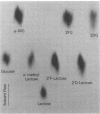
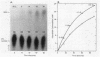
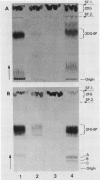
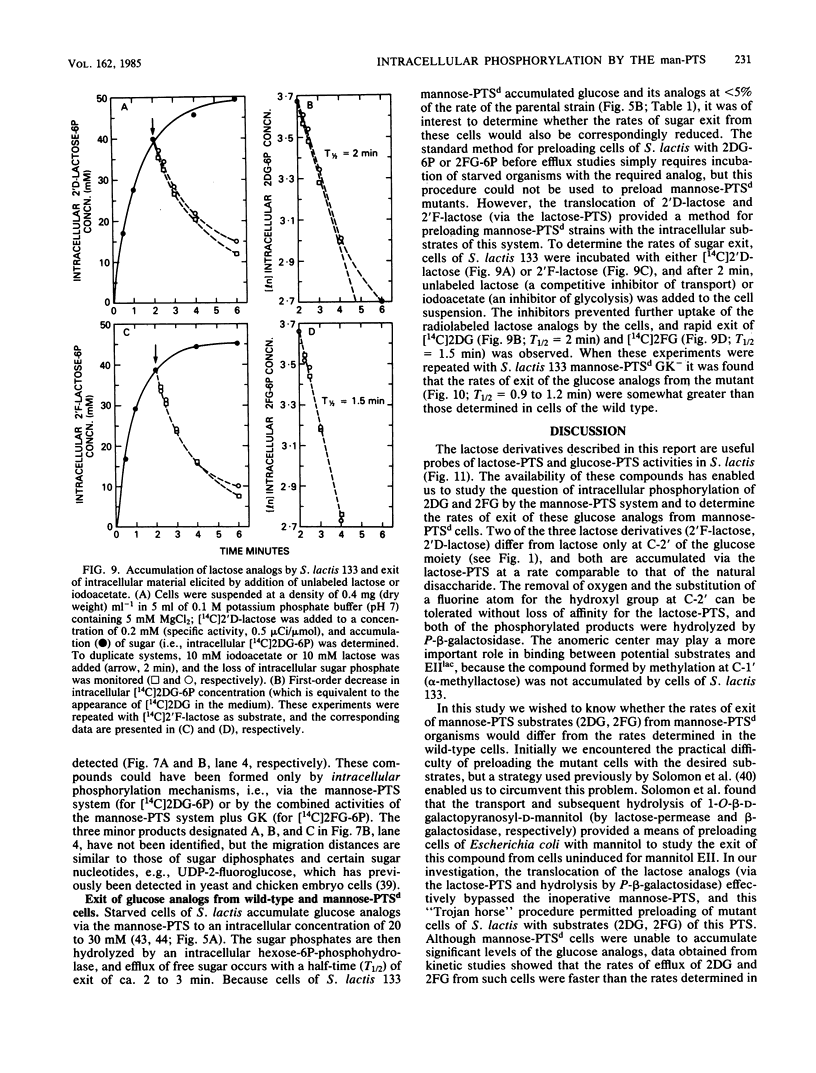
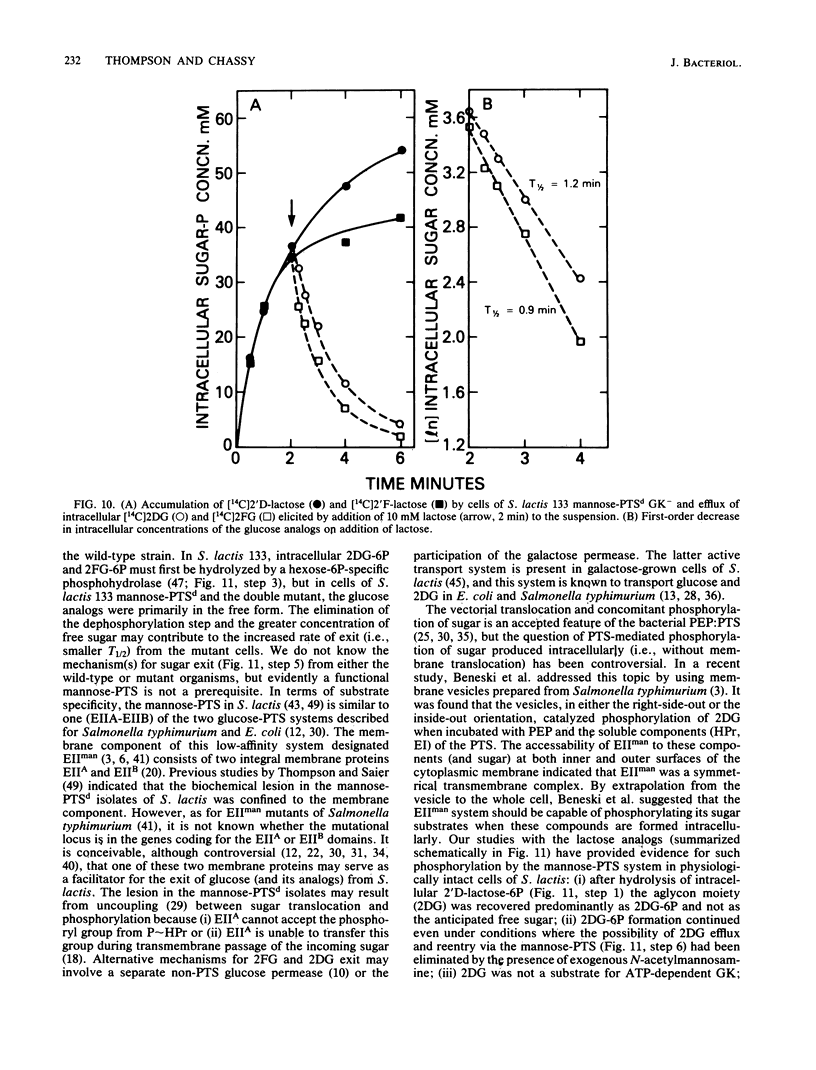
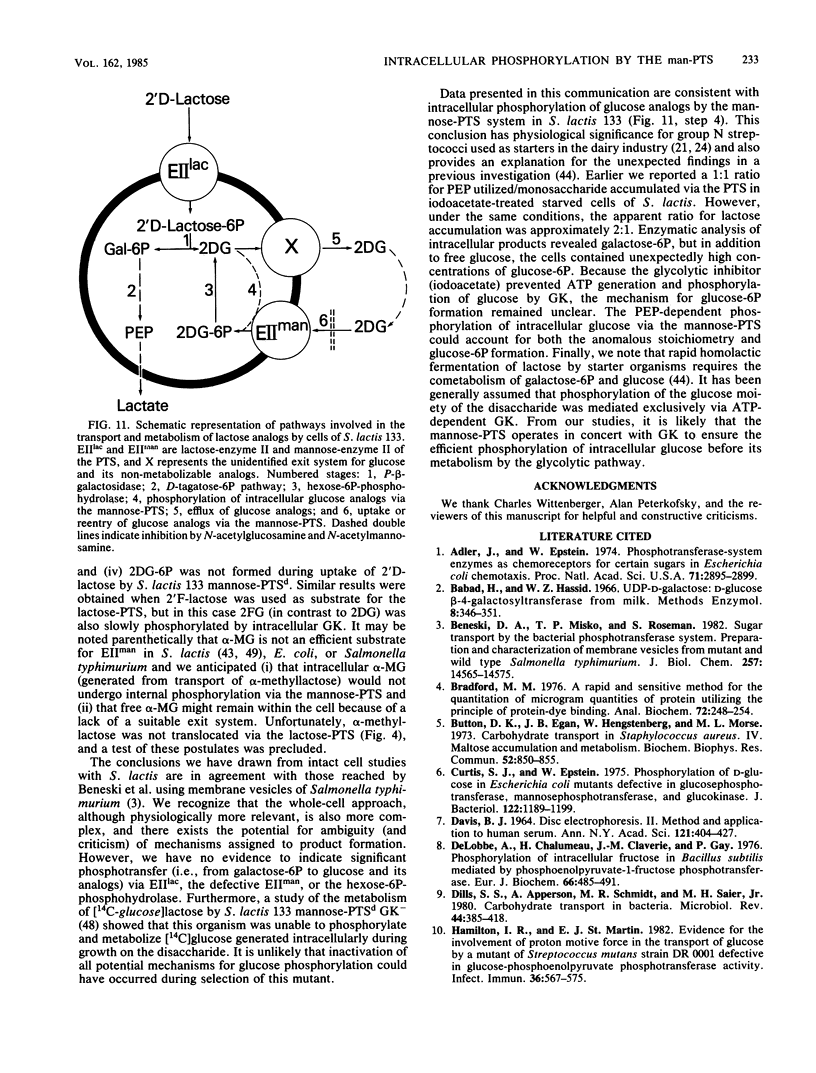
Abstract
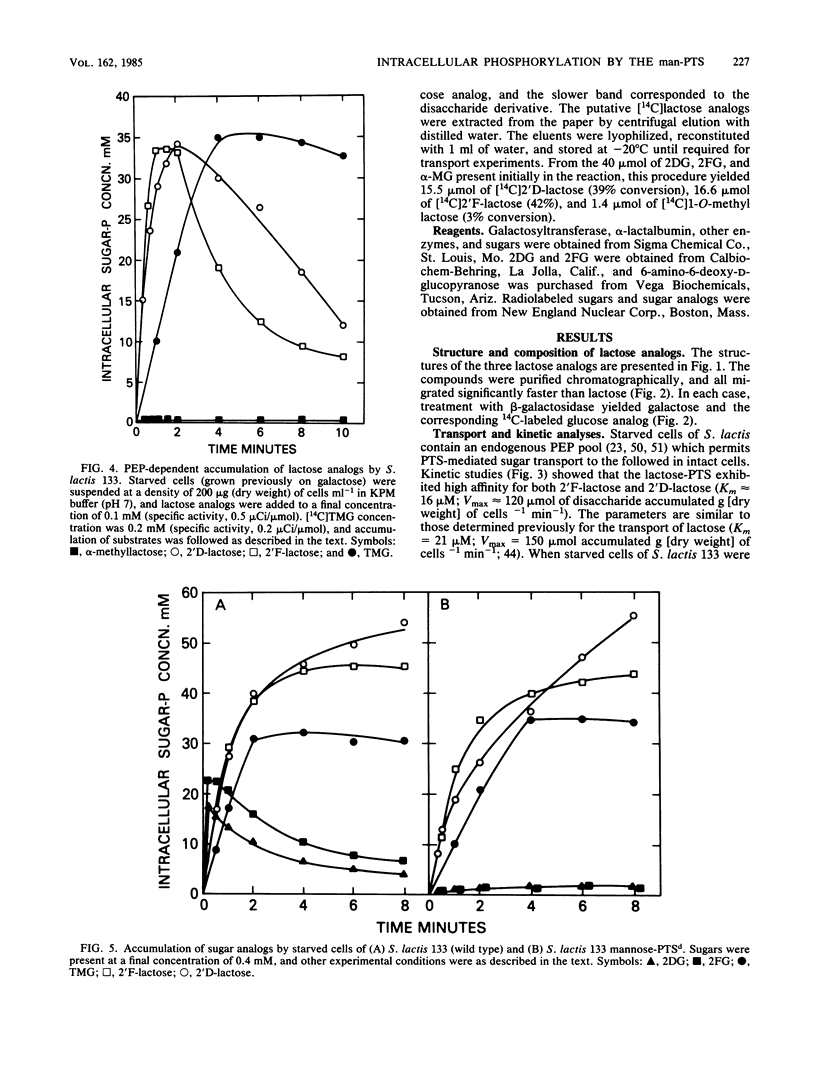
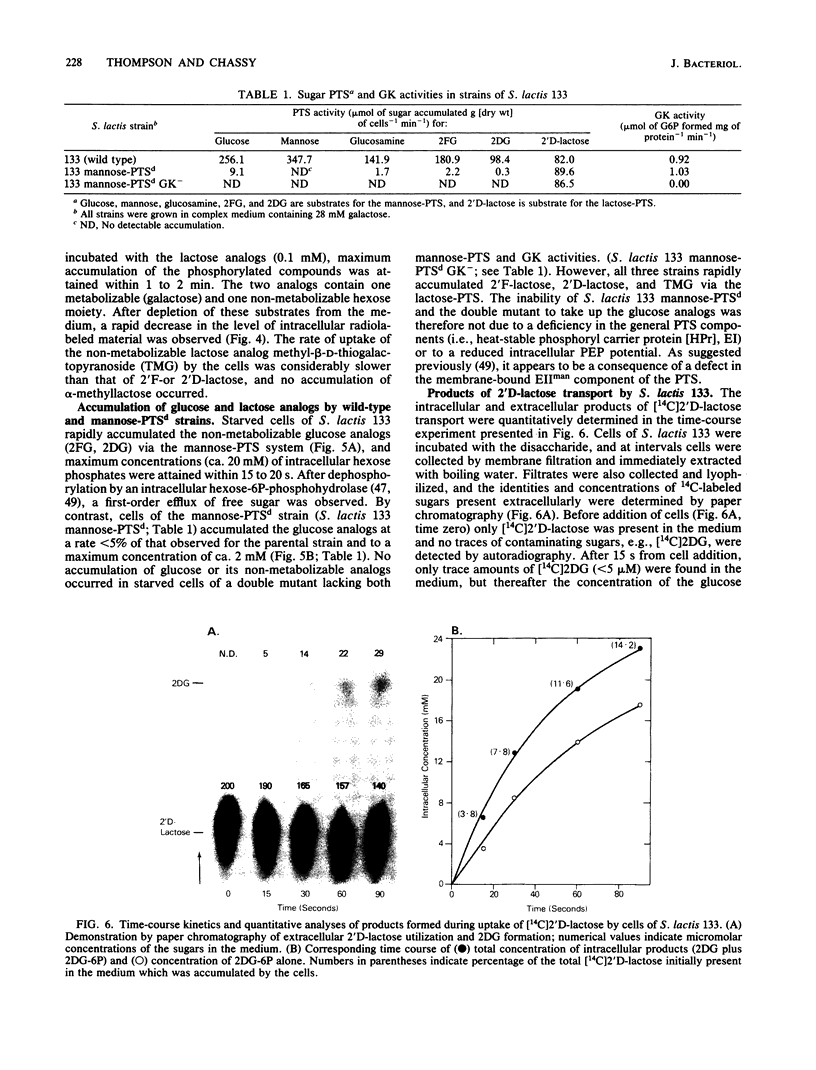
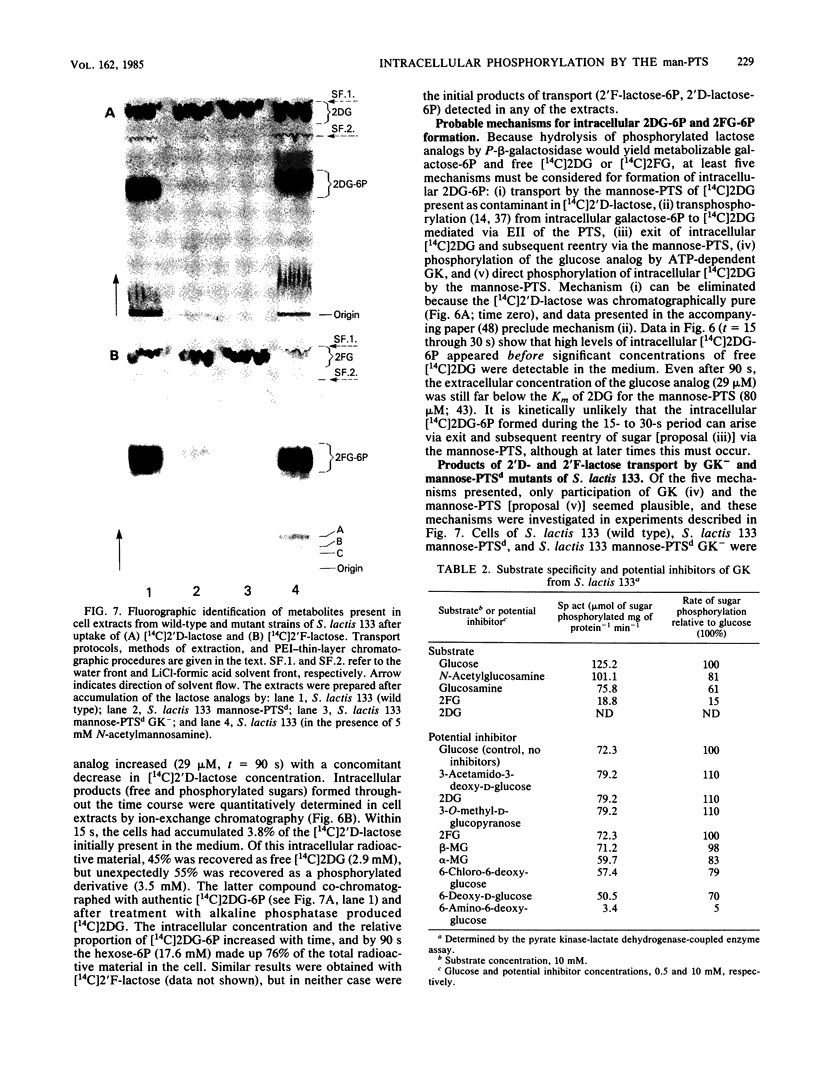
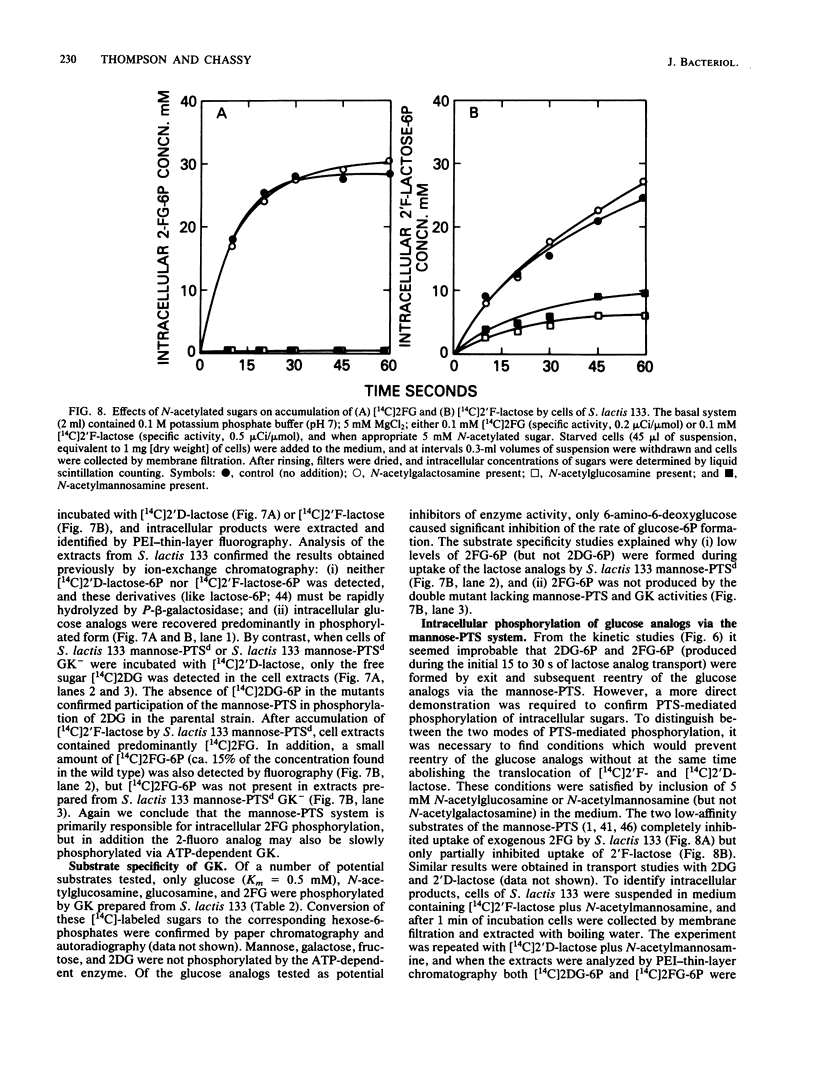
The bacterial phosphoenolpyruvate:sugar-phosphotransferase system (PTS) mediates the vectorial translocation and concomitant phosphorylation of sugars. The question arises of whether the PTS can also mediate the phosphorylation of intracellular sugars. To investigate this possibility in Streptococcus lactis 133, lactose derivatives have been prepared containing 14C-labeled 2-deoxy-glucose (2DG), 2-deoxy-2-fluoro-D-glucose (2FG), or alpha-methylglucoside as the aglycon substituent of the disaccharide. Two of the compounds, beta-O-D-galactopyranosyl-(1,4')-2'-deoxy-D-glucopyranose (2'D-lactose) and beta-O-D-galactopyranosyl-(1,4')-2'-deoxy-2'-fluoro-D-glucopyranose (2'F-lactose), were high-affinity substrates of the lactose-PTS. After translocation, the radiolabeled 2'F-lactose 6-phosphate (2'F-lactose-6P) and 2'D-lactose-6P derivatives were hydrolyzed by P-beta-galactoside-galactohydrolase to galactose-6P and either [14C]2FG or [14C]2DG, respectively. Thereafter, the glucose analogs appeared in the medium, but the rates of sugar exit from mannose-PTS-defective mutants were greater than those determined in the parent strain. Unexpectedly, the results of kinetic studies and quantitative analyses of intracellular products in S. lactis 133 showed that initially (and before exit) the glucose analogs existed primarily in