Abstract
Background and purpose:
A new concept for the inhibition of CGRP signalling has been developed by interaction with the CGRP molecule per se by using a CGRP antibody or a CGRP binding RNA-Spiegelmer (NOX-C89). We have compared these CGRP scavengers with two known receptor antagonists (CGRP8–37 and BIBN4096BS) on CGRP-induced relaxations in the rat middle cerebral artery (MCA). Furthermore, the role of the endothelial barrier has been studied.
Experimental approach:
We used the luminally perfused MCA in an arteriograph, pressurized to 85 mm Hg and myograph studies of isolated ring segments of the MCA.
Key results:
In myograph studies and in the perfusion system during abluminal application, αCGRP and βCGRP induced concentration-dependent dilatation of the MCA. Given luminally neither peptide was significantly vasodilator. Adrenomedullin and amylin induced weak dilatations. In myograph experiments, relaxation induced by αCGRP was prevented by the four CGRP blockers (CGRP8–37, BIBN4096BS, the CGRP antibody and NOX-C89.). In abluminal perfusion experiments, the relaxant response to αCGRP was prevented by these agents to a varying degree. Dilatation induced by abluminal application of αCGRP was inhibited by luminal CGRP8–37 but not by luminal BIBN4096BS, CGRP antibody or NOX-C89.
Conclusions and Implications:
α or βCGRP acted on smooth muscle cell CGRP receptors in rat MCA and were effectively prevented from reaching these receptors by the endothelial barrier. The CGRP blockers significantly inhibited αCGRP induced relaxation but were also prevented from reaching the CGRP receptors by the arterial endothelium.
Keywords: migraine, CGRP, amylin, adrenomedullin, CGRP-antibody, CGRP8–37, BIBN4096BS, middle cerebral artery, RNA-Spiegelmer
Introduction
Trigeminal sensory C-fibres innervating the cranial vasculature contain several vasoactive peptides such as calcitonin gene-related peptide (CGRP), neurokinin A (NKA), substance P (SP) and pituitary adenylate cyclase activating peptide (PACAP) (Gulbenkian et al., 2001). Stimulation of the trigeminal ganglion liberates vasoactive peptides into the perivascular space. CGRP may thus interact mainly with smooth muscle cells, as all receptor elements are expressed on the smooth muscle cells (Oliver et al., 2002), whereas pre-synaptic receptors on the perivascular nerves have only been inferred indirectly (Tajti et al., 1999). CGRP is a potent dilator of cerebral (pial) (Edvinsson et al., 1987) and dural blood vessels (Williamson et al., 1997; Jansen-Olesen et al., 2003), and participates as a vasodilator but not to induce plasma extravasation in dural neurogenic inflammation (Grant et al., 2005). Furthermore, CGRP levels are increased during acute migraine and cluster headache attacks (Goadsby et al., 1990; Edvinsson and Goadsby, 1994; Goadsby and Edvinsson, 1994) and the direct infusion of CGRP induces headache and migraine attacks in migraine patients (Goadsby et al., 1990; Goadsby and Edvinsson, 1994; Lassen et al., 2002).
BIBN4096BS is a novel, well-characterized CGRP receptor antagonist that is highly selective and potent in both animal and human experiments (Doods et al., 2000; Edvinsson et al., 2002; Petersen et al., 2005). As this compound is safe for human administration and, in a proof of concept phase-II study, effective in treating acute migraine attacks (Olesen et al., 2004; Petersen et al., 2005), research has focused on the development and understanding of the site of action of CGRP antagonists for migraine treatment. Both peptide (CGRP8–37) and non-peptide (‘compound 1' and SB-273779) molecules effectively antagonize CGRP in vivo and in vitro (Aiyar et al., 2001; Edvinsson et al., 2001; Williamson et al., 2001). These antagonists are, for a variety of reasons, however, not suitable for human use.
Recently, a new concept for the inhibition of CGRP signalling was developed, namely a CGRP-binding RNA-Spiegelmer. This is a single-stranded mirror-image oligonucleotide that is highly resistant to nuclease degradation and is capable of tightly and specifically binding to CGRP and thus to inhibit its function (Vater et al., 2003). In addition, a humanized CGRP-binding monoclonal antibody has been raised in rabbits for treatment of CGRP-related disorders such as migraine.
In the present study, we have compared the functional effects of the CGRP receptor antagonists (BIBN4096BS and CGRP8–37), the CGRP antibody and the CGRP-binding RNA-Spiegelmer on the vasodilator response to αCGRP in isolated cerebral arteries. We have used two methods: (i) a sensitive myograph to study isolated ring segments of rat middle cerebral artery (MCA) (Hogestatt et al., 1983) and (ii) a pressure myograph to examine the effect of intraluminal and abluminal administration of agonists and antagonists (Bryan et al., 1996; You et al., 1997). The results suggest that CGRP receptors are located to the smooth muscle cells and that the blood–brain barrier prevents luminal CGRP and its blockers from reaching the arterial smooth muscle cells.
Materials and methods
Tissue preparation
The Animal Protocol Review committee at the University of Lund approved the experimental protocol (M111-04).
Male Sprague–Dawley rats (250–350 g) were anaesthetized using CO2 and decapitated. The brain was immediately removed and placed in cold (4°C) buffer solution of the following composition (mM):NaCl 119, NaHCO3 15, KCl 4.6, MgCl2 1.2, NaH2PO4 1.2, CaCl2 1.5 and glucose 5.5. With the aid of a dissecting microscope, MCA segments were carefully harvested from each animal beginning at the circle of Willis and extending 5–8 mm distally.
