Abstract
Background and purpose:
To follow up in vitro evidence that Δ9-tetrahydrocannabivarin extracted from cannabis (eΔ9-THCV) is a CB1 receptor antagonist by establishing whether synthetic Δ9-tetrahydrocannabivarin (O-4394) and Δ8-tetrahydrocannabivarin (O-4395) behave as CB1 antagonists in vivo.
Experimental approach:
O-4394 and O-4395 were compared with eΔ9-THCV as displacers of [3H]-CP55940 from specific CB1 binding sites on mouse brain membranes and as antagonists of CP55940 in [35S]GTPγS binding assays performed with mouse brain membranes and of R-(+)-WIN55212 in mouse isolated vasa deferentia. Their ability to antagonize in vivo effects of 3 or 10 mg kg−1 (i.v.) Δ9-tetrahydrocannabinol in mice was then investigated.
Key results:
O-4394 and O-4395 exhibited similar potencies to eΔ9-THCV as displacers of [3H]-CP55940 (K i=46.6 and 64.4 nM, respectively) and as antagonists of CP55940 in the [35S]GTPγS binding assay (apparent K B=82.1 and 125.9 nM, respectively) and R-(+)-WIN55212 in the vas deferens (apparent K B=4.8 and 3.9 nM respectively). At i.v. doses of 0.1, 0.3, 1.0 and/or 3 mg kg−1 O-4394 and O-4395 attenuated Δ9-tetrahydrocannabinol-induced anti-nociception (tail-flick test) and hypothermia (rectal temperature). O-4395 but not O-4394 also antagonized Δ9-tetrahydrocannabinol-induced ring immobility. By themselves, O-4395 and O-4394 induced ring immobility at 3 or 10 mg kg−1 (i.v.) and antinociception at doses above 10 mg kg−1 (i.v.). O-4395 also induced hypothermia at 3 mg kg−1 (i.v.) and above.
Conclusions and implications:
O-4394 and O-4395 exhibit similar in vitro potencies to eΔ9-THCV as CB1 receptor ligands and as antagonists of cannabinoid receptor agonists and can antagonize Δ9-tetrahydrocannabinol in vivo.
Keywords: Tetrahydrocannabivarin, tetrahydrocannabinol, CP55940, R-(+)-WIN55212, cannabinoid CB1 receptor antagonist, mouse vas deferens, anti-nociception, tail-flick test, ring test, hypothermia
Introduction
We have demonstrated previously that (–)-Δ9-tetrahydrocannabivarin (Δ9-THCV), a constituent of cannabis and the propyl homologue of Δ9-tetrahydrocannabinol (Δ9-THC), behaves as a competitive surmountable antagonist of (R)-(+)-[2,3-dihydro-5-methyl-3-(4-morpholinylmethyl)pyrrolo-[1,2,3-de]-1,4-benzoxazin-6-yl]-1-naphthalenylmethanone (R-(+)-WIN55212), anandamide and certain other cannabinoid receptor agonists in the mouse isolated vas deferens, with a potency similar to that exhibited in this tissue by the established CB1 receptor antagonist, N-(piperidin-1-yl)-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide hydrochloride (SR1417161A) (Pertwee et al., 1995; Thomas et al., 2005). Δ9-THCV has also been found to antagonize R-(+)-WIN55212-induced stimulation of [35S]GTPγS binding to mouse brain membranes, albeit significantly less potently than it antagonized this agonist in the vas deferens (Thomas et al., 2005), and in the same investigation another CB1/CB2 receptor agonist, (–)-cis-3-[2-hydroxy-4-(1,1-dimethylheptyl)phenyl]-trans-4-(3-hydroxypropyl)cyclohexanol (CP55940), was shown to be equally susceptible to antagonism by Δ9-THCV in this assay. The potency exhibited by Δ9-THCV against R-(+)-WIN55212 and CP55940 in the [35S]GTPγS-binding assay was also similar to the potency with which it displaced [3H]-CP55940 from specific CB1-binding sites on mouse brain membranes, suggesting that Δ9-THCV was competing with R-(+)-WIN55212 and CP55940 for CB1 receptors on brain membranes, but acting through some other as yet unidentified mechanism in the vas deferens.
In spite of the structural similarity between Δ9-THCV and Δ9-tetrahydrocannabinol (Δ9-THC), it is not unexpected that THCV can block the effects of CB1 receptor agonists, even though THC is a CB1 receptor partial agonist (reviewed in Howlett et al., 2002). Thus, data from structure–activity investigations suggest that, because its alkyl side chain (propyl) is shorter than that of Δ9-THC (pentyl), Δ9-THCV should indeed exhibit even less efficacy than Δ9-THC as a CB1 receptor agonist (reviewed in Howlett et al., 2002). Even so, although Δ9-THCV behaves as a CB1 receptor antagonist in vitro, it has been found to exhibit CB1 agonist-like activity in vivo. Thus, in the first ever pharmacological experiments with Δ9-THCV, this compound was found to induce immobility in the mouse ring test (Gill et al., 1970), an effect that is also known to be produced by established CB1 receptor agonists such as R-(+)-WIN55212, CP55940 and Δ9-THC acting through CB1 receptors (reviewed in Howlett et al., 2002).
Even though, CB1 receptor activation can induce immobility in the ring test, it remains possible that Δ9-THCV did not produce this effect by acting through CB1 receptors, as certain compounds that do not activate these receptors are also known to induce immobility in this assay (Wiley and Martin, 2003). In addition, although the potency exhibited by Δ9-THCV in the ring test was 4.8 times less than that of Δ9-THC (Gill et al., 1970), there is little difference between the potencies of these two ligands as displacers of [3H]-CP55940 from CB1-binding sites (Howlett et al., 2002; Thomas et al., 2005). These findings raise the possibility that Δ9-THCV might behave as a CB1 receptor antagonist in vivo at doses below those at which it induces ring immobility, and the main objective of the present investigation was to investigate this possibility. This was achieved by establishing whether there are any in vivo doses at which Δ9-THCV shares the ability of the CB1-selective antagonist, SR141716A (Compton et al., 1996; Adams et al., 1998), to attenuate the production by Δ9-THC in mice of anti-nociception in the tail-flick test, immobility in the ring test and hypothermia.