phosphorylated form. Furthermore, the production of intracellular [14C]2FG-6P and [14C]2DG-6P (during uptake of the lactose analogs) continued when the possibility for reentry of [14C]2FG and 2DG was precluded by addition of mannose-PTS inhibitors (N-acetylglucosamine or N-acetylmannosamine) to the medium. By contrast, (i) only [14C]2DG, [14C]2FG, and trace amounts of [14C]2FG-6P were found in cells of a mannose-PTS-defective mutant, and (ii) only [14C]2FG and [14C]2DG were present in cells of a double mutant lacking both mannose-PTS and glucokinase activities. We conclude from these data that the mannose-PTS can effect the intracellular phosphorylation of glucose and its analogs in S. lactis 133.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler J., Epstein W. Phosphotransferase-system enzymes as chemoreceptors for certain sugars in Escherichia coli chemotaxis. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2895–2899. doi: 10.1073/pnas.71.7.2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beneski D. A., Misko T. P., Roseman S. Sugar transport by the bacterial phosphotransferase system. Preparation and characterization of membrane vesicles from mutant and wild type Salmonella typhimurium. J Biol Chem. 1982 Dec 10;257(23):14565–14575. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Button D. K., Egan J. B., Hengstenberg W., Morse M. L. Carbohydrate transport in Staphylococcus aureus. IV. Maltose accumulation and metabolism. Biochem Biophys Res Commun. 1973 Jun 8;52(3):850–855. doi: 10.1016/0006-291x(73)91015-2. [DOI] [PubMed] [Google Scholar]
