Abstract
Background and purpose:
Obesity is a risk factor for several inflammation-based diseases including arthritis. We investigated the anti-nociceptive and anti-inflammatory effects of the cannabinoid CB1 receptor antagonist rimonabant in lean and diet-induced obese female rats with arthritis induced by complete Freund's adjuvant (CFA) injected in the right hind-paw.
Experimental approach:
The effect of oral rimonabant was assessed in rat paws on thermal hyperalgesia, mechanical allodynia, oedema, global arthritis score, nitrite/nitrate levels and ankle widths.
Key results:
After 7 but not after 14 days, the inflammatory response to CFA was significantly higher in obese than lean rats; however, the nociceptive response (thermal hyperalgesia and mechanical allodynia) was similar. Oral rimonabant (3 or 10 mg kg−1, once a day for 1 week from day 7 after CFA) only reduced the global arthritic score and joint width in obese rats, with no effect on the paw oedema. It also markedly reduced thermal hyperalgesia and mechanical allodynia in both lean and obese rats, with a greater effect in the latter.
Conclusion and implications:
Rimonabant appears to be a potent inhibitor of sensorial hypersensitivity associated with CFA-induced arthritis in obese rats, in which the inflammatory reaction is more severe than in lean rats. It may thus have therapeutic potential in obesity-associated inflammatory diseases, particularly in the treatment of the pain associated with arthritis.
Keywords: rimonabant, cannabinoid, obesity, arthritis, hyperalgesia, allodynia, inflammation, rats, SR141716, pain
Introduction
During the last few years, there has been increasing evidence that obesity is associated with chronic systemic low-grade inflammation (Berg and Scherer, 2005; Gimeno and Klaman, 2005). Adipocytes secrete several pro-inflammatory cytokines (adipokines), including interleukin-6 (IL-6) and tumour necrosis factor-α (TNFα), the production of which rises with the mass of adipose tissue (Fantuzzi, 2005; Hauner, 2005; Trayhurn, 2005). It is also well known that obesity is a risk factor for a number of serious chronic diseases having a common inflammatory basis, with arthritis being one of the most frequent, after diabetes (Mokdad et al., 2001; Mehrotra et al., 2004).
Rimonabant (SR141716) (N-piperidino-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4methyl-pyrazole-3-carboxamide) is a potent and selective cannabinoid CB1 receptor antagonist possessing food intake inhibiting and antiobesity activity (Rinaldi-Carmona et al., 1994, 1995; Colombo et al., 1998; Simiand et al., 1998; Ravinet Trillou et al., 2003; Carai et al., 2005; Jbilo et al., 2005). It is widely used as a tool to investigate the mechanisms by which cannabinoid agonists produce their pharmacological effects and it may exert several intrinsic actions possibly by blocking the activation of cannabinoid CB1 receptors by the endocannabinoid system, which is tonically activated under certain pathophysiological conditions (Di Marzo and Matias, 2005; Engeli et al., 2005; Matias et al., 2006).
A recent clinical study in obese patients with dyslipidemia (Després et al., 2005) found rimonabant reduced not only body weight, but also a number of cardiovascular metabolic risk factors. Besides its antiobesity action and inhibitory effect on food intake, rimonabant reduced nociceptive responses and inflammation in several experimental animal models; in rats and mice, the drug prevented the rise in TNFα serum levels induced by Escherichia coli lipopolysaccharide, reduced indomethacin-induced small intestinal lesions and relieved neuropathic pain after sciatic nerve ligature (Smith et al., 2000; Croci et al., 2003; Costa et al., 2005). The antinociceptive effect after repeated treatment is an important finding, as pain owing to peripheral nerve injury from a number of causes is still a major challenge for clinicians. This type of pain is generally unresponsive to most analgesics (MacFarlane et al., 1997; Harden, 2005) and less responsive than physiological pain to opioids (Mao et al., 1995; Ossipov et al., 1995; Idanpaan-Heikkila and Guilbaud, 1999).
Considering its antiobesity, anti-inflammatory and antinociceptive actions, we compared the effect of this cannabinoid CB1 antagonist on inflammation and pain in a model of adjuvant-induced arthritis in lean and diet-induced obese female rats (DIO rats). Arthritis was induced unilaterally by injecting complete Freund's adjuvant (CFA) into the right hind paw. This model is useful for studying neurogenic inflammation and the associated sensorial hypersensitivity (McDougall et al., 1994; Decaris et al., 1999; Naeini et al., 2005) that are often difficult to measure in models of polyarthritis (Schaible et al., 2005).
Methods
Animals and treatments
The experiments were carried out on either lean or obese female Crl:CD BR rats (Charles River, Lecco, Italy) of the same age (40 weeks), weighing 320±3.3 and 530±12 g, respectively. They were conducted in accordance with internationally accepted principles for care of laboratory animals (EEC council Directive 86/609, OJ L 358, 1,12 December, 1987) and with the guidelines for the study of pain in conscious animals established by the International Association for the Study of Pain. The experimental protocol was approved at corporate level by the Animal Care and Use Committee of Sanofi-aventis Research.
Obesity was induced by a high-fat diet (4.7 kcal g−1, 49% of calories from fats, 19% from proteins and 32% from carbohydrates; TD97366, Teklad, USA) administered from the age of 4 weeks, whereas the lean rats were given a conventional pellet diet (2.7 kcal g−1, 6.5% of calories from fats, 27% from proteins, 60% from carbohydrates and 6.5% from fibre; 4RF 21, Mucedola, Italy). The animals were housed under controlled environmental conditions (22±1°C, 55±15% relative humidity, 12 h light from 06 h 30 min to 18 h 30 min) with water and food ad libitum.
