group.
Artemether inhibited T-cell division and cell cycle progression through G1/S transition
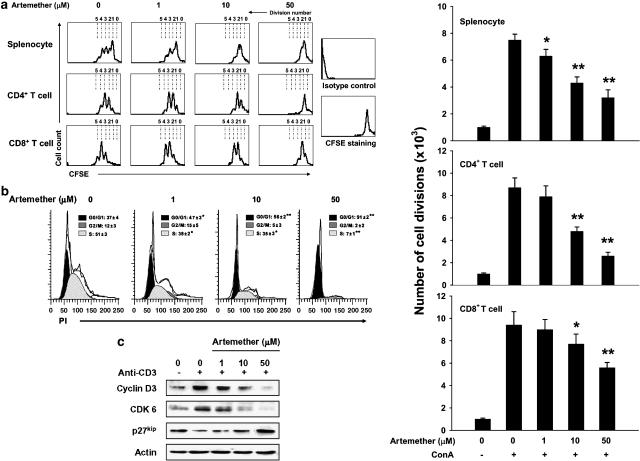
For monitoring the effect of artemether on cell division, splenocytes were pre-labeled with CFSE, as this marker will attach to free amine groups of cytoplasmic cell proteins. As the cell divides, the retained CFSE label is distributed to each daughter cell. According to preliminary assays, CFSE-labeled cells divide extensively after stimulation with ConA for 72 h. We analyzed the effect of artemether on CD4 T-cell and CD8 T-cell division by gating of CD4+ and CD8+ subsets of T cells, respectively. As shown in Figure 2a, artemether treatment (1, 10 and 50 μM) resulted in a dose-dependent inhibition of ConA-induced splenocyte, CD4+T- and CD8+ T-cell divisions. Artemether at 50 μM almost completely blocked cell division.
Figure 2.
Artemether inhibited T-cell division and cell cycle progression through the G1/S transition. (a) CFSE-labeled splenocytes were stimulated with ConA (5 μg ml−1) for 72 h to achieve the full cycling in the absence or presence of artemether (1, 10 and 50 μM), as described in Materials and methods. The cell signals were acquired with or without gating on CD4+ or CD8+ T subsets in flow cytometry. The number of cell divisions (from three independent experiments) is shown by the histograms. Results are mean±s.e.m., n=3. *P< 0.05, **P< 0.01 versus vehicle-treated ConA-stimulated group. (b) T lymphocytes from lymph nodes were stimulated with ConA (5 μg ml−1) for 16 h in the absence or presence of artemether (1, 10 and 50 μM). The cells were then stained with PI for cell cycle distribution analysis in flow cytometry. The frequency (%) for different phases of cell cycle was generated from three independent experiments and is presented as mean±s.e.m., n=3. *P< 0.05, **P< 0.01 versus vehicle-treated ConA-stimulated group. (c) T lymphocytes from lymph nodes were activated with anti-CD3 mAb and harvested after 24 h culture with or without artemether (1, 10 and 50 μM). Whole-cell extracts were obtained and probed for cyclin D3, CDK6 and p27kip in Western blotting assay. The results presented are from one experiment, which is representative of two others performed.
Because artemether inhibited DNA synthesis and cell division, the effects of artemether were then examined on cell cycle distribution by staining for DNA content (PI staining) and using flow cytometry. As presented in Figure 2b, 37% of ConA-stimulated T cells were at G0/G1 phases and artemether at 1, 10 and 50 μM arrested 47, 56 and 91% of the cells at G0/G1 phases, respectively.
Cell entry into the cell cycle and progression through the G1 phase is dictated by the presence of D-type cyclins (cyclin D1, cyclin D2 and cyclin D3) in complex with the G1-phase cyclin-dependent kinases CDK4/CDK6 (Ajchenbaum et al., 1993; Lucas et al., 1995). It has been reported that cyclin D2/3, CDK6 and CDK inhibitor p27kip play a major role in T cells and are markedly induced by TCR ligation (Zhang et al., 2001; Lea et al., 2003). For further demonstration of the effect of artemether on cell cycle, we investigated the influence of artemether on the levels of cyclin D2/3, CDK6 and p27kip in purified T cells. Consistent with the observations on cell cycle distribution, artemether (1, 10 and 50 μM) dose-dependently inhibited the increase of cyclin D3 and CDK6 induced by TCR ligation and suppressed degradation of p27kip. The results showed that artemether influenced the cell cycle regulatory molecules of G1 phase and blocked cell cycle progression through G1/S transition (Figure 2c).
