Abstract
Background and purpose:
Neuroblastoma is the most common solid tumour in infants characterized by a high resistance to apoptosis. Recently, the cyclo-oxygenase pathway has been considered a potential target in the treatment of different kinds of tumours. The aim of the present work was to investigate a possible relationship between cyclo-oxygenase pathway and stauroporine-induced apoptosis in the neuroblastoma cell line SH-SY5Y.
Experimental approach:
Cellular viability was measured by release of LDH. DNA fragmentation was visualized by electrophoresis on agarose gel containing ethidium bromide. Cyclo-oxygenase activity was measured in microsomal fractions obtained from cells by quantification of its final product PGE2 by RIA. Caspase-3 activity was measured fluorimetrically and Western blot analysis was performed to assess cytochrome c expression.
Key results:
We have found that staurosporine (500 nM) induced cellular death in a time-dependent manner in SH-SY5Y human neuroblastoma cells. Cyclo-oxygenase enzymatic activity was present in SH-SY5Y human neuroblastoma cells under basal conditions and pharmacological experiments using COX inhibitors indicate that cyclo-oxygenase-1 and cyclo-oxygenase-3 are the active isoforms in these cells. Co-incubation of SH-SY5Y cells with staurosporine (500 nM) and acetaminophen for 24 h potentiated staurosporine-mediated cellular death in a concentration-dependent manner. This process is mediated by an increase in cytochrome c release and caspase 3 activation and is prevented by N-acetylcysteine or the superoxide dismutase mimetic, MnTBAP.
Conclusions and implications:
Acetaminophen potentiates staurosporine-mediated neuroblastoma cell death. The mechanism of action of acetaminophen seems to be related to production of reactive oxygen species and decreased intracellular glutathione levels.
Keywords: neuroblastoma, apoptosis, cyclooxygenase, mitochondria, glutathione, free radicals
Introduction
Neuroblastoma is the single most common extracranial solid tumour in infants younger than 1 year of age. Tumours originate from neural crest cells in the sympathetic nervous system, and it has been proposed that resistance to apoptosis is one of the mechanisms that contribute to the aggressive behaviour of advanced-stage neuroblastoma, particularly in older children. In fact, a large percentage of neuroblastomas do not express apical caspases 8 and 10 and do not respond to the extrinsic apoptotic pathway (Haase et al., 1999).
Prostaglandins are a family of structurally related molecules derived from fatty acids, primarily arachidonate, which are released from membrane phospholipids by the action of phospholipases. Arachidonate is first converted into an unstable endoperoxide intermediate by cyclooxygenase (COX) and then is subsequently converted into one of several related products through the action of specific prostaglandin synthetases (Smith, 1989; Smith, 1992). It has been reported that COX activity as well as its major final product, prostaglandin E2 (PGE2), protect cells against apoptosis (Dempke et al., 2001; Wang and Dubois, 2004; Minghetti et al., 2005). In fact, rat intestinal epithelial cells over expressing COX-2 were resistant to butyrate-induced apoptosis and had elevated Bcl-2 protein expression (Tsujii and DuBois, 1995). On the other hand, PGE2 prevented apoptosis caused by a COX inhibitor and induced Bcl-2 expression in human colon cancer cells (Sheng et al., 1998). In addition, it has been described that human lung adenocarcinoma CL 1.0 cells with high levels of COX-2 showed a remarkable resistance to apoptosis induced by vinblastine (Lin et al., 2001). It has also been reported that several neuroblastomas expressed COX-2 and that treatment with non-steroidal anti-inflammatory drugs for 48 h induced apoptosis and inhibited growth of neuroblastoma cells in vitro (Johnsen et al., 2004).
Staurosporine, a non-selective protein kinase C (PKC) inhibitor, is an effective inducer of apoptosis and also promotes neuritogenesis in many different cell types including human neuroblastomas and neurons from different animal species (Jalava et al., 1993; Koh et al., 1995; Prehn et al., 1997; Scarlett et al., 2000). However, although staurosporine-induced apoptosis has been extensively characterized, its molecular targets are not well known. It has been described that staurosporine at concentrations up to 50 nM is able to induce SH-SY5Y differentiation and that cell treatment with higher concentrations results in cell death by a classical apoptotic process (Boix et al., 1997; Posmantur et al., 1997). Mitochondria seem to play a central role in staurosporine-induced apoptosis. In fact, Bcl-xl overexpression confers resistance to staurosporine-induced apoptosis in SH-SY5Y cells by preventing cytochrome c release from mitochondria (Yuste et al., 2002) and it has been described that cytochrome c−/− stem cells are resistant to cell-death induced by staurosporine (Li et al., 2000).
One of the possible targets for staurosporine-induced cell death might be COX activity. Recently, it has been reported that PKC regulates COX-2 expression in the heart (Xuan et al., 2005) and in lipopolysaccharide-activated primary rat microglia (Akundi et al., 2005), and it is known that activation of PKC by phorbol esters impairs endothelium-dependent relaxation via release of vasoconstrictor prostanoids (Tesfamariam et al., 1991).
The aim of the present work was to examine a possible involvement of the COX pathway in staurosporine-induced apoptosis in SH-SY5Y neuroblastoma cells. We have found that staurosporine decreases PGE2 production and that acetaminophen, a COX-3 inhibitor, markedly potentiated staurosporine-induced cellular death by a mechanism related to a decrease in glutathione (GSH) levels and impairment of mitochondrial function.
Methods
Cell culture
SH-SY5Y neuroblastoma cell line was grown in Dulbecco's modified Eagle's medium supplemented with 2 mM L-glutamine, penicillin (20 U ml−1), streptomycin (5 μg ml−1) and 15% heat-inactivated foetal calf serum as reported previously by Boix et al. (1997). Cells were maintained at 37°C in a saturated humidity atmosphere containing 95% air and 5% CO2. For viability experiments, cells were cultured in 24-well culture plates until 80% confluence was reached and then treated with vehicle (dimethyl sulfoxide (DMSO) 1%), staurosporine (500 nM) or staurosporine+COX inhibitors for different times. Supernatants were collected and cells were washed with phosphate-buffered saline (PBS) and lysed with 0.9% Triton X-100 (v/v) in saline. Lactate dehydrogenase (LDH) activity was measured as an index of cellular death and mortality was assessed by the percentage of cellular LDH released into culture media. LDH was measured spectrophotometrically at 490 nm on a 96-well plate reader by using the Cytotox 96 Kit according to the manufacturer instructions. Supernatants collected were also used to quantify PGE2 levels by radioimmunoassay (Moroney et al., 1988).
