Abstract
During the past 18 years, sildenafil has evolved from a potential anti-angina drug to an on-demand treatment for erectile dysfunction and more recently to a new orally active treatment for pulmonary hypertension. Recent studies suggest that the drug has powerful cardioprotective effect against ischemia/reperfusion injury, doxorubicin-induced cardiomyopathy and anti-hypertensive effect induced by chronic inhibition of nitric oxide synthase in animals. Based on several recent basic and clinical studies, it is clear that sildenafil and other clinically approved type-5 phosphodiesterase-5 inhibitors including vardenafil and tadalafil will eventually be developed for several cardiovascular indications including essential hypertension, endothelial dysfunction, ischemia/reperfusion injury, myocardial infarction, ventricular remodeling and heart failure.
Keywords: Phosphodiesterase-5, hypertension, nitric oxide, hypertrophy, ischaemia, heart failure, endothelial function, cGMP, reperfusion injury, erectile dysfunction
Phosphodiesterase type-5 (PDE-5) inhibitors are a class of vasoactive drugs that have been developed for treatment of erectile dysfunction (ED) (Boolell et al., 1996; Porst et al., 2001). These compounds antagonize, PDE-5, which is found in most vascular beds as well as cardiac myocytes (Senzaki et al., 2001; Das et al., 2005). PDE-5 inhibitors prevent the breakdown of nitric oxide (NO)-driven cyclic guanosine monophosphate (cGMP), primarily in vascular smooth muscle cells, and therefore are potent vasodilators. PDE-5 enzyme is found in high abundance in the corpus cavernosum and in pulmonary artery smooth muscle, where its inhibition produces an increase in penile blood flow and a reduction in pulmonary vascular resistance, respectively (Sebkhi et al., 2003). Corbin and Francis (1999) showed that the regulatory domain in the amino-terminal portion of PDE-5 contains a phosphorylation site (Ser 92), two allosteric cGMP-binding sites and at least a portion of the dimerization domain. The catalytic domain in the carboxy-terminal portion of the protein contains the two Zn2+-binding motifs and a cGMP substrate-binding site. Most known PDE-5 inhibitors compete with the substrate cGMP for binding to the protein at the catalytic site. Although cGMP binding to the catalytic site stimulates cyclic-nucleotide binding to the allosteric sites, inhibitors do not elicit the same property, and Ser 92 phosphorylation has no effect on inhibitor binding (Corbin and Francis, 1999). Sildenafil citrate (Viagra) is the first PDE-5 inhibitor approved for treatment of ED. Two additional agents in this class tadalafil (Cialis) and vardenafil (Levitra) are also in clinical use for management of ED. Vardenafil has been shown in a rabbit model to increase intracavernosal pressure more quickly and to a greater extent than sildenafil (Saenz de Tejada et al., 2001). Vardenafil has a similar duration of action to sildenafil, but is more potent and selective biochemically. It has also been shown in clinical trials to have a high efficacy and low adverse-event profile in a population with mixed ED aetiologies (Porst et al., 2001). Tadalafil is a long-acting PDE-5 inhibitor which is effective for up to 36 h in the majority of men (Eardley and Cartledge, 2002). Except for the marked hypotensive effect of these drugs in men when combined with nitrates and α-blockers used to treat essential hypertension, a large body of evidence has shown that PDE-5 inhibitors are generally safe, being used by millions of patients around the world.
In recent years, there has been considerable interest in studying the effect of sildenafil and other PDE-5 inhibitors in protection against ischaemia/reperfusion injury (reviewed in Kukreja et al., 2004, 2005). We hypothesized that the vasodilator action of PDE-5 inhibitors could potentially release endogenous mediators of cardioprotection, including adenosine and bradykinin from endothelial cells, that may trigger a signalling cascade (through the action of kinases including protein kinase C and other mitogen-activated protein kinases) and generation of NO by phosphorylation of endothelial NO synthase (eNOS). NO activates guanlylate cyclase resulting in enhanced formation of cGMP, which activates protein kinase G (PKG). PKG can subsequently open mitochondrial KATP channels resulting in cardioprotective effects against ischaemia/reperfusion injury (Kukreja et al., 2004, 2005; Das et al., 2006). We initially showed that rabbits treated with sildenafil or vardenafil had reduced infarct size after ischaemia/reperfusion (Ockaili et al., 2002; Salloum et al., 2006). Recently, Sesti et al. (2006) also confirmed these results with tadalafil. The protective effect of sildenafil has been duplicated in several other experimental models including infant rabbits (Bremer et al., 2005), the mouse isolated perfused heart (Salloum et al., 2003), adult cardiac myocytes subjected to simulated ischaemia and reoxygenation in vitro (Das et al., 2005) and doxorubicin-induced cardiomyopathy in mice (Fisher et al., 2005). Sildenafil preconditioning has also been shown to reduce serious ventricular arrhythmias during ischaemia in dogs (Nagy et al., 2004) . Very recently, we demonstrated that administration of sildenafil or vardenafil at reperfusion after lethal ischaemia reduced infarct size by opening the mitochondrial KATP channel, similar to the preconditioning-like effect (Salloum et al., 2006). In contrast, the antianginal drug, nitroglycerine failed to exhibit similar infarct-limiting effects, suggesting that the protection at reperfusion was selectively due to inhibition of PDE-5. In general, there is overwhelming evidence for the cardioprotective effect of PDE-5 inhibitors against ischaemia/reperfusion injury.