Unilateral arthritis was induced by intraplantar (i.pl.) injection of CFA into the right hind paw. CFA consisted of a paraffin oil suspension (1 mg ml−1) of heat-killed Mycobacterium tuberculosis (Sigma, St Louis, MI, USA). Test animals received 0.15 ml CFA and control rats were injected with the same volume of saline. CFA induced a unilateral paw arthritis at the site of injection, with no involvement of other joints.
Rimonabant was administered orally daily for 7 days, at doses of 3 or 10 mg kg−1, starting on the 7th day after the CFA injection. The compound was suspended in 10% Tween 80 in distilled water and administered by gavage in a volume of 2 ml kg−1 b.w.
Evaluation of inflammatory reaction and arthritis
The inflammatory reaction to CFA was evaluated by measuring (a) paw oedema volume on day 7 after the CFA injection and at the end of the week's rimonabant treatment (day 14); (b) ankle width on day 14, 24 h following the last drug treatment; (c) arbitrary arthritis score on day 7 before the first dose of rimonabant, day 10 before the fourth rimonabant treatment and day 14, 24 h following the last drug treatment.
The oedema volume was defined as the difference between the volume of the CFA-injected paw and the contralateral hind paw, measured with a plethysmometer (2Biological Instruments, Varese, Italy). Ankle widths of injected and contralateral hind paws were measured using a digital caliper (0.01 mm resolution; 2 Biological Instruments, Italy).
The arthritis was rated in a blind manner using the following arbitrary scale: 0 is negative, 1 is swollen paw, 2 is swollen paw and joint plus erythema and 3 is pronounced oedematous paw and swollen joint (Williams et al., 1992).
Nociceptive tests
Thermal hyperalgesia was evaluated by recording the paw withdrawal latencies to a radiant thermal stimulus according to Hargreaves' method (plantar test), using a specific instrument (Ugo Basile, Varese, Italy) (Hargreaves et al., 1988). Animals were placed in a clear acrylic box on a glass platform and allowed to acclimatize. A constant-intensity radiant-heat source was applied through the platform to the plantar surface of the hind paw. The response to the stimulus was recorded by a photocell detecting withdrawal of the paw and the latency expressed in seconds. Thermal hyperalgesia was evaluated in the injected and contralateral hind paw before, 7 and 14 days (24 h after the last drug treatment) following CFA injection.
Mechanical allodynia was assessed according to the method described by von Frey (Villetti et al., 2003), using an instrument that measures the reaction to a mechanical stimulus (Plantar Aesthesiometer, Ugo Basile, Varese, Italy). Briefly, rats were placed on a metal mesh table and after acclimatization, an electronic needle was pushed against the plantar foot surface with ascending mechanical force (50 g over a 20 s period) until the rat withdrew the paw. Mechanical allodynia was evaluated by measuring the withdrawal threshold (in grams) in the injected and contralateral hind paw before, 7 and 14 days (24 h after the last drug treatment) following CFA injection.
Nitrites/nitrates
Nitric oxide (NO) production in the rat paw was assessed, after the rat had been killed on day 14, on the basis of the levels of nitrites/nitrates, which are the oxidation end-products of NO. The paw tissue was crushed and homogenized with a T25, 18N Ultra-Turrax in 1 : 4 (w : v) phosphate buffer (50 mM, pH 7.4) then centrifuged at 10 000 g for 20 min at 4°C. The supernatant fraction was centrifuged at 100 000 g for 15 min, 4°C and the new supernatant was filtered through a Vivaspin 10 000 MWCO (10 000 g for 30 min, 4°C) (Vivascience, Hannover, Germany). Nitrite/nitrate concentrations were measured using a colorimetric assay kit purchased from Cayman Chemicals (Ann Arbor, MI, USA). The optical density was determined at 540 nm, employing a microplate reader (Spectra Count, Packard, PerkinElmer, Wellesley, MA, USA), and nitrite/nitrate content in the paw was calculated from a standard curve and expressed as nmol g−1 wet tissue.
Data analysis
Data are expressed as the mean±s.e.m. and analysed using one-way analysis of variance (ANOVA) or two-way ANOVA followed by Newman–Keuls' test, as indicated in the figure legends. A probability level <0.05 was accepted as significant.
Drug
Rimonabant (SR141716) was synthesized at Sanofi-aventis Recherche, Montpellier, France.
Results
Body weight
CFA injection induced a slight but not significant decrease in body weight in both lean and obese animals.
Rimonabant (3 and 10 mg kg−1 day−1) after 7 days treatment reduced significantly body weight only in obese rats (Table 1).
Table 1.
Effect of rimonabant on body weight (Δ%) in control and CFA-injected rats
| Lean rat | Obese rat | |
|---|---|---|
| Controls | 2.60±2.08 | 0.59±0.84 |
| Controls+ R 3 mg kg−1 | −1.05±0.75 | −6.50±0.79* |
| Controls+ R 10 mg kg−1 | −0.95±1.37 | −7.09±0.50* |
| CFA | 0.94±0.79 | −0.75±0.27 |
| CFA+ R 3 mg kg−1 | 2.59±0.82 | −8.13±0.34° |
| CFA+ R 10 mg kg−1 | −2.79±1.20 | −9.52±0.45° |
Abbreviation: CFA, complete Freund's adjuvant.
Data are mean±s.e.m. from 5 to 12 rats. Body weight was expressed as a Δ% from mean body weight of day 7. Rats body weight was on day 0: 320±3.3 g lean, 514.0±6.1 g obese; on day 7 after CFA injection was 314.0±0.9 g lean controls, 311.0±0.87 g lean CFA, 518.0±7.8 g obese control, 507.7±4.5 g obese CFA.