DNA fragmentation
Cells were grown in 25 cm2 culture flask until 80% confluence was reached and then treated with vehicle (DMSO 1‰) or staurosporine (500 nM). Twenty-four hours later, cells were collected by scraping and centrifuged at 800 × g for 10 min. The pellet was washed twice with PBS–MgCl2 5 mM and then resuspended in lysis buffer (50 mM Tris-HCl, 50 mM NaCl, 10 mM ethylenediaminetetraacetic acid (EDTA), 0.5% sodium dodecyl sulfate (SDS) pH 7.4) containing 0.125% (w/v) proteinase K and maintained at 50°C overnight. After centrifugation at 10 000 × g for 10 min at 4°C, fragmented DNA in the supernatant was extracted by adding a mixture of phenol:chloroform:isoamyl alcohol (25:24:1) and centrifuged at 10 000 × g for 10 min at 4°C. Fragmented DNA in the aqueous phase was precipitated by adding 3 M sodium acetate and 800 μl of absolute ethanol and then isolated by centrifugation at 10 000 × g for 20 min. The DNA pellet was dissolved in 25 μl of a 10 mM Tris-HCl, pH 7.4 solution containing 1 mM EDTA. The DNA samples were subjected to electrophoresis on 1.5% agarose gel and then visualized under ultraviolet light after staining with ethidium bromide. Densitometric analysis of low-molecular weight (<1 Kbp) was performed by using ImageQuant 5.2 program.
Caspase 3 activity
Cells were grown in six-well culture plates until 80% confluence was reached. Then, cells were treated with vehicle (DMSO 1‰), staurosporine (500 nM) alone or in combination with acetaminophen for different times. Afterwards, cells were washed twice with cold PBS and lysed in lysis buffer containing 100 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulphonic acid (HEPES), 5 mM dithiothreitol (DTT), 5 mM ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′,-tetraacetic acid (EGTA), 0.04% Nonidet P-40 and 20% glycerol; pH 7.4. Extracts were then centrifuged at 5000 × g for 10 min at 4°C, and protein content was determined by using the bicinchoninic acid (BCA) protein assay according to the manufacturer instructions. Cell extracts (40 μg of protein) were incubated in reaction buffer (25 mM HEPES, 10% sucrose, 0.1% 3-[(3-cholamido propyl)-dimethylammonio]-2-hydroxy-1-propanesulfonic acid, 10 mM DTT) containing 50 μM fluorescence substrate Asp-Glu-Val-Asp-7-amino-4 trifluoromethyl-coumaryl (Z-DEVD-AFC) at 37°C for 1 h. Cleavage of the AFC fluorophore was determined in a spectrofluorometer at excitation wavelength of 400 nm and fluorescence was detected at an emission wavelength of 505 nm. Caspase 3 activity was expressed as units of fluorescence (mg of protein h−1).
COX assay
Cells were grown in six-well culture plates until 80% confluence was reached and then treated with vehicle (DMSO 1‰) or staurosporine (500 nM). Cells were then collected and resuspended in homogenization buffer (10 mM HEPES, 0.32 M sucrose, 100 μM EDTA, 1 mM DTT, 0.1 mM phenylmethylsulphonyl fluoride (PMSF), 40 μg ml−1 aprotinin, 20 μg ml−1 leupeptin; pH 7.4). Cells were homogenized using a Polytron (two cycles, 10 s at maximum speed). Homogenates were centrifuged at 100 000 × g for 60 min and the pellet (microsomal fraction) was resuspended and used to determine COX activity. Microsomal fractions (40 μg) obtained from cells after treatment with vehicle or staurosporine were incubated with 4 μM hematin, 1 mM L-tryptophan and 5 μM arachidonic acid at 37°C for 15 min. In another set of experiments, microsomal fractions (40 μg) obtained from vehicle-treated cells were preincubated with indomethacin (a non-selective inhibitor of COX), NS-398 (a selective inhibitor of COX-2) or acetaminophen (an inhibitor of COX-3) for 5 min, and then with 4 μM hematin, 1 mM L-tryptophan and 5 μm arachidonic acid at 37°C for 15 min. Reaction was finished by heating the samples at 90°C for 5 min and then centrifuging at 10 000 × g for 15 min. Supernatants were used to quantify PGE2 production by radioimmunoassay (Moroney et al., 1988).
Cytochrome c determination
Immunoblot analysis was performed on cytosolic and mitochondrial fractions from vehicle-, staurosporine- and staurosporine+acetaminophen-treated cells. After different times of incubation, cells were washed twice with PBS, scraped and collected by centrifugation at 1500 × g for 10 min. Cell pellets were resuspended in 200 μl of extraction buffer (250 mM sucrose, 50 mM Tris-HCl, 1 mM EGTA, 2.5 mM EDTA, 50 μM Na3VO4, 1 mM DTT, 0.1 mM PMSF, 40 μg ml−1 aprotinin, 20 μg ml−1 leupeptin; pH 7.4) and homogenized with a pellet pestle (Sigma, St Louis, MO, USA) (15 strokes) and, after 15 min on ice, centrifuged at 20 000 × g for 30 min at 4°C. The supernatants, that is, cytosolic fractions, were removed and stored at −80°C until analysed by gel electrophoresis. Pellets containing mitochondria were resuspended in 50 μl of extraction buffer, homogenized with a pestle (five strokes) and then centrifuged at 20 000 × g for 60 min at 4°C. The supernatants, that is, mitochondrial fractions, were removed and analysed by gel electrophoresis. Protein samples (30 μg) were loaded on 15% SDS polyacrylamide gel electrophoresis and transferred onto nitrocellulose membranes. Membranes were blocked in PBS-Tween 20 (0.1%) containing 5% non-fat dry milk and 0.1% BSA for 1 h at 4°C and incubated with anti-cytochrome c polyclonal antibody (1:1000), α-tubulin polyclonal antibody (1:2000) or anti-OxPhos Complex IV subunit IV monoclonal antibody (1:1000) overnight at 4°C. Afterwards, blots were washed with PBS-Tween 20 (0.1%) and incubated with horseradish peroxidase conjugated (HRP) anti-mouse immunoglobulin (Ig)G (1:10 000) for 2 h at 4°C. Immunoreactive bands were visualized using an enhanced chemiluminescence system (Amersham Biosciences, Barcelona, Spain).
GSH measurement
Cells were grown in six-well culture plates until 80% confluence was reached and then treated with vehicle (DMSO 1‰), staurosporine (500 nM) alone or in combination with acetaminophen for 18 h. Then, cells were washed twice with cold PBS and scraped in 1 ml of PBS. Cells collected were counted using a microscope Neubauer counting chamber and after centrifugation at 1500 × g for 10 min, the pellet was resuspended in 100 μl of 5-sulphosalicylic acid (3.33%) containing 0.25 mM EDTA to prevent oxidation of GSH and to inhibit GSH-utilizing enzymes. Tubes were frozen and thawed three times to break the cells and release GSH. The lysate was then centrifuged (10 000 × g for 5 min at 4°C) and the supernatants transferred to Eppendorf tubes kept in dry ice until assayed for GSH content. GSH measurements were performed as previously described (Griffith, 1980). GSH reacts non-enzymically with 5,5′-dithiobis-2-nitrobenzoic acid (DTNB) to generate oxidized GSH (GSSG) and the highly coloured 5-thio-2-nitrobenzoic acid (peak absorbance 420 nm); the formed GSSG is back reduced to GSH by GSH reductase coupled to nicotinamide adenine dinucleotide phosphate (reduced form) (NADPH) oxidation. In this cycling assay, concentrations of the reactants are chosen so that the rate of colour formation is linear with time, the slope being (Δabsorbance/Δtime (min)) directly proportional to total glutathione (GSH+GSSG; GSt) concentration. This allows computer-assisted on-line construction of standard curves relating GSt concentrations to the slopes of their assays. As this relationship is linear, it is possible to measure the concentration of GSt in identically treated test sample by interpolation. Concentration obtained was normalized by the number of living cells 18 h after treatments. Standards and cell homogenates were assayed in triplicate. Assay mixture contained: NADPH 0.21 mM, DTNB 0.6 mM and EDTA 6.3 mM in a final volume of 1 ml of phosphate buffer 0.125 M; pH 7.5. Reaction was started by adding the sample and GSH reductase (2 U).