For previous pharmacological experiments with Δ9-THCV (Gill et al., 1970; Thomas et al., 2005), this compound was obtained by extracting it from cannabis. As a result, the samples of Δ9-THCV used most likely contained traces of other cannabis constituents that could perhaps have contributed to the pharmacological effects that these samples produced. To avoid this possibility, the experiments described in the present paper were performed with synthetic Δ9-THCV (O-4394). To facilitate future structure–activity studies, synthetic (–)-Δ8-tetrahydrocannabivarin (O-4395) was also investigated, it being easier to synthesize analogues of Δ8- than Δ9-tetrahydrocannabivarin. Consequently, our initial experiments were directed at establishing whether O-4394 and O-4395 possess similar potencies to those found previously for Δ9-THCV extracted from cannabis as displacers of [3H]-CP55940 from specific-binding sites on mouse brain membranes, as antagonists of CP55940-induced stimulation of [35S]GTPγS binding to mouse brain membranes and as antagonists of R-(+)-WIN55212-induced inhibition of electrically evoked contractions of the mouse isolated vas deferens. Δ9-THCV extracted from cannabis is referred to in the remainder of this paper as eΔ9-THCV.
The results indicate first that O-4394 and O-4395 exhibit similar potencies to eΔ9-THCV as displacers of [3H]-CP55940 and as antagonists of CP55940 and R-(+)-WIN55212 in mouse brain membrane and vas deferens assays and second that they both can antagonize Δ9-THC in vivo at doses of 0.1, 0.3, 1.0 and/or 3 mg kg−1, intravenously (i.v.).
Methods
The methods used comply with the UK Animals (Scientific Procedures) Act, 1986 and associated guidelines for the use of experimental animals. All in vivo animal protocols were also approved by the Virginia Commonwealth University Institutional Animal Care and Use Committee.
Membrane preparation
Binding assays with [3H]-CP55940 and [35S]GTPγS were performed with mouse whole brain membranes, prepared as described by Thomas et al. (2004). Protein assays were carried out using a Bio-Rad Dc kit (Bio-Rad, Hercules, CA, USA).
Radioligand displacement assay
The assays were carried out with [3H]-CP55940, Tris-binding buffer (50 mM Tris-HCl; 50 mM Tris Base; 0.1% bovine serum albumin (BSA)), total assay volume 500 μl, using the filtration procedure described previously by Ross et al. (1999b). Binding was initiated by the addition of the brain membranes (33 μg protein per well). All assays were performed at 37°C for 60 min before termination by addition of ice-cold Tris-binding buffer and vacuum filtration using a 24-well sampling manifold (cell harvester; Brandel Inc., Gaitherburg, MD, USA) and GF/B filters (Whatman, Maidstone, UK), that had been soaked in wash buffer at 4°C for at least 24 h. Each reaction well was washed six times with a 1.2 ml aliquot of Tris-binding buffer. The filters were oven-dried for at least 60 min and then placed in 5 ml of scintillation fluid (Ultima Gold XR, Perkin-Elmer Life Sciences Inc., Boston, MA, USA). Radioactivity was quantified by liquid scintillation spectrometry. Specific binding was defined as the difference between the binding that occurred in the presence or absence of 1 μM unlabelled CP55940. The concentration of [3H]-CP55940 used in our displacement assays was 0.7 nM. O-4394 and O-4395 were stored as a stock solution of 10 mM in dimethylsulphoxide (DMSO), the vehicle concentration in all assay wells being 0.1% DMSO. The binding parameters for [3H]-CP55940 in the mouse brain membranes, determined by fitting data from saturation binding experiments to a one-site saturation plot using GraphPad Prism, were 2336 fmol mg−1 protein (Bmax) and 2.31 nM (Kd) (Thomas et al., 2004).
[35S]GTPγS binding assay
The procedure for measuring agonist-stimulated [35S]GTPγS binding to cannabinoid CB1 receptors was adapted from the methods of Kurkinen et al. (1997) and Breivogel et al. (2001) as described previously (Thomas et al., 2005). Assays were carried out with GTPγS-binding buffer (50 mM Tris-HCl; 50 mM Tris base; 5 mM MgCl2; 1 mM ethylenediaminetetraacetic acid (EDTA); 100 mM NaCl; 1 mM dithiothreitol; 0.1% BSA) in the presence of 0.1 nM [35S]GTPγS and 30 μM guanosine 5′-diphosphate (GDP) and in a final volume of 500 μl. Membranes (5 μg protein per well) were preincubated for 30 min at 30°C with 0.5 U ml−1 adenosine deaminase (200 U mg−1) to remove endogenous adenosine. Binding was initiated by the addition of [35S]GTPγS to the wells. Non-specific binding was measured in the presence of 30 μM GTPγS. The drugs were incubated in the assay for 60 min at 30°C. The reaction was terminated by a rapid vacuum filtration method using Tris-binding buffer as described previously (Ross et al., 1999a), and the radioactivity was quantified by liquid scintillation spectrometry. Agonists and antagonists were stored as a stock solution of 1 or 10 mM in DMSO, the vehicle concentration in all assay wells being 0.11% DMSO.
Vas deferens experiments
Vasa deferentia were obtained from albino MF1 mice weighing 32–44 g. The tissues were mounted vertically in 4 ml organ baths. They were then subjected to electrical stimulation of progressively greater intensity, followed by an equilibration procedure in which they were exposed to alternate periods of stimulation (2 min) and rest (10 min) until contractions with consistent amplitudes were obtained (Thomas et al., 2004). These contractions were monophasic and isometric and were evoked by 0.5 s trains of pulses of 110% maximal voltage (train frequency 0.1 Hz; pulse frequency 5 Hz; pulse duration 0.5 ms). All drug additions were made to the organ baths after the equilibration period and there was no washout between these additions. There was an initial application of a potential antagonist or its vehicle and this was followed 28 min later by a 2 min period of electrical stimulation at the end of which the lowest of a series of concentrations of the cannabinoid receptor agonist, R-(+)-WIN55212 was applied. After a 13 min period of rest, the tissues were electrically stimulated for 2 min and then subjected to a further addition of R-(+)-WIN55212. This cycle of drug addition, 13 min rest and 2 min stimulation was repeated, to obtain cumulative concentration–response curves, only one of which was constructed per tissue (Pertwee et al., 1996).