P<0.05 vs controls
P<0.05 vs CFA (one-way ANOVA plus Newman–Keuls' test).
Inflammation
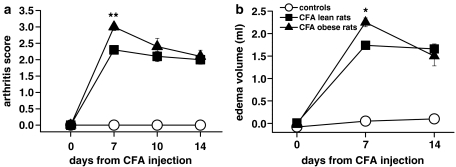
An intra-plantar injection of CFA into the right paw produced a more marked inflammatory response in the obese than in the lean rats (Figure 1). Seven days after CFA injection, the arthritis score and the oedema volume were significantly higher in obese than in lean rats (by 21 and 26%, respectively: Figure 1a and b). From days 7–14 the inflammatory reaction tended to subside in obese but not in lean rats, so that 2 weeks after the injection both oedema volume and arthritis score were similar in the two groups.
Figure 1.
Arthritis score (a) and paw oedema (b) in lean and obese rats injected with CFA in the right hind paw. Rats received CFA or saline on day 0. The severity of arthritis was rated using an arbitrary score, as described in the Methods. The oedema volume was measured with a plethysmometer. Data are mean±s.e.m. (vertical lines) from 6 to 12 rats. *P<0.05, **P<0.001 vs CFA lean rats (two-way ANOVA plus Newman–Keuls test).
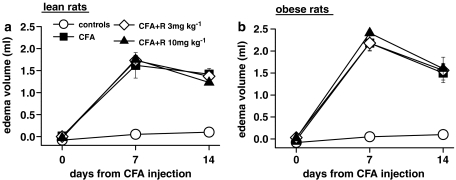
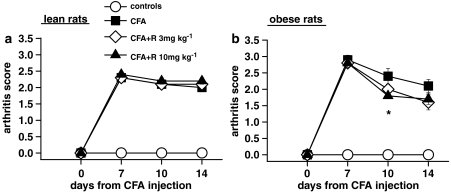
Rimonabant (3 and 10 mg kg−1 day−1 for 7 days) had no effect on paw oedema in lean or obese rats (Figure 2a and b). It also had no effect on the global arthritis score in lean rats (Figure 3a), but it significantly reduced the severity of arthritis in obese rats after 3 days of treatment at 10 mg kg−1 (Figure 3b). This effect was also greater in obese compared to lean rats at the end of treatment, but it did not reach the same level of significance.
Figure 2.
Effect of a 7-day treatment with rimonabant (R) on paw oedema in lean (a) and obese (b) rats injected with CFA in the right hind paw. Rats received CFA or saline on day 0 and were given rimonabant daily from day 7 to day 14. The oedema volume was measured with a plethysmometer. Data are mean±s.e.m. (vertical lines) from 5 to 12 rats.
Figure 3.
Effect of a 7-day treatment with R on arthritis score in lean (a) and obese (b) rats injected with CFA in the right hind paw. The severity of arthritis was rated using an arbitrary score, as described in the Methods. Rats received CFA or saline on day 0 and were given rimonabant daily from day 7 to day 14. Data are mean±s.e.m. (vertical lines) from 5–12 rats. *P<0.05 vs CFA only (two-way ANOVA plus Newman–Keuls test).
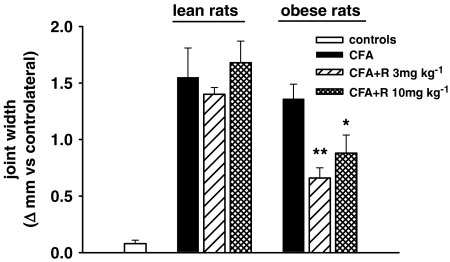
After 7 days of treatment, rimonabant significantly reduced the ankle width of the arthritic paw, but only in the obese rats (Figure 4). Rimonabant had no effect on paw volume and ankle width in either lean and obese control rats (paw volume and ankle width, mean±s.e.m.: in lean rats 1.85±0.01 ml and 7.07±0.10 mm at 3 mg kg−1, 1.94±0.02 ml and 7.02±0.05 mm at 10 mg kg−1, 1.90±0.02 ml and 6.84±0.16 mm vehicle; in obese rats 2.31±0.06 ml and 4.63±0.24 mm at 3 mg kg−1, 2.17±0.05 ml and 5.00±0.16 mm at 10 mg kg−1, 2.49±0.17 ml and 4.75±0.25 mm vehicle).
Figure 4.
Effect of a 7-day treatment with rimonabant (R) on the ankle joint width in lean and obese rats injected with CFA in the right hind paw. Columns indicate the ankle width expressed as Δ mm from the contralateral joint after 7 days of rimonabant treatment (day 14). Data are mean±s.e.m. (vertical lines) from 5 to 12 rats. *P<0.05, **P<0.001 vs CFA only (one-way ANOVA plus Newman–Keuls test).
