Abstract
Background and purpose:
To study the importance of endothelium-derived contracting factors (EDCFs) in arteries of rats with type I diabetes.
Experimental approach:
Rat femoral arteries were collected four or twelve weeks after induction of diabetes with streptozotocin. Rings, with or without endothelium, were suspended in organ chambers for isometric tension measurement. COX protein levels were determined by Western blotting.
Key results:
Four weeks after the injection of streptozotocin, the endothelium-dependent relaxations (during contractions to phenylephrine) to A23817 were attenuated, but the endothelium-dependent contractions (quiescent preparations) to the ionophore were augmented. Indomethacin and S18886 prevented the endothelium-dependent contractions, while dazoxiben reduced them in rings from streptozotocin-treated rats, suggesting that thromboxane A2, activating TP- receptors, is involved. Twelve weeks after the injection of streptozotocin, the changes in endothelium-dependent relaxations and contractions to A23187 were even more noticeable. The protein expression of COX-1 was increased in femoral arteries of the diabetic rats. Valeryl salicylate and SC560 inhibited the contractions, suggesting that the EDCFs are produced by COX-1. At that time, a combination of S18886 with EP1-blockers was required to abolish the contractions, suggesting that the EDCFs involved act at both TP- and EP-receptors. Rings without endothelium from streptozotocin-treated rats exhibited a reduced maximal contraction to potassium chloride and U46619, combined with hyper-responsiveness to the latter, suggesting that more prolonged diabetes also alters the responsiveness of vascular smooth muscle.
Conclusion and Implications:
The production of EDCFs is progressively increased in the course of type I diabetes. Eventually, the disease also damages vascular smooth muscle.
Keywords: cyclooxygenase, endothelium, endothelium-derived contracting factor(s), endothelium-dependent contractions, smooth muscle, streptozotocin-induced diabetes, thromboxane-prostanoid receptor
Introduction
Endothelial dysfunction is characterized by a reduced release and/or bioavailability of nitric oxide (NO) as well as by an enhanced production of endothelium-derived contracting factor (s; EDCF) (Vanhoutte et al., 2005). Endothelium-dependent contractions were observed first in isolated veins of the dog (De Mey and Vanhoutte, 1982) and then in various arteries including the rat aorta (Lüscher and Vanhoutte, 1986, Auch-Schwelk et al., 1992; Yang et al., 2004a, 2004b; Tang et al., 2005a), the canine basilar artery (Katusic et al., 1988; Shirahase et al., 1988), the rabbit pulmonary artery (Russell and Rohrbach 1989) and the mouse aorta (Tang et al., 2005b). In the main, they are due to a product(s) of endothelial cyclooxygenase, which activates thromboxane-prostanoid receptor (TP receptors) on vascular smooth muscle (Lüscher and Vanhoutte, 1986; Auch-Schwelk et al., 1990; Yang et al., 2003; Tang et al., 2005b). The production of EDCF(s) is potentiated by aging (Koga et al., 1993) and during pathophysiological conditions, such as hypertension (Lüscher and Vanhoutte, 1986; Koga et al., 1989) and hyperglycemia (Tesfamariam et al., 1990).
Endothelial dysfunction has been reported in diabetic people and experimental animals with diabetes (De Vriese et al., 2000; Schalkwijk and Stehouwer, 2005). The purpose of the present study was to assess whether or not endothelium-dependent contractions occur in isolated arteries of streptozotocin-treated rats, an experimental model resembling type I diabetes in humans and, if so, to determine the mechanisms underlying this phenomenon.
Methods
Experimental animals
Type I diabetes was induced in 16-week-old male Sprague–Dawley rats (450–600 g) by a single administration of streptozotocin (55 mg kg−1, given intravenously) dissolved in citric acid-trisodium citrate (0.2 mM) buffer (pH 4.0–4.5). After 72 h, blood samples were obtained from the tail and the glucose concentration measured using a one-touch glucometer (LifeScan Inc., Milpitas, CA, USA). Induction of diabetes was considered successful when the glucose level was higher than 300 mg dl−1. Another group of rats was injected with vehicle alone and kept under identical conditions as non-diabetic controls. The animals were housed in the laboratory animal unit of the University of Hong Kong, fed with regular chow and given free access to water. Rats were studied either 4 or 12 weeks after induction of type I diabetes. They were anaesthetized with sodium pentobarbitone (70 mg kg−1, i.p.), administered the anticoagulant heparin (0.5 U kg−1) and exsanguinated. The non-fasting glucose level was measured again on the day of death (Table 1). Control rats with a glucose level higher than 200 mg dl−1 were excluded from the study. All procedures and protocols were approved by the institutional animal care committee.
Table 1.
Body weight, glucose level in plasma and response to 60 mM potassium chloride in rings of femoral arteries from control and STZ rats
| Before treatment (16 weeks) |
4 weeks after treatment |
12 weeks after treatment |
|||
|---|---|---|---|---|---|
| Control | STZ-treated | Control | STZ-treated | ||
| Body weight (g) | 542.9±4.4 | 684.5±23.6* | 456.1±12.5*# | 694.4±9.6* | 416.5±4.0*# |
| Glucose (mg dl−1) | 183.0±5.6 | 182.7±7.2 | 526.9±12.6# | 183.0±4.8 | 524.7±4.7# |
| 60 mM KCl (g) | 1.94±0.15 | 2.04±0.13 | 1.75±0.08 | 1.79±0.11 | 1.15±0.06# |
Abbreviation: STZ, streptozotocin-treated.