Artemether attenuated the DNFB-induced DTH reaction
To assess the immunosuppressive property of artemether in vivo, we used the DNFB-induced DTH model in BALB/c mice. The DTH reaction is based on a cell-mediated pathologic response involved with CD4+ T-cell activation and the production of many cytokines (Kobayashi et al., 2001). The result showed that oral administration of artemether at 50 and 100 mg kg−1 dose-dependently suppressed ear swelling (Table 2). The results suggested that artemether inhibits T-cell-mediated immune responses in vivo.
Table 2.
Artemether suppressed DNFB-induced DTH reaction
| Group | Dosage (mg kg−1) | Increase in ear thickness (mm) | Increase in ear patch weight (mg) |
|---|---|---|---|
| Vehicle | — | 0.21±0.04 | 29.3±6.0 |
| CsA | 2 | 0.14±0.04** | 18.1±3.9 |
| Artemether | 5 | 0.19±0.02 | 28.9±5.3 |
| Artemether | 50 | 0.13±0.02** | 20.4±4.9** |
| Artemether | 100 | 0.05±0.01** | 12.5±4.8** |
Abbreviations: DNFB, 2,4-dinitrofluorobenzene; DTH, delayed-type hypersensitivity.
BALB/c mice were sensitized with 0.5% DNFB on days 0 and 1 and then challenged by DNFB on day 9. Vehicle, CsA (2 mg kg−1, i.p.) and artemether (5, 50 or 100 mg kg−1, p.o.) was administered 1 day before challenge and then once a day for a total of 3 days. Ear swelling was calculated as the increase in ear thickness (mm) and ear patch weight (mg) between left (DNFB-untreated) and right (DNFB-treated) ear 40 h after challenge. The baseline ear thickness and ear patch weight were 0.20±0.02 mm and 18.4±2.9 mg, respectively. Results are presented as mean±s.e.m, n=10.
P< 0.01, versus vehicle group.
Artemether inhibited OVA-specific T-cell responses
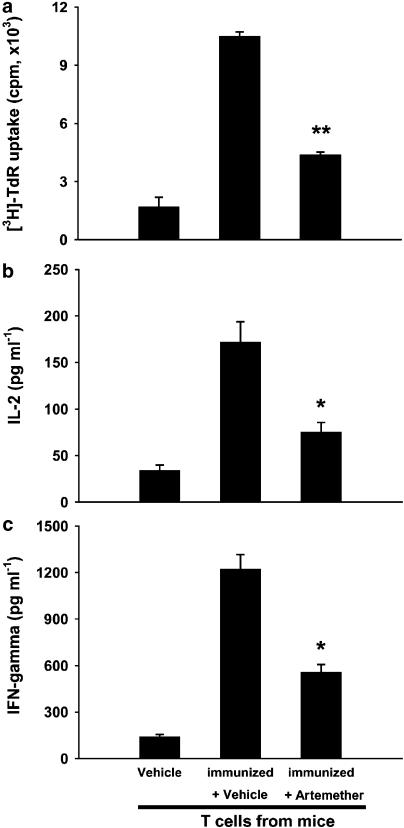
We then investigated whether the suppression induced by artemether was directed toward T cells in vivo. For this purpose, ovalbumin, a T-cell-dependent antigen, was used to induce immune response in BALB/c mice. The capacity of ovalbumin-specific T-cell responses in ovalbumin-immunized mice with or without artemether treatment was measured in an antigen-presenting assay. As shown in Figure 3, compared with T cells from naïve mice, T cells from ovalbumin-immunized mice exhibited a marked antigen-specific recall response to ovalbumin. Treatment with artemether (100 mg kg−1) significantly suppressed the ovalbumin-specific T-cell proliferation and cytokine production (IL-2 and IFN-γ). The results demonstrated that artemether directly inhibited T-cell responses in vivo.
Figure 3.
Artemether impaired ovalbumin-specific T-cell responses in vivo. BALB/c mice were immunized with ovalbumin (100 μg per mouse) on day 1. Vehicle and artemether (100 mg kg−1) were given orally, once daily for 7 days. (a) For proliferation assay, purified T cells from each group were incubated with ovalbumin (100 μg ml−1) in the presence of γ-irradiated APC from naïve mice in triplicate for 72 h. Cytokine levels of IL-2 (b) and IFN-γ (c) were determined after 48 h culture. Results presented are mean±s.e.m., n=3. *P< 0.05, **P< 0.01, versus vehicle-treated ovalbumin-immunized group.