Myograph experiments
Each MCA was cut into 1–2 mm long circular segments and placed in an ice-cold buffer solution gassed with 5% CO2 in O2 (Hogestatt et al., 1983). In order to determine vessel tension, each segment was mounted on two metal wires 40 μm in diameter in a myograph (Model 610M; Danish Myo Technology, Aarhus, Denmark). The artery segments were bathed in the buffer solution described above and were throughout the experiment continuously aerated with 95% O2:5% CO2 gas mixtures further stabilizing the pH at 7.4. The artery segments were allowed to equilibrate for approximately 30 min. The vessels were stretched to the internal circumference the vessels would have if relaxed and exposed to a passive transmural pressure of 75 mm Hg (10 kPa). This was carried out in order to achieve maximal active force development. Following a 30-min equilibration period, the vessels were constricted twice by 63 mM of KCl in a modified buffer solution in which NaCl was substituted for KCl on an equimolar basis. The force of these contractions amounted to 0.83±0.07 mN (n=44). In order to study the relaxant effect of CGRP, the MCA was pre-contracted with 3 × 10−6 M prostaglandin F2α (PGF2α). This resulted in a stable tension of 0.87±0.08 mN (n=44) lasting for at least 30 min without significant fall in tone, to which agonists were added in increasing concentrations. In blockade experiments, a single concentration of a blocker (see below) was added 30 min before the addition of CGRP. Out of four segments run in parallel, two served as controls (i.e. without blocker) and the others were treated with blocker.
Pressurized arteriograph experiments
A section of the MCA (1–2 mm in length) was mounted in a pressurized arteriograph (Living Systems, Burlington, VT, USA) as described previously (Bryan et al., 1996; You et al., 1997). Micropipettes were inserted into both ends of the MCA and secured with 11-0 nylon ties. The MCA was bathed in the buffer solution (37°C) equilibrated with a gas mixture consisting of 5% CO2 and 95% O2, resulting in a pH of 7.4. Transmural pressure of the MCA was maintained at 85 mm Hg by raising reservoirs connected to the micropipettes to the appropriate height above the MCA. Luminal perfusion flow was adjusted to 100 μl min−1 (range 0.1–0.09 ml min) by setting the two reservoirs at different heights. Pressure transducers on either side of the MCA provided direct measurement of perfusion pressure across the MCA.
The vessel was magnified 600-fold using a microscope coupled to a digital camera (Axis, Lund, Sweden) connected to a computer. The program Mary (Nihil KB, Lund, Sweden) saved the pictures at intervals of 1 s during the experiment as well as measuring the outer diameter of the vessels.
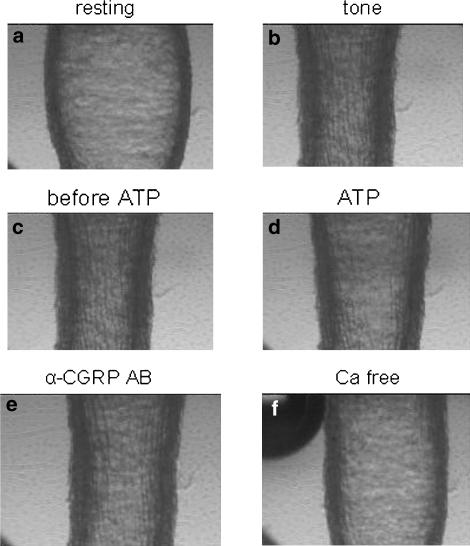
Following a resting period, the pressurized vessels attained a stable tone, usually considered to be due to shear stress. In the present study the initial vessel diameter was 197.3±4.9 μm (n=67) and after tone 135.1±3.0 μm, which represents contraction by 30.8±1.0% (Figure 1). Any MCA that did not develop spontaneous tone of at least 10% compared to the initial diameter within 1 h was excluded from the experiment. After another period of 30 min, the MCA segments were exposed to luminal ATP (10−5 M) in order to evaluate the function of the endothelium. A dilatation of at least 15% of the resting diameter was considered indicative of a functional endothelium. In the present study, the mean dilatation of the MCA segments to ATP was 99.0±6.1% (n=67). This was considered a positive sign for a functional endothelium and patency of the blood–brain barrier (Bryan et al., 1996; You et al., 1997). Experimental protocols were not initiated until the MCA diameter was stable over a 15-min period. The vascular response to stimulation of endothelial receptors was carried out by adding αCGRP, βCGRP, adrenomedullin or amylin to the luminal perfusate (in the concentration range 10−12–10−6 M). In addition, either CGRP peptide was added abluminally in the same concentration range to test the direct smooth muscle effect; this route of administration avoids interaction with the endothelium.
Figure 1.
Illustration of the perfusion studies. (a) Baseline tone just after mounting of the vessel. (b) Following perfusion, tone was induced by the shear stress induced by perfusate flow through the lumen (0.1 ml/min). (c) Tone just before and (d) when ATP (10−5 M) was applied. (e) Tone when αCGRP (10−6 M) was applied abluminally and (f) when calcium-free buffer was perfused.
To characterize the responses to luminal and abluminal stimulation further, the two antagonists BIBN4096BS or CGRP8–37 in doses up to 1 μM were used either alone or in the presence of increasing concentrations of αCGRP. In addition, the CGRP antibody (2.4 μg ml−1 when given abluminally and 12 μg ml−1 when given luminally) and the RNA-Spiegelmer (10−6 M) were used as above. Inhibitors were added either to the abluminal and the luminal perfusate 30 min before commencing application of αCGRP. At the end of each experiment, the vessel segments were exposed to a calcium-free buffer solution, which provided a measure of the maximum relaxant capability of the MCA segment (38.0±2.3 %) (Figure 1).