Data shown as means±s.e.m.; n=6–12.
Statistically significant difference with control at the induction day (P<0.05).
Statistically significant difference with control rat at the same stage (P<0.05).
Preparation of arteries
Both femoral arteries were dissected free, excised and placed into ice-cold modified Krebs–Ringer solution of the following composition (mM): NaCl 118, KCl 4.7, CaCl2 2.5, MgSO4 1.2, NaHCO3 25.0, KH2PO4 1.18 and calcium disodium ethylenediaminetetraacetic acid 0.026, glucose 11.1 (control solution). The blood vessels were cut into rings (1.5–2 mm in length). In some preparations, the endothelial cell layer was removed by the injection of 1 ml of saponin solution (1 mg ml−1; diluted with Krebs–Ringer solution; Samata et al., 1986) in the artery before cutting the rings. The preparations were suspended in organ chambers containing control solution (37°C) aerated with 95% of O2 and 5% of CO2. They were connected to a force transducer (Powerlab Model ML785 and ML119). Changes in isometric tension were recorded. The rings were stretched progressively to 1.0 g of optimal resting tension (determined in preliminary experiment, data not shown) and were allowed to equilibrate for 90 min.
The incubation time with drugs was 30 min and concentration–response curves were obtained in a cumulative manner. Increases in tension were expressed as a percentage of the reference contraction to 60 mM KCl before drug incubation. To study endothelium-dependent relaxations, the preparations were exposed to phenylephrine (10 nM–1 μM; in order to obtain 50–70% of response to KCl (60 mM)). Sodium nitroprusside (10 μM) was added at the end of the experiments and the relaxations were expressed as a percentage of the maximal relaxation induced by the nitrovasodilator. To study endothelium-dependent contractions, all experiments were performed in the presence of Nω-nitro-L-arginine methyl ester hydrochloride (L-NAME) (0.3 mM; Auch-Schwelk et al., 1992).
Protein preparation and Western blot analysis
Femoral arteries, harvested 4 or 12 weeks after the injection of streptozotocin, were dissected in ice-cold modified Krebs–Ringer solution. The tissue samples were homogenized at 4°C in lysis buffer (Tris HCl 20 mM, NaCl 150 mM, ethylenediaminetetraacetic acid (EDTA) 1 mM, sodium pyrophosphate 25 mM, β-glycophosphate 1 mM, sodium orthovanadate 1 mM, leupeptin 2.1 μM, aprotinin 1 mg ml−1 and phenylmethylsulphonyl fluoride 1 mM, Triton X-100 1%) and incubated on ice for ten minutes. Samples were then centrifuged at 3000 g for 10 min at 4°C, and the supernatant was collected. Protein concentrations were determined using the Bradford assay. Lysates were used immediately or stored at −70°C. In all immunoblot experiments, 30 μg of total protein were loaded in each lane of 7.5% sodium dodecyl sulfate-polyacrylamide gel electrophoresis gel. Molecular masses of immunoreactive bands were determined by loading a biotinylated molecular mass standard (Bio-Rad Laboratories, Hercules, CA, USA). After electrophoresis, protein was transferred to nitrocellulose membranes (Bio-Rad Laboratories) and blocked with 5% non-fat dry milk in Tris-buffered saline (TBS) (pH 7.4, 20 mM Tris base, 137 mM NaCl). Membranes were then incubated with the corresponding primary antibodies (anti-COX-1 monoclonal antibody (1:300), anti-COX-2 polyclonal antibody (1:500) and anti-β-actin monoclonal antibody (1:6000)) overnight at room temperature, followed by three 10 min washes in 0.1% Tween-20-TBS (T-TBS). Blots were then incubated in secondary antibody at 1:1000 dilution for 2 h at room temperature and were then washed three times for 10 min in T-TBS. Membranes were then incubated with an enhanced chemiluminescence reagent (Amersham Biosciences, Buchinghamshire, UK) for min and exposed to X-ray films (Fuji Super RX medical X-ray films; Fuji Photo Film, Dusseldorf, Germany) for optimal time, depending on signal strength. Software for electrophoresis analysis (Multi-analyst (Bio-rad Laboratories) was used for the densitometric analysis of immunoreactive bands. The presence of protein was normalized to β-actin. All protein concentrations are presented as a percentage of control.
Data analysis
Data are presented as means±s.e.m.; n refers to the number of rats. Statistical analysis was carried out by one-way analysis of variance (ANOVA) followed by post hoc comparison using Bonferroni's test or two-way ANOVA, as appropriate (Prism version 3a, GraphPad Software, San Diego, CA, USA). Differences were considered to be statistically significant when P>0.05.
Drugs
A23187, AH 6809 (6-isopropoxy-9-oxoxanthene-2-carboxylic acid), acetylcholine; anti-β-actin monoclonal antibody indomethacin, L-NAME, phenylephrine, saponin, sodium nitroprusside, SC 19220 (2-acetylhydrazide 10(11 H)-carboxylic acid 8-chloro-dibenz [b,f][1,4]oxazepine-10(11 H)-carboxylic acid) and SC560 (5-(4-chlorophenyl)-1-(4-methoxyphenyl)-3-trifluoromethyl pyrazole) were purchased from Sigma Chemical Company (St Louis, MO, USA). U46619 (15(S)-hydroxy-11,9-(epoxymethano)prostadienoic acid), NS-398 (N[-2-(cyclohexyloxy)-4-nitrophenyl]-methanesulphonamide) and valeryl salicylate (2-[(1-oxopenytyl)oxy]-benzoic acid), COX-1 monoclonal antibody and COX-2 polyclonal antibody were purchased from Cayman Chemical Company (Ann Arbor, MI, USA). Heparin was purchased from LEO Pharm (Ballerup, Denmark). Secondary antibodies (enhanced chemilumiscence (ECL) anti-rabbit IgG and ECL anti-mouse IgG) were bought from Amersham Biosciences. S18886 (3-[(6-amino-(4-chlorobenzensulphonyl)-2-methyl-5,6,7,8-tetrahydronapht]-1-yl)propionic acid) and dazoxiben were kind gifts of the Institut de Recherches Servier (Paris, France). Drugs concentrations are given as final molar concentrations in the bath solution.