Response to nociceptive tests
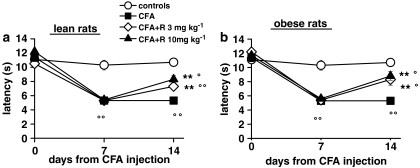
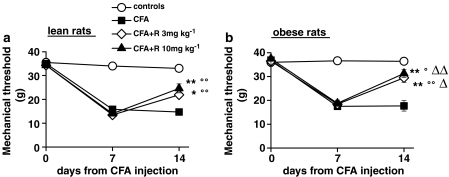
The results of CFA-induced sensorial hypersensitivity measured by the plantar test (thermal hyperalgesia) or von Frey test (mechanical allodynia) in lean and obese rats are shown in Figures 5 and 6. The response latency to thermal hyperalgesia and the threshold to mechanical stimulation were similar in both lean and obese rats before, 7 and 14 days after CFA. Rimonabant (10 mg kg−1 24 h before the behavioural tests) had no effect on thermal hyperalgesia and allodynia after acute administration in CFA injected lean and obese animals (data not showed). After 7 days of treatment (day 14), rimonabant (3 and 10 mg kg−1 day−1) markedly reduced thermal hyperalgesia, similarly in lean and obese rats (Figure 5). The recovery of latency values towards control levels, calculated as the difference between vehicle and CFA-injected animals for 3 and 10 mg kg−1 rimonabant, was shown to be 44 and 62% in lean and 53 and 69% in obese rats. Unlike thermal hyperalgesia, in the mechanical allodynia test, rimonabant was significantly more effective in obese (recovery from control levels 68 and 78%, respectively) than lean rats (39 and 59%) (Figure 6a and b). Rimonabant repeated treatment did not affect pain responses in either lean or obese control animals (mechanical threshold and thermal latency, mean±s.e.m.: in lean rats, 36.0±2.5 g and 10.4±0.4 s at 3 mg kg−1, 34.7±1.9 g and 10.4±0.2 s at 10 mg kg−1, 33.0±1.3 g and 10.2±0.3 s vehicle; in obese rats, 37.5±2.0 g and 11.5±0.5 s at 3 mg kg−1, 37.2±0.9 g and 9.9±0.4 s at 10 mg kg−1, 35.5±1.5 g and 10.8±0.6 s vehicle).
Figure 5.
Effect of a 7-day treatment with rimonabant (R) on thermal hyperalgesia in lean (a) and obese (b) rats injected with CFA in the right hind paw. Thermal hyperalgesia was tested by the plantar test as described in the Methods. Results are expressed as response latency in seconds. Rats were given rimonabant daily from day 7 to 14. Data are means±s.e.m. (vertical lines) from 5 to 12 rats. **P<0.001 vs CFA only; °P<0.05, °°P<0.001 vs controls (two-way ANOVA plus Newman–Keuls test).
Figure 6.
Effect of a 7-day treatment with rimonabant (R) on mechanical allodynia in lean (a) and obese (b) rats injected with CFA in the right hind paw. Mechanical allodynia was tested by the von Frey test as described in the Methods. Results are expressed as the mechanical response threshold in grams. Rats were given rimonabant daily from day 7 to 14. Data are means±s.e.m. (vertical lines) from 5 to 12 rats. *P<0.05, **P<0.001 vs CFA only; °P<0.05, °°P<0.001 vs controls; ΔP<0.05, ΔΔP<0.001 vs rimonabant treated lean rats (two-way ANOVA plus Newman–Keuls test).
Effect on nitrates/nitrites
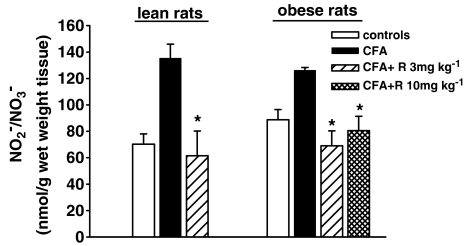
As shown in Figure 7, the end products of NO oxidation reached slightly higher levels in obese than lean rats, but the difference was not significant. Fourteen days after CFA injection, nitrates/nitrites in the inflamed hind paw were about double control levels in lean rats and about 40% higher in obese rats. The physiological levels of nitrites/nitrates in control animals were not affected by repeated doses of rimonabant (data not shown). In lean and obese CFA-treated rats rimonabant significantly reduced nitrite/nitrate levels.
Figure 7.
Effect of a 7-day treatment with rimonabant (R) on nitrates and nitrites in the right hind paw injected with CFA in lean and obese rats. Data are mean±s.e.m. (vertical lines) from 5 to 12 rats. *P<0.05 vs CFA only (one-way ANOVA plus Newman–Keuls test).
Discussion
The main aim of this study was to compare the anti-inflammatory and antinociceptive activities of the cannabinoid CB1 receptor antagonist rimonabant in lean and obese rats with unilateral arthritis induced by injecting CFA in the right hind paw plantar area. Lean and obese arthritic animals showed a similar nociceptive response to painful thermal stimulus and mechanical allodynia. However, 7 days after the adjuvant injection, obese rats had a higher arthritic score and developed more severe paw oedema than lean rats. After 14 days, the inflammation slightly decreased and there was no significant difference between lean and obese animals.
These findings are in line with the current view that obesity is a mild inflammatory condition (Fantuzzi, 2005; Gimeno and Klaman, 2005; Trayhurn, 2005) and, as in our study, it may increase the susceptibility to an inflammatory stimulus or cause the symptoms to be worse in obese arthritic animals compared to lean ones. An increased vascular inflammatory reaction has also been demonstrated in obese rodents (Barbato et al., 2005; Vachharajani et al., 2005).
In man, obesity facilitates the development of chronic inflammatory diseases (Berg and Scherer, 2005; Gimeno and Klaman, 2005) such as arthritis and Crohn's disease (Mokdad et al., 2001; Mehrotra et al., 2004). It has been suggested that the elevated TNFα-plasma levels in obese subjects may contribute to their increased susceptibility to inflammation (Hauner, 2005). It is also worth mentioning that in patients with dyslipidemia, rimonabant reduced a number of cardiovascular risk factors that may eventually predispose the patients to chronic inflammatory diseases (Després et al., 2005).
After 7 days of repeated oral treatment, rimonabant (3 and 10 mg kg−1) reduced the body weight but only slightly decreased the severity of arthritis (lower global arthritic score and joint width, with no effect on paw oedema) in obese but not in lean rats. The drug also reduced NO production in the inflamed right hind paw, as indirectly indicated by the lower concentrations of nitrates and nitrites. A role for NO in pain has been suggested by many other studies (Tedesco et al., 2002; Laurido et al., 2003).