Data analysis
For data obtained in myograph experiments on isolated ring segments of the MCA, the amount of relaxation induced by each concentration of CGRP receptor agonist was calculated as percentage of PGF2α precontraction. For the experiments performed on the pressurized arteriograph, the changes in measured diameters of the vessel segments are expressed as a percentage of the resting diameter. Dilatation is given as a percentage of the spontaneous tension obtained through perfusion. Values are given as mean±s.e.m. The number of experiments=n, which in the perfusion experiments equals the number of rats. Emax denotes the maximum response elicited by an agonist whereas pEC50 denotes the negative logarithm of the concentration needed to elicit half the maximum response. All concentrations expressed indicate the final concentration in the tissue baths and in the luminal or abluminal compartments of the pressurized arteriograph. Sigmoidal curve fitting was carried out using the computer program GraphPad Prism (GraphPad Software, San Diego, CA, USA). Based on the principal equation for a sigmoidal curve, the program makes iterated computations to derive a best fit based upon the actual experimental values. The non-parametric, Mann–Whitney U-test was used to determine statistical significance between two groups of data. Statistical significance was assumed when P<0.05.
Drugs
ATP, Rat αCGRP, βCGRP, adrenomedullin, amylin and CGRP8–37 (Sigma, St Louis, MO, USA) were dissolved in distilled water. The CGRP Spiegelmer NOX-C89 (a gift from NOXXON Pharma AG, Berlin, Germany) was dissolved in the same buffer solution as used throughout the experiments. BIBN4096BS was a gift synthesized by Merck (Terlings Park, UK). BIBN4096BS was dissolved in a small volume (20 μl) of 1 N HCl, further diluted with saline (0.9%) and finally adjusted to pH 6.5–7.0 by 1 N NaOH. Stock solutions of each compound were aliquoted and frozen at –20°C. A stock solution of the humanized CGRP binding monoclonal antibody raised in rabbit was obtained from Rinat Neuroscience (San Fransisco, CA, USA). Stock solutions were stored frozen in small aliquots until use. For experiments, drugs were diluted in the buffer solution immediately before use. All chemicals were obtained from Merck (Darmstadt, Germany). Only double distilled water was used throughout the experiments.
Results
Concentration–effect relations of CGRP, adrenomedullin and amylin
Pressurized arteriograph experiments
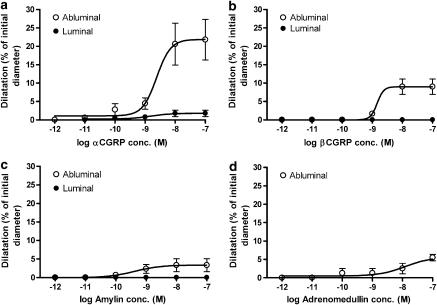
Abluminally applied αCGRP and βCGRP caused significant relaxations of 23±2.4% (n=12) and 11±1.1% (n=3), respectively (Figure 2). The pEC50 values for abluminal αCGRP and βCGRP were 9.31±0.35 and 9.02±0.43, respectively. Luminal administration of αCGRP and βCGRP showed no significant effects (1.8±0.85, and 0.0±0.0%, respectively). Slight relaxations were seen following abluminal application of amylin (Emax: 7.0±0.6%; pEC50: 9.39±0.74) and adrenomedullin (Emax: 7.5±1.3%; pEC50: 7.90±0.35). The response to luminally applied amylin was weak (Emax: 4.7±1.5%; n=3) and that of adrenomedullin did not differ from baseline (Figure 2).
Figure 2.
Concentration–dilation curves in perfused MCA for abluminally or luminally administered (a) αCGRP, (b) βCGRP, (c) amylin and (d) adrenomedullin. Values are given as means±s.e.m., n=3–12.
Myograph experiments
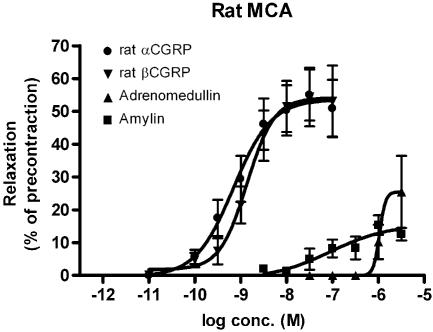
The addition of increasing concentrations of αCGRP and βCGRP (10−10–10−7 M) resulted in concentration-dependent relaxations of the MCA (αCGRP, Emax: 54±5%; pEC50: 9.16±0.21) and βCGRP (Emax: 54±5%; pEC50: 8.86±0.16) (Figure 3). The pEC50 values for amylin – and adrenomedullin-induced relaxations were 7.05±0.89 and 6.00±1.32, respectively. Emax value for amylin was 16±7% and for adrenomedullin 26±15% (Figure 3).
Figure 3.
The concentration–effect relationship of rat αCGRP, βCGRP, adrenomedullin and amylin on rings of MCA, pre-contracted with PGF2α (3 × 10−6 M). Values are given as means±s.e.m., n=4–15.
Effect of CGRP inhibitors on αCGRP induced vasodilatation
Pressurized arteriograph experiments
When applied per se in increasing concentrations up to 1 μM none of the four CGRP blockers, BIBN4096BS, CGRP8–37, the CGRP antibodies or NOX-C89, given luminally or abluminally had any effect on the resting tone of MCA (data not shown).