Results
General conditions
At the age of 16 weeks, the body weight of the rats averaged 542.9±4.4 g. Four weeks later, the control rats had gained body weight whereas streptozotocin-treated rats had lost weight significantly. The streptozotocin-treated group had a significantly higher glucose level than the controls. Similar results were obtained after 12 weeks (Table 1).
Vascular responsiveness
Contracted preparations
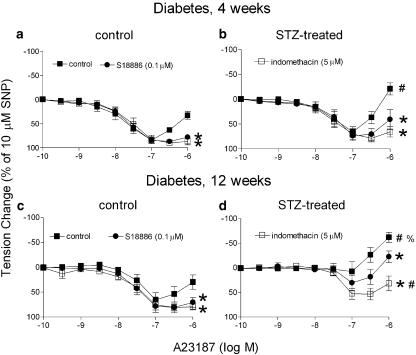
Four weeks after streptozotocin. Four weeks after the injection of vehicle or streptozotocin, during contractions to phenylephrine, the calcium ionophore A23187 (0.1 nM–0.1 μM) induced a concentration- and endothelium-dependent relaxation in arteries from both control and streptozotocin-treated rats. Higher concentrations of A23187 (0.3–1 μM) induced a secondary increase in tension which was inhibited by both indomethacin (inhibitor of cyclooxygenase; 5 μM) and S18886 (selective blocker of TP-receptors; 0.1 μM; Figure 1a and b). In the rings without endothelium, the calcium ionophore A23187 (0.1–1 μM) induced similar contractions in femoral arteries from streptozotocin-treated rats to those from control rats, but not relaxations (data not shown). The streptozotocin-treated group exhibited significantly higher secondary contractions than the controls in the rings with endothelium. In the presence of indomethacin, the endothelium-dependent relaxations to A23187, in the control group were comparable to those in the streptozotocin-treated groups (Figure 1a and b).
Figure 1.
Relaxations to A23187 in femoral arteries from control and streptozotocin-treated rats after 4 (a and b) and 12 (c and d) weeks. Data shown are means±s.e.m. (vertical lines); n=7. *Statistically significant difference from control rings (P<0.05). #Statistically significant difference from rings of control rats under identical conditions (P<0.05). % indicates a statistically significant difference from rings of 4 weeks streptozotocin-treated rats under identical conditions (P<0.05).
Twelve weeks after streptozotocin. Twelve weeks after the injection of vehicle or streptozotocin, rings from the control rats exhibited relaxations and secondary contractions comparable to those observed in the 4 weeks control rats. Contracted rings from the streptozotocin-treated group did not exhibit a significant relaxation to A23187 but a significantly greater secondary contraction. Indomethacin reversed the secondary contractile response to a relaxation. The relaxant responses restored by indomethacin were greater in arteries from control rats than in those from streptozotocin-treated animals (Figure 1c and d). S18886 partially restored relaxations and reduced the secondary contraction (Figure 1c and d). The rings without endothelium exhibited similar responses to those observed in the 4-week treated rats (data not shown).
Quiescent preparations
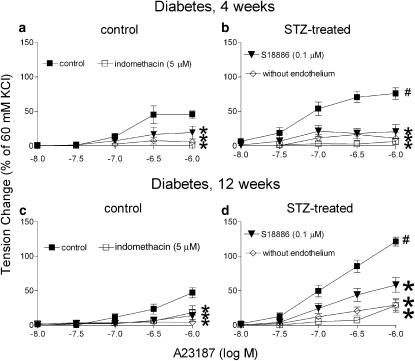
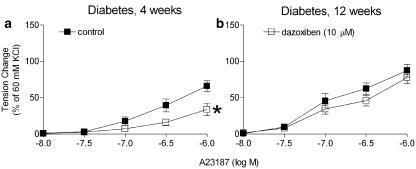
Four weeks after streptozotocin. In rings from the control group, A23187 induced a concentration-dependent contraction that was not observed in rings without endothelium. Indomethacin and S18886 inhibited this response completely. In the rings from the streptozotocin-treated group, A23187 induced a significantly greater endothelium-dependent contraction that was abolished by either indomethacin or S18886 (Figure 2a and b). Dazoxiben (inhibitor of thromboxane A2 synthase; 10 μM) significantly reduced the endothelium-dependent contraction (Figure 3a).
Figure 2.
Contractions to A23187 in femoral arteries from control and streptozotocin-treated rats in the presence of L-NAME (0.3 mM) after 4 (a and b) and 12 (c and d) weeks. Data shown are means±s.e.m. (vertical lines); n=7. *Statistically significant difference from control rings (P<0.05). #Statistically significant difference from rings of control rats under identical conditions (P<0.05).
Figure 3.