- Curtis S. J., Epstein W. Phosphorylation of D-glucose in Escherichia coli mutants defective in glucosephosphotransferase, mannosephosphotransferase, and glucokinase. J Bacteriol. 1975 Jun;122(3):1189–1199. doi: 10.1128/jb.122.3.1189-1199.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Delobbe A., Chalumeau H., Claverie J. M., Gay P. Phosphorylation of intracellular fructose in Bacillus subtilis mediated by phosphoenolpyruvate-1-fructose phosphotransferase. Eur J Biochem. 1976 Jul 15;66(3):485–491. doi: 10.1111/j.1432-1033.1976.tb10573.x. [DOI] [PubMed] [Google Scholar]
- Dills S. S., Apperson A., Schmidt M. R., Saier M. H., Jr Carbohydrate transport in bacteria. Microbiol Rev. 1980 Sep;44(3):385–418. doi: 10.1128/mr.44.3.385-418.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton I. R., St Martin E. J. Evidence for the involvement of proton motive force in the transport of glucose by a mutant of Streptococcus mutans strain DR0001 defective in glucose-phosphoenolpyruvate phosphotransferase activity. Infect Immun. 1982 May;36(2):567–575. doi: 10.1128/iai.36.2.567-575.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausman S. Z., Thompson J., London J. Futile xylitol cycle in Lactobacillus casei. J Bacteriol. 1984 Oct;160(1):211–215. doi: 10.1128/jb.160.1.211-215.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson P. J., Giddens R. A., Jones-Mortimer M. C. Transport of galactose, glucose and their molecular analogues by Escherichia coli K12. Biochem J. 1977 Feb 15;162(2):309–320. doi: 10.1042/bj1620309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hüdig H., Hengstenberg W. The bacterial phosphoenolpyruvate dependent phosphotransferase system (PTS): solubilisation and kinetic parameters of the glucose-specific membrane bound enzyme II component of Streptococcus faecalis. FEBS Lett. 1980 May 19;114(1):103–106. doi: 10.1016/0014-5793(80)80869-6. [DOI] [PubMed] [Google Scholar]