Statistical analysis
Data are expressed as mean±s.e.m. Statistical analyses were carried out using the one-way analysis of variance and Bonferroni's t-test for multiple comparisons. Statistical results are given in the figure legends.
Drugs and reagents
BCA protein assay kit was from Pierce Biotechnology Inc. (IL, USA). Cytotox 96 kit was from Promega Biotech Iberica SL (Madrid, Spain). Foetal calf serum was from Invitrogen (Barcelona, Spain). NS-398 and Z-DEVD-AFC were from Calbiochem (Madrid, Spain). [3H-PGE2] was from Amersham Biosciences (Barcelona, Spain). The antibody against cytochrome c was from BD Pharmingen (Madrid, Spain). The antibody against OxPhos Complex IV subunit IV was from Molecular Probes (Barcelona, Spain). The HRP-conjugated IgG antibody was purchased from DakoCytomation SA (Barcelona, Spain). All other reagents were obtained from Sigma-Aldrich (Madrid, Spain).
Results
Effect of staurosporine on SH-SY5Y viability
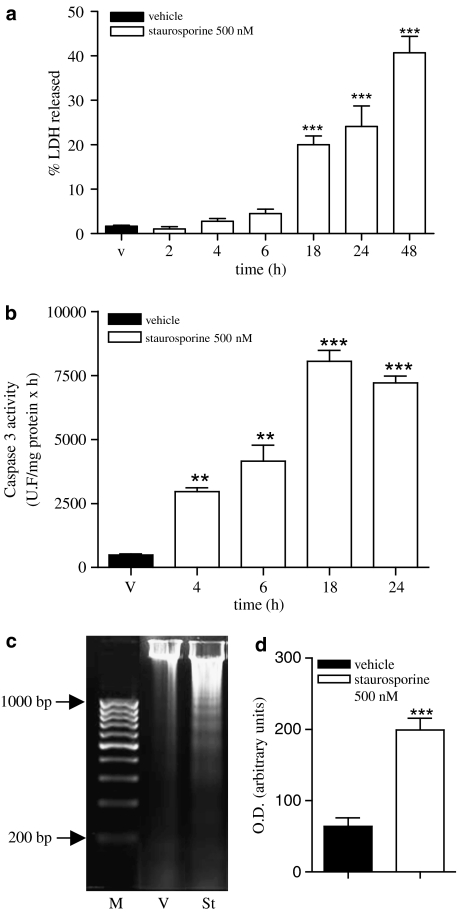
SH-SY5Y human neuroblastoma cells were treated with staurosporine (500 nM) to determine its effect on cellular viability. After different incubation periods, supernatants were collected and cells were lysed. LDH activity was measured in supernatants as well as in cell lysates and the percentage of LDH released (% LDH released) was used as an index of cell death. Staurosporine treatment induced an increase in cellular mortality that was time-dependent. Eighteen hours after the addition of staurosporine, the percentage of LDH released rose to about 20 versus 3% in vehicle-treated cells (Figure 1a). This increase in cell mortality was accompanied by an increase in caspase 3 activity that preceded LDH release (Figure 1b). In addition, staurosporine treatment induced DNA fragmentation (Figure 1c) as described previously (Yuste et al., 2002). Densitometric analysis of low-molecular weight DNA fragments showed that staurosporine induced a fourfold increase in fragmentation compared to vehicle-treated cells (Figure 1c).
Figure 1.
Effect of staurosporine (500 nM) on SH-SY5Y neuroblastoma cell line. (a) Time course of staurosporine-induced cell death. Release of LDH is expressed as percentage of total LDH content in the cell at the beginning of the experiment. Data represent mean+s.e.m. of 12 experiments. **P<0.01 as compared to vehicle-treated cells (v). (b) Time course of staurosporine-induced caspase 3 activity in total lysates. Caspase 3 activity was measured as indicated in Methods. Data are expressed as mean±s.e.m. of 12 experiments. **P<0.01 ***P<0.001, compared to vehicle-treated cells. (c) DNA fragmentation in SH-SY5Y cells treated with vehicle (v) or staurosporine (St) 500 nM for 24 h. Nucleosomal fragmentation was visualized by agarose gel electrophoresis. M indicates DNA size markers. Data shown are representative of three different experiments. (d) Densitometric analysis of low-molecular weight DNA fragments extracted from vehicle- or staurosporine-treated cells. Optical density is expressed in arbitrary units. Data are expressed as mean±s.e.m. of three experiments. ***P<0.001 compared to vehicle-treated cells.
COX activity
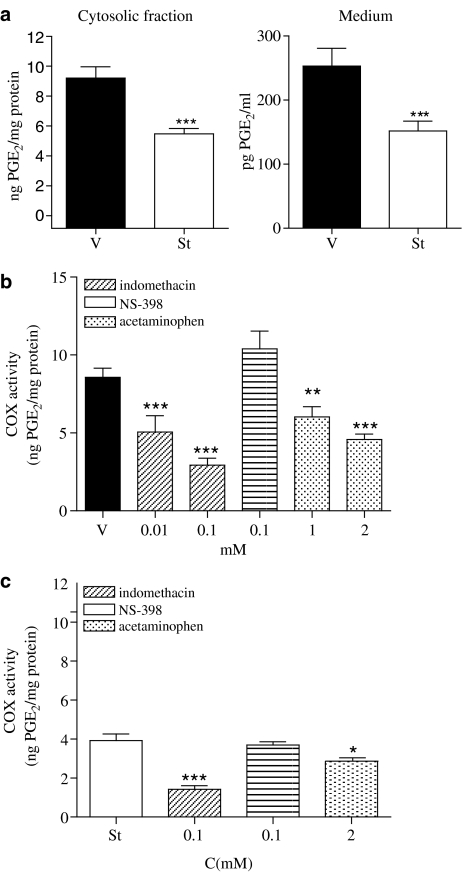
We then examined COX activity in the SH-SY5Y neuroblastoma cells. For this purpose, SH-SY5Y cells were treated with vehicle or staurosporine (500 nM) for 24 h. Afterwards cells were lysed and microsomal fractions were obtained. Assay for PGE2, as the final product of COX activity showed that treatment with staurosporine significantly reduced COX activity when compared with vehicle-treated cells (Figure 2a, left panel). Similarly, the amount of PGE2 released into the cell culture medium 24 h after vehicle- or staurosporine-treatment showed a reduction in the staurosporine-treated cells (Figure 2a, right panel).
Figure 2.