Effects of the inhibitor of thromboxane synthase dazoxiben (10 μM) on the response to A23187, in the presence of L-NAME (0.3 mM), in femoral arteries with endothelium of rats 4 weeks (a) and 12 weeks (b) after injection of streptozotocin. Data shown are means±s.e.m. (vertical lines); n=7. *Statistically significant difference from rings under control conditions (P<0.05).
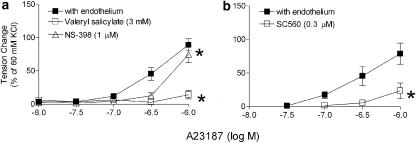
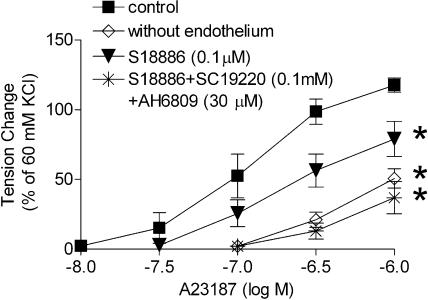
Twelve weeks after streptozotocin. The rings from the control rats exhibited a comparable endothelium- and concentration-dependent contraction to A23187 as those from the 4 weeks control rats. Indomethacin and S18886 abolished the response. The rings from the streptozotocin-treated group exhibited a significantly greater contraction to A23187. The response of the rings without endothelium was comparable to that from controls (Figure 2c and d). Indomethacin abolished the endothelium-dependent contraction (Figure 2d). Valeryl salicylate as well as SC 560 prevented the contraction whereas NS-398 did not (Figure 4). S18886 only partially reduced the endothelium-dependent contraction (Figure 2d). AH 6809 (nonspecific blocker of prostaglandin E receptors; 30 μM) and SC 19220 (specific blocker of the subtype 1 of prostaglandin E receptors; 0.1 mM) alone reduced the endothelium-dependent contraction to A23187 significantly (Table 2). The combination of these blockers plus S18886 abolished this contraction (Figure 5; Table 2). Dazoxiben no longer significantly inhibited the endothelium-dependent contraction (Figure 3b).
Figure 4.
Effects of inhibitors of cyclooxygenase on the response to A23187 in quiescent femoral arteries of rats 12 weeks after the injection of streptozotocin. The experiments were performed in the presence of L-NAME (0.3 mM). Data shown as means±s.e.m. (vertical lines); n=7–8. *Statistically significant difference from rings (with endothelium) under control conditions (COX-1 inhibitors: valeryl salicylate (a) and SC560 (b); COX-2 inhibitor: NS-398 (a)).
Table 2.
Effects of antagonists at TP and EP1 receptors on the response to A23187 in quiescent femoral arteries 12 weeks after the injection of streptozotocin
| Control | S18886 (0.1 μM) | AH6809 (30 μM) | SC19220 (0.1 mM) | SC18886+AH6809+SC19220 | |
|---|---|---|---|---|---|
| Emax (% of 60 mM KCl) | 112.77±4.95 | 70.70±13.60 | 69.08±9.95 | 52.74±13.54* | 37.00±11.54* |
| pEC50 (−log M) | 6.84±0.12 | 6.66±0.12 | 6.56±0.12 | 6.57±0.12 | 6.44±0.12 |
Abbreviations: EP-receptor, prostaglandin receptors E; TP, thromboxane-prostanoid.
Data shown as means±s.e.m.; n=6–9.
Statistically significant differences with control rings in the presence of L-NAME (one-way ANOVA; P<0.05).
Figure 5.
Effects of antagonists of TP and EP1 receptors on the response to A23187 in quiescent femoral arteries 12 weeks after the injection of streptozotocin. The experiments were performed in the presence of L-NAME (0.3 mM). Data shown are means±s.e.m. (vertical lines); n=6–9. *Statistically significant difference from rings under control conditions (P<0.05).
Smooth muscle
Four weeks after the injection of streptozotocin, rings from both the control and treated group exhibited comparable contractions to 60 mM potassium chloride, which were not significantly different from those obtained in rings from 16-week-old untreated rats. After 12 weeks of diabetes, the rings from the streptozotocin-treated group had a significantly reduced contraction to 60 mM potassium chloride (Table 1).
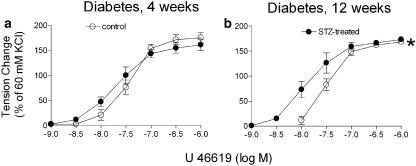
Four weeks after the injection of streptozotocin, the rings without endothelium from both the control and treated group showed comparable responses to U46619. In rings without endothelium from the 12-week streptozotocin-treated group, the maximal contraction to U46619 was reduced to a similar extent as that to 60 mM potassium chloride, but the concentration–response curve to the TP-agonist was shifted significantly to the left (Figure 6a and b).
Figure 6.
Response to U46619 in rings of femoral arteries without endothelium from control rats and those 4 (a) and 12 (b) weeks after an injection of streptozotocin. Data shown are means±s.e.m. (vertical lines); n=7. *Statistically significant difference from rings of control rats (P<0.05).
Cyclooxygenases
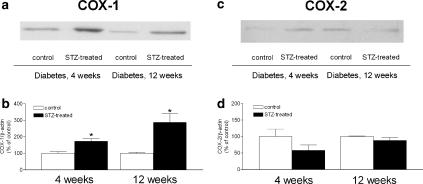
Four weeks after the injection of streptozotocin, the protein level of COX-1 was significantly greater in arteries from the streptozotocin-treated group than those from the control rats. Twelve weeks after the injection of streptozotocin, it was enhanced further. The level of COX-2 was similar after 12 weeks treatment to that after 4 weeks of treatment (Figure 7).
