Abstract
The effects of injection of 3,4-methylenedioxymethamphetamine (MDMA), 3,4-methylenedioxyamphetamine (MDA) and N-ethyl-3,4-methylenedioxyamphetamine (MDEA) (all 20 mg kg−1) on blood pressure, heart rate, core body temperature and locomotor activity in conscious rats were investigated using radiotelemetry.
MDMA and MDA produced a prolonged increase in both systolic and diastolic pressures, with MDA causing the most marked rise. MDEA produced a transient but nonsignificant fall in diastolic pressure. The pressor response produced by MDA was accompanied by bradycardia.
All three amphetamine derivatives caused an initial hypothermic response; however, MDA also produced a subsequent hyperthermia, and the speed of recovery from hypothermia was MDA>MDMA>MDEA. The α2A-adrenoceptor antagonist 2-((4,5-dihydro-1H-imidazol-2-yl)methyl)-2,3-dihydro-1-methyl-1H-isoindole (BRL 44408) (1 mg kg−1) prolonged the hypothermic response to MDMA.
Only MDA induced locomotor activity when given alone, but in the presence of BRL 44408, MDMA produced increased locomotor activity.
The order of potency for producing isometric contractions of rat aorta (α1D) and vas deferens (α1A) was MDA>MDMA>MDEA, with MDEA acting as an α1-adrenoceptor antagonist with a pKB of 4.79±0.12 (n=4) in aorta.
The order of potency for prejunctional inhibition of stimulation-evoked contractions in rat vas deferens (α2A-adrenoceptor mediated) was MDA>MDMA>MDEA.
Blood pressure actions of the three amphetamine derivatives may be at least partly due to α1-adrenoceptor agonism or antagonism. The reversal of the hypothermic actions are at least partly due to α2A-adrenoceptor agonism since the hypothermic response was more prolonged with MDEA which exhibits low α2A-adrenoceptor potency, and effects of MDMA after α2A-adrenoceptor antagonism were similar to those of MDEA.
Keywords: MDMA, MDEA, MDA, α1-adrenoceptors, α2A-adrenoceptors, BRL 44408, hypothermia, hyperthermia, rat aorta, rat vas deferens
Introduction
Substance abuse is widespread, especially in the young adult population, and is associated with numerous complications (see Ghuran et al., 2001). Ingestion of amphetamine derivatives has been associated with acute adverse reactions such as hyperthermia, hypertension, tachycardia, acute myocardial infarction and increased motor activities such as jaw clenching (Hegadoren et al., 1999; Cole & Sumnall, 2003a). Consistent with clinical signs, animal studies have also shown increases in blood pressure, disruption in thermoregulation, inhibition of the jaw opening reflex and increased locomotor activity (Gold et al., 1988; Colado et al., 1995; 1999; Daws et al., 2000; Boules et al., 2001; Arrue et al., 2004). Furthermore, it has been demonstrated that repeated treatment with amphetamines in experimental animals can produce either tolerance or sensitization to their behavioural actions (Foltin & Schuster, 1982; Kalivas et al., 1998).
Although much of the research into the actions of amphetamine derivatives has focused on serotonergic and dopaminergic systems, there is evidence also for the involvement of the noradrenergic system, particularly in the periphery. Users of methylenedioxymethamphetamine (MDMA) are reported to have elevated plasma catecholamine levels, which may be due to noradrenergic hyperactivity and may be linked to cardiovascular complications (Stuerenburg et al., 2002). Urinary retention reported with MDMA (McCann et al., 1996) may also involve peripheral α-adrenoceptor-mediated actions. The jaw clenching reported with the use of MDMA (Hayner & McKinney, 1986; McCann et al., 1996) may involve α2-adrenoceptor-mediated inhibition of the jaw opening reflex (Arrue et al., 2004), presumably by a central action. Acute psychiatric complications of MDMA, including panic attacks (McCann et al., 1996), as well as temperature changes (Bexis & Docherty, 2005), may also involve noradrenergic mechanisms.
We have previously demonstrated that MDMA has agonist actions at both α1- and α2-adrenoceptors both in vivo and in vitro (Lavelle et al., 1999; McDaid & Docherty, 2001), that α1- and α2-adrenoceptor actions contribute to the blood pressure effects in rat (McDaid & Docherty, 2001) and that α2A-adrenoceptor agonist actions contribute to the hyperthermia in mice (Bexis & Docherty, 2005). In the present study, using radiotelemetry, we sought to determine whether the differing effects of three amphetamine derivatives, the drugs of abuse MDMA (‘Ecstasy'), N-ethyl-3,4-methylenedioxyamphetamine (MDEA, ‘Eve') and their metabolite 3,4-methylenedioxyamphetamine (MDA, ‘Love') (Hayner & McKinney, 1986; Gouzoulis et al., 1993; Hegadoren et al., 1999), on blood pressure, heart rate, core body temperature and locomotor activity in conscious rats can be explained, at least in part, in terms of differing affinities for α-adrenoceptor subtypes, particularly α1A-, α1D- and α2A-adrenoceptors.
Methods
Male Wistar rats (250–350 g) were obtained from Trinity College Dublin. All studies conform to the