COX activity in SH-SY5Y neuroblastoma cell line. (a) Left panel, COX activity is expressed as PGE2 generated by the microsomal fraction obtained from SH-SY5Y cells treated with vehicle (v) or staurosporine (st; 500 nM; 24 h). (a) Right panel, the PGE2 levels released into supernatants obtained from vehicle-(v) or staurosporine (St; 500 nM)-treated SH-SY5Y cells for 24 h. Data represent mean+s.e.m. of 12 experiments. ***P<0.001 as compared with vehicle. (b) Effect of indomethacin, NS-398 and acetaminophen on COX activity measured in microsomal fraction obtained from vehicle-treated SH-SY5Y cells. Data represent mean+s.e.m. of 12 experiments. ***P<0.001 **P<0.01, compared to vehicle-treated cells. (c) Effect of indomethacin, NS-398 and acetaminophen on COX activity measured in microsomal fraction obtained from staurosporine (500 nM)-treated SH-SY5Y cells for 24 h. Data represent mean±s.e.m. of 12 experiments. ***P<0.001; *P<0.05 in comparison to staurosporine-treated cells.
To determine which isoforms of COX were functional in SH-SY5Y cells, microsomal fractions from vehicle- or staurosporine-treated cells were incubated with different inhibitors of the COX pathway. In vehicle-treated cells, indomethacin as well as acetaminophen, but not NS-398 inhibited COX activity in a concentration-dependent manner, suggesting that COX-3, but not COX-2 was contributing to the observed COX activity in SH-SY5Y neuroblastoma cells (Figure 2b). On the other hand, in staurosporine-treated cells again indomethacin and acetaminophen further inhibited COX activity but only at the highest concentration assayed (Figure 2c) whereas NS-398 did not have any effect suggesting that following staurosporine treatment for 24 h, COX-3 activity was still observed in the SH-SY5Y neuroblastoma cell line (Figure 2c).
Effect of COX inhibitors on SH-SY5Y viability
As staurosporine-treatment reduced COX activity in SH-SY5Y cells, we decided to investigate the effect of COX inhibitors on cellular viability. When SH-SY5Y neuroblastoma cells were incubated in the presence of different concentrations of indomethacin, NS-398 or acetaminophen for 24 h, cellular viability was not modified as compared to vehicle-treated cells (Table 1). Co-incubation of neuroblastoma cells with staurosporine+indomethacin or staurosporine+NS-398 at different concentrations for 24 h did not modify the observed effect of staurosporine on cellular viability (Table 1). However, the combination of staurosporine+acetaminophen for 24 h significantly increased the percentage of LDH released from SH-SY5Y cells as compared with staurosporine alone (Table 1).
Table 1.
Effect of COX inhibitors on viability of SH-SY5Y cells
| +Indo 0.1 | +Indo 0.01 | +NS 0.1 | +NS 0.01 | +APP 2 | +APP 1 | +APP 0.5 | ||
|---|---|---|---|---|---|---|---|---|
| V | 3.6±0.3 | 2.7±0.3 | 3.1±0.4 | 2.7±0.2 | 3.1±0.5 | 2.1±0.5 | 3.3±0.2 | N.D. |
| St | 24.4±1.0 | 23.2±4.4 | 28.0±3.7 | 24.0±3.7 | 28.0±2.3 | 49.3±5.1*** | 35.8±2.3** | 29.9±2.8 |
Abbreviations: APP, acetaminophen; COX, cyclooxygenase; indo, indomethacin; St, staurosporine.
The values in the Table are the percentage of cellular LDH released from SH-SY5Y human neuroblastoma cells incubated in the presence of Indo, NS-398 (NS) and APP alone (V) or in combination with St; 500 nM for 24 h. Drug concentration is expressed in mM. Data represents mean+s.e.m. of six to nine experiments.
P<0.01
P<0.001.
Caspase 3 activity and cytochrome c release
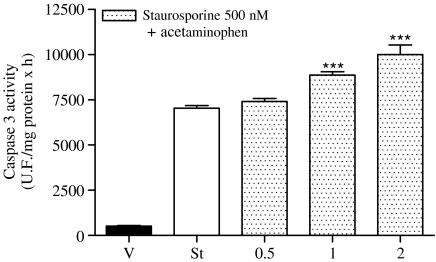
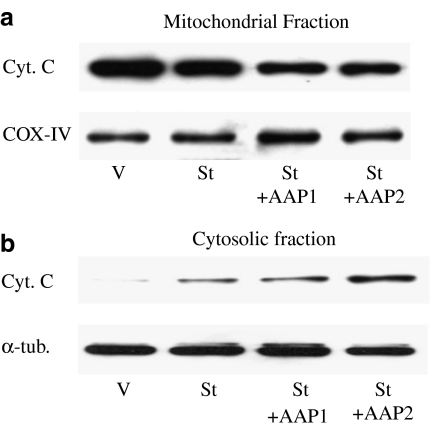
SH-SY5Y cells were treated with vehicle, staurosporine and staurosporine+acetaminophen for 18 or 24 h and, then, caspase 3 activity as well as cytochrome c release from mitochondria were analysed. The combination of staurosporine+acetaminophen enhanced caspase 3 activity when compared with staurosporine treatment alone (Figure 3). This increase in caspase 3 activity was apparent at an acetaminophen concentration of 1 mM that correlated very well with the observed effect on cellular viability. Western blot analysis of mitochondrial and cytosolic fractions of SH-SY5Y cells showed that staurosporine-induced cytochrome c release from the mitochondria and that the combination of staurosporine+acetaminophen increased the effect of staurosporine alone in a concentration-dependent manner (Figure 4). In both cases, acetaminophen treatment alone for 24 h neither activated caspase 3 nor induced cytochrome c release from mitochondria (data not shown).
Figure 3.
Caspase 3 activity in SH-SY5Y neuroblastoma cell line. Caspase 3 activity measured in total lysates obtained from SH-SY5Y cells treated with vehicle, staurosporine 500 nM or staurosporine 500 nM+different acetaminophen concentrations (in mM) for 18 h. Data are expressed as mean±s.e.m. (n=12). ***P<0.001 compared to staurosporine-treated cells.
Figure 4.
Effect of staurosporine (St) and staurosporine+acetaminophen (2 mM; St+AAP) on cytochrome c (Cyt C) released from mitochondria 24 h after treatment. (a) Cyt C levels in mitochondrial fractions; OxPhos Complex IV subunit IV (COX-IV) protein levels were used as mitochondrial protein loading controls. (b) Cyt C levels in cytosolic fractions; α-tubulin protein levels were used as cytosolic protein loading controls. Figures are representative of three separate experiments.