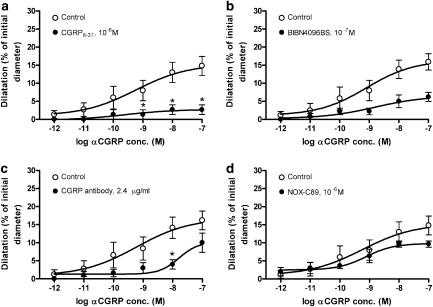
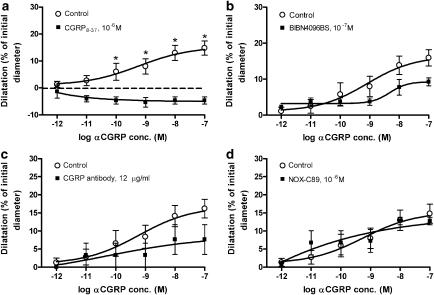
The effect of these blockers was studied only on the concentration-dependent dilatation of the pressurized MCA induced by abluminal administration of αCGRP (10−9–10−7 M) (Figure 4). The αCGRP response was significantly inhibited by abluminally applied CGRP8–37 (10−6 M) and BIBN4096BS (P<0.05). Luminal CGRP8–37 also inhibited the abluminal αCGRP responses (Figure 5a), whereas luminal BIBN4096BS did not (Figures 4b and 5b).
Figure 4.
Relaxant effect of abluminal αCGRP in perfused MCA after abluminal application of (a) 10−6 M CGRP8–37, (b) 10−7 M BIBN4096BS, (c) 2.4 μg ml−1 CGRP antibody and (d) 10−6 M NOX-C89. Values are given as means±s.e.m. Statistical analysis by Mann–Whitney U-test, *P<0.01, n=3–7.
Figure 5.
Relaxant effect of abluminal αCGRP in perfused MCA after luminal application of (a) 10−6 M CGRP8–37, (b) 10−7 M BIBN4096BS, (c) 12 μg ml−1 CGRP antibody and (d) 10−6 M NOX-C89. Values are given as means±s.e.m. In (a), there was a significant effect of the CGRP antagonist CGRP8–37 as indicated (P<0.05); the dashed line represents the baseline values. For the other antagonists (b, c), there were no statistically significant effects on dilatation induced by αCGRP (Mann–Whitney U-test, *P>0.05, n=3–7).
The CGRP antibody was examined in a concentration of 2.4 μg/ml when given abluminally and 12 μg ml−1 when given luminally. When given abluminally, the antibody caused a significant inhibition of the αCGRP-induced relaxation only at 10−8 M of αCGRP (Figure 4c). There was no significant blockade of abluminal αCGRP by luminal administration of the CGRP antibody (Figure 5c).
Neither abluminal nor luminal administration of 10−6 M NOX-C89 showed a significant inhibition of the αCGRP induced relaxation (Figures 4d and 5d).
Myograph experiments
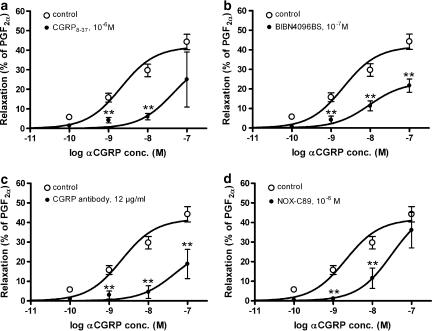
The responses to αCGRP were significantly attenuated by CGRP8–37 (10−6 M), BIBN4096BS (10−7 M) and NOX-C89 (10−6 M) (Figure 6a, b and d) ml−1. The CGRP antibody, given in a concentration of 4.8 μg, did not decrease the relaxation induced by αCGRP (data not shown). However, increasing the CGRP antibody concentration to 12 μg/ml significantly inhibited the αCGRP-induced relaxation, as shown in Figure 6c. In concentration–response experiments, NOX-C89 (10−6 M) and 12 μg ml−1 CGRP antibody produced significantly lower pEC50 values of αCGRP (10−10–10−7 M) mediated relaxation. However, the pEC50 values were not affected by the presence of BIBN4096BS (10−7 M) (Table 1). In these experiments, 12 μg/ml of the CGRP antibody and BIBN4096BS (10−7 M), but not CGRP8–37 (10−6 M) and NOX-C89 (10−6 M), significantly lowered maximum responses to α-CGRP (Table 1).
Figure 6.
The effect of (a) 10−6 M CGRP8–37, (b) 10−7 M BIBN4096BS, (c) 12 μg CGRP antibody and (d) 10−6 M NOX-C89 on relaxation of rings of MCA, pre-contracted with PGF2α (3 × 10−6 M). Values are given as means±s.e.m. Statistical analysis by Mann–Whitney U-test, **P<0.01, n=5–7.
Table 1.
Relaxant effect of αCGRP on rat middle cerebral arteries pre-contracted by 3 × 10−6 M PGF2α
| Blocker tested | pEC50 | Emax | n |
|---|---|---|---|
| None | 8.75±0.16 | 43±4 | 15 |
| AB, 4.8 μg ml−1 | 8.13±0.27NS | 44±19NS. | 6 |
| AB, 12 μg ml−1 | 7.65±0.09** | 19±7* | 6 |
| NOX-C89, 10−6 M | 7.74±0.07** | 36±9NS | 7 |
| BIBN4096BS, 10−7 M | 8.09±0.16NS | 14±3*** | 6 |
| CGRP8–37, 10−6 M | 8.55±0.34NS | 25±14NS | 5 |
Abbreviations: CGRP, calcitonin gene-related peptide; NS, not significant.
Data are given as means±s.e.m.; n=number of vessel segments examined. Emax=maximum relaxation in % of pre-contraction; pEC50=the negative logarithmic concentration (M) of an agonist eliciting half-maximum relaxation.