Figure 7.
The presence of COX-1 (a and b) and COX-2 (c and d) in intact femoral artery from control and streptozotocin-treated rats, 4 weeks and 12 weeks after the injection of streptozotocin. Data presented are a percentage of control and shown as means±s.e.m.; n=6. *Statistically significant difference from control rats (P<0.05).
Discussion
In the present experiments, the rats treated with streptozotocin had a lower body weight and a higher glucose level, suggesting that diabetes was successfully induced. The diabetes was maintained for at least 12 weeks, without further changes in weight and glucose level.
A23187 induced a concentration-dependent relaxation in arteries from both control and streptozotocin-treated groups that was not observed in the rings without endothelium, confirming that the calcium ionophore A23187 is an endothelium-dependent vasodilator (Singer and Peach, 1982). However, at higher concentrations (0.3–1 μM), A23187 caused a secondary contraction that was not observed in the rings without endothelium. This endothelium-dependent contraction was abolished by indomethacin and S18886. Thus, A23187 releases an endothelium-dependent contracting factor produced by cyclooxygenase and activating TP-receptors (Lüscher and Vanhoutte, 1986; Auch-Schwelk et al., 1990, Yang et al., 2004a, 2004b). The observation that the contraction to A23187 was greater in the arteries from streptozotocin-treated rats suggests that under comparable conditions, the endothelial cells from diabetic rats must produce more EDCF(s). At a later stage of streptozotocin-induced diabetes, A23187 failed to induce relaxation during contraction to phenylephrine, but evoked an augmented endothelium-dependent contraction instead. Indomethacin abolished the contraction, while S18886 reversed it only partially. This indicates that the endothelial dysfunction is augmented with continued exposure to diabetes. Reduced relaxant responses to A23187 have been observed after arterial grafting (Miller et al., 1987), in diabetes (Gebermedhin et al., 1988; Cameron and Cotter 1992) and in hypertension (Yang et al., 2004a, 2004b). The present experiments support the hypothesis that in the case of diseases such as diabetes, the inhibition of the relaxation to endothelium-dependent dilators can be attributed, at least in part to the enhanced production of an EDCF, produced by cyclooxygenase, that then activates mainly, but not exclusively TP- receptors (Vanhoutte et al., 2005).
To study endothelium-dependent contractions directly, experiments were performed in quiescent arteries in the presence of L-NAME, which optimizes such responses (Auch-Schwelk et al., 1992; Yang et al., 2004a, 2004b; Tang et al., 2005a; Zhou et al., 2005). The finding that the A23187-induced endothelium- and concentration-dependent contractions in the arteries from streptozotocin-treated rats were larger in the presence of the NOS-inhibitor demonstrates that streptozotocin-induced diabetes does indeed potentiate the production or the action of EDCFs.
In the present experiments, the selective inhibitors of COX-1, valeryl salicylate (Bhattacharyya et al., 1995) and SC560 (Smith et al., 1998), but not NS-398, a preferential inhibitor of COX-2 (Futaki et al., 1993), abolished the endothelium-dependent contraction to A23187 (Ge et al., 1999; Yang et al., 2002; Tang et al., 2005b; Gluais et al., 2006). Furthermore, the Western blot analysis revealed that the presence of COX-1 is significantly augmented in the arteries from the streptozotocin-treated group compared to control preparations, whereas that of COX-2 is unaffected. These data confirm the importance of COX-1, the constitutive isoform of the enzyme for the production of EDCFs (Ge et al., 1999; Yang et al., 2002; Tang et al., 2005b).
The exact nature of the endothelium-derived, cyclooxygenase-dependent contracting factor depends on the experimental model studied and the stimulating agents used. In the aorta of the spontaneously hypertensive rat, in response to acetylcholine, endoperoxides and prostacyclin are involved in the activation of TP receptors (Kato et al., 1990; Ge et al., 1995; Rapoport and Williams, 1996; Gluais et al., 2005), whereas in response to A23187, thromboxane A2, along with prostacyclin and endoperoxides, is implicated (Gluais et al., 2006). Similarly in the aorta of diabetic rabbits, thromboxane A2 or possibly its precursor, endoperoxide, is the EDCF involved (Tesfamariam et al., 1989). The present experiments support the hypothesis that thromboxane A2 is responsible for the endothelium-dependent contractions (Ingerman-Wojenski et al., 1981; Gonzales et al., 2005), at least in femoral arteries from animals with an early stage of type I diabetes, as dazoxiben, an inhibitor of thromboxane A2 synthase (Vermylen et al., 1981) reduced the contractions to the ionophore. However, dazoxiben is less effective at a later stage of the disease and this is also associated with a reduced efficacy of S18886 to prevent the endothelium-dependent contractions evoked by A23187. Antagonists at prostaglandin receptors E (EP-receptors) (SC 19220 (Armstrong et al., 1986) and AH 6809 (Sanner, 1969)) given alone partially inhibited the endothelium-dependent contractions, but when combined with S18886 abolished them. Accumulation of endoperoxides favours the metabolism to prostaglandin E2 (Zou et al., 1999). Prostaglandin E2 is involved in the cyclooxygenase-dependent contractions of rat resistance arteries (Bolla et al., 2004). Therefore, the present results suggest that after 12, but not 4 weeks of streptozotocin-induced diabetes, the endothelium-dependent contractions induced by A23187 are owing to the combined activation of TP- and EP-receptors.