Effect of redox status on acetaminophen potentiation of staurosporine-induced SH-SY5Y apoptosis
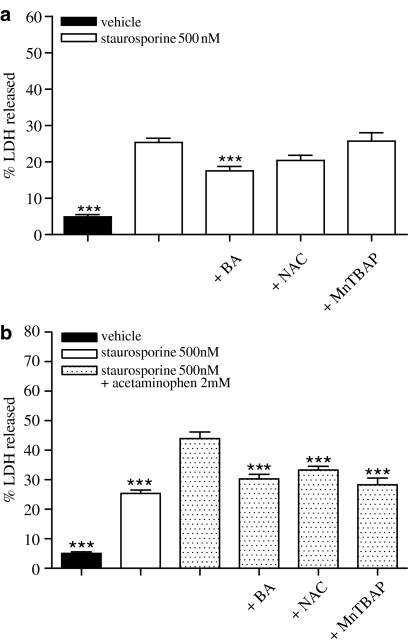
To further investigate the possible mechanism by which acetaminophen potentiates staurosporine-induced SH-SY5Y apoptosis, we investigated the effect of different compounds that affect cellular redox status on cell viability. Incubation of SH-SY5Y cells with staurosporine (500 nM)+Mn(III)-tetrakis(4-benzoic acid)-porphyrin chloride (MnTBAP) (1 μM) or with staurosporine (500 nM)+N-acetylcysteine (NAC) (100 μM) did not prevent cellular death induced by staurosporine alone (Figure 5a). However, both MnTBAP and NAC inhibited similarly (about 40 and 30%, respectively) staurosporine (500 nM)+acetaminophen (2 mM)-induced SH-SY5Y death (Figure 5b) to the levels observed in the presence of staurosporine alone. On the other hand, bongkrekic acid (BA), a blocker of the mitochondrial permeability transition pore, inhibited, by 40%, cellular death induced by staurosporine (500 nM) in the presence or absence of acetaminophen (2 mM) (Figure 5a and b).
Figure 5.
Effect of BA, NAC and MnTBAP on SH-SY5Y cell viability. (a) Percentage of LDH released from cells treated with vehicle or staurosporine 500 nM alone or in the presence of MnTBAP 1 μM, NAC 100 μM or BA 2 μM for 24 h. Data represent mean+s.e.m. of 12 experiments. ***P<0.001 as compared to staurosporine-treated cells. (b) Percentage of LDH released from cells treated with vehicle, staurosporine 500 nM+acetaminophen 2 mM or staurosporine 500 nM+acetaminophen 2 mM in the presence of MnTBAP 1 μM, NAC 100 μM or BA 2 μM for 24 h. Data represent mean±s.e.m. of 12 experiments. ***P<0.001 as compared to staurosporine+acetaminophen-treated cells.
GSH levels
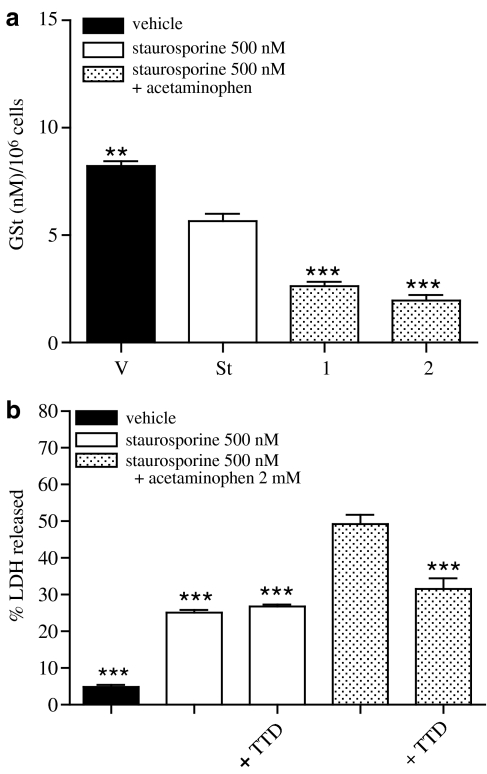
As NAC was able to prevent the effect of staurosporine+acetaminophen on SH-SY5Y viability, and it is well known that acetaminophen when metabolized by cytochrome P450 forms an intermediate metabolite (N-acetyl-p-benzoquinone), which reacts with GSH, we explored whether GSH levels in these cells could be affected by staurosporine or staurosporine+acetaminophen. Incubation of neuroblastoma cells with staurosporine for 18 h induced a weak reduction in GSt content that was enhanced by co-incubation with acetaminophen in a concentration-dependent manner (Figure 6a).
Figure 6.
Effect of staurosporine (St) and staurosporine+acetaminophen (St+AAP) on GSt content 18 h after treatment. (a) GSt was measured in total lysates obtained from SH-SY5Y cells treated with vehicle, staurosporine 500 nM or staurosporine 500 nM+different acetaminophen concentrations (in mM) for 18 h. Data are expressed as mean±s.e.m (n=12). **P<0.01; ***P<0.001 compared to staurosporine-treated cells. (b) Percentage of LDH released from cells treated with vehicle, staurosporine 500 nM, staurosporine 500 nM+acetaminophen 2 mM in the presence of disulfiram 100 nM for 24 h. Data are expressed as mean±s.e.m. (n=12). ***P<0.001 compared to staurosporine+acetaminophen-treated cells.
Effect of disulfiram on acetaminophen potentiation of staurosporine-induced SH-SY5Y apoptosis
To confirm the participation of cytochrome P450 in the mechanism by which acetaminophen potentiates staurosporine-induced SH-SY5Y apoptosis, the effect of disulfiram, an inhibitor of cytochrome P450, on cellular death was studied. Cells were treated with staurosporine (500 nM) or with staurosporine (500 nM)+acetaminophen (2 mM) in the presence or absence of disulfiram (100 nM) for 24 h and percentage of LDH release was measured. SH-SY5Y co-incubation with staurosporine (500 nM)+disulfiram (100 nM) for 24 h did not modify cellular death induced by staurosporine alone (Figure 6b) whereas disulfiram reduced, by approximately 35%, neuroblastoma cell death induced by staurosporine (500 nM)+acetaminophen (2 mM) for 24 h (Figure 6b).
Discussion
In the present work we have studied the mechanism involved in the acetaminophen-mediated potentiation of staurosporine-induced cell death in SH-SY5Y human neuroblastoma cells. Staurosporine, a non-selective PKC inhibitor, is a potent inducer of apoptosis in several cell types (Koh et al., 1995; Prehn et al., 1997; Scarlett et al., 2000). We have found, in agreement with data previously reported, that staurosporine (500 nM) induced apoptosis in SH-SY5Y human neuroblastoma cells owing to impaired mitochondrial function causing cytochrome c release and caspase 3 activation (Boix et al., 1997; Yuste et al., 2002). Further, we have demonstrated that, following 24 h treatment, staurosporine decreases COX activity. The presence of two isoforms of COX enzyme, COX-1 constitutively expressed in most tissues, and COX-2, the inducible isoform, which is upregulated during tissue inflammation enhancing prostaglandin production, is widely accepted (Fitzpatrick, 2004; Simmons et al., 2004). Recently, a third COX isoform, COX-3, has been described being highly expressed in heart and nervous system (Willoughby et al., 2000; Chandrasekharan et al., 2002). Under basal conditions, COX activity in the SH-SY5Y neuroblastoma cell line was inhibited in a concentration-dependent manner by both the non-selective COX inhibitor indomethacin and acetaminophen that preferentially inhibits COX-3 (Chandrasekharan et al., 2002). However, the selective COX-2 inhibitor, NS-398 did not have any effect at concentrations that have been described to inhibit COX-2 activity (Coffey et al., 1997; Wallace, 1999), suggesting that, under basal conditions, the SH-SY5Y neuroblastoma cells did not express the inducible isoform COX-2. Recently, Johnsen et al. (2004) have described the presence of COX-2, detected by Western blot analysis, in several neuroblastoma cell lines including SH-SY5Y. However, we found that NS-398 did not inhibit COX activity in these cells suggesting that, even if COX-2 was present, it did not seem to be responsible for PGE2 production. Moreover, COX activity measured in microsomal fractions obtained from 24-h staurosporine-treated cells was inhibited by indomethacin and acetaminophen (only at the highest concentration tested), but not by NS-398 suggesting that COX-2 activity was negligible in these cells.