Artemether inhibited activation of ERK in T cells
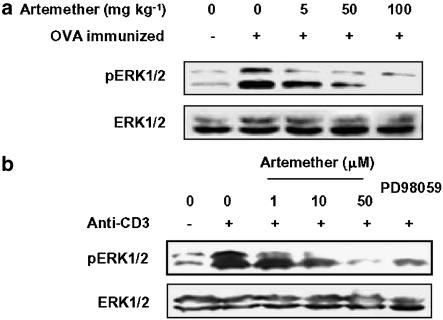
From the above studies, it is evident that artemether has a strong inhibitory effect on T-cell responses both in vitro and in vivo. We next examined the possible mechanisms involved in the inhibitory effect of artemether. Because it is well recognized that ERK participates in T-cell activation and plays a pivotal role in T-cell proliferation and cytokine production (Whitehurst et al., 1992), we measured the effect of artemether on activation of ERK in ovalbumin-stimulated T cells purified from ovalbumin-immunized mice in vivo (Figure 4a) and in anti-CD3-stimulated T cells purified from normal mice in vitro (Figure 4b). The level of phosphorylated ERK1/2 was measured to determine ERK1/2 activation, and the MEK1 inhibitor PD98059 was used as a control. The result showed that both in vivo and in vitro treatment with artemether dose-dependently inhibited the increased activation of ERK1/2 induced by either antigen presented by APC or anti-CD3 crosslinking.
Figure 4.
Artemether inhibited activation of ERK in T cells. (a) T cells (1 × 107 cells ml−1) purified from draining lymph nodes of naïve mice, vehicle- and artemether-treated, ovalbumin-immunized mice were exposed to ovalbumin (100 μg ml−1) in the presence of γ-irradiated APC from naïve mice for 10 min. Cells were then harvested, lysed and assayed for phospho-ERK and total ERK. (b) T cells were pretreated with artemether (1, 10 and 50 μM) or PD98059 (30 μM) for 2 h followed by stimulation with anti-CD3 (5 μg ml−1) for 10 min and then lysed in SDS sample buffer. The lysates were then probed for phospho-ERK and total ERK by Western blotting assay. The results presented are from one experiment, which is representative of two others performed.
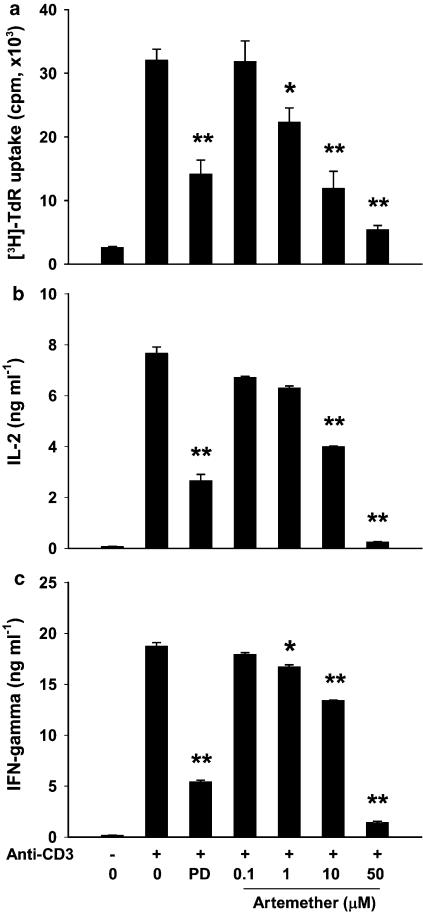
Artemether inhibited anti-CD3-stimulated primary T-cell responses
The effect of artemether (0.1, 1, 10 and 50 μM) on CD3-mediated T-cell proliferation and cytokine production was investigated. Purified T cells from normal mice responded vigorously to plate-coated anti-CD3 monoclonal antibody (5 μg ml−1; Figure 5). Artemether (1, 10 and 50 μM) and PD98059 (30 μM) significantly inhibited the T-cell proliferation and cytokine production (IL-2 and IFN-γ). The results demonstrated the direct inhibitory effect of artemether on T cells and indicated that its immunosuppressive activity in T cells was closely related to its inhibitory effect on ERK activation.
Figure 5.
Artemether inhibited anti-CD3-induced primary T-cell responses. (a) For proliferation assays, T cells (4 × 105 cells well−1) from lymph nodes were cultured with anti-CD3 monoclonal antibody (5 μg ml−1) in the absence or presence of artemether (0.1, 1, 10 and 50 μM) or PD98059 (30 μM) in 96-well flat-bottom plates in triplicate for 48 h. Cytokine levels of IL-2 (b) and IFN-γ (c) were measured at 16 and 24 h, respectively. Results presented are mean±s.e.m., n=3. *P<0.05, **P< 0.01 versus vehicle-treated anti-CD3-stimulated control.