With the progression of diabetes the maximal contractions to potassium chloride and U46619 were reduced to a comparable extent, but the concentration–contraction response curve to U46619 was shifted to the left, in agreement with previous findings demonstrating enhanced contractions in response to U46619 or endothelin 1 in arteries from streptozotocin-treated rats (Hattori et al., 1999). These data indicate that after 12 weeks of diabetes the vascular smooth muscle exhibits both a reduced intrinsic ability to contract but at the same time a hypersensitivity to TP agonists. The present experiments do not allow us to conclude whether or not at the later stage of streptozotocin-induced diabetes, this hyper-responsiveness of the smooth muscle cells contributes to the exaggerated endothelium-dependent contractions.
Most studies of endothelium-dependent contractions have been performed using acetylcholine (Lüscher and Vanhoutte, 1986; Auch-Schwelk et al., 1992; Yang et al., 2004a, 2004b; Tang et al., 2005a, 2005b; Zhou et al., 2005). Acetylcholine activates endothelial muscarinic receptors which when expressed in blood vessels control both relaxation and contraction (Boulanger et al., 1994; Ehlert 2003). However, in the femoral artery of the diabetic rat, although the acetylcholine-induced relaxation is impaired (Shi et al., 2006), acetylcholine failed to induce contractions (data not shown). The calcium ionophore, A23187, is more potent than acetylcholine at inducing endothelium-dependent contractions (Yang et al., 2004a, 2004b; Tang et al., 2005b; Gluais et al., 2006). Therefore, A23187 was used as a tool to amplify EDCF-mediated responses and to allow a proper identification of the mediators involved.
Conclusion
The present study suggests a progressive change in the nature of the EDCF occurs during the course of type I diabetes. In addition, as it progresses, the disease also damages vascular smooth muscle.
Acknowledgments
The present study was supported in part by Research Grants Council Grant HKU 7524.
Abbreviations
- EP receptor
prostaglandin receptors E
- TP receptor
thromboxane-prostanoid receptor
Conflict of Interest
The authors state no conflict of interest.
References
- Armstrong RA, Jones RL, Macdermot J, Wilson NH. Prostaglandin endoperoxide analogues which are both thromboxane receptor antagonists and prostacyclin mimetics. Br J Pharmacol. 1986;87:543–551. doi: 10.1111/j.1476-5381.1986.tb10196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auch-schwelk W, Katusic ZS, Vanhoutte PM. Thromboxane A2 receptor antagonists inhibit endothelium-dependent contractions. Hypertension. 1990;15:699–703. doi: 10.1161/01.hyp.15.6.699. [DOI] [PubMed] [Google Scholar]
- Auch-schwelk W, Katusic ZS, Vanhoutte PM. Nitric oxide inactivates endothelium-derived contracting factor in the rat aorta. Hypertension. 1992;19:442–445. doi: 10.1161/01.hyp.19.5.442. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya DK, Lecomte M, Dunn J, Morgans DJ, Smith WL. Selective inhibition of prostaglandin endoperoxide synthase-1 (cyclooxygenase-1) by valerylsalicylic acid. Arch Biochem Biophys. 1995;317:19–24. doi: 10.1006/abbi.1995.1130. [DOI] [PubMed] [Google Scholar]
- Bolla M, You D, Loufrani L, Levy BI, Levy-Toledano S, Habib A, et al. Cyclooxygenase involvement in thromboxane-dependent contraction in rat mesenteric resistance arteries. Hypertension. 2004;43:1264–1269. doi: 10.1161/01.HYP.0000127438.39744.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulanger CM, Morrison KJ, Vanhoutte PM. Mediation by M3-muscarinic receptors of both endothelium-dependent contraction and relaxation to acetylcholine in the aorta of the spontaneously hypertensive rat. Br J Pharmacol. 1994;112:519–524. doi: 10.1111/j.1476-5381.1994.tb13104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron NE, Cotter MA. Impaired contraction and relaxation in aorta from streptozotocin-diabetic rats: role of polyol pathway. Diabetologia. 1992;35:1011–1019. doi: 10.1007/BF02221675. [DOI] [PubMed] [Google Scholar]
- De Mey JG, Vanhoutte PM. Heterogeneous behavior of the canine arterial and venous wall. Importance of the endothelium. Circ Res. 1982;51:439–447. doi: 10.1161/01.res.51.4.439. [DOI] [PubMed] [Google Scholar]
- De Vriese AS, Verbeuren TJ, Van De Voorde J, Lameire NH, Vanhoutte PM. Endothelial dysfunction in diabetes. Br J Pharmacol. 2000;130:963–974. doi: 10.1038/sj.bjp.0703393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlert FJ. Pharmacological analysis of the contractile role of M2 and M3 muscarinic receptors in smooth muscle. Receptors Channels. 2003;9:261–277. [PubMed] [Google Scholar]