PDE-5 inhibitors are also highly effective vasodilators in the systemic circulation. A recent clinical study suggested that PDE-5 inhibitors might be a new class of drug that can potentially be used for the treatment of essential hypertension (Oliver et al., 2006). Active treatment with sildenafil significantly reduced both ambulatory and clinical blood pressure by an extent similar to that observed with the other classes of antihypertensive drugs. PDE-5 inhibitors may also be effective in the treatment of patients with heart failure because of their effects on preload and afterload. Endothelial function is severely limited in chronic congestive heart failure and sildenafil has been shown to improve endothelial function in such patients (Katz et al., 2000).
An important property of sildenafil is its ability to increase eNOS and inducible NO synthase proteins in the heart (Salloum et al., 2003) and cardiomyocytes (Das et al., 2005), and this has a direct cause and effect relationship in protection against myocardial infarction (Salloum et al., 2003) and simulated ischaemia/reoxygenation-induced necrosis, as well as apoptosis in cardiomyocytes (Das et al., 2005). Because of the ability of sildenafil to augment NO synthase (NOS) and cGMP levels, it is logical to hypothesize that cardiovascular dysfunction induced by chronic NOS inhibition would be alleviated by concomitant treatment with sildenafil. In this issue of the British Journal of Pharmacology, Rossoni et al. (2007) describe the beneficial effect of chronic sildenafil therapy on cardiovascular alterations induced by NOS inhibition with a non-selective NOS inhibitor, Nω-nitro-L-arginine methyl ester (L-NAME). Sildenafil was given orally at doses of 0.37, 0.75 or 1.5 mg kg−1 day−1 for 4 weeks, either alone or with L-NAME (35–40 mg kg−1 day−1) in the drinking water. The results show that sildenafil dose-dependently prevented the increase of systolic blood pressure, improved post-ischaemic functional recovery and reduced the release of creatine kinase and lactate dehydrogenase in the L-NAME-treated rats. Moreover, sildenafil restored the drop in myocardial cGMP and cAMP, the urinary levels of NO and attenuated the excretion of the vasoconstrictor metabolites of arachidonic acid including thromboxane B2, 6-keto-PGF1α and 8-iso-PGF2α in L-NAME-treated rats. These results support the concept that sildenafil and possibly other PDE-5 inhibitors have antihypertensive as well as cardioprotective effects against ischaemia/reperfusion injury. The authors further showed that sildenafil therapy restored the vasorelaxant effect of acetylcholine in L-NAME-treated rats, which is in line with previous studies showing improvement of endothelium-dependent vasodilatation with sildenafil (Katz et al., 2000; Gori et al., 2005). In general, these data are very interesting because the authors were the first to provide evidence for the beneficial effect of sildenafil against cardiovascular injury in an experimental setting where NOS was chronically inhibited.
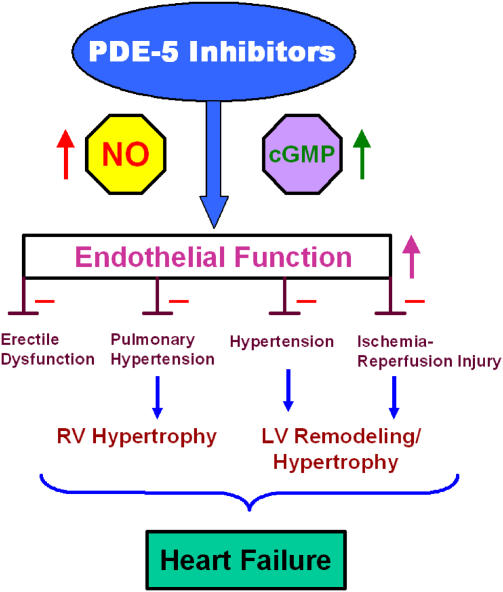
Since 1989, sildenafil has evolved from a potential antiangina drug to an on-demand oral treatment for ED and more recently to a new orally active treatment for pulmonary hypertension (Revatio) (Ghofrani et al., 2006). Based on several clinical and animal studies including those of Rossoni et al. (2007), it is clear that sildenafil and other PDE-5 inhibitors will eventually be developed for a number of cardiovascular indications including essential hypertension, endothelial dysfunction, ischaemia/reperfusion injury, myocardial infarction, ventricular remodelling and heart failure as outlined in Figure 1.