- KUNDIG W., GHOSH S., ROSEMAN S. PHOSPHATE BOUND TO HISTIDINE IN A PROTEIN AS AN INTERMEDIATE IN A NOVEL PHOSPHO-TRANSFERASE SYSTEM. Proc Natl Acad Sci U S A. 1964 Oct;52:1067–1074. doi: 10.1073/pnas.52.4.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaback H. R. The role of the phosphoenolpyruvate-phosphotransferase system in the transport of sugars by isolated membrane preparations of Escherichia coli. J Biol Chem. 1968 Jul 10;243(13):3711–3724. [PubMed] [Google Scholar]
- Kashket E. R., Wilson T. H. Isolation and properties of mutants of Escherichia coli with increased phosphorylations of thiomethyl-beta-galactoside. Biochim Biophys Acta. 1969;193(2):294–307. doi: 10.1016/0005-2736(69)90190-4. [DOI] [PubMed] [Google Scholar]
- Kelker N. E., Anderson R. L. Evidence for vectorial phosphorylation of D-fructose by intact cells of Aerobacter aerogenes. J Bacteriol. 1972 Dec;112(3):1441–1443. doi: 10.1128/jb.112.3.1441-1443.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundig W. Molecular interactions in the bacterial phosphoenolpyruvate-phosphotransferase system (PTS). J Supramol Struct. 1974;2(5-6):695–814. doi: 10.1002/jss.400020514. [DOI] [PubMed] [Google Scholar]
- Kundig W., Roseman S. Sugar transport. II. Characterization of constitutive membrane-bound enzymes II of the Escherichia coli phosphotransferase system. J Biol Chem. 1971 Mar 10;246(5):1407–1418. [PubMed] [Google Scholar]
- Lengeler J., Steinberger H. Analysis of the regulatory mechanisms controlling the synthesis of the hexitol transport systems in Escherichia coli K12. Mol Gen Genet. 1978 Aug 17;164(2):163–169. doi: 10.1007/BF00267381. [DOI] [PubMed] [Google Scholar]