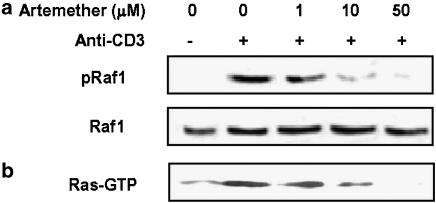
Artemether inhibited anti-CD3-induced activation of Ras and phosphorylation of Raf1 in primary T cells
To delineate further the molecular mechanisms following TCR activation in T cells, we explored the possible role of artemether on the Ras–Raf–ERK protein kinase cascades in T-cell activation. The results showed that very low levels of pRaf1 and Ras-GTP were detected before T-cell activation and that stimulation with anti-CD3 significantly increased the levels of pRaf1 (Figure 6a) and Ras-GTP (Figure 6b). Artemether (1, 10 and 50 μM) dose-dependently inhibited CD3-mediated phosporylation of Raf1 and activation of Ras. Thus, artemether appears to be able to interfere with the activation of Ras–Raf1–ERK1/2 cascade during T-cell activation.
Figure 6.
Artemether inhibited CD3-mediated activation of Ras and phosphorylation of Raf1. (a) Primary T cells (1 × 107 cells ml−1) were pretreated with artemether (1, 10 and 50 μM) for 2 h followed by stimulation with anti-CD3 (5 μg ml−1) for 10 min. Cells were lysed and assayed for phospho-Raf1 and total Raf1 by Western blotting. (b) T cells were treated with artemether (1, 10 and 50 μM) for 2 h, washed and then stimulated with anti-CD3 (5 μg ml−1) for 5 min. Cell lysates were incubated with a GST-fusion protein coupled to the RBD of Raf1. Affinity-precipitated active Ras-GTP proteins were detected by Western blotting with anti-Ras monoclonal antibody. The results presented are from one experiment, which is representative of two others performed.
Discussion
Artemisinin and its derivatives exhibit potent immunosuppressive activity and have been tested as treatments for autoimmune diseases in clinical trials (Chen and Maibach, 1994; Yu and Gao, 1997; Gladman et al., 1998; Tam et al., 2000; Zhang et al., 2002). In our laboratory, we screened a series of artemisinin derivatives with the goal of developing potent immunosuppressive agents, and several of the compounds with promising results are being studied both in vitro and in vivo (Yang et al., 2005; Zhou et al., 2005; Zhang et al., 2006). Artemisinin and its derivatives may have a direct inhibitory action on T cells but their underlying molecular mechanisms are still not well known. Artemether is widely known as an anti-malarial agent. It has improved efficacy in malaria treatment and was found, in our preliminary assay, to exhibit more potent anti-proliferation activity than its parent compound, artemisinin. Therefore, in the present study, we investigated the immunosuppressive activity of artemether on T lymphocytes to understand better the mode of action of artemisinin and its derivatives.
T lymphocytes play a pivotal role in the pathogenesis of cell-mediated autoimmune diseases and the chronic inflammatory disorders (Perkins, 1998). Previous studies suggested that artemisinin derivatives could influence T-cell-mediated immune response (Zhou et al., 2005). In this study, we focused on the action of artemether on T lymphocytes. We investigated mitogen- (ConA) and alloantigen-induced T-cell proliferation and production of cytokines (IL-2 and IFN-γ) in vitro. The proliferative response induced with each of the two inducers was dose-dependently suppressed by artemether. Consistent with these observations, CFSE-labeled cell division data indicated that artemether inhibited T-cell division, and we found that T-cell proliferation was generally controlled by artemether at the G0/G1 phases. Cell progression through G1 to S transition was markedly inhibited by artemether, which was correlated with an artemether-mediated reduction of the protein levels of G1-phase cell cycle molecules (cyclin D3 and CDK6) and an increase in CDK inhibitor p27kip. These findings suggested that artemether influenced T-cell activation and inhibited T-cell cycle progression in vitro.