Statistical analysis by Mann–Whitney U-test
P<0.05
P<0.01
P<0.005 vs values in the absence of blocker.
The relaxant response was studied in the absence or presence of one of four different CGRP blockers.
Discussion
The present study has demonstrated that the rat MCA contains smooth muscle cell CGRP1 receptors, the CGRP family of peptides do not pass the endothelial barrier, the four CGRP blockers can antagonize CGRP receptor activation in myograph experiments and dilatation induced by abluminal αCGRP was inhibited by abluminal CGRP8–37, BIBN4096BS and the CGRP antibody in the perfused MCA.
Effect of CGRP, amylin and adrenomedullin
CGRP, amylin and adrenomedullin receptors are generated by the genes CALCR that codes for the 7 transmembrane (TM) calcitonin receptor, whereas CALCRL codes for the 7 TM calcitonin receptor-like receptor. Their function and pharmacology are determined by the presence of receptor activity-modifying proteins (RAMPs) (McLatchie et al., 1998). RAMPs are single TM domain proteins identified as a family of three members: RAMP1, RAMP2 and RAMP3. RAMP1, 2 and 3 in combination with CALCR constitute the respective amylin 1, 2 and 3 receptors (Muff et al., 1999; Tilakaratne et al., 2000; Zumpe et al., 2000). The CALCRL in combination with RAMP1 represents the CGRP receptor whereas CALCRL in combination with RAMP2 and 3 constitutes the two adrenomedullin (1 and 2) receptors (Buhlmann et al., 1999; Fraser et al., 1999; Sexton et al., 2001).
In the present study, the order of relaxant potency in the rat MCA was: αCGRP=βCGRP>> amylin> adrenomedullin in myograph studies. This order of potency is consistent with that of the CGRP1 receptor (CGRP> amylin⩾ adrenomedullin) and differs from the adrenomedullin (AM1: adrenomedullin>> CGRP> amylin, AM2; adrenomedullin⩾ CGRP> amylin) or amylin receptors (amylin⩾ CGRP> adrenomedullin) (Alexander et al., 2005).
This definition of the receptors in the MCA is further supported by the weak relaxant effects of adrenomedullin and amylin. Therefore, our data showed the presence of the CGRP1 receptor in rat MCA. Our experiments further revealed that the CGRP1 receptors are situated in the smooth muscle cells, as only abluminal administration of the peptides had effects. This is supported by data in which removal of the endothelium did not alter the CGRP-induced relaxation in brain vessels and the relaxation occurred in parallel with activation of adenylyl cyclase in the vessel walls (Jansen-Olesen et al., 1996; Edvinsson et al., 1998).
CGRP has been found to provoke migraine attacks in a human experimental model of migraine (Lassen et al., 2002). It is released during migraine or cluster headache attacks (Goadsby et al., 1990; Goadsby and Edvinsson, 1994; Juhasz et al., 2003); CGRP is released in parallel with increasing degree of pain during migraine attacks (Juhasz et al., 2005) and with enhanced intracranial vasoconstriction during subarachnoid haemorrhage (Juul et al., 1990). The systemic administration of CGRP can reduce cerebrovascular vasoconstriction following subarachnoid haemorrhage in man (Juul et al., 1994). We therefore, addressed the question of whether circulating CGRP or its family of peptides can influence the tone of the MCA. We also intended to examine the role of the endothelial barriers in brain vessels. Abluminally applied αCGRP and βCGRP concentration-dependently relaxed the MCA. However, CGRP given luminally had only minor effects on MCA diameter, which suggested that it did not pass the endothelial barrier readily. Adrenomedullin and amylin behaved in much the same way, but induced weaker responses than CGRP. This agrees with recent in vivo data where Petersen et al. (2004) observed that systemic αCGRP and βCGRP had no direct effect on cortex pial artery diameter but relaxed the middle meningeal artery, which lacks a blood–brain barrier.
The effect of CGRP on cerebral blood flow (CBF) is not clear. In most species, including rat, the peptide increases CBF upon intravenous administration (Suzuki et al., 1989; Baskaya et al., 1995). The above-mentioned study, reported a dose-dependent increase in local CBFFlux with infusion of both αCGRP and βCGRP, however; it was only significant at the highest doses given (Petersen et al., 2004). These results are in concert with the view that there might be some minor passage of the peptide across the endothelium of cerebral blood vessels but not as much as through the endothelium of the vessels in the dura mater, where there is no blood–brain barrier. In addition, the method for studies of CBF using laser Doppler flowmetry does not measure true tissue blood flow but rather changes in cerebral vessel tone, which could be elicited by several mechanisms (Edvinsson and Krause, 2002).
Mechanism and site of action of the antagonists
BIBN4096BS is a hydrophilic dipeptide derivative (consisting of two amino acids) and is structurally smaller than the other antagonists and, therefore, more likely to pass the blood–brain barrier, to some extent. There are, to our knowledge, no studies available that have directly measured the passage of BIBN4096BS across the blood–brain barrier. In contrast to the pial vessels, the meningeal vessels have no barrier and BIBN4096BS is likely to diffuse freely into the wall of these vessels (Knudsen et al., 1988; Faraci et al., 1989). Thus, it was observed in the closed cranial window experiments that BIBN4096BS had antagonistic effects on the middle meningeal artery following systemic administration of αCGRP and βCGRP, but only a weak effect on endogenously released CGRP in pial arteries and on CBF (Petersen et al., 2004). These results agree with the present data that BIBN4096BS showed an inhibitory effect when applied abluminally but none after luminal application. Thus, the blood–brain barrier seems to inhibit the passage of BIBN4096BS from the luminal to the abluminal side of the brain vessel. However, the larger hydrophilic peptide antagonist CGRP8–37 had significant inhibitory effects on abluminal and luminal αCGRP-induced dilatation. The reason for this is unclear.