- Mason P. W., Carbone D. P., Cushman R. A., Waggoner A. S. The importance of inorganic phosphate in regulation of energy metabolism of Streptococcus lactis. J Biol Chem. 1981 Feb 25;256(4):1861–1866. [PubMed] [Google Scholar]
- Misset O., Blaauw M., Postma P. W., Robillard G. T. Bacterial phosphoenolpyruvate-dependent phosphotransferase system. Mechanism of the transmembrane sugar translocation and phosphorylation. Biochemistry. 1983 Dec 20;22(26):6163–6170. doi: 10.1021/bi00295a019. [DOI] [PubMed] [Google Scholar]
- Peterkofsky A., Gazdar C. Escherichia coli adenylate cyclase complex: regulation by the proton electrochemical gradient. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1099–1103. doi: 10.1073/pnas.76.3.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter E. V., Chassy B. M., Holmlund C. E. Purification and kinetic characterization of a specific glucokinase from Streptococcus mutans OMZ70 cells. Biochim Biophys Acta. 1982 Dec 20;709(2):178–186. doi: 10.1016/0167-4838(82)90459-9. [DOI] [PubMed] [Google Scholar]
- Postma P. W. Defective enzyme II-BGlc of the phosphoenolpyruvate:sugar phosphotransferase system leading to uncoupling of transport and phosphorylation in Salmonella typhimurium. J Bacteriol. 1981 Aug;147(2):382–389. doi: 10.1128/jb.147.2.382-389.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postma P. W. Galactose transport in Salmonella typhimurium. J Bacteriol. 1977 Feb;129(2):630–639. doi: 10.1128/jb.129.2.630-639.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postma P. W., Roseman S. The bacterial phosphoenolpyruvate: sugar phosphotransferase system. Biochim Biophys Acta. 1976 Dec 14;457(3-4):213–257. doi: 10.1016/0304-4157(76)90001-0. [DOI] [PubMed] [Google Scholar]