- Futaki N, Yoshikawa K, Hamasaka Y, Arai I, Higuchi S, Iizuka H, et al. NS-398, a novel non-steroidal anti-inflammatory drug with potent analgesic and antipyretic effects, which causes minimal stomach lesions. Gen Pharmacol. 1993;24:105–110. doi: 10.1016/0306-3623(93)90018-s. [DOI] [PubMed] [Google Scholar]
- Ge T, Hughes H, Junquero DC, Wu KK, Vanhoutte PM, Boulanger CM. Endothelium-dependent contractions are associated with both augmented expression of prostaglandin H synthase-1 and hypersensitivity to prostaglandin H2 in the SHR aorta. Circ Res. 1995;76:1003–1010. doi: 10.1161/01.res.76.6.1003. [DOI] [PubMed] [Google Scholar]
- Ge T, Vanhoutte PM, Boulanger CM. Increased response to prostaglandin H2 precedes changes in PGH synthase-1 expression in the SHR aorta. Zhongguo Yao Li Xue Bao. 1999;20:1087–1092. [PubMed] [Google Scholar]
- Gebermedhin D, Koltai MZ, Pogatsa G, Magyar K, Hadhazy P. Influence of experimental diabetes on the mechanical responses of canine coronary arteries: role of endothelium. Cardiovasc Res. 1988;22:537–544. doi: 10.1093/cvr/22.8.537. [DOI] [PubMed] [Google Scholar]
- Gluais P, Lonchampt M, Morrow JD, Vanhoutte PM, Feletou M. Acetylcholine-induced endothelium-dependent contractions in the SHR aorta: the Janus face of prostacyclin. Br J Pharmacol. 2005;146:834–845. doi: 10.1038/sj.bjp.0706390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluais P, Paysant J, Badier-Commander C, Verbeuren T, Vanhoutte PM, Feletou M. In the SHR aorta, the calcium ionophore A 23187 releases prostacyclin and thromboxane A2 as endothelium-derived contracting factors (EDCF) Am J Physiol Heart Circ Physiol. 2006;291:H2255–H2264. doi: 10.1152/ajpheart.01115.2005. [DOI] [PubMed] [Google Scholar]
- Gonzales RJ, Ghaffari AA, Duckls SP, Krause DN. Testosterone treatment increases thromboxane function in rat cerebral arteries. Am J Physiol Heart Circ Physiol. 2005;289:578–585. doi: 10.1152/ajpheart.00958.2004. [DOI] [PubMed] [Google Scholar]
- Hattori Y, Kawasaki H, Kanno M. Increased contractile responses to endothelin-1 and U46619 via a protein kinase C-mediated nifedipine-sensitive pathway in diabetic rat aorta. Res Commun Mol Pathol Pharmacol. 1999;104:73–80. [PubMed] [Google Scholar]
- Ingerman-Wojenski C, Silver MJ, Smith JB, Macarak E. Bovine endothelial cells in culture produce thromboxane as well as prostacyclin. J Clin Invest. 1981;67:1292–1296. doi: 10.1172/JCI110157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato T, Iwama Y, Okumura K, Hashimoto H, Ito T, Satake T. Prostaglandin H2 may be the endothelium-derived contracting factor released by acetylcholine in the aorta of the rat. Hypertension. 1990;15:475–481. doi: 10.1161/01.hyp.15.5.475. [DOI] [PubMed] [Google Scholar]
- Katusic ZS, Shepherd JT, Vanhoutte PM. Endothelium-dependent contractions to calcium ionophore A23187, arachidonic acid, and acetylcholine in canine basilar arteries. Stroke. 1988;19:476–479. doi: 10.1161/01.str.19.4.476. [DOI] [PubMed] [Google Scholar]
- Koga T, Takata Y, Kobayashi K, Takishita S, Yamashita Y, Fujishima M. Age and hypertension promote endothelium-dependent contractions to acetylcholine in the aorta of the rat. Hypertension. 1989;14:542–548. doi: 10.1161/01.hyp.14.5.542. [DOI] [PubMed] [Google Scholar]
- Koga T, Takata Y, Kobayshi K, Takishita S, Yamashita Y, Fujishima M. Age and hypertension promote endothelium-dependent contractions to acetylcholine in the aorta of the rat. Hypertension. 1993;22:577–583. doi: 10.1161/01.hyp.14.5.542. [DOI] [PubMed] [Google Scholar]
- Lüscher TF, Vanhoutte PM. Endothelium-dependent contractions to acetylcholine in the aorta of the spontaneously hypertensive rat. Hypertension. 1986;8:344–348. doi: 10.1161/01.hyp.8.4.344. [DOI] [PubMed] [Google Scholar]
- Miller VM, Reigel MM, Hollier LH, Vanhoutte PM. Endothelium-dependent responses in autogenous femoral veins grafted into the arterial circulation of the dog. J Clin Invest. 1987;80:1350–1357. doi: 10.1172/JCI113212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport RM, Williams SP. Role of prostaglandins in acetylcholine-induced contraction of aorta from spontaneously hypertensive and Wistar–Kyoto rats. Hypertension. 1996;28:64–75. doi: 10.1161/01.hyp.28.1.64. [DOI] [PubMed] [Google Scholar]
- Russell JA, Rohrbach MS. Tannin induces endothelium-dependent contraction and relaxation of rabbit pulmonary artery. Am Rev Respir Dis. 1989;139:498–503. doi: 10.1164/ajrccm/139.2.498. [DOI] [PubMed] [Google Scholar]
- Samata K, Kimura T, Satoh S, Watanabe H. Chemical removal of the endothelium by saponin in the isolated dog femoral artery. Eur J Pharmacol. 1986;128:85–91. doi: 10.1016/0014-2999(86)90561-3. [DOI] [PubMed] [Google Scholar]