As receptors with a CGRP receptor-like pharmacological profile are situated primarily on the smooth muscle cells of cerebral arteries in man (Edvinsson et al., 2002; Moreno et al., 2002; Oliver et al., 2002; Petersen et al., 2005), systemically administered CGRP8–37 might have to pass the blood–brain barrier to inhibit vasodilatation induced by CGRP released from sensory nerves in pial arteries and arterioles.
The hydrophilic, high molecular weight CGRP scavengers had slight antagonistic effects on relaxation to abluminal αCGRP, when given abluminally but not luminally. The CGRP-binding RNA-Spiegelmer in the concentrations used, was less effective than the CGRP antibody in the perfusion study, but not in the myograph study. However, in an in vivo study, we have shown that intravenous infusions of the CGRP antibodies and the CGRP-binding RNA-Spiegelmer are able to inhibit the effect of a subsequent infusion of CGRP but not of CGRP released from perivascular sensory nerves after electrical stimulation in the dura mater (Juhl et al., in preparation). Thus, the two novel CGRP scavengers seem to be not able to penetrate the cerebral vessel wall.
The CGRP peptide agonists were effectively prevented from reaching the CGRP1 receptors in the smooth muscle cells by the endothelial barrier. The CGRP blockers significantly inhibited αCGRP-induced relaxation but were similarly prevented from reaching the receptors by the endothelial barrier. Studies of isolated human brain vessels have revealed pA2 values for BIBN4096BS of about 0.1 nM (Edvinsson et al., 2002). The low penetration across the blood–brain barrier of BIBN4096BS and the antimigraine effect seen at 200 nM (Olesen et al., 2004) suggest that the antimigraine effect of BIBN4096BS takes place at the abluminal side of the blood–brain barrier in the MCA as BIBN4096BS passes the dural artery freely.
Acknowledgments
This work was supported by the Swedish Medical Research Council (Grant no. 5958), The Danish Medical Research Council, The Lundbeck Foundation Center for Neurovascular Signalling, the Heart–Lung foundation, the Lundbeck foundation and the McKinney Møller foundation. We acknowledge NOXXON Pharma AG for the kind gift of NOX-C89 and Rinat Neuroscience for the kind gift of the CGRP antibody. We also thank Dr Sven Klussmann and Dr Axel Vater for helpful discussions.
Abbreviations
- CBF
cerebral blood flow
- CGRP
calcitonin gene-related peptide
- MCA
middle cerebral artery
Conflict of interest
The authors state no conflict of interest.
References
- Aiyar N, Daines RA, Disa J, Chambers PA, Sauermelch CF, Quiniou M, et al. Pharmacology of SB-273779, a nonpeptide calcitonin gene-related peptide 1 receptor antagonist. J Pharmacol Exp Ther. 2001;296:768–775. [PubMed] [Google Scholar]
- Alexander SP, Mathie A, Peters JA. 7 TM Receptors. Br J Pharmacol. 2005;1 Suppl 144:S4–S62. doi: 10.1038/sj.bjp.0706158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baskaya MK, Suzuki Y, Anzai M, Seki Y, Saito K, Takayasu M, et al. Effects of adrenomedullin, calcitonin gene-related peptide, and amylin on cerebral circulation in dogs. J Cereb Blood Flow Metab. 1995;15:827–834. doi: 10.1038/jcbfm.1995.103. [DOI] [PubMed] [Google Scholar]
- Bryan RM, Jr, Eichler MY, Swafford MW, Johnson TD, Suresh MS, Childres WF. Stimulation of alpha 2 adrenoceptors dilates the rat middle cerebral artery. Anesthesiology. 1996;85:82–90. doi: 10.1097/00000542-199607000-00012. [DOI] [PubMed] [Google Scholar]
- Buhlmann N, Leuthauser K, Muff R, Fischer JA, Born W. A receptor activity modifying protein (RAMP)2-dependent adrenomedullin receptor is a calcitonin gene-related peptide receptor when coexpressed with human RAMP1. Endocrinology. 1999;140:2883–2890. doi: 10.1210/endo.140.6.6783. [DOI] [PubMed] [Google Scholar]
- Doods H, Hallermayer G, Wu D, Entzeroth M, Rudolf K, Engel W, et al. Pharmacological profile of BIBN4096BS, the first selective small molecule CGRP antagonist. Br J Pharmacol. 2000;129:420–423. doi: 10.1038/sj.bjp.0703110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edvinsson L, Alm R, Shaw D, Rutledge RZ, Koblan KS, Longmore J, et al. Effect of the CGRP receptor antagonist BIBN4096BS in human cerebral, coronary and omental arteries and in SK-N-MC cells. Eur J Pharmacol. 2002;434:49–53. doi: 10.1016/s0014-2999(01)01532-1. [DOI] [PubMed] [Google Scholar]
- Edvinsson L, Ekman R, Jansen I, McCulloch J, Uddman R. Calcitonin gene-related peptide and cerebral blood vessels: distribution and vasomotor effects. J Cereb Blood Flow Metab. 1987;7:720–728. doi: 10.1038/jcbfm.1987.126. [DOI] [PubMed] [Google Scholar]