- Postma P. W., Stock J. B. Enzymes II of the phosphotransferase system do not catalyze sugar transport in the absence of phosphorylation. J Bacteriol. 1980 Feb;141(2):476–484. doi: 10.1128/jb.141.2.476-484.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reizer J., Novotny M. J., Panos C., Saier M. H., Jr Mechanism of inducer expulsion in Streptococcus pyogenes: a two-step process activated by ATP. J Bacteriol. 1983 Oct;156(1):354–361. doi: 10.1128/jb.156.1.354-361.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reizer J., Panos C. Regulation of beta-galactoside phosphate accumulation in Streptococcus pyogenes by an expulsion mechanism. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5497–5501. doi: 10.1073/pnas.77.9.5497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reizer J., Saier M. H., Jr Involvement of lactose enzyme II of the phosphotransferase system in rapid expulsion of free galactosides from Streptococcus pyogenes. J Bacteriol. 1983 Oct;156(1):236–242. doi: 10.1128/jb.156.1.236-242.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robillard G. T. The enzymology of the bacterial phosphoenolpyruvate-dependent sugar transport systems. Mol Cell Biochem. 1982 Jul 7;46(1):3–24. doi: 10.1007/BF00215577. [DOI] [PubMed] [Google Scholar]
- Saier M. H., Jr, Bromberg F. G., Roseman S. Characterization of constitutive galactose permease mutants in Salmonella typhimurium. J Bacteriol. 1973 Jan;113(1):512–514. doi: 10.1128/jb.113.1.512-514.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saier M. H., Jr, Feucht B. U., Mora W. K. Sugar phosphate: sugar transphosphorylation and exchange group translocation catalyzed by the enzyme 11 complexes of the bacterial phosphoenolpyruvate: sugar phosphotransferase system. J Biol Chem. 1977 Dec 25;252(24):8899–8907. [PubMed] [Google Scholar]