The in vivo properties of artemether were illustrated in a T-cell-mediated DTH response. DTH is a CD4+ T-cell-mediated immunity response and is associated with T-cell activation and production of Th1-type cytokines (Kobayashi et al., 2001). Artemether showed a prominent inhibitory effect on DTH response. During the whole in vivo experiment period, there were no death or any signs of illness observed in mice treated with artemether (LD50=977 mg kg−1, p.o.) (You et al., 1992; Xiao et al., 1998), at the doses of 5, 50 and 100 mg kg−1. Moreover, artemether did not influence mice body weight, spleen weight, splenocyte number and T-cell number in vivo (Supplementary Table 1).
Further studies then elucidated the direct action of artemether on T cells both in vivo and in vitro. In ovalbumin-immunized mice, artemether treatment resulted in an impaired T-cell response to antigen in the presence of γ-irradiated APC from naïve mice. In purified T cells from normal mice, artemether dose-dependently suppressed TCR ligation-induced T-cell proliferation and cytokine production. These observations indicated that artemether exerted immunosuppressive effect through its inhibition of T-cell activation and proliferation. The results suggested that artemisinin derivatives have a potential for the treatment of T-cell-mediated immune diseases.
The mechanisms of the inhibitory activity of artemether on T cell were partially identified. The results indicated that artemether markedly inhibited CD3-mediated activation of Ras and phosphorylation of Raf1 in primary T cells and suppressed ERK activation in T lymphocytes. The Ras–Raf–ERK protein kinase cascade is essential for cell cycle progression and for the production of cytokines by primary T lymphocytes (Whitehurst et al., 1992; Alberola-Ila et al., 1997; Aktas et al., 1997). Ras and its effectors, particularly the Raf–ERK pathway, are activated within minutes after stimulation of TCR and play an essential role in T-cell activation (Rayter et al., 1992; Izquierdo et al., 1993). The effective inhibitory concentration of artemether on the Ras–Raf1–ERK1/2 protein kinase cascade was comparable to that for T-cell proliferation, cell cycle progression and cytokine production. The results are also consistent with the effects of the artemisinin derivative, eupatilin, in Ras-transformed epithelial cells (Kim et al., 2004).
Our study, which was focused on the activation of the Ras–Raf–ERK protein kinase cascade activation, does not exclude other molecular pathways from being influenced by artemisinin derivatives. For instance, artemisinin affected calmodulin activity (Noori et al., 2004). Calmodulin regulates calcineurin, which in turn dephosphorylates NF-AT, leading to its nuclear translocation and activation. Moreover, dihydroarteannuin, a semi-synthetic derivative of artemisinin, effectively blocked IκB degradation and inhibited the nuclear translocation of necrosis factor (NF)-κB in peritoneal macrophages of BXSB mice (Li et al., 2006). It seems therefore that artemisinin derivatives have a wide range of effects on intracellular signaling pathways. Our result regarding artemether inhibition of the Ras-ERK pathway in primary T-cell activation is new and interesting. This pathway has been implicated in regulating the molecular events (cyclin D and p27kip) that drive cell entry into G1 phase and progression through G1/S transition (Aktas et al., 1997; Cheng et al., 1998). It is known that mitogen-activated protein (MAP) kinases are composed of three principal family members, ERK, c-jun N-terminal kinase (JNK) and p38. JNK and p38 MAP kinases are two relatively new components of MAP kinase family and are also involved in T-cell activation (Whitehurst et al., 1992; Su et al., 1994; Zhang et al., 1999). Activated MAP kinases induce various transcription factors (Karin, 1995; Su and Karin, 1996; Whitmarsh and Davis, 1996), which, in turn, lead to T-cell proliferation and cytokine production (Liu et al., 1997). In a study of a new water-soluble artemisinin derivative, we observed that the activities of JNK and p38 MAP kinases were also modified (unpublished observations). The results of this study suggested that the inhibitory effects of artemether on T-cell responses were partially mediated through the Ras–Raf1–ERK1/2 signaling pathway.
Here, we have demonstrated that artemether inhibited T-cell activation and proliferation both in vitro and in vivo. The immunosuppressant mechanism of artemether correlated with inhibition of the Ras–Raf1–ERK1/2 cascade activation in T cells. These findings extended our understanding of the immunosuppressive effect of artemisinin and its derivatives. They have also suggested the potential of artemisinin derivatives as new and effective treatments for the treatment of T-cell-mediated autoimmune diseases.
External data objects
Acknowledgments
This work was supported by the grants of the Knowledge Innovation Program of Chinese Academy of Sciences (No. KSCX2-SW-202) and Shanghai Science and Technology Committee (No. 06DZ19006).