In vivo experiments
Male ICR mice weighing 22–30 g (Harlan, Indianapolis, IN, USA) were housed in groups of five in 28 × 16 cm plastic cages with steel mesh tops in a temperature-controlled vivarium and were maintained on a 12 h light/dark cycle. Food and water were available ad libitum. Vehicle or 0.03, 0.1, 0.3, 1, 3, 10, 30 or 56 mg kg−1 O-4394 or O-4395 were injected into a tail vein. In some experiments, mice were pretreated intraperitoneally (i.p.) with 3 mg kg−1 SR141716A 10 min before the intravenous administration of 56 mg kg−1 O-4394 or O-4395 or 3 mg kg−1 Δ9-THC. In other antagonism experiments, mice were pretreated (i.v.) with 0.03, 0.1, 0.3, 1 or 3 mg kg−1 O-4394 or O-4395 or with the vehicle immediately before i.v. administration of Δ9-THC either at 10 mg kg−1, the lowest dose required to produce maximal effects in the three assays that were used, or at 3 mg kg−1. The measured responses were tail-flick latency in the radiant heat nociceptive test (D'Amour and Smith, 1941), immobility in the ring test (Pertwee, 1972) and core temperature. For the ring test, each mouse was placed on a ring (5.5 cm diameter) that was elevated 16 cm from a table top for a 5-min observation period. The amount of time each animal remained motionless, except for respiratory movements, was recorded to the nearest second. Core temperatures were measured to the nearest 0.1°C by inserting a rectal probe connected to a telethermometer (YSI Inc., Yellow Springs, OH, USA) to a depth of 1.8 cm. As decribed previously (Varvel et al., 2005), control tail-flick latencies and core body temperatures were assessed in each mouse before i.v. administration of any drugs. The intensity of the stimulus from the heat lamp in the tail-flick test was adjusted to yield control latencies of 2–4 s and an automatic 10 s cut-off was used. At 20 min post exposure, tail-flick latencies were reassessed and, at 40 min mice were subjected to the ring test. Core temperatures were reassessed at 60 min. All measures were taken in each animal. The ambient temperature was approximately 22°C.
Drugs and chemicals
Δ9-THC was supplied by the National Institute on Drug Abuse (Bethesda, MD, USA) and O-4394 and O-4395 by Dr Raj Razdan (Organix Inc, MA, USA). SR141716A was obtained from Sanofi-Aventis (Montpellier, France) and from the National Institute on Drug Abuse (Bethesda, MD, USA) and R-(+)-WIN55212 and CP55940 were purchased from Tocris (Bristol, UK). For the binding experiments, [3H]-CP55940 (160 Ci mmol−1) and [35S]GTPγS (1250 Ci mmol−1) were obtained from Perkin-Elmer Life Sciences Inc. (Boston, MA, USA), GTPγS and adenosine deaminase from Roche Diagnostic (Indianapolis, IN, USA) and GDP from Sigma-Aldrich (St Louis, MO, USA). For mouse vas deferens experiments, R-(+)-WIN55212 was dissolved in a 50% (v v−1) solution of DMSO and a 0.9% aqueous solution of NaCl (saline) and all other drugs were dissolved in pure DMSO. Drugs were added to organ baths in a volume of 10 μl. For the in vivo experiments, O-4394, O-4395, Δ9-THC and SR141716A were dissolved in a 1:1 mixture of absolute ethanol and alkamuls-620 (Aventis, Strasbourg, France) and diluted with saline to a final ratio of 1:1:18 (ethanol/alkamuls/saline). Injections were given in a volume of 10 ml kg−1.
Analysis of data
Values have been expressed as means and variability as s.e.m. or as 95% confidence limits. The concentrations of O-4394 and O-4395 that produced a 50% displacement of radioligand from specific binding sites (IC50 values) were calculated using GraphPad Prism 4. Their dissociation constants (Ki values) were calculated using the equation of Cheng and Prusoff (1973). Net agonist-stimulated [35S]GTPγS binding values were calculated by subtracting basal binding values (obtained in the absence of agonist) from agonist-stimulated values (obtained in the presence of agonist), as detailed elsewhere (Ross et al., 1999a). Inhibition of the electrically evoked twitch response of the vas deferens has been expressed in percentage terms and this has been calculated by comparing the amplitude of the twitch response after each addition of a twitch inhibitor with its amplitude immediately before the first addition of the inhibitor.
Rectal temperatures have been expressed as the difference between pre- and post-injection values obtained from each mouse, and anti-nociception has been calculated by transforming the tail-flick data to the percentage of maximum possible effect (%MPE), where %MPE=100 × ([post-injection latency−pre-injection latency]/[cutoff time−pre-injection latency]) (Varvel et al., 2005). Anti-nociceptive ED50 values have been determined by least-squares linear regression and the 95% confidence limits of these values were also calculated (Bliss, 1967).
For in vitro data, values for EC50 and for the s.e.m. or 95% confidence limits of these values have been calculated by nonlinear regression analysis using the equation for a sigmoid concentration–response curve (GraphPad Prism). The apparent dissociation constant (KB) values for antagonism of agonists by O-4394 or O-4395 in the vas deferens or [35S]GTPγS-binding assay have been calculated by Schild analysis from the concentration–ratio, defined as the concentration of an agonist that elicits a response of a particular size in the presence of a competitive reversible antagonist at a concentration, B, divided by the concentration of the same agonist that produces an identical response in the absence of the antagonist. The methods used to determine concentration–ratio and apparent KB values and to establish whether log concentration–response plots deviated significantly from parallelism are detailed elsewhere (Pertwee et al., 2002).
Mean values obtained in vitro have been compared with zero using the one-sample t-test or with each other using Student's two-tailed t-test for unpaired data or one-way analysis of variance (ANOVA), followed by Dunnett's test (GraphPad Prism). For in vivo data, the significance of any differences from controls (the vehicle group in tests of agonism and the vehicle+Δ9-THC group in tests of antagonism) was assessed by one-way ANOVA, followed by Fisher's protected least significant difference (PLSD) test post hoc (Bliss, 1967). A P-value <0.05 was considered to be significant.