Although both indomethacin and acetaminophen inhibited prostaglandin production, only acetaminophen potentiated staurosporine-induced cell death in a concentration-dependent manner that was accompanied by an increase in caspase 3 activity. In addition, Western blot analysis of cytochrome c release from mitochondria showed that acetaminophen in combination with staurosporine potentiated the release of this protein from mitochondria to the cytosol and this effect was concentration-related. As acetaminophen but not indomethacin increased staurosporine-induced SH-SY5Y neuroblastoma cell death, it seemed that the COX pathway and its metabolites were not involved in acetaminophen-mediated potentiation of staurosporine-induced death, suggesting a different mechanism of action.
Staurosporine-induced apoptosis in neuroblastoma cells has been related to mitochondrial depolarization associated with swelling, outer membrane disruption and release of cytochrome c from mitochondria (Boix et al., 1997; Scarlett et al., 2000). BA, an inhibitor of adenine nucleotide translocase, prevents mitochondria depolarization and the release of apoptogenic proteins such as cytochrome c by inhibiting the opening of the mitochondrial permeability transition pore complex (Furlong et al., 1998; Gross et al., 1999). Accordingly, BA prevented the toxic effect of either staurosporine or staurosporine+acetaminophen treatments in SH-SY5Y cells.
Mitochondrial dysfunction and uncoupling of electron transport chain is associated with an increased superoxide production, which is a major cause of the oxidative damage to cells (Echtay et al., 2003; Brand et al., 2004). To investigate if production of reactive oxygen species (ROS) such as superoxide, was related to staurosporine- and staurosporine+acetaminophen-induced cellular death, we tested the effect of these compounds in the presence of MnTBAP, a cell-permeable superoxide dismutase mimetic compound that catalyses the dismutation of superoxide radicals to molecular oxygen and hydrogen peroxide that is subsequently metabolized to water and oxygen by catalase (Szabo et al., 1996; Keller et al., 1998). We also used NAC, an antioxidant that increases intracellular GSH, the main component of the pathways by which cells are protected from oxidative stress (Lauterburg et al., 1983; Ferrari et al., 1995) and that has been widely used to prevent liver damage by acetaminophen overdose. Co-treatment of SH-SY5Y cells with MnTBAP or with NAC did not prevent staurosporine-mediated cell death. However, both MnTBAP and NAC prevented the acetaminophen-mediated potentiation of staurosporine-induced cellular death, suggesting that acetaminophen potentitated staurosporine-mediated cell death through a mechanism related to ROS production. It seems that GSH depletion does not play a major role in the mechanism of action of staurosporine as NAC does not prevent staurosporine-induced neuroblastoma cell death. However, NAC blocks acetaminophen-mediated potentiation of staurosporine-induced SH-SY5Y cell death, suggesting that acetaminophen modifies GSH levels in the cells. It is well established that acetaminophen overdose causes severe hepatotoxicity leading to liver failure (Dargan and Jones, 2002). In hepatocytes, acetaminophen inhibits mitochondrial oxidative phosphorylation (Meyers et al., 1988; Jaeschke 1990; Nazareth et al., 1991) and depletes both cytosolic and mitochondrial GSH (Burcham and Harman, 1991; Knight et al., 2002). Acetaminophen is mainly metabolized via conjugation with glucuronic acid and sulphate and then excreted, but a small proportion of acetaminophen is metabolized by cytochrome P450 (Manyike et al., 2000) forming a chemically reactive metabolite, N-acetyl-p-benzoquinone imine (NAPQI) which reacts with GSH to form a non-toxic conjugate that can be excreted. Once GSH is exhausted, NAPQI binds to cellular proteins, including mitochondrial proteins, leading to hepatocellular death (Jaeschke et al., 2003; James et al., 2003). Recently the presence of cytochrome P450 has been described in human neuroblastoma SH-SY5Y cells (Guarneri et al., 2000). The blockade by NAC of the acetaminophen potentiation of staurosporine-induced neuroblastoma cell death might be explained by the fact that acetaminophen can be converted by cytochrome P450 to NAPQI which, once the cellular levels of GSH are exhausted, will react with different mitochondrial proteins potentiating the toxic effect of staurosporine. In agreement with this hypothesis, our experiments showed that staurosporine weakly decreased GSt content 18 h after treatment and that this reduction was strongly potentiated by acetaminophen. Besides, co-incubation of neuroblastoma cells with staurosporine+acetaminophen+disulfiram, a cytochrome P450 2E1 isoform inhibitor (Emery et al., 1999), reduced acetaminophen mediated potentation of staurosporine-induced apoptosis, corroborating the involvement of cytochrome P450 in the mechanism by which acetaminophen increases staurosporine-induced neuroblastoma death.
In summary, our results show that acetaminophen potentiates staurosporine-mediated death of SH-SY5Y human neuroblastoma cells through a mechanism that is independent of the COX pathway and might be related to a reduction in GSH content, consequent on acetaminophen metabolism through the cytochrome P450 pathway.
Acknowledgments
This work has been supported by the Programa Ramón y Cajal from Ministerio de Educación y Ciencia (Spain) and UCLM-CCM to IP and by Grants PI52112 to IP, G02-019 and SAN-03-23 from JCCM and from Fundació ‘la Caixa' to VC.
Abbreviations
- COX
cyclooxygenase
- DTNB
5,5′-dithiobis-2-nitrobenzoic acid
- GSH
glutathione
- GSSG
oxidized glutathione
- GSt
total glutathione
- LDH
lactate dehydrogenase
- MnTBAP
Mn(III)-tetrakis(4-benzoic acid)-porphyrin chloride
- NAC
N-acetylcysteine
- NAPQI
N-acetyl-p-benzoquinone imine
- PGE2
prostaglandin E2
- PKC
protein kinase C
- ROS
reactive oxygen species
- Z-DEVD-AFC
Asp-Glu-Val-Asp-7-amino-4 trifluoromethyl-coumaryl
Conflict of interest
The authors state no conflict of interest.