The principle finding of this study is that chronic treatment with rimonabant has an antinociceptive action and this occurs at doses similar to those shown to inhibit a number of central and/or peripheral cannabinoid CB1 receptor-mediated responses, such as those involved in food intake, gastrointestinal transit, TNFα production, indomethacin lesions and neuropathic pain (Rinaldi-Carmona et al., 1994; Smith et al., 2000; Croci et al., 2003; Costa et al., 2005). The drug potently reduced thermal hyperalgesia and mechanical allodynia in both lean and obese arthritic animals. These effects were more prominent in obese than lean rats; the maximal effect of rimonabant either on inflammation, nociception or body weight loss was apparently reached at the dose of 3 mg kg−1. The antinociceptive activity is noteworthy, as rimonabant treatment was started on the 7th day after the onset of the disease with nociceptive response recorded 24 h after the last administration of the drug, therefore suggesting that the drug's ability to improve established disease may have therapeutic implications.
In this study, rimonabant alone had no intrinsic effect on nociception in either control lean or control obese female rats that were not injected with CFA. This result is in apparent contrast with those from our previous study (Costa et al., 2005), where it was shown that a slight but significant hyperalgesic response to rimonabant occurs in male Wistar rats. However, strain and gender differences might explain this discrepancy. It is also noteworthy that this intrinsic effect of rimonabant was not observed in mice (Rinaldi-Carmona et al., 1994; Welch et al., 1998; Costa et al., 2005).
The antinociceptive effect of rimonabant in chronic constriction injury of the sciatic nerve (CCI) in the rat (Costa et al., 2005) and in CFA-induced monoarthritis (present study) was time dependent and only observed after repeated dosing, suggesting that the drug-induced pain relief was not, owing to a direct action on the pain signalling system (Costa et al., 2005). This antinociceptive action markedly differs from that shown by cannabinoid CB1 agonists, which appear to have analgesic activity after both acute and chronic treatment (Fox et al., 2001; Lim et al., 2003; Cravatt and Lichtman, 2004). It thus seems paradoxical that rimonabant, which inhibits the analgesic effect of CB1 agonists with no intrinsic agonist activity at native CB1 receptors, was effective in reducing a similar type of pain in CCI and CFA models after repeated treatment. As the drug selectively antagonized cannabinoid CB1 receptors, its inhibition of sensorial hyper-reactivity is possibly related to the antagonism at this cannabinoid receptor subtype. Furthermore, CB1 receptors seem to be required for the antihyperalgesic action of rimonabant, as it was not able to provide pain relief in CB1 knockout mice with chronic injury of the sciatic nerve (Costa et al., 2005). Both CB1 and CB2 receptors have been shown to be involved in nerve injury hyperalgesia in several animal models (Fox et al., 2001; Lim et al., 2003; Cravatt and Lichtman, 2004; LaBuda et al., 2005; Whiteside et al., 2005). Chronic pain is a constant symptom in arthritis, often neurogenic in nature, resulting from injury of the local nerves through inflammation and tissue damage (Zimmermann, 2001; Ueda, 2006). In experimental arthritic models, the neurogenic component of inflammation is involved in the genesis and maintenance of the disease (for a review, see Schaible et al., 2005). In the rat model of CFA-induced monoarthritis, electrophysiological and morphological alterations of somato-sensory synaptic function have been described (Naeini et al., 2005). Several clinical observations also support the theory that neurogenic inflammation plays a significant role in the pathogenesis of rheumatoid arthritis (Schaible et al., 2005).
In neurogenic inflammatory pain, including that of arthritis, many cytokines, especially TNFα, play key roles in the generation and maintenance of hyperalgesia (Schafers et al., 2003; Sommer and Kress, 2004; Inglis et al., 2005; Schaible et al., 2005; Ueda, 2006) and TNFα-blocking drugs, now used in arthritis therapy, reduce hyperalgesia in peripheral neuropathy (Sommer et al., 2001; Choo-Kang et al., 2005). In rats with sciatic nerve constriction injury, the antihyperalgesic activity of rimonabant was also associated with reversal of the lesion-induced rise in spinal cord TNFα levels and degeneration of myelinated fibers (Costa et al., 2005). It is conceivable that, in addition to its TNFα-inhibitory effect, rimonabant has a direct trophic action on nerves in both the CCI and CFA models. Recently, it was shown that rimonabant also has neuroprotective effects after acute administration in gerbils and rodents (Hansen et al., 2002; Pegorini et al., 2006).
It is difficult to explain the apparently selective anti-inflammatory and antiallodynic action of rimonabant in obese animals. We can only assume that the ability of the drug to act as a TNFα inhibitor (Smith et al., 2000; Croci et al., 2003; Costa et al., 2005), plays some role in this selective action, as it has been suggested that the increased TNFα plasma levels observed in obese subjects may contribute to their increased susceptibility to inflammation (Hauner, 2005). Furthermore, the higher tone of the endocannabinoid system in obese rats and during inflammation (Di Marzo and Matias, 2005; Oka et al., 2005; Matias et al., 2006) might be involved in the antihyperalgersic effects of rimonabant and its higher efficacy in obese rather than lean rats. It is possible that in the presence of CB1 receptor inhibition, the endocannabinoids might promote activation of CB2 receptors and/or TRPV1 receptors desensitization contributing to antinociceptive and anti-inflammatory activity.