Results
Experiments with brain membranes or the isolated vas deferens
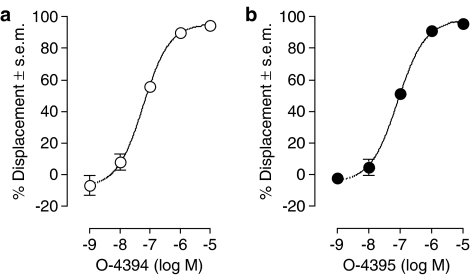
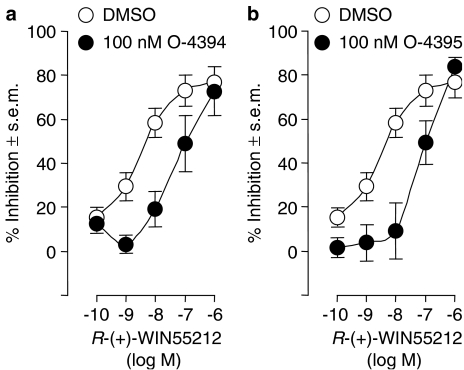
O-4394 and O-4395 each displaced [3H]-CP55940 from specific-binding sites on mouse brain membranes (Figure 1). This they did in a manner that fitted better to a one-site rather than a two-site competition curve (P<0.05; GraphPad Prism 4). The mean Ki-values of both these compounds are listed in Table 1. It was also found that at 1 μM, O-4394 and O-4395 each attenuated the ability of CP55940 to stimulate [35S]GTPγS binding to mouse brain membranes (Figure 2), and that at 100 nM they each opposed the ability of R-(+)-WIN55212 to reduce the amplitude of electrically evoked contractions of the vas deferens (Figure 3). In both these tissue preparations this antagonism was surmountable. It was probably also competitive in nature, as each antagonist induced a dextral shift in the log concentration–response curves of CP55940 and R-(+)-WIN55212 that did not deviate significantly from parallelism (Figures 2 and 3). The mean apparent KB values of O-4394 and O-4395 for their antagonism of CP55940 and R-(+)-WIN55212 are shown in Table 1. These values indicate that, as found previously for eΔ9-THCV (Table 1), O-4394 and O-4395 are both markedly more potent as antagonists of R-(+)-WIN55212 in the vas deferens than of CP55940 in brain membranes.
Figure 1.
Displacement of [3H]-CP55940 by (a) O-4394 and (b) O-4395 from specific binding sites on mouse whole brain membranes. Each symbol represents the mean percent displacement±s.e.m. Mean Ki-values for this displacement have been calculated from these data and these values are listed in Table 1.
Table 1.
Ki-values for displacement of [3H]-CP55940 from mouse brain membranes and apparent KB-values for antagonism of CP55940-induced stimulation of [35S]GTPγS binding to mouse brain membranes and of R-(+)-WIN55212-induced inhibition of electrically evoked contractions of the mouse isolated vas deferens
| Compound | Mouse tissue | Mean values (nM) | 95% confidence limits | n |
|---|---|---|---|---|
| Ki | ||||
| O-4394 | Brain membranes | 46.6 | 31.3 and 69.4 | 5 |
| O-4395 | 64.4 | 49.0 and 84.7 | 5 | |
| eΔ9-THCVa | 75.4 | 53.4 and 106.3 | 4–8 | |
| Apparent KB | ||||
| O-4394 (1 μM) | Brain membranes | 82.1 | 54.1 and 124.4 | 5 |
| O-4395 (1 μM) | 125.9 | 83.1 and 195.9 | 5 | |
| eΔ9-THCV (1 μM)a | 93.1 | 66.5 and 130.6 | 6 | |
| Apparent KB | ||||
| O-4394 (100 nM) | Vas deferens | 4.8 | 0.3 and 30.3 | 7 |
| O-4395 (100 nM) | 3.9 | 0.7 and 13.3 | 7 | |
| eΔ9-THCV (100 nM)a | 1.5 | 1.1 and 2.3 | 6–9 |
Abbreviation: eΔ9-THCV, Δ9-tetrahydrocannabivarin extracted from cannabis; R-(+)-WIN55212, (R)-(+)-[2,3-dihydro-5-methyl-3-(4-morpholinylmethyl)pyrrolo-[1,2,3-de]-1,4-benzoxazin-6-yl]-1-naphthalenylmethanone; CP55940, (–)-cis-3-[2-hydroxy-4-(1,1-dimethylheptyl)phenyl]-trans-4-(3-hydroxypropyl)cyclohexanol.
From Thomas et al. (2005).
Figure 2.
The effect of 1 μM O-4394 and O-4395 on the mean log concentration–response curve of CP55940 for stimulation of [35S]GTPγS binding (n=4 or 5; upper panels) and on [35S]GTPγS binding in the absence of CP55940 (n=6; lower panels). Each symbol represents the mean percentage change in [35S]GTPγS binding to mouse whole brain membranes and vertical lines show s.e.m. Mean apparent KB values of O-4394 and O-4395 for their antagonism of CP55940 have been calculated from the data in the upper panels and these values are listed in Table 1. The asterisks in the lower panel denote significant differences from zero (*P<0.05; ***P<0.001; one-sample t-test).
Figure 3.
The effect of pretreatment with (a) 100 nM O-4394 or (b) 100 nM O-4395 on the mean log concentration–response curve of R-(+)-WIN55212 in the mouse isolated vas deferens. Each symbol represents the mean value (and vertical lines show s.e.m.) for inhibition of electrically evoked contractions expressed as a percentage of the amplitude of the twitch response measured immediately before the first addition of R-(+)-WIN55212 to the organ bath. O-4394, O-4395 or DMSO was added 30 min before the first addition of R-(+)-WIN55212, further additions of which were made at 15 min intervals. Each log concentration–response curve was constructed cumulatively without washout (n=7). Mean apparent KB values of O-4394 and O-4395 for their antagonism of R-(+)-WIN55212 have been calculated from these data and these values are listed in Table 1.
Effects of O-4394 and O-4395, administered by themselves, on [35S]GTPγS binding to brain membranes were also investigated (Figure 2). Binding was stimulated by O-4395 at 0.1 and 1 μM, although not significantly affected by this compound at 1 or 10 nM or at 10 μM. In contrast, O-4394 did not affect [35S]GTPγS binding at any of these concentrations. The amplitude of electrically evoked contractions of the vas deferens was not affected by either O-4394 or O-4395 administered at the concentration that antagonized R-(+)-WIN55212. Thus, the mean amplitude was no different when measured 30 min after 100 nM O-4394 or O-4395 than when measured 30 min after DMSO (P>0.05; ANOVA followed by Dunnett's test; n=7).
In vivo experiments
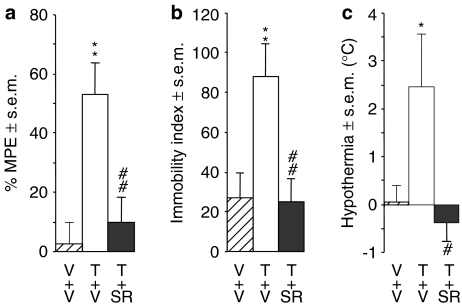
In experiments with 10 mg kg−1 Δ9-THC, anti-nociception induced by this cannabinoid was significantly opposed by O-4394 at 3 mg kg−1 and by O-4395 at 0.3, 1 and 3 mg kg−1 (Figure 4). Although O-4394 also seemed to oppose the antinociceptive effect of Δ9-THC at 0.3 and 1 mg kg−1, the apparent antagonism induced by these lower doses was not significant. In the ring test, O-4395 significantly reduced the ability of Δ9-THC to induce immobility when administered at a dose of 0.3 or 3 mg kg−1, although not when administered at 1 mg kg−1 (Figure 4). In contrast, O-4394 did not antagonize Δ9-THC in this bioassay at 0.3, 1 or 3 mg kg−1. However, both O-4394 and O-4395 significantly antagonized Δ9-THC-induced hypothermia at 0.3 and 3 mg kg−1, although not at 1 mg kg−1 (Figure 4).