- Schanbacher F. L., Ebner K. E. Galactosyltransferase acceptor specificity of the lactose synthetase A protein. J Biol Chem. 1970 Oct 10;245(19):5057–5061. [PubMed] [Google Scholar]
- Schmidt M. F., Biely P., Krátký Z., Schwarz R. T. Metabolism of 2-deoxy-2-fluoro-D-[3H]glucose and 2-deoxy-2-fluoro-D-[3H]mannose in yeast and chick-embryo cells. Eur J Biochem. 1978 Jun 1;87(1):55–68. doi: 10.1111/j.1432-1033.1978.tb12351.x. [DOI] [PubMed] [Google Scholar]
- Solomon E., Miyal K., Lin E. C. Membrane translocation of mannitol in Escherichia coli without phosphorylation. J Bacteriol. 1973 May;114(2):723–728. doi: 10.1128/jb.114.2.723-728.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stock J. B., Waygood E. B., Meadow N. D., Postma P. W., Roseman S. Sugar transport by the bacterial phosphotransferase system. The glucose receptors of the Salmonella typhimurium phosphotransferase system. J Biol Chem. 1982 Dec 10;257(23):14543–14552. [PubMed] [Google Scholar]
- Thompson J. Characteristics and energy requirements of an alpha-aminoisobutyric acid transport system in Streptococcus lactis. J Bacteriol. 1976 Aug;127(2):719–730. doi: 10.1128/jb.127.2.719-730.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J., Chassy B. M., Egan W. Lactose metabolism in Streptococcus lactis: studies with a mutant lacking glucokinase and mannose-phosphotransferase activities. J Bacteriol. 1985 Apr;162(1):217–223. doi: 10.1128/jb.162.1.217-223.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J., Chassy B. M. Intracellular hexose-6-phosphate:phosphohydrolase from Streptococcus lactis: purification, properties, and function. J Bacteriol. 1983 Oct;156(1):70–80. doi: 10.1128/jb.156.1.70-80.