Abbreviations
- APC
antigen-presenting cell
- CC50
concentration that reduced cell viability by 50%
- CFA
complete Freund's adjuvant
- DNFB
2,4-dinitrofluorobenzene
- CFSE
5-carboxyfluorescein diacetate succinimide ester
- ConA
concanavalin A
- DTH
delayed-type hypersensitivity
- ELISA
enzyme-linked immunosorbent assay
- ERK
extracellular signal-regulated kinase
- IC50
the inhibitory concentration of the compound that reduced cell proliferation by 50%
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide
- TCR
T-cell receptor
Conflict of interest
The authors state no conflict of interest.
Supplementary Information accompanies the paper on British Journal of Pharmacology website (http://www.nature.com/bjp)
References
- Ajchenbaum F, Ando K, Decaprio JA, Griffin JD. Independent regulation of human D-type cyclin gene expression during G1 phase in primary human T lymphocytes. J Biol Chem. 1993;268:4113–4119. [PubMed] [Google Scholar]
- Aktas H, Cai H, Cooper GM. Ras links growth factor signaling to the cell cycle machinery via regulation of cyclin D1 and the Cdk inhibitor p27KIP1. Mol Cell Biol. 1997;17:3850–3857. doi: 10.1128/mcb.17.7.3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberola-Ila J, Takaki S, Kerner JD, Perlmutter RM. Differential signaling by lymphocyte antigen receptors. Annu Rev Immunol. 1997;15:125–154. doi: 10.1146/annurev.immunol.15.1.125. [DOI] [PubMed] [Google Scholar]
- Balint GA. Artemisinin and its derivatives: an important new class of antimalarial agents. Pharmacol Ther. 2001;90:261–265. doi: 10.1016/s0163-7258(01)00140-1. [DOI] [PubMed] [Google Scholar]
- Chen H, Gao YX. Mechanism of immunological action of artesunate. Zhongguo Pi Fu Xing Bing Xue Za Zhi. 1990;23:250–252. [Google Scholar]
- Chen H, Maibach HI. Topical application of artesunate on guinea pig allergic contact dermatitis. Contact Dermatitis. 1994;30:280–282. doi: 10.1111/j.1600-0536.1994.tb00598.x. [DOI] [PubMed] [Google Scholar]
- Cheng M, Sexl V, Sherr CJ, Roussel MF. Assembly of cyclin D-dependent kinase and titration of p27Kip1 regulated by mitogen-activated protein kinase kinase (MEK1) Proc Natl Acad Sci USA. 1998;95:1091–1096. doi: 10.1073/pnas.95.3.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabtree GR. Contingent genetic regulatory events in T lymphocyte activation. Science. 1989;243:355–361. doi: 10.1126/science.2783497. [DOI] [PubMed] [Google Scholar]
- Geginat J, Bossi G, Bender JR, Pardi R. Anchorage dependence of mitogen-induced G1 to S transition in primary T lymphocytes. J Immunol. 1999;162:5085–5093. [PubMed] [Google Scholar]
- Gladman DD, Urowitz MB, Senecal JL, Fortin PJ, Petty RE, Esdaile JM, et al. Aspects of use of antimalarials in systemic lupus erythematosus. J Rheumatol. 1998;25:983–985. [PubMed] [Google Scholar]
- Izquierdo M, Leevers SJ, Marshall CJ, Cantrell D. p21ras couples the T cell antigen receptor to extracellular signal-regulated kinase 2 in T lymphocytes. J Exp Med. 1993;178:1199–1208. doi: 10.1084/jem.178.4.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M. The regulation of AP-1 activity by mitogen-activated protein kinases. J Biol Chem. 1995;270:16483–16486. doi: 10.1074/jbc.270.28.16483. [DOI] [PubMed] [Google Scholar]
- Kim DH, Na HK, Oh TY, Kim WB, Surh YJ. Eupatilin, a pharmacologically active flavone derived from Artemisia plants, induces cell cycle arrest in ras-transformed human mammary epithelial cells. Biochemical Pharmacology. 2004;68:1081–1087. doi: 10.1016/j.bcp.2004.04.027. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Kaneda K, Kasama T. Immunopathogenesis of delayed-type hypersensitivity. Microsc Res Tech. 2001;53:241–245. doi: 10.1002/jemt.1090. [DOI] [PubMed] [Google Scholar]