Figure 4.
Effects of O-4394 (n=6 or 12; left-hand panels) and O-4395 (n=12 or 15; right-hand panels) on the ability of Δ9-THC, 10 mg kg−1 i.v., to induce (a) anti-nociception at +20 min, (b) ring immobility at +40 min and (c) hypothermia at +60 min in mice. Each column represents the mean value and vertical lines show s.e.m. O-4394 and O-4395 were injected i.v. immediately before Δ9-THC. %MPE is the percentage of maximum possible effect in the tail-flick test. The symbol # denotes a significantly greater response to Δ9-THC (T) than to its vehicle (V; P<0.001; ANOVA followed by Fisher's PLSD test) and asterisks indicate significant differences between responses to Δ9-THC+vehicle and responses to Δ9-THC+O-4394 or Δ9-THC+O-4395 (*P<0.05; **P<0.01; ***P<0.001; ANOVA followed by Fisher's PLSD test).
When a lower dose of Δ9-THC was used (3 mg kg−1, i.v.), i.v. injected O-4395 significantly attenuated Δ9-THC-induced anti-nociception at 0.1, 0.3 and 1 mg kg−1 but not at 0.03 or 3 mg kg−1; Δ9-THC-induced hypothermia at 0.1 and 0.3 mg kg−1 but not at 0.03, 1 or 3 mg kg−1; and Δ9-THC-induced immobility at 0.03 mg kg−1 but not at 0.1, 0.3, 1 or 3 mg kg−1 (n=6 or 10; P<0.05; ANOVA followed by Fisher's PLSD test; data not shown).
Effects of injecting O-4394 and O-4395 by themselves are shown in Figure 5. Neither O-4394 nor O-4395 induced a significant degree of anti-nociception at a dose of 3 or 10 mg kg−1. However, both compounds were antinociceptive at 30 and 56 mg kg−1. As to ring immobility, this was increased significantly by O-4395 at 3 mg kg−1, by O-4394 at 10 mg kg−1, and by both O-4394 and O-4395 at 30 and 56 mg kg−1. O-4395 but not O-4394 also induced a significant degree of hypothermia at 3, 10, 30 and 56 mg kg−1. When administered i.p. at a dose of 3 mg kg−1, the CB1-selective antagonist, SR141716A, markedly attenuated anti-nociception induced by O-4394 and O-4395 at 56 mg kg−1 (Figure 5) and also anti-nociception, hypothermia and ring immobility induced by Δ9-THC at 3 mg kg−1 (Figure 6). However, the same dose of SR141716A did not antagonize hypothermia or ring immobility induced by 56 mg kg−1 O-4394 or O-4395 (Figure 5).
Figure 5.
Effects of i.v. injections of O-4394 (n=6–10; left-hand panels) and O-4395 (n=6–11; right-hand panels) on (a) anti-nociception at +20 min, (b) ring immobility at +40 min and (c) hypothermia at +60 min in mice. Each column represents the mean value and vertical lines show s.e.m. In some experiments, injection of O-4394 or O-4395 at 56 mg kg−1 i.v. was preceded at −10 min by an i.p. injection of 3 mg kg−1 SR141716A (solid columns). %MPE is the percentage of maximum possible effect in the tail-flick test. Asterisks denote significant differences between the effects of vehicle (V) and O-4394 or O-4395 (*P<0.05; **P<0.01; ***P<0.001; ANOVA followed by Fisher's PLSD test) and the symbol # denotes significant differences between responses to O-4394 or O-4395 alone at 56 mg kg−1 i.v. and responses to this dose of O-4394 or O-4395 following pretreatment with SR141716A (P<0.05; ANOVA followed by Fisher's PLSD test). Mean antinociceptive ED50 values with 95% confidence limits shown in parentheses were 43 mg kg−1 (22 and 83 mg kg−1) for O-4394 and 44 mg kg−1 (23 and 82 mg kg−1) for O-4395.
Figure 6.
Effects of i.v. injections of Δ9-THC (T) at 3 mg kg−1 (n=5 or 6) on (a) anti-nociception at +20 min, (b) ring immobility at +40 min and (c) hypothermia at +60 min in mice. Each column represents the mean value and vertical lines show s.e.m. Injection of Δ9-THC was preceded at −10 min by an i.p. injection of either the vehicle (V) or 3 mg kg−1 SR141716A (SR). %MPE is the percentage of maximum possible effect in the tail-flick test. Asterisks denote significant differences between the effects of vehicle and Δ9-THC (*P<0.05; **P<0.01; ANOVA followed by Fisher's PLSD test) and the symbol # denotes significant differences between effects of Δ9-THC following pretreatment with vehicle and its effects following pretreatment with SR141716A (#P<0.05; ##P<0.01; ANOVA followed by Fisher's PLSD test).
Discussion
The results obtained in this investigation indicate that both O-4394 and O-4395 exhibit pharmacological properties in vitro similar to those demonstrated for eTHCV by Thomas et al. (2005). More specifically, as shown in Figure 1 and Table 1, each synthetic compound displaced [3H]-CP55940 from specific sites on mouse brain membranes with a Ki value that does not differ significantly from that of the corresponding Ki value of eTHCV determined previously. It was also found that the ability of eTHCV to behave as a competitive surmountable antagonist of R-(+)-WIN55212 in the mouse isolated vas deferens and of CP55940 in the [35S]GTPγS binding assay performed with mouse brain membranes (Thomas et al., 2005) was shared by both O-4394 and O-4395. The apparent KB-values of O-4394 and O-4395 for this antagonism of R-(+)-WIN55212 and CP55940 do not deviate significantly from the corresponding apparent KB-values of eTHCV determined previously (Table 1). O-4394 and O-4395 also resemble eTHCV in being markedly more potent as antagonists of R-(+)-WIN55212 in the vas deferens than of CP55940-induced stimulation of [35S]GTPγS binding to brain membranes (Table 1). The apparent KB-values of all three compounds for their antagonism of CP55940 in the [35S]GTPγS binding assay are not significantly different from their Ki-values for displacement of [3H]-CP55940 from specific sites on brain membranes, suggesting that they were all antagonizing CP55940 by interacting with this ligand at cannabinoid receptors. These are most likely CB1 receptors. Thus, although CP55940 and eTHCV each binds as readily to the CB1 as to the CB2 receptor (Howlett et al., 2002; Thomas et al., 2005), and both these cannabinoid receptor subtypes are present in the brain (Van Sickle et al., 2005), brain tissue is much more densely populated with the CB1 subtype.