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J., Chassy B. M. Novel phosphoenolpyruvate-dependent futile cycle in Streptococcus lactis: 2-deoxy-D-glucose uncouples energy production from growth. J Bacteriol. 1982 Sep;151(3):1454–1465. doi: 10.1128/jb.151.3.1454-1465.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J. Galactose transport systems in Streptococcus lactis. J Bacteriol. 1980 Nov;144(2):683–691. doi: 10.1128/jb.144.2.683-691.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J. In vivo regulation of glycolysis and characterization of sugar: phosphotransferase systems in Streptococcus lactis. J Bacteriol. 1978 Nov;136(2):465–476. doi: 10.1128/jb.136.2.465-476.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J. Lactose metabolism in Streptococcus lactis: phosphorylation of galactose and glucose moieties in vivo. J Bacteriol. 1979 Dec;140(3):774–785. doi: 10.1128/jb.140.3.774-785.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J., Saier M. H., Jr Regulation of methyl-beta-d-thiogalactopyranoside-6-phosphate accumulation in Streptococcus lactis by exclusion and expulsion mechanisms. J Bacteriol. 1981 Jun;146(3):885–894. doi: 10.1128/jb.146.3.885-894.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J., Thomas T. D. Phosphoenolpyruvate and 2-phosphoglycerate: endogenous energy source(s) for sugar accumulation by starved cells of Streptococcus lactis. J Bacteriol. 1977 May;130(2):583–595. doi: 10.1128/jb.130.2.583-595.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J., Torchia D. A. Use of 31P nuclear magnetic resonance spectroscopy and 14C fluorography in studies of glycolysis and regulation of pyruvate kinase in Streptococcus lactis. J Bacteriol. 1984 Jun;158(3):791–800. doi: 10.1128/jb.158.3.791-800.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]