- Edvinsson L, Goadsby PJ. Neuropeptides in migraine and cluster headache. Cephalalgia. 1994;14:320–327. doi: 10.1046/j.1468-2982.1994.1405320.x. [DOI] [PubMed] [Google Scholar]
- Edvinsson L, Krause D. Lippincott, Williams & Wilkins: Philadelphia; 2002. Cerebral Blood Flow and Metabolism. [Google Scholar]
- Edvinsson L, Mulder H, Goadsby PJ, Uddman R. Calcitonin gene-related peptide and nitric oxide in the trigeminal ganglion: cerebral vasodilatation from trigeminal nerve stimulation involves mainly calcitonin gene-related peptide. J Auton Nerv Syst. 1998;70:15–22. doi: 10.1016/s0165-1838(98)00033-2. [DOI] [PubMed] [Google Scholar]
- Edvinsson L, Sams A, Jansen-Olesen I, Tajti J, Kane SA, Rutledge RZ, et al. Characterisation of the effects of a non-peptide CGRP receptor antagonist in SK-N-MC cells and isolated human cerebral arteries. Eur J Pharmacol. 2001;415:39–44. doi: 10.1016/s0014-2999(00)00934-1. [DOI] [PubMed] [Google Scholar]
- Faraci FM, Kadel KA, Heistad DD. Vascular responses of dura mater. Am J Physiol. 1989;257:H157–H161. doi: 10.1152/ajpheart.1989.257.1.H157. [DOI] [PubMed] [Google Scholar]
- Fraser NJ, Wise A, Brown J, McLatchie LM, Main MJ, Foord SM. The amino terminus of receptor activity modifying proteins is a critical determinant of glycosylation state and ligand binding of calcitonin receptor-like receptor. Mol Pharmacol. 1999;55:1054–1059. doi: 10.1124/mol.55.6.1054. [DOI] [PubMed] [Google Scholar]
- Goadsby PJ, Edvinsson L. Human in vivo evidence for trigeminovascular activation in cluster headache. Neuropeptide changes and effects of acute attacks therapies. Brain. 1994;117 Part 3:427–434. doi: 10.1093/brain/117.3.427. [DOI] [PubMed] [Google Scholar]
- Goadsby PJ, Edvinsson L, Ekman R. Vasoactive peptide release in the extracerebral circulation of humans during migraine headache. Ann Neurol. 1990;28:183–187. doi: 10.1002/ana.410280213. [DOI] [PubMed] [Google Scholar]
- Grant AD, Pinter E, Salmon AM, Brain SD. An examination of neurogenic mechanisms involved in mustard oil-induced inflammation in the mouse. Eur J Pharmacol. 2005;507:273–280. doi: 10.1016/j.ejphar.2004.11.026. [DOI] [PubMed] [Google Scholar]
- Gulbenkian S, Uddman R, Edvinsson L. Neuronal messengers in the human cerebral circulation. Peptides. 2001;22:995–1007. doi: 10.1016/s0196-9781(01)00408-9. [DOI] [PubMed] [Google Scholar]
- Hogestatt ED, Andersson KE, Edvinsson L. Mechanical properties of rat cerebral arteries as studied by a sensitive device for recording of mechanical activity in isolated small blood vessels. Acta Physiol Scand. 1983;117:49–61. doi: 10.1111/j.1748-1716.1983.tb07178.x. [DOI] [PubMed] [Google Scholar]
- Jansen-Olesen I, Jorgensen L, Engel U, Edvinsson L. In-depth characterization of CGRP receptors in human intracranial arteries. Eur J Pharmacol. 2003;481:207–216. doi: 10.1016/j.ejphar.2003.09.021. [DOI] [PubMed] [Google Scholar]
- Jansen-Olesen I, Mortensen A, Edvinsson L. Calcitonin gene-related peptide is released from capsaicin-sensitive nerve fibres and induces vasodilatation of human cerebral arteries concomitant with activation of adenylyl cyclase. Cephalalgia. 1996;16:310–316. doi: 10.1046/j.1468-2982.1996.1605310.x. [DOI] [PubMed] [Google Scholar]
- Juhasz G, Zsombok T, Jakab B, Nemeth J, Szolcsanyi J, Bagdy G. Sumatriptan causes parallel decrease in plasma calcitonin gene-related peptide (CGRP) concentration and migraine headache during nitroglycerin induced migraine attack. Cephalalgia. 2005;25:179–183. doi: 10.1111/j.1468-2982.2005.00836.x. [DOI] [PubMed] [Google Scholar]
- Juhasz G, Zsombok T, Modos EA, Olajos S, Jakab B, Nemeth J, et al. NO-induced migraine attack: strong increase in plasma calcitonin gene-related peptide (CGRP) concentration and negative correlation with platelet serotonin release. Pain. 2003;106:461–470. doi: 10.1016/j.pain.2003.09.008. [DOI] [PubMed] [Google Scholar]
- Juul R, Aakhus S, Bjornstad K, Gisvold SE, Brubakk A, Edvinsson L. Calcitonin gene-related peptide (human α-CGRP) counteracts vasoconstriction in human subarachnoid haemorre. Neurosci Lett. 1994;170:67–70. doi: 10.1016/0304-3940(94)90240-2. [DOI] [PubMed] [Google Scholar]
- Juul R, Edvinsson L, Gisvold SE, Ekman R, Brubakk AO, Fredriksen TA. Calcitonin gene-related peptide-LI in subarachnoid haemorrhage in man. Signs of activation of the trigemino-cerebrovascular system. Br J Neurosurg. 1990;4:171–179. doi: 10.3109/02688699008992720. [DOI] [PubMed] [Google Scholar]
- Knudsen G, Juhler M, Paulsen O.Morphology, physiology and pathophysiology of the blood brain barrier Basic Mechanisms of Migraine 1988Elsevier Science Publisher: Amsterdam; 49–60.In: Olesen J, Edvinsson L (eds) [Google Scholar]