Figure 1.
Cardiovascular protection with PDE-5 inhibitors. See text for description. Abbreviations: RV, right ventricle; LV, left ventricle.
Acknowledgments
This work was supported by grants HL51045, HL59469 and HL079424 from the National Institutes of Health.
Abbreviations
- cAMP
cyclic adenosine monophosphate
- cGMP
cyclic gunosine monophosphate
- ED
erectile dysfunction
- eNOS
endothelial nitric oxide synthase
- L-NAME
Nω-nitro-L-arginine methyl ester
- NO
nitric oxide
- NOS
nitric oxide synthase
- PDE-5
phosphodiesterase-5
- PKG
protein kinase G
References
- Boolell M, Allen MJ, Ballard SA, Gepi-Attee S, Muirhead GJ, Naylor AM, et al. Sildenafil: an orally active type 5 cyclic GMP-specific phosphodiesterase inhibitor for the treatment of penile erectile dysfunction. Int J Impot Res. 1996;8:47–52. [PubMed] [Google Scholar]
- Bremer YA, Salloum F, Ockaili R, Chou E, Moskowitz WB, Kukreja RC. Sildenafil citrate (Viagra) induces cardioprotective effects after ischemia/reperfusion injury in infant rabbits. Pediat Res. 2005;57:22–27. doi: 10.1203/01.PDR.0000147736.27672.15. [DOI] [PubMed] [Google Scholar]
- Corbin JD, Francis SH. Cyclic GMP phosphodiesterase-5: target of sildenafil. J Biol Chem. 1999;274:13729–13732. doi: 10.1074/jbc.274.20.13729. [DOI] [PubMed] [Google Scholar]
- Das A, Smolenski A, Lohmann SM, Kukreja RC. Cyclic GMP-dependent protein kinase Iá attenuates necrosis and apoptosis following ischemia/reoxygenation in cardiomyocytes. J Biol Chem. 2006;281:38644–38652. doi: 10.1074/jbc.M606142200. [DOI] [PubMed] [Google Scholar]
- Das A, Xi L, Kukreja RC. Phosphodiesterase-5 inhibitor, sildenafil preconditions adult cardiac myocytes against necrosis and apoptosis: essential role of NO signaling. J Biol Chem. 2005;280:12944–12955. doi: 10.1074/jbc.M404706200. [DOI] [PubMed] [Google Scholar]
- Eardley I, Cartledge J. Tadalafil (Cialis) for men with erectile dysfunction. Int J Clin Pract. 2002;56:300–304. [PubMed] [Google Scholar]
- Fisher P, Salloum F, Das A, Hyder H, Kukreja RC. Phosphodiesterase-5 Inhibition with sildenafil attenuates cardiomyocyte apoptosis and left ventricular dysfunction in a chronic model of doxorubicin cardiotoxicity. Circulation. 2005;42:219–232. doi: 10.1161/01.CIR.0000160359.49478.C2. [DOI] [PubMed] [Google Scholar]
- Ghofrani HA, Osterloh IH, Grimminger F. Sildenafil: from angina to erectile dysfunction to pulmonary hypertension and beyond. Nat Rev Drug Discov. 2006;5:689–702. doi: 10.1038/nrd2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gori T, Sicuro S, Dragoni S, Donati G, Forconi S, Parker JD. Sildenafil prevents endothelial dysfunction induced by ischemia and reperfusion via opening of adenosine triphosphate-sensitive potassium channels: a human in vivo study. Circulation. 2005;111:742–746. doi: 10.1161/01.CIR.0000155252.23933.2D. [DOI] [PubMed] [Google Scholar]
- Katz SD, Balidemaj K, Homma S, Wu H, Wang J, Maybaum S. Acute type 5 phosphodiesterase inhibition with sildenafil enhances flow-mediated vasodilation in patients with chronic heart failure. J Am Coll Cardiol. 2000;36:845–851. doi: 10.1016/s0735-1097(00)00790-7. [DOI] [PubMed] [Google Scholar]