In conclusion, in the present study rimonabant reduced joint inflammation in a model of adjuvant-induced unilateral arthritis in rats made obese by a fat-rich diet, a condition in which inflammation appears to be severe. Rimonabant markedly prevented the arthritis-associated nociceptive responses; it inhibited thermal and mechanical hyperalgesia in obese as well as in lean arthritic rats.
Rimonabant may therefore have potential for the treatment and prevention of arthritis and other chronic inflammatory diseases, in view of its ability to relieve pain and improve inflammatory symptoms, particularly in obese patients.
Acknowledgments
The authors thank MG Marongiu, A Ronghi, E Salvatori for their technical support and Dr A Bianchetti for help in writing the manuscript.
Abbreviations
- CCI
chronic constriction injury of sciatic nerve
- CFA
complete Freund's adjuvant
- rimonabant (SR141716)
N-piperidino-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4methyl-pyrazole-3-carboxamide
Conflict of interest
The authors state no conflict of interest.
References
- Barbato JE, Zuckerbraun BS, Overhaus M, Raman KG, Tzeng E. Nitric oxide modulates vascular inflammation and intimal hyperplasia in insulin resistance and the metabolic syndrome. Am J Physiol Heart Circ Physiol. 2005;289:H228–H236. doi: 10.1152/ajpheart.00982.2004. [DOI] [PubMed] [Google Scholar]
- Berg AH, Scherer PE. Adipose tissue, inflammation, and cardiovascular disease. Circ Res. 2005;96:939–949. doi: 10.1161/01.RES.0000163635.62927.34. [DOI] [PubMed] [Google Scholar]
- Carai MA, Colombo G, Gessa GL. Rimonabant: the first therapeutically relevant cannabinoid antagonist. Life Sci. 2005;77:2339–2350. doi: 10.1016/j.lfs.2005.04.017. [DOI] [PubMed] [Google Scholar]
- Choo-Kang BS, Hutchison S, Nickdel MB, Bundick RV, Leishman AJ, Brewer JM, et al. TNF-blocking therapies: an alternative mode of action. Trends Immunol. 2005;26:518–522. doi: 10.1016/j.it.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Colombo G, Agabio R, Diaz G, Lobina C, Reali R, Gessa GL. Appetite suppression and weight loss after the cannabinoid antagonist SR 141716. Life Sci. 1998;63:PL113–PL117. doi: 10.1016/s0024-3205(98)00322-1. [DOI] [PubMed] [Google Scholar]
- Costa B, Trovato AE, Colleoni M, Giagnoni G, Zarini E, Croci T. Effect of the cannabinoid CB1 receptor antagonist, SR141716, on nociceptive response and nerve demyelination in rodents with chronic constriction injury of the sciatic nerve. Pain. 2005;116:52–61. doi: 10.1016/j.pain.2005.03.043. [DOI] [PubMed] [Google Scholar]
- Cravatt BF, Lichtman AH. The endogenous cannabinoid system and its role in nociceptive behavior. J Neurobiol. 2004;61:149–160. doi: 10.1002/neu.20080. [DOI] [PubMed] [Google Scholar]
- Croci T, Landi M, Galzin AM, Marini P. Role of cannabinoid CB1 receptors and tumor necrosis factor-α in the gut and systemic anti-inflammatory activity of SR 141716 (rimonabant) in rodents. Br J Pharmacol. 2003;140:115–122. doi: 10.1038/sj.bjp.0705412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decaris E, Guingamp C, Chat M, Philippe L, Grillasca JP, Abid A, et al. Evidence for neurogenic transmission inducing degenerative cartilage damage distant from local inflammation. Arthritis Rheum. 1999;42:1951–1960. doi: 10.1002/1529-0131(199909)42:9<1951::AID-ANR22>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Després JP, Golay A, Sjöström L, for the Rimonabant in Obesity–Lipids Study Group Effects of rimonabant on metabolic risk factors in overweight patients with dyslipidemia. N Engl J Med. 2005;353:2121–2134. doi: 10.1056/NEJMoa044537. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Matias I. Endocannabinoid control of food intake and energy balance. Nat Neurosci. 2005;8:585–589. doi: 10.1038/nn1457. [DOI] [PubMed] [Google Scholar]
- Engeli S, Bohnke J, Feldpausch M, Gorzelniak K, Janke J, Batkai S, et al. Activation of the peripheral endocannabinoid system in human obesity. Diabetes. 2005;54:2838–2843. doi: 10.2337/diabetes.54.10.2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantuzzi G. Adipose tissue, adipokines, and inflammation. J Allergy Clin Immunol. 2005;115:911–919. doi: 10.1016/j.jaci.2005.02.023. [DOI] [PubMed] [Google Scholar]
- Fox A, Kesingland A, Gentry C, McNair K, Patel S, Urban L, et al. The role of central and peripheral cannabinoid1 receptors in the antihyperalgesic activity of cannabinoids in a model of neuropathic pain. Pain. 2001;92:91–100. doi: 10.1016/s0304-3959(00)00474-7. [DOI] [PubMed] [Google Scholar]
- Gimeno RE, Klaman LD. Adipose tissue as an active endocrine organ: recent advances. Curr Opin Pharmacol. 2005;5:122–128. doi: 10.1016/j.coph.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Hansen HH, Azcoitia I, Pons S, Romero J, Garcia-Segura LM, Ramos JA, et al. Blockade of cannabinoid CB1 receptor function protects against in vivo disseminating brain damage following NMDA-induced excitotoxicity. J Neurochem. 2002;82:154–158. doi: 10.1046/j.1471-4159.2002.00961.x. [DOI] [PubMed] [Google Scholar]