- Lea NC, Orr SJ, Stoeber K, Williams GH, Lam EW, Ibrahim MA, et al. Commitment point during G0 → G1 that controls entry into the cell cycle. Mol Cell Biol. 2003;23:2351–2361. doi: 10.1128/MCB.23.7.2351-2361.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li WD, Dong YJ, Tu YY, Lin ZB. Dihydroarteannuin ameliorates lupus symptom of BXSB mice by inhibiting production of TNF-alpha and blocking the signaling pathway NF-kappa B translocation. Int Immunopharmacol. 2006;6:1243–1250. doi: 10.1016/j.intimp.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Li Y, Wu YL. An over four millennium story behind qinghaosu (artemisinin): a fantastic antimalarial drug from a traditional Chinese herb. Curr Med Chem. 2003;10:2197–2230. doi: 10.2174/0929867033456710. [DOI] [PubMed] [Google Scholar]
- Liang J, Li Y. Synthesis of the aryl ethers derivatives of artemisinin. Zhongguo Yao Wu Hua Xue Za Zhi. 1996;6:22–25. [Google Scholar]
- Liu B, Carle KW, Whisler RL. Reductions in the activation of ERK and JNK are associated with decreased IL-2 production in T cells from elderly humans stimulated by the TCR/CD3 complex and costimulatory signals. Cell Immunol. 1997;182:79–88. doi: 10.1006/cimm.1997.1226. [DOI] [PubMed] [Google Scholar]
- Lucas JJ, Szepesi A, Modiano JF, Domenico J, Gelfand EW. Regulation of synthesis and activity of the PLSTIRE protein (cyclin-dependent kinase 6 (cdk6)), a major cyclin D-associated cdk4 homologue in normal human T lymphocytes. J Immunol. 1995;154:6275–6284. [PubMed] [Google Scholar]
- Moodie SA, Willumsen BM, Weber MJ, Wolfman A. Complexes of Ras. GTP with Raf-1 and mitogen-activated protein kinase kinase. Science. 1993;260:1658–1661. doi: 10.1126/science.8503013. [DOI] [PubMed] [Google Scholar]
- Noori S, Naderi GA, Hassan ZM, Habibi Z, Bathaie SZ, Hashemi SM. Immunosuppressive activity of a molecule isolated from Artemisia annua on DTH responses compared with cyclosporin A. Int Immunopharmacol. 2004;4:1301–1306. doi: 10.1016/j.intimp.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Perkins DL. T-cell activation in autoimmune and inflammatory diseases. Curr Opin Nephrol Hypertens. 1998;7:297–303. doi: 10.1097/00041552-199805000-00010. [DOI] [PubMed] [Google Scholar]
- Rayter SI, Woodrow M, Lucas SC, Cantrell DA, Downward J. p21ras mediates control of IL2 gene promoter function in T cell activation. EMBO J. 1992;11:4549–4556. doi: 10.1002/j.1460-2075.1992.tb05556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen M, Ge HL, He YX, Song QL, Zhang HZ. Immunosuppressive action of Qinghaosu. Sci Sin [B] 1984;27:398–406. [PubMed] [Google Scholar]
- Su B, Karin M. Mitogen-activated protein kinase cascades and regulation of gene expression. Curr Opin Immunol. 1996;8:402–411. doi: 10.1016/s0952-7915(96)80131-2. [DOI] [PubMed] [Google Scholar]
- Su B, Jacinto E, Hibi M, Kallunki T, Karin M, Ben-Neriah Y. JNK is involved in signal integration during costimulation of T lymphocytes. Cell. 1994;77:727–736. doi: 10.1016/0092-8674(94)90056-6. [DOI] [PubMed] [Google Scholar]
- Tam LS, Gladman DD, Hallett DC, Rahman P, Urowitz MB. Effect of antimalarial agents on the fasting lipid profile in systemic lupus erythematosus. J Rheumatol. 2000;27:2142–2145. [PubMed] [Google Scholar]
- VanderBorght A, Geusens P, Raus J, Stinissen P. The autoimmune pathogenesis of rheumatoid arthritis: role of autoreactive T cells and new immunotherapies. Semin Arthritis Rheu. 2001;31:160–175. doi: 10.1053/sarh.2001.27736. [DOI] [PubMed] [Google Scholar]
- van-Leeuwen JE, Samelson LE. T-cell antigen-receptor signal transduction. Curr Opin Immunol. 1999;11:242–248. doi: 10.1016/s0952-7915(99)80040-5. [DOI] [PubMed] [Google Scholar]