O-4394 and O-4395 also behaved as cannabinoid receptor antagonists in vivo. Thus, at i.v. doses of 3 mg kg−1 or less, both these compounds attenuated Δ9-THC-induced anti-nociception and hypothermia and O-4395 also attenuated the ability of Δ9-THC to induce immobility in the ring test. It is likely that O-4394 and O-4395 produced their antagonism of Δ9-THC by interacting with this compound at cannabinoid CB1 receptors. Thus first, antagonism of these responses to Δ9-THC can also readily be induced by the CB1-selective antagonist, SR141716A (Compton et al., 1996; Adams et al., 1998; see also Figure 6), and, second, as already discussed, the results we obtained in brain membrane experiments with CP55940 suggest that both O-4394 and O-4395 are CB1 receptor antagonists. Moreover, previous experiments with mice have shown that the ability of synthetic cannabinoid receptor agonists to induce anti-nociception in the tail-flick test and hypothermia is not opposed by the CB2-selective antagonist, SR144528 (Wiley et al., 2002). There are also studies showing that Δ9-THC fails to induce ring immobility and/or hypothermia in two lines of CB1 knockout mice (Ledent et al., 1999; Zimmer et al., 1999), although interestingly, in one of these transgenic strains, Δ9-THC did retain the ability to display antinociceptive activity in the tail-flick test (Zimmer et al., 1999).
The dose range within which O-4394 or O-4395 was found to antagonize Δ9-THC in vivo (0.03–3 mg kg−1; 0.1–10.5 μmol kg−1) indicates that the potency with which these compounds induce such antagonism is of the same order as the potency exhibited by Δ9-THC as a CB1 receptor agonist when this is assessed by its ability to induce anti-nociception, ring immobility or hypothermia in mice. Thus, when injected i.v., Δ9-THC has been shown to exhibit agonist activity in these assays with ED50 values of 1.4 or 1.5 mg kg−1 (4.5 or 4.8 μmol kg−1; Compton et al., 1992). These data lend further support to the hypothesis that O-4394 and O-4395 produced their antagonism of Δ9-THC in vivo by interacting with Δ9-THC at CB1 cannabinoid receptors, as both O-4394 and O-4395 (Table 1) exhibit a similar potency to that of Δ9-THC (Ki=40.7 nM; Compton et al., 1993) as displacers of [3H]-CP55940 from specific binding sites on rodent brain membranes.
When administered alone, albeit only at doses above those at which they attenuated the ability of Δ9-THC to induce anti-nociception and hypothermia in mice, both O-4394 and O-4395 elicited antinociceptive responses, and O-4395 but not O-4394 induced hypothermia. O-4395 also antagonized Δ9-THC in the ring test with greater potency than it induced ring immobility when injected by itself. Although in most of the bioassays used in this investigation O-4394 and O-4395 exhibited less potency in vivo as agonists than as antagonists of Δ9-THC, the highest dose at which O-4395 antagonized Δ9-THC-induced anti-nociception and hypothermia (3 mg kg−1 i.v.) did produce a significant degree of hypothermia and ring immobility. It is likely that the antinociceptive effects of O-4394 and O-4395 were CB1 receptor-mediated, as they were attenuated by SR141716A when this was administered i.p. at 3 mg kg−1, a dose that additionally antagonized not only anti-nociception but also ring immobility and hypothermia induced in mice by Δ9-THC (Figure 6). In contrast, the ring immobility induced by O-4394 and O-4395 and the hypothermia induced by O-4395 were not significantly attenuated by SR141716A at 3 mg kg−1 i.p. This may have been because this dose of SR141716A, an established surmountable CB1 receptor antagonist, was insufficient to attenuate responses to what appears to be a supramaximal dose, at least of O-4394 in the ring test and of O-4395 for its production of hypothermia (Figure 5). Another possible explanation is that O-4394 and O-4395 did not induce their effects on ring immobility and core temperature by activating CB1 receptors. It is noteworthy, therefore, that experiments with the mouse isolated vas deferens have already provided evidence that eΔ9-THCV can produce effects that are not mediated by CB1 receptors. These experiments showed that, at concentrations above those at which it antagonized R-(+)-WIN55212, anandamide, CP55940 or Δ9-THC, eΔ9-THCV inhibited electrically evoked contractions of this tissue in an SR141716A-independent manner and reduced contractile responses to both phenylephrine and β,γ-methylene-ATP (Thomas et al., 2005). If, on the one hand, O-4394 and O-4395 are CB1 receptor antagonists and, on the other hand, they can induce ring immobility through a CB1 receptor-independent mechanism, this could explain why the ability of a low dose of O-4395 to attenuate ring immobility induced by 3 mg kg−1 Δ9-THC disappeared when higher doses of O-4395 were administered. Also, this could be the reason why none of the doses of O-4394 used in our experiments produced any detectable attenuation of the ring immobility induced by 10 mg kg−1 Δ9-THC.
It is currently unclear why O-4394 and O-4395 behave as CB1 receptor antagonists in the tail-flick assay at doses of 3 mg kg−1 or less, and as CB1 receptor agonists in this assay at doses above 10 mg kg−1. One possibility is that O-4394 and/or O-4395 lack significant efficacy as cannabinoid receptor agonists, but can be metabolized in vivo to compounds that are capable of inducing signs of anti-nociception in this assay when they are present at sufficiently high concentrations. Given the structural similarities between THC and THCV, this hypothesis is supported by evidence that Δ8-THC and Δ9-THC exhibit less potency than their 11-hydroxy metabolites as antinociceptive agents in the mouse hot plate test (Wilson and May, 1975), and that Δ8-THC and Δ9-THC are also less potent than 11-hydroxy-Δ8-THC and 11-hydroxy-Δ9-THC, respectively, as inducers of tachycardia and conjunctival reddening in human subjects (Hollister, 1974). There is already evidence that O-4394 and O-4395 are metabolized to 11-hydroxy metabolites (Brown and Harvey, 1988). Clearly, however, further experiments are required to establish firstly, whether unmetabolized O-4394 and O-4395 lack significant antinociceptive activity at the doses used in this investigation, and second, whether the 11-hydroxy metabolites of these cannabinoids induce signs of anti-nociception in the tail-flick test. In the meantime, it is worth noting that the structural similarity that exists both between Δ9-THC and O-4394 and between Δ8-THC and O-4395 makes it very likely that the 11-hydroxy metabolites of O-4394 and O-4395 exhibit greater antinociceptive activity than their parent compounds. For O-4394, there is further evidence that it lacks significant activity as a CB1 receptor agonist. Thus, O-4394 was found not to share the ability of CP55940 to stimulate [35S]GTPγS binding to brain membranes when applied at concentrations of up to 10 μM. In contrast, O-4395 did induce a small but significant stimulation of [35S]GTPγS binding to brain membranes at some concentrations. It is noteworthy, however, that the maximal degree of stimulation of [35S]GTPγS-binding produced by O-4395 was markedly less than that induced by CP55940, an indication that O-4395 may be a low-efficacy partial agonist for the CB1 receptor. In view of our finding that O-4395 exhibits agonist activity over quite a narrow concentration range, it remains possible that O-4394 would also exhibit such activity if administered at concentrations other than those used in this investigation. That O-4394 should resemble O-4395 in this way would not be unexpected, given the marked similarity that exists between the pharmacology of Δ8- and Δ9-THC, the pentyl analogues of O-4395 and O-4394, respectively (reviewed in Howlett et al., 2002).