- Harden RN. Chronic neuropathic pain. Mechanisms, diagnosis, and treatment. Neurologist. 2005;11:111–122. doi: 10.1097/01.nrl.0000155180.60057.8e. [DOI] [PubMed] [Google Scholar]
- Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- Hauner H. Secretory factors from human adipose tissue and their functional role. Proc Nutr Soc. 2005;64:163–169. doi: 10.1079/pns2005428. [DOI] [PubMed] [Google Scholar]
- Idanpaan-Heikkila JJ, Guilbaud G. Pharmacological studies on a rat model of trigeminal neuropathic pain: baclofen, but not carbamazepine, morphine or tricyclic antidepressants, attenuates the allodynia-like behaviou. Pain. 1999;79:281–290. doi: 10.1016/s0304-3959(98)00172-9. [DOI] [PubMed] [Google Scholar]
- Inglis JJ, Nissim A, Lees DM, Hunt SP, Chernajovsky Y, Kidd BL. The differential contribution of tumour necrosis factor to thermal and mechanical hyperalgesia during chronic inflammation. Arthritis Res Ther. 2005;7:R807–R816. doi: 10.1186/ar1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jbilo O, Ravinet-Trillou C, Arnone M, Buisson I, Bribes E, Peleraux A, et al. The CB1 receptor antagonist rimonabant reverses the diet-induced obesity phenotype through the regulation of lipolysis and energy balance. FASEB J. 2005;19:1567–1569. doi: 10.1096/fj.04-3177fje. [DOI] [PubMed] [Google Scholar]
- LaBuda CJ, Koblish M, Little PJ. Cannabinoid CB2 receptor agonist activity in the hind paw incision model of postoperative pain. Eur J Pharmacol. 2005;527:172–174. doi: 10.1016/j.ejphar.2005.10.020. [DOI] [PubMed] [Google Scholar]
- Laurido C, Hernandez A, Constandil L, Pelissier T. Nitric oxide synthase and soluble guanylate cyclase are involved in spinal cord wind-up activity of monoarthritic, but not of normal rats. Neurosci Lett. 2003;352:64–66. doi: 10.1016/j.neulet.2003.08.014. [DOI] [PubMed] [Google Scholar]
- Lim G, Sung B, Ji RR, Mao J. Upregulation of spinal cannabinoid-1-receptors following nerve injury enhances the effects of WIN 55,212-2 on neuropathic pain behaviors in rats. Pain. 2003;105:275–283. doi: 10.1016/s0304-3959(03)00242-2. [DOI] [PubMed] [Google Scholar]
- MacFarlane BV, Wright A, O'Callaghan J, Benson HA. Chronic neuropathic pain and its control by drugs. Pharmacol Ther. 1997;75:1–19. doi: 10.1016/s0163-7258(97)00019-3. [DOI] [PubMed] [Google Scholar]
- Mao J, Price DD, Mayer DJ. Experimental mononeuropathy reduces the antinociceptive effects of morphine: implications for common intracellular mechanisms involved in morphine tolerance and neuropathic pain. Pain. 1995;61:353–364. doi: 10.1016/0304-3959(95)00022-K. [DOI] [PubMed] [Google Scholar]
- Matias I, Gonthier MP, Orlando P, Martiadis V, De Petrocellis L, Cervino C, et al. Regulation, function, and dysregulation of endocannabinoids in models of adipose and beta-pancreatic cells and in obesity and hyperglycemia. J Clin Endocrinol Metab. 2006;91:3171–3180. doi: 10.1210/jc.2005-2679. [DOI] [PubMed] [Google Scholar]
- McDougall JJ, Karimian SM, Ferrell WR. Alteration of substance P-mediated vasodilatation and sympathetic vasoconstriction in the rat knee joint by adjuvant-induced inflammation. Neurosci Lett. 1994;174:127–129. doi: 10.1016/0304-3940(94)90002-7. [DOI] [PubMed] [Google Scholar]
- Mehrotra C, Naimi TS, Serdula M, Bolen J, Pearson K. Arthritis, body mass index, and professional advice to lose weight: implications for clinical medicine and public health. Am J Prev Med. 2004;27:16–21. doi: 10.1016/j.amepre.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Mokdad AH, Ford ES, Bowman BA, Dietz WH, Vinicor F, Bales VS, et al. Prevalence of obesity, diabetes, and obesity-related health risk factors. JAMA. 2001;289:76–79. doi: 10.1001/jama.289.1.76. [DOI] [PubMed] [Google Scholar]
- Naeini RS, Cahill CM, Ribeiro-da-Silva A, Ménard HA, Henry L. Remodelling of spinal nociceptive mechanisms in an animal model of monoarthritis. Eur J Neurosci. 2005;22:2005–2015. doi: 10.1111/j.1460-9568.2005.04382.x. [DOI] [PubMed] [Google Scholar]
- Oka S, Yanagimoto S, Ikeda S, Gokoh M, Kishimoto S, Waku K, et al. Evidence for the involvement of the cannabinoid CB2 receptor and its endogenous ligand 2-arachidonoylglycerol in 12-O-tetradecanoylphorbol-13-acetate-induced acute inflammation in mouse ear. J Biol Chem. 2005;280:18488–18497. doi: 10.1074/jbc.M413260200. [DOI] [PubMed] [Google Scholar]
- Ossipov MH, Lopez Y, Nichols ML, Bian D, Porreca F. Inhibition by spinal morphine of the tail-flick response is attenuated in rats with nerve ligation injury. Neurosci Lett. 1995;199:83–86. doi: 10.1016/0304-3940(95)12026-z. [DOI] [PubMed] [Google Scholar]