References
- Akundi RS, Candelario-Jalil E, Hess S, Hull M, Lieb K, Gebicke-Haerter PJ, et al. Signal transduction pathways regulating cyclooxygenase-2 in lipopolysaccharide-activated primary rat microglia. Glia. 2005;51:199–208. doi: 10.1002/glia.20198. [DOI] [PubMed] [Google Scholar]
- Boix J, Llecha N, Yuste VJ, Comella JX. Characterization of the cell death process induced by staurosporine in human neuroblastoma cell lines. Neuropharmacology. 1997;36:811–821. doi: 10.1016/s0028-3908(97)00030-0. [DOI] [PubMed] [Google Scholar]
- Brand MD, Affourtit C, Esteves TC, Green K, Lambert AJ, Miwa S, et al. Mitochondrial superoxide: production, biological effects, and activation of uncoupling proteins. Free Radic Biol Med. 2004;37:755–767. doi: 10.1016/j.freeradbiomed.2004.05.034. [DOI] [PubMed] [Google Scholar]
- Burcham PC, Harman AW. Acetaminophen toxicity results in site-specific mitochondrial damage in isolated mouse hepatocytes. J Biol Chem. 1991;266:5049–5054. [PubMed] [Google Scholar]
- Chandrasekharan NV, Dai H, Roos KL, Evanson NK, Tomsik J, Elton TS, et al. COX-3, a cyclooxygenase-1 variant inhibited by acetaminophen and other analgesic/antipyretic drugs: cloning, structure, and expression. Proc Natl Acad Sci USA. 2002;99:13926–13931. doi: 10.1073/pnas.162468699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey RJ, Hawkey CJ, Damstrup L, Graves-Deal R, Daniel VC, Dempsey PJ, et al. Epidermal growth factor receptor activation induces nuclear targeting of cyclooxygenase-2, basolateral release of prostaglandins, and mitogenesis in polarizing colon cancer cells. Proc Natl Acad Sci USA. 1997;94:657–662. doi: 10.1073/pnas.94.2.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dargan PI, Jones AL. Acetaminophen poisoning: an update for the intensivist. Crit Care. 2002;6:108–110. doi: 10.1186/cc1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempke W, Rie C, Grothey A, Schmoll HJ. Cyclooxygenase-2: a novel target for cancer chemotherapy. J Cancer Res Clin Oncol. 2001;127:411–417. doi: 10.1007/s004320000225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echtay KS, Esteves TC, Pakay JL, Jekabsons MB, Lambert AJ, Portero-Otin M, et al. A signalling role for 4-hydroxy-2-nonenal in regulation of mitochondrial uncoupling. EMBO J. 2003;22:4103–4110. doi: 10.1093/emboj/cdg412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery MG, Jubert C, Thummel KE, Kharasch ED. Duration of cytochrome P-450 2E1 (CYP2E1) inhibition and estimation of functional CYP2E1 enzyme half-life after single-dose disulfiram administration in humans. J Pharmacol Exp Ther. 1999;291:213–219. [PubMed] [Google Scholar]
- Ferrari G, Yan CY, Greene LA. N-acetylcysteine (D- and L-stereoisomers) prevents apoptotic death of neuronal cells. J Neurosci. 1995;15:2857–2866. doi: 10.1523/JNEUROSCI.15-04-02857.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick FA. Cyclooxygenase enzymes: regulation and function. Curr Pharm Des. 2004;10:577–588. doi: 10.2174/1381612043453144. [DOI] [PubMed] [Google Scholar]
- Furlong IJ, Lopez MC, Ascaso R, Lopez RA, Collins MK. Induction of apoptosis by valinomycin: mitochondrial permeability transition causes intracellular acidification. Cell Death Differ. 1998;5:214–221. doi: 10.1038/sj.cdd.4400335. [DOI] [PubMed] [Google Scholar]
- Griffith OW. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal Biochem. 1980;106:207–212. doi: 10.1016/0003-2697(80)90139-6. [DOI] [PubMed] [Google Scholar]
- Gross A, McDonnell JM, Korsmeyer SJ. BCL-2 family members and the mitochondria in apoptosis. Genes Dev. 1999;13:1899–1911. doi: 10.1101/gad.13.15.1899. [DOI] [PubMed] [Google Scholar]
- Guarneri P, Cascio C, Piccoli T, Piccoli F, Guarneri R. Human neuroblastoma SH-SY5Y cell line: neurosteroid-producing cell line relying on cytoskeletal organization. J Neurosci Res. 2000;60:656–665. doi: 10.1002/(SICI)1097-4547(20000601)60:5<656::AID-JNR11>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Haase GM, Perez C, Atkinson JB. Current aspects of biology, risk assessment, and treatment of neuroblastoma. Semin Surg Oncol. 1999;16:91–104. doi: 10.1002/(sici)1098-2388(199903)16:2<91::aid-ssu3>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Jaeschke H. Glutathione disulfide formation and oxidant stress during acetaminophen-induced hepatotoxicity in mice in vivo: the protective effect of allopurinol. J Pharmacol Exp Ther. 1990;255:935–941. [PubMed] [Google Scholar]
- Jaeschke H, Knight TR, Bajt ML. The role of oxidant stress and reactive nitrogen species in acetaminophen hepatotoxicity. Toxicol Lett. 2003;144:279–288. doi: 10.1016/s0378-4274(03)00239-x. [DOI] [PubMed] [Google Scholar]
- Jalava A, Akerman K, Heikkila J. Protein kinase inhibitor, staurosporine, induces a mature neuronal phenotype in SH-SY5Y human neuroblastoma cells through an alpha-, beta-, and zeta-protein kinase C-independent pathway. J Cell Physiol. 1993;155:301–312. doi: 10.1002/jcp.1041550211. [DOI] [PubMed] [Google Scholar]
- James LP, Mayeux PR, Hinson JA. Acetaminophen-induced hepatotoxicity. Drug Metab Dispos. 2003;31:1499–1506. doi: 10.1124/dmd.31.12.1499. [DOI] [PubMed] [Google Scholar]
- Johnsen JI, Lindskog M, Ponthan F, Pettersen I, Elfman L, Orrego A, et al. Cyclooxygenase-2 is expressed in neuroblastoma, and nonsteroidal anti-inflammatory drugs induce apoptosis and inhibit tumor growth in vivo. Cancer Res. 2004;64:7210–7215. doi: 10.1158/0008-5472.CAN-04-1795. [DOI] [PubMed] [Google Scholar]
- Keller JN, Kindy MS, Holtsberg FW, St Clair DK, Yen HC, Germeyer A, et al. Mitochondrial manganese superoxide dismutase prevents neural apoptosis and reduces ischemic brain injury: suppression of peroxynitrite production, lipid peroxidation, and mitochondrial dysfunction. J Neurosci. 1998;18:687–697. doi: 10.1523/JNEUROSCI.18-02-00687.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight TR, Ho YS, Farhood A, Jaeschke H. Peroxynitrite is a critical mediator of acetaminophen hepatotoxicity in murine livers: protection by glutathione. J Pharmacol Exp Ther. 2002;303:468–475. doi: 10.1124/jpet.102.038968. [DOI] [PubMed] [Google Scholar]