- Kukreja RC, Ockaili R, Salloum F, Yin C, Hawkins J, Das A, et al. Cardioprotection with phosphodiesterase-5 inhibition – a novel preconditioning strategy. J Mol Cell Cardiol. 2004;36:165–173. doi: 10.1016/j.yjmcc.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Kukreja RC, Salloum F, Das A, Ockaili R, Yin C, Bremer YA, et al. Pharmacological preconditioning with sildenafil: basic mechanisms and clinical implications. Vascul Pharmacol. 2005;42:219–232. doi: 10.1016/j.vph.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Nagy O, Hajnal A, Parratt JR, Agnes V. Sildenafil (Viagra) reduces arrhythmia severity during ischaemia 24 h after oral administration in dogs. Br J Pharmacol. 2004;141:549–551. doi: 10.1038/sj.bjp.0705658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ockaili R, Salloum F, Hawkins J, Kukreja RC. Sildenafil (Viagra) induces powerful cardioprotective effect via opening of mitochondrial KATP channels in rabbits. Am J Physiol. 2002;283:H1263–H1269. doi: 10.1152/ajpheart.00324.2002. [DOI] [PubMed] [Google Scholar]
- Oliver JJ, Melville VP, Webb DJ. Effect of regular phosphodiesterase type 5 inhibition in hypertension. Hypertension. 2006;48:622–627. doi: 10.1161/01.HYP.0000239816.13007.c9. [DOI] [PubMed] [Google Scholar]
- Porst H, Rosen R, Padma-Nathan H, Goldstein I, Giuliano F, Ulbrich E, et al. The efficacy and tolerability of vardenafil, a new, oral, selective phosphodiesterase type 5 inhibitor, in patients with erectile dysfunction: the first at-home clinical trial. Int J Impot Res. 2001;13:192–199. doi: 10.1038/sj.ijir.3900713. [DOI] [PubMed] [Google Scholar]
- Rossoni G, Manfredi B, De Gennaro Colonna V, Berti M, Guazzi M, Berti F.Sildenafil reduces L-NAME-induced severe hypertension and worsening of myocardial ischaemia-reperfusion damage in the rat Br J Pharmacol 2007150567–576.(this issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saenz de Tejada I, Angulo J, Cuevas P, Fernandez A, Moncada I, Allona A, et al. The phosphodiesterase inhibitory selectivity and the in vitro and in vivo potency of the new PDE-5 inhibitor vardenafil. Int J Impot Res. 2001;13:282–290. doi: 10.1038/sj.ijir.3900726. [DOI] [PubMed] [Google Scholar]
- Salloum F, Ockaili R, Michael Wittkamp M, Marwaha VR, Kukreja RC. Vardenafil: a novel type 5 phosphodiesterase inhibitor reduces myocardial infarct size following ischemia/reperfusion injury via opening of mitochondrial KATP channels in rabbits. J Mol Cell Cardiol. 2006;40:405–411. doi: 10.1016/j.yjmcc.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Salloum F, Takenoshita Y, Ockaili R, Daoud VP, Chou E, Yoshida KI, et al. Sildenafil and vardenafil but not nitroglycerin limit myocardial infarction through opening of mitochondrial KATP channels when administered at reperfusion following ischemia in rabbits J Mol Cell Cardiol 2006. Dec 6 [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- Salloum F, Yin C, Xi L, Kukreja RC. Sildenafil (Viagra) induces delayed preconditioning through iNOS-dependent pathway in mouse heart. Circ Res. 2003;92:595–597. doi: 10.1161/01.RES.0000066853.09821.98. [DOI] [PubMed] [Google Scholar]
- Sebkhi A, Strange JW, Phillips SC, Wharton J, Wilkins MR. Phosphodiesterase type 5 as a target for the treatment of hypoxia-induced pulmonary hypertension. Circulation. 2003;107:3230–3235. doi: 10.1161/01.CIR.0000074226.20466.B1. [DOI] [PubMed] [Google Scholar]
- Senzaki H, Smith CJ, Juang GJ, Isoda T, Ohler A, Paolocci N, et al. Cardiac phosphodiesterase 5 (cGMP specific) modulates beta-adrenergic signaling in vivo and is down-regulated in heart failure. FASEB J. 2001;15:1718–1726. doi: 10.1096/fj.00-0538com. [DOI] [PubMed] [Google Scholar]
- Sesti C, Florio V, Johnson EG, Kloner RA.The phosphodiesterase-5 inhibitor tadalafil reduces myocardial infarct size Int J Impot Res 2006. July 20 [Epub ahead of print] [DOI] [PubMed]