- Pegorini S, Zani A, Braida D, Guerini-Rocco C, Sala M. Vanilloid VR1 receptor is involved in rimonabant-induced neuroprotection. Br J Pharmacol. 2006;147:552–559. doi: 10.1038/sj.bjp.0706656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravinet Trillou C, Arnone M, Delgorge C, Gonalons N, Keane P, Maffrand JP, et al. Anti-obesity effect of SR 141716, a CB1 receptor antagonist, in diet-induced obese mice. Am J Physiol Regul Integr Comp Physiol. 2003;284:R345–R353. doi: 10.1152/ajpregu.00545.2002. [DOI] [PubMed] [Google Scholar]
- Rinaldi-Carmona M, Barth F, Héaulme M, Alonso R, Shire D, Congy C, et al. Biochemical and pharmacological characterisation of SR 141716A, the first potent and selective brain cannabinoid receptor antagonist. Life Sci. 1995;56:1941–1947. doi: 10.1016/0024-3205(95)00174-5. [DOI] [PubMed] [Google Scholar]
- Rinaldi-Carmona M, Barth F, Heaulme M, Shire D, Calandra B, Congy C, et al. SR141716A, a potent and selective antagonist of the brain cannabinoid receptor. FEBS Lett. 1994;350:240–244. doi: 10.1016/0014-5793(94)00773-x. [DOI] [PubMed] [Google Scholar]
- Schafers M, Geis C, Svensson CI, Luo ZD, Sommer C. Selective increase of tumour necrosis factor-alpha in injured and spared myelinated primary afferents after chronic constrictive injury of rat sciatic nerve. Eur J Neurosci. 2003;17:791–804. doi: 10.1046/j.1460-9568.2003.02504.x. [DOI] [PubMed] [Google Scholar]
- Schaible HG, Del Rosso A, Matucci-Cerinic M. Neurogenic aspects of inflammation. Rheum Dis Clin North Am. 2005;31:77–101. doi: 10.1016/j.rdc.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Simiand J, Keane M, Keane PE, Soubrié P. SR 141716, a CB1 cannabinoid receptor antagonist, selectively reduces sweet food intake in marmoset. Behav Pharmacol. 1998;9:179–181. [PubMed] [Google Scholar]
- Smith SR, Terminelli C, Denhardt G. Effects of cannabinoid receptor agonist and antagonist ligands on production of inflammatory cytokines and anti-inflammatory interleukin-10 in endotoxemic mice. J Pharmacol Exp Ther. 2000;293:136–150. [PubMed] [Google Scholar]
- Sommer C, Kress M. Recent findings on how proinflammatory cytokines cause pain: peripheral mechanisms in inflammatory and neuropathic hyperalgesia. Neurosci Lett. 2004;361:184–187. doi: 10.1016/j.neulet.2003.12.007. [DOI] [PubMed] [Google Scholar]
- Sommer C, Schafers M, Marziniak M, Toyka KV. Etanercept reduces hyperalgesia in experimental painful neuropathy. J Peripher Nerv Syst. 2001;6:67–72. doi: 10.1046/j.1529-8027.2001.01010.x. [DOI] [PubMed] [Google Scholar]
- Tedesco LS, Fuseler J, Grisham M, Wolf R, Roerig SC. Therapeutic administration of nitric oxide synthase inhibitors reverses hyperalgesia but not inflammation in a rat model of polyarthritis. Pain. 2002;95:215–223. doi: 10.1016/S0304-3959(01)00402-X. [DOI] [PubMed] [Google Scholar]
- Trayhurn P. Endocrine and signalling role of adipose tissue: new perspectives on fat. Acta Physiol Scand. 2005;184:285–293. doi: 10.1111/j.1365-201X.2005.01468.x. [DOI] [PubMed] [Google Scholar]
- Ueda H. Molecular mechanisms of neuropathic pain-phenotypic switch and initiation mechanisms. Pharmacol Ther. 2006;109:57–77. doi: 10.1016/j.pharmthera.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Vachharajani V, Russell JM, Scott KL, Conrad S, Stokes KY, Tallam L, et al. Obesity exacerbates sepsis-induced inflammation and microvascular dysfunction in mouse brain. Microcirculation. 2005;12:183–194. doi: 10.1080/10739680590904982. [DOI] [PubMed] [Google Scholar]
- Villetti G, Bergamaschi M, Bassani F, Bolzoni PT, Maiorino M, Pietra C, et al. Antinociceptive activity of the N-methyl-D-aspartate receptor antagonist N-(2-Indanyl)-glycinamide hydrochloride (CHF3381) in experimental models of inflammatory and neuropathic pain. J Pharmacol Exp Ther. 2003;306:804–814. doi: 10.1124/jpet.103.050039. [DOI] [PubMed] [Google Scholar]
- Welch SP, Huffman JW, Lowe J. Differential blockade of the antinociceptive effects of centrally administered cannabinoids by SR141716A. J Pharmacol Exp Ther. 1998;286:1301–1308. [PubMed] [Google Scholar]
- Whiteside GT, Gottshall SL, Boulet JM, Chaffer SM, Harrison JE, Pearson MS, et al. A role for cannabinoid receptors, but not endogenous opioids, in the antinociceptive activity of the CB2-selective agonist, GW405833. Eur J Pharmacol. 2005;528:65–72. doi: 10.1016/j.ejphar.2005.10.043. [DOI] [PubMed] [Google Scholar]
- Williams RO, Feldmann M, Maini RN. Anti-tumor necrosis factor ameliorates joint disease in murine collagen-induced arthritis. Proc Natl Acad Sci USA. 1992;89:9784–9788. doi: 10.1073/pnas.89.20.9784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann M. Pathobiology of neuropathic pain. Eur J Pharmacol. 2001;429:23–37. doi: 10.1016/s0014-2999(01)01303-6. [DOI] [PubMed] [Google Scholar]