- Koh JY, Wie MB, Gwag BJ, Sensi SL, Canzoniero LM, Demaro J, et al. Staurosporine-induced neuronal apoptosis. Exp Neurol. 1995;135:153–159. doi: 10.1006/exnr.1995.1074. [DOI] [PubMed] [Google Scholar]
- Lauterburg BH, Corcoran GB, Mitchell JR. Mechanism of action of N-acetylcysteine in the protection against the hepatotoxicity of acetaminophen in rats in vivo. J Clin Invest. 1983;71:980–991. doi: 10.1172/JCI110853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K, Li Y, Shelton JM, Richardson JA, Spencer E, Chen ZJ, et al. Cytochrome c deficiency causes embryonic lethality and attenuates stress-induced apoptosis. Cell. 2000;101:389–399. doi: 10.1016/s0092-8674(00)80849-1. [DOI] [PubMed] [Google Scholar]
- Lin MT, Lee RC, Yang PC, Ho FM, Kuo ML. Cyclooxygenase-2 inducing Mcl-1-dependent survival mechanism in human lung adenocarcinoma CL1.0 cells. Involvement of phosphatidylinositol 3-kinase/Akt pathway. J Biol Chem. 2001;276:48997–49002. doi: 10.1074/jbc.M107829200. [DOI] [PubMed] [Google Scholar]
- Manyike PT, Kharasch ED, Kalhorn TF, Slattery JT. Contribution of CYP2E1 and CYP3A to acetaminophen reactive metabolite formation. Clin Pharmacol Ther. 2000;67:275–282. doi: 10.1067/mcp.2000.104736. [DOI] [PubMed] [Google Scholar]
- Meyers LL, Beierschmitt WP, Khairallah EA, Cohen SD. Acetaminophen-induced inhibition of hepatic mitochondrial respiration in mice. Toxicol Appl Pharmacol. 1988;93:378–387. doi: 10.1016/0041-008x(88)90040-3. [DOI] [PubMed] [Google Scholar]
- Minghetti L, jmone-Cat MA, De Berardinis MA, De SR. Microglial activation in chronic neurodegenerative diseases: roles of apoptotic neurons and chronic stimulation. Brain Res Brain Res Rev. 2005;48:251–256. doi: 10.1016/j.brainresrev.2004.12.015. [DOI] [PubMed] [Google Scholar]
- Moroney MA, Alcaraz MJ, Forder RA, Carey F, Hoult JR. Selectivity of neutrophil 5-lipoxygenase and cyclo-oxygenase inhibition by an anti-inflammatory flavonoid glycoside and related aglycone flavonoids. J Pharm Pharmacol. 1988;40:787–792. doi: 10.1111/j.2042-7158.1988.tb05173.x. [DOI] [PubMed] [Google Scholar]
- Nazareth WM, Sethi JK, McLean AE. Effect of paracetamol on mitochondrial membrane function in rat liver slices. Biochem Pharmacol. 1991;42:931–936. doi: 10.1016/0006-2952(91)90055-a. [DOI] [PubMed] [Google Scholar]
- Posmantur R, McGinnis K, Nadimpalli R, Gilbertsen RB, Wang KK. Characterization of CPP32-like protease activity following apoptotic challenge in SH-SY5Y neuroblastoma cells. J Neurochem. 1997;68:2328–2337. doi: 10.1046/j.1471-4159.1997.68062328.x. [DOI] [PubMed] [Google Scholar]
- Prehn JH, Jordan J, Ghadge GD, Preis E, Galindo MF, Roos RP, et al. Ca2+ and reactive oxygen species in staurosporine-induced neuronal apoptosis. J Neurochem. 1997;68:1679–1685. doi: 10.1046/j.1471-4159.1997.68041679.x. [DOI] [PubMed] [Google Scholar]
- Scarlett JL, Sheard PW, Hughes G, Ledgerwood EC, Ku HH, Murphy MP. Changes in mitochondrial membrane potential during staurosporine-induced apoptosis in Jurkat cells. FEBS Lett. 2000;475:267–272. doi: 10.1016/s0014-5793(00)01681-1. [DOI] [PubMed] [Google Scholar]
- Sheng H, Shao J, Morrow JD, Beauchamp RD, DuBois RN. Modulation of apoptosis and Bcl-2 expression by prostaglandin E2 in human colon cancer cells. Cancer Res. 1998;58:362–366. [PubMed] [Google Scholar]
- Simmons DL, Botting RM, Hla T. Cyclooxygenase isozymes: the biology of prostaglandin synthesis and inhibition. Pharmacol Rev. 2004;56:387–437. doi: 10.1124/pr.56.3.3. [DOI] [PubMed] [Google Scholar]
- Smith WL. The eicosanoids and their biochemical mechanisms of action. Biochem J. 1989;259:315–324. doi: 10.1042/bj2590315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith WL. Prostanoid biosynthesis and mechanisms of action. Am J Physiol. 1992;263:F181–F191. doi: 10.1152/ajprenal.1992.263.2.F181. [DOI] [PubMed] [Google Scholar]
- Szabo C, Day BJ, Salzman AL. Evaluation of the relative contribution of nitric oxide and peroxynitrite to the suppression of mitochondrial respiration in immunostimulated macrophages using a manganese mesoporphyrin superoxide dismutase mimetic and peroxynitrite scavenger. FEBS Lett. 1996;381:82–86. doi: 10.1016/0014-5793(96)00087-7. [DOI] [PubMed] [Google Scholar]
- Tesfamariam B, Brown ML, Cohen RA. Elevated glucose impairs endothelium-dependent relaxation by activating protein kinase C. J Clin Invest. 1991;87:1643–1648. doi: 10.1172/JCI115179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujii M, DuBois RN. Alterations in cellular adhesion and apoptosis in epithelial cells overexpressing prostaglandin endoperoxide synthase 2. Cell. 1995;83:493–501. doi: 10.1016/0092-8674(95)90127-2. [DOI] [PubMed] [Google Scholar]
- Wallace JL. Distribution and expression of cyclooxygenase (COX) isoenzymes, their physiological roles, and the categorization of nonsteroidal anti-inflammatory drugs (NSAIDs) Am J Med. 1999;107:11S–16S. doi: 10.1016/s0002-9343(99)00363-0. [DOI] [PubMed] [Google Scholar]
- Wang D, DuBois RN. Cyclooxygenase-2: a potential target in breast cancer. Semin Oncol. 2004;31:64–73. doi: 10.1053/j.seminoncol.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Willoughby DA, Moore AR, Colville-Nash PR. COX-1, COX-2, and COX-3 and the future treatment of chronic inflammatory disease. Lancet. 2000;355:646–648. doi: 10.1016/S0140-6736(99)12031-2. [DOI] [PubMed] [Google Scholar]
- Xuan YT, Guo Y, Zhu Y, Wang OL, Rokosh G, Messing RO, et al. Role of the protein kinase C-epsilon-Raf-1-MEK-1/2-p44/42 MAPK signaling cascade in the activation of signal transducers and activators of transcription 1 and 3 and induction of cyclooxygenase-2 after ischemic preconditioning. Circulation. 2005;112:1971–1978. doi: 10.1161/CIRCULATIONAHA.105.561522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuste VJ, Sanchez-Lopez I, Sole C, Encinas M, Bayascas JR, Boix J, et al. The prevention of the staurosporine-induced apoptosis by Bcl-X(L), but not by Bcl-2 or caspase inhibitors, allows the extensive differentiation of human neuroblastoma cells. J Neurochem. 2002;80:126–139. doi: 10.1046/j.0022-3042.2001.00695.x. [DOI] [PubMed] [Google Scholar]