- Van-OERS NS. T-cell receptor-mediated signs and signals governing T-cell development. Semin Immunol. 1999;11:227–237. doi: 10.1006/smim.1999.0179. [DOI] [PubMed] [Google Scholar]
- Whitehurst CE, Boulton TG, Cobb MH, Geppert TD. Extracellular signal-regulated kinases in T cells: anti-CD3 and 4 beta-phorbol 12-myristate 13-acetate-induced phosphorylation and activation. J. Immunol. 1992;148:3230–3237. [PubMed] [Google Scholar]
- Whitmarsh AJ, Davis RJ. Transcription factor AP-1 regulation by mitogen-activated protein kinase signal transduction pathways. J Mol Med. 1996;74:589–607. doi: 10.1007/s001090050063. [DOI] [PubMed] [Google Scholar]
- Wu QL, Fu YF, Zhou WL, Wang JX, Feng YH, Liu J, et al. Inhibition of S-adenosyl-L-homocysteine hydrolase induces immunosuppression. J Pharmacol Exp Ther. 2005;313:705–711. doi: 10.1124/jpet.104.080416. [DOI] [PubMed] [Google Scholar]
- Xiao BG, Link H. Antigen-specific T cells in autoimmune diseases with a focus on multiple sclerosis and experimental allergic encephalomyelitis. Cell Mol Life Sci. 1999;56:5–21. doi: 10.1007/s000180050002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao SH, You JQ, Mei JY, Jiao PY, Guo HF, Feng Z. Preventive effect of artemether in rabbits infected with Schistosoma japonicum cercariae. Zhongguo Yao Li Xue Bao. 1998;19:63–66. [PubMed] [Google Scholar]
- Yang ZS, Wang JX, Zhou Y, Zuo JP, Li Y. Synthesis and immunosuppressive activity of new artemisinin derivatives. Part 2:2-[12 (b or a)-dihydroartemisinoxymethyl-(or 1′-ethyl)]phenoxyl propionic acids and esters. Bioorg Med Chem. 2006;14:8043–8049. doi: 10.1016/j.bmc.2006.07.038. [DOI] [PubMed] [Google Scholar]
- Yang ZS, Zhou WL, Sui Y, Wang JX, Wu JM, Zhou Y, et al. Synthesis and immunosuppressive activity of new artemisinin derivatives. 1. [12(beta or alpha)-dihydroartemisininoxy]phen(ox)yl aliphatic acids and esters. J Med Chem. 2005;48:4608–4617. doi: 10.1021/jm048979c. [DOI] [PubMed] [Google Scholar]
- You JQ, Mei JY, Xiao SH. Effect of artemether against Schistosoma japonicum. Zhongguo Yao Li Xue Bao. 1992;13:280–284. [PubMed] [Google Scholar]
- Yu Q, Gao Y. Systemic lupus erythematosus treated with artesunate. Zhongguo Pi Fu Xing Bing Xue Za Zhi. 1997;30:51–52. [Google Scholar]
- Zhang J, Kabra NH, Cado D, Kang C, Winoto A. FADD-deficient T cells exhibit a disaccord in regulation of the cell cycle machinery. J Biol Chem. 2001;276:29815–29818. doi: 10.1074/jbc.M103838200. [DOI] [PubMed] [Google Scholar]
- Zhang J, Salojin KV, Gao JX, Cameron MJ, Bergerot I, Delovitch TL. p38 mitogen-activated protein kinase mediates signal integration of TCR/CD28 costimulation in primary murine T cells. J Immunol. 1999;162:3819–3829. [PubMed] [Google Scholar]
- Zhang J, Zhong J, Shi Z, Dai X. Effects of lingdan and artesunate on T cell populations in human SLE. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2002;7:489–491. [Google Scholar]
- Zhang JX, Wang JX, Zhang Y, Zuo JP, Wu JM, Sui Y, et al. Synthesis and immunosuppressive activity of new artemisinin derivatives containing polyethylene glycol group. Yao Xue Xue Bao. 2006;41:65–70. [PubMed] [Google Scholar]
- Zhou WL, Wu JM, Wu QL, Wang JX, Zhou Y, Zhou R, et al. A novel artemisinin derivative, 3-(12-beta-artemisininoxy) phenoxyl succinic acid (SM735), mediates immunosuppressive effects in vitro and in vivo. Acta Pharmacol Sin. 2005;26:1352–1358. doi: 10.1111/j.1745-7254.2005.00232.x. [DOI] [PubMed] [Google Scholar]
- Zhu YN, Zhao WM, Yang YF, Liu QF, Zhou Y, Tian J, et al. Periplocoside E, an effective compound from Periploca sepium Bge, inhibited T cell activation in vitro and in vivo. J Pharmacol Exp Ther. 2006;316:662–669. doi: 10.1124/jpet.105.093732. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.