- Sanner JH. Antagonism of prostaglandin E2 by 1-acetyl-2-(8-chloro-10,11-dihydrodibenz (b,f) (1,4) oxazepine-10-carbonyl) hydrazine (SC-19220) Arch Int Pharmacodyn Ther. 1969;180:46–56. [PubMed] [Google Scholar]
- Schalkwijk CG, Stehouwer CD. Vascular complications in diabetes mellitus: the role of endothelial dysfunction. Clin Sci (Lond) 2005;109:143–159. doi: 10.1042/CS20050025. [DOI] [PubMed] [Google Scholar]
- Shi Y, Ku DD, Man RY, Vanhoutte PM. Augmented EDHF-mediated relaxations attenuate endothelial dysfunction in femoral and mesenteric, but not in carotid arteries from type I diabetic rats. J Pharmacol Exp Ther. 2006;318:276–281. doi: 10.1124/jpet.105.099739. [DOI] [PubMed] [Google Scholar]
- Shirahase H, Usui H, Kurahashi K, Fujiwara M, Fukui K. Endothelium-dependent contraction induced by nicotine in isolated canine basilar artery – possible involvement of a thromboxane A2 (TXA2) like substance. Life Sci. 1988;42:437–445. doi: 10.1016/0024-3205(88)90082-3. [DOI] [PubMed] [Google Scholar]
- Singer HA, Peach MJ. Calcium- and endothelial-mediated vascular smooth muscle relaxation in rabbit aorta. Hypertension. 1982;4:19–25. [PubMed] [Google Scholar]
- Smith CJ, Zhang Y, Koboldt CM, Muhammad J, Zweifel BS, Shaffer A, et al. Pharmacological analysis of cyclooxygenase-1 in inflammation. Proc Natl Acad Sci USA. 1998;95:13313–13318. doi: 10.1073/pnas.95.22.13313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang EH, Feletou M, Huang Y, Man RY, Vanhoutte PM. Acetylcholine and sodium nitroprusside cause long-term inhibition of EDCF-mediated contractions. Am J Physiol Heart Circ Physiol. 2005a;289:H2434–H2440. doi: 10.1152/ajpheart.00568.2005. [DOI] [PubMed] [Google Scholar]
- Tang EH, Ku DD, Tipoe GL, Feletou M, Man RY, Vanhoutte PM. Endothelium-dependent contractions occur in the aorta of wild-type and COX2−/− knockout but not COX1−/− knockout mice. J Cardiovasc Pharmacol. 2005b;46:761–765. doi: 10.1097/01.fjc.0000187174.67661.67. [DOI] [PubMed] [Google Scholar]
- Tesfamariam B, Brown ML, Deykin D, Cohen RA. Elevated glucose promotes generation of endothelium-derived vasoconstrictor prostanoids in rabbit aorta. J Clin Invest. 1990;85:929–932. doi: 10.1172/JCI114521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesfamariam B, Jakubowski JA, Cohen RA. Contraction of diabetic rabbit aorta caused by endothelium-derived PGH2-TxA2. Am J Physiol. 1989;257:H1327–H1333. doi: 10.1152/ajpheart.1989.257.5.H1327. [DOI] [PubMed] [Google Scholar]
- Vanhoutte PM, Feleou M, Taddei S. Endothelium-dependent contractions in hypertension. Br J Pharmacol. 2005;144:449–458. doi: 10.1038/sj.bjp.0706042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermylen J, Defreyn G, Carreras LO, Machin SJ, Van Schaeren J, Verstraete M. Thromboxane synthetase inhibition as antithrombotic strategy. Lancet. 1981;1:1073–1075. doi: 10.1016/s0140-6736(81)92241-8. [DOI] [PubMed] [Google Scholar]
- Yang D, Feletou M, Boulanger CM, Wu HF, Levens N, Zhang JN, Vanhoutte PM. Oxygen-derived free radicals mediate endothelium-dependent contractions to acetylcholine in aortas from spontaneously hypertensive rats. Br J Pharmacol. 2002;136:104–110. doi: 10.1038/sj.bjp.0704669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D, Feletou M, Levens N, Zhang JN, Vanhoutte PM. A diffusible substance(s) mediates endothelium-dependent contractions in the aorta of SHR. Hypertension. 2003;41:143–148. doi: 10.1161/01.hyp.0000047651.45322.16. [DOI] [PubMed] [Google Scholar]
- Yang D, Gluais P, Zhang JN, Vanhoutte PM, Feletou M. Endothelium-dependent contractions to acetylcholine, ATP and the calcium ionophore A 23187 in aortas from spontaneously hypertensive and normotensive rats. Fundam Clin Pharmacol. 2004a;18:321–326. doi: 10.1111/j.1472-8206.2004.00247.x. [DOI] [PubMed] [Google Scholar]
- Yang D, Gluais P, Zhang JN, Vanhoutte PM, Feletou M. Nitric oxide and inactivation of the endothelium-dependent contracting factor released by acetylcholine in spontaneously hypertensive rat. J Cardiovasc Pharmacol. 2004b;43:815–820. doi: 10.1097/00005344-200406000-00011. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Varadharaj S, Zhao X, Parinandi N., Flavahan NA, Zweier JL. Acetylcholine causes endothelium-dependent contraction of mouse arteries. Am J Physiol Heart Circ Physiol. 2005;289:H1027–1032. doi: 10.1152/ajpheart.00226.2005. [DOI] [PubMed] [Google Scholar]
- Zou M.H, Leist M, Ullich V. Selective nitration of prostacyclin synthase and defective vasorelaxation in atherosclerotic bovine coronary arteries. Am J Pathol. 1999;154:1359–1365. doi: 10.1016/S0002-9440(10)65390-4. [DOI] [PMC free article] [PubMed] [Google Scholar]