- Lassen LH, Haderslev PA, Jacobsen VB, Iversen HK, Sperling B, Olesen J. CGRP may play a causative role in migraine. Cephalalgia. 2002;22:54–61. doi: 10.1046/j.1468-2982.2002.00310.x. [DOI] [PubMed] [Google Scholar]
- McLatchie LM, Fraser NJ, Main MJ, Wise A, Brown J, Thompson N, et al. RAMPs regulate the transport and ligand specificity of the calcitonin-receptor-like receptor. Nature. 1998;393:333–339. doi: 10.1038/30666. [DOI] [PubMed] [Google Scholar]
- Moreno MJ, Abounader R, Hebert E, Doods H, Hamel E. Efficacy of the non-peptide CGRP receptor antagonist BIBN4096BS in blocking CGRP-induced dilations in human and bovine cerebral arteries: potential implications in acute migraine treatment. Neuropharmacology. 2002;42:568–576. doi: 10.1016/s0028-3908(02)00008-4. [DOI] [PubMed] [Google Scholar]
- Muff R, Buhlmann N, Fischer JA, Born W. An amylin receptor is revealed following co-transfection of a calcitonin receptor with receptor activity modifying proteins-1 or -3. Endocrinology. 1999;140:2924–2927. doi: 10.1210/endo.140.6.6930. [DOI] [PubMed] [Google Scholar]
- Olesen J, Diener HC, Husstedt IW, Goadsby PJ, Hall D, Meier U, et al. Calcitonin gene-related peptide receptor antagonist BIBN4096BS for the acute treatment of migraine. N Engl J Med. 2004;350:1104–1110. doi: 10.1056/NEJMoa030505. [DOI] [PubMed] [Google Scholar]
- Oliver KR, Wainwright A, Edvinsson L, Pickard JD, Hill RG. Immunohistochemical localization of calcitonin receptor-like receptor and receptor activity-modifying proteins in the human cerebral vasculature. J Cereb Blood Flow Metab. 2002;22:620–629. doi: 10.1097/00004647-200205000-00014. [DOI] [PubMed] [Google Scholar]
- Petersen KA, Birk S, Doods H, Edvinsson L, Olesen J. Inhibitory effect of BIBN4096BS on cephalic vasodilatation induced by CGRP or transcranial electrical stimulation in the rat. Br J Pharmacol. 2004;143:697–704. doi: 10.1038/sj.bjp.0705966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen KA, Birk S, Lassen LH, Kruuse C, Jonassen O, Lesko L, et al. The CGRP-antagonist, BIBN4096BS does not affect cerebral or systemic haemodynamics in healthy volunteers. Cephalalgia. 2005;25:139–147. doi: 10.1111/j.1468-2982.2004.00830.x. [DOI] [PubMed] [Google Scholar]
- Sexton PM, Albiston A, Morfis M, Tilakaratne N. Receptor activity modifying proteins. Cell Signal. 2001;13:73–83. doi: 10.1016/s0898-6568(00)00143-1. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Satoh S, Ikegaki I, Okada T, Shibuya M, Sugita K, et al. Effects of neuropeptide Y and calcitonin gene-related peptide on local cerebral blood flow in rat striatum. J Cereb Blood Flow Metab. 1989;9:268–270. doi: 10.1038/jcbfm.1989.44. [DOI] [PubMed] [Google Scholar]
- Tajti J, Uddman R, Moller S, Sundler F, Edvinsson L. Messenger molecules and receptor mRNA in the human trigeminal ganglion. J Auton Nerv Syst. 1999;76:176–183. doi: 10.1016/s0165-1838(99)00024-7. [DOI] [PubMed] [Google Scholar]
- Tilakaratne N, Christopoulos G, Zumpe ET, Foord SM, Sexton PM. Amylin receptor phenotypes derived from human calcitonin receptor/RAMP coexpression exhibit pharmacological differences dependent on receptor isoform and host cell environment. J Pharmacol Exp Ther. 2000;294:61–72. [PubMed] [Google Scholar]
- Vater A, Jarosch F, Buchner K, Klussmann S. Short bioactive Spiegelmers to migraine-associated calcitonin gene-related peptide rapidly identified by a novel approach: tailored-SELEX. Nucleic Acids Res. 2003;31:e130. doi: 10.1093/nar/gng130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson DJ, Hargreaves RJ, Hill RG, Shepheard SL. Intravital microscope studies on the effects of neurokinin agonists and calcitonin gene-related peptide on dural vessel diameter in the anaesthetized rat. Cephalalgia. 1997;17:518–524. doi: 10.1046/j.1468-2982.1997.1704518.x. [DOI] [PubMed] [Google Scholar]
- Williamson DJ, Hill RG, Shepheard SL, Hargreaves RJ. The anti-migraine 5-HT(1B/1D) agonist rizatriptan inhibits neurogenic dural vasodilation in anaesthetized guinea-pigs. Br J Pharmacol. 2001;133:1029–1034. doi: 10.1038/sj.bjp.0704162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You J, Johnson TD, Childres WF, Bryan RM., Jr Endothelial-mediated dilations of rat middle cerebral arteries by ATP and ADP. Am J Physiol. 1997;273:H1472–H1477. doi: 10.1152/ajpheart.1997.273.3.H1472. [DOI] [PubMed] [Google Scholar]
- Zumpe ET, Tilakaratne N, Fraser NJ, Christopoulos G, Foord SM, Sexton PM. Multiple ramp domains are required for generation of amylin receptor phenotype from the calcitonin receptor gene product. Biochem Biophys Res Commun. 2000;267:368–372. doi: 10.1006/bbrc.1999.1943. [DOI] [PubMed] [Google Scholar]