In conclusion, this investigation has provided evidence that O-4394 and O-4395 exhibit similar pharmacological properties to eΔ9-THCV in vitro and that they can antagonize the CB1/CB2 receptor agonist, Δ9-THC, in vivo. Our results showing that O-4394 and O-4395 behave in vivo as antagonists in one dose range, but as agonists in another are in line with previous findings; for example, Sulcova et al. (1998) and Sañudo-Peña et al. (2000) demonstrated that some cannabinoid receptor agonists exhibit biphasic or even triphasic in vivo effects in a dose-dependent manner (reviewed in Pertwee, 1985; Dewey, 1986). As eΔ9-THCV exhibits unexpectedly high potency as an antagonist of anandamide and R-(+)-WIN55212 in the mouse vas deferens (Thomas et al., 2005), it will be of interest to establish in future experiments whether there are any responses to exogenously administered anandamide or R-(+)-WIN55212, or to endogenously released anandamide that are particularly sensitive to antagonism by O-4394 or O-4395, or indeed by eΔ9-THCV, when it is administered in vivo. The ability of O-4394 and O-4395 to antagonize CB1-mediated in vivo effects of Δ9-THC other than anti-nociception, ring immobility or hypothermia, for example Δ9-THC-induced inhibition of intestinal motility (reviewed in Pertwee, 2001), also merits future investigation.
Acknowledgments
This investigation was supported by grants from GW Pharmaceuticals and the National Institute on Drug Abuse (DA-09789, DA-02396 & DA-03672).
Abbreviations
- BSA
bovine serum albumin
- CHO
Chinese hamster ovary
- CP55940
(–)-cis-3-[2-hydroxy-4-(1,1-dimethylheptyl)phenyl]-trans-4-(3-hydroxypropyl)cyclohexanol
- Δ9-THC
Δ9-tetrahydrocannabinol
- DMSO
dimethyl sulphoxide
- eΔ9-THCV
Δ9-tetrahydrocannabivarin extracted from cannabis
- GDP
guanosine 5′-diphosphate
- GTPγS
guanosine-5′-O-(3-thiotriphosphate)
- MPE
maximum possible effect
- O-4394
Δ9-tetrahydrocannabivarin
- O-4395
Δ8-tetrahydrocannabivarin
- PLSD
protected least significant difference
- R-(+)-WIN55212
(R)-(+)-[2,3-dihydro-5-methyl-3-(4-morpholinylmethyl)pyrrolo-[1,2,3-de]-1,4-benzoxazin-6-yl]-1-naphthalenylmethanone
- SR141716A
N-(piperidin-1-yl)-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide hydrochloride.
Conflict of interest
The authors state no conflict of interest.
References
- Adams IB, Compton DR, Martin BR. Assessment of anandamide interaction with the cannabinoid brain receptor: SR 141716A antagonism studies in mice and autoradiographic analysis of receptor binding in rat brain. J Pharmacol Exp Ther. 1998;284:1209–1217. [PubMed] [Google Scholar]
- Bliss CI. Statistics in Biology: Statistical Methods for Research in the Natural Sciences. McGraw-Hill: New York; 1967. [Google Scholar]
- Breivogel CS, Griffin G, Di Marzo V, Martin BR. Evidence for a new G protein-coupled cannabinoid receptor in mouse brain. Mol Pharmacol. 2001;60:155–163. [PubMed] [Google Scholar]
- Cheng Y-C, Prusoff WH. Relationship between the inhibition constant (KI) and the concentration of inhibitor which causes 50 percent inhibition (IC50) of an enzymatic reaction. Biochem Pharmacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- Compton DR, Aceto MD, Lowe J, Martin BR. In vivo characterization of a specific cannabinoid receptor antagonist (SR141716A): inhibition of Δ9-tetrahydrocannabinol-induced responses and apparent agonist activity. J Pharmacol Exp Ther. 1996;277:586–594. [PubMed] [Google Scholar]
- Compton DR, Johnson MR, Melvin LS, Martin BR. Pharmacological profile of a series of bicyclic cannabinoid analogs: classification as cannabimimetic agents. J Pharmacol Exp Ther. 1992;260:201–209. [PubMed] [Google Scholar]
- Compton DR, Rice KC, de Costa BR, Razdan RK, Melvin LS, Johnson MR, et al. Cannabinoid structure-activity relationships: correlation of receptor binding and in vivo activities. J Pharmacol Exp Ther. 1993;265:218–226. [PubMed] [Google Scholar]
- D'Amour FE, Smith DL. A method for determining loss of pain sensation. J Pharmacol Exp Ther. 1941;72:74–79. [Google Scholar]
- Dewey WL. Cannabinoid Pharmacology. Pharmacol Rev. 1986;38:151–178. [PubMed] [Google Scholar]
- Gill EW, Paton WDM, Pertwee RG. Preliminary experiments on the chemistry and pharmacology of cannabis. Nature. 1970;228:134–136. doi: 10.1038/228134a0. [DOI] [PubMed] [Google Scholar]
- Harvey DJ, Brown NK.Metabolism of delta-8- and delta-9-tetrahydrocannabinol homologues in mice Marijuana: An International Research Report, Monograph Series No. 7 1988Australian Gov. Publ. Service: Canberra, Australia; 253–258.In Chesher G, Consroe P, Musty R (eds) [Google Scholar]
- Hollister LE. Structure-activity relationships in man of cannabis constituents and homologs and metabolites of Δ9-tetrahydrocannabinol. Pharmacology. 1974;11:3–11. doi: 10.1159/000136462. [DOI] [PubMed] [Google Scholar]
- Howlett AC, Barth F, Bonner TI, Cabral G, Casellas P, Devane WA, et al. International Union of Pharmacology. XXVII. Classification of Cannabinoid Receptors. Pharmacol Rev. 2002;54:161–202. doi: 10.1124/pr.54.2.161. [DOI] [PubMed] [Google Scholar]
- Kurkinen KMA, Koistinaho J, Laitinen JT. [γ-35S]GTP autoradiography allows region-specific detection of muscarinic receptor-dependent G-protein activation in the chick optic tectum. Brain Res. 1997;769:21–28. doi: 10.1016/s0006-8993(97)00663-x. [DOI] [PubMed] [Google Scholar]
- Ledent C, Valverde O, Cossu G, Petitet F, Aubert J-F, Beslot F, et al. Unresponsiveness to cannabinoids and reduced addictive effects of opiates in CB1 receptor knockout mice. Science. 1999;283:401–404. doi: 10.1126/science.283.5400.401. [DOI] [PubMed] [Google Scholar]
- Pertwee RG. The ring test: a quantitative method for assessing the ‘cataleptic' effect of cannabis in mice. Br J Pharmacol. 1972;46:753–763. doi: 10.1111/j.1476-5381.1972.tb06900.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee RG.Effects of cannabinoids on thermoregulation: a brief review Marihuana ‘84 1985IRL Press: Oxford; 263–277.In: Harvey DJ (ed) [Google Scholar]
- Pertwee RG. Cannabinoids and the gastrointestinal tract. Gut. 2001;48:859–867. doi: 10.1136/gut.48.6.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee RG, Fernando SR, Griffin G, Ryan W, Razdan RK, Compton DR, et al. Agonist-antagonist characterization of 6′-cyanohex-2′-yne-Δ8-tetrahydrocannabinol in two isolated tissue preparations. Eur J Pharmacol. 1996;315:195–201. doi: 10.1016/s0014-2999(96)00631-0. [DOI] [PubMed] [Google Scholar]
- Pertwee RG, Griffin G, Lainton JAH, Huffman JW. Pharmacological characterization of three novel cannabinoid receptor agonists in the mouse isolated vas deferens. Eur J Pharmacol. 1995;284:241–247. doi: 10.1016/0014-2999(95)00318-f. [DOI] [PubMed] [Google Scholar]
- Pertwee RG, Ross RA, Craib SJ, Thomas A. Cannabidiol antagonizes cannabinoid receptor agonists and noradrenaline in the mouse vas deferens. Eur J Pharmacol. 2002;456:99–106. doi: 10.1016/s0014-2999(02)02624-9. [DOI] [PubMed] [Google Scholar]
- Ross RA, Brockie HC, Stevenson LA, Murphy VL, Templeton F, Makriyannis A, et al. Agonist-inverse agonist characterization at CB1 and CB2 cannabinoid receptors of L759633, L759656 and AM630. Br J Pharmacol. 1999a;126:665–672. doi: 10.1038/sj.bjp.0702351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross RA, Gibson TM, Stevenson LA, Saha B, Crocker P, Razdan RK, et al. Structural determinants of the partial agonist-inverse agonist properties of 6′-azidohex-2′-yne-Δ8-tetrahydrocannabinol at cannabinoid receptors. Br J Pharmacol. 1999b;128:735–743. doi: 10.1038/sj.bjp.0702836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sañudo-Peña MC, Romero J, Seale GE, Fernández-Ruiz JJ, Walker JM. Activational role of cannabinoids on movement. Eur J Pharmacol. 2000;391:269–274. doi: 10.1016/s0014-2999(00)00044-3. [DOI] [PubMed] [Google Scholar]
- Sulcova E, Mechoulam R, Fride E. Biphasic effects of anandamide. Pharmacol Biochem Behav. 1998;59:347–352. doi: 10.1016/s0091-3057(97)00422-x. [DOI] [PubMed] [Google Scholar]
- Thomas A, Ross RA, Saha B, Mahadevan A, Razdan RK, Pertwee RG. 6′′-Azidohex-2′′-yne-cannabidiol: a potential neutral, competitive cannabinoid CB1 receptor antagonist. Eur J Pharmacol. 2004;487:213–221. doi: 10.1016/j.ejphar.2004.01.023. [DOI] [PubMed] [Google Scholar]
- Thomas A, Stevenson LA, Wease KN, Price MR, Baillie G, Ross RA, et al. Evidence that the plant cannabinoid Δ9-tetrahydrocannabivarin is a cannabinoid CB1 and CB2 receptor antagonist. Br J Pharmacol. 2005;146:917–926. doi: 10.1038/sj.bjp.0706414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Sickle MD, Duncan M, Kingsley PJ, Mouihate A, Urbani P, Mackie K, et al. Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science. 2005;310:329–332. doi: 10.1126/science.1115740. [DOI] [PubMed] [Google Scholar]
- Varvel SA, Bridgen DT, Tao Q, Thomas BF, Martin BR, Lichtman AH. Δ9-tetrahydrocannbinol accounts for the antinociceptive, hypothermic, and cataleptic effects of marijuana in mice. J Pharmacol Exp Ther. 2005;314:329–337. doi: 10.1124/jpet.104.080739. [DOI] [PubMed] [Google Scholar]
- Wiley JL, Jefferson RG, Griffin G, Liddle J, Yu S, Huffman JW, Martin BR. Paradoxical pharmacological effects of deoxy-tetrahydrocannabinol analogs lacking high CB1 receptor affinity. Pharmacology. 2002;66:89–99. doi: 10.1159/000065631. [DOI] [PubMed] [Google Scholar]
- Wiley JL, Martin BR. Cannabinoid pharmacological properties common to other centrally acting drugs. Eur J Pharmacol. 2003;471:185–193. doi: 10.1016/s0014-2999(03)01856-9. [DOI] [PubMed] [Google Scholar]
- Wilson RS, May EL. Analgesic properties of the tetrahydrocannabinols, their metabolites and analogs. J Med Chem. 1975;18:700–703. doi: 10.1021/jm00241a012. [DOI] [PubMed] [Google Scholar]
- Zimmer A, Zimmer AM, Hohmann AG, Herkenham M, Bonner TI. Increased mortality, hypoactivity, and hypoalgesia in cannabinoid CB1 receptor knockout mice. Proc Natl Acad Sci USA. 1999;96:5780–5785. doi: 10.1073/pnas.96.10.5780. [DOI] [PMC free article] [PubMed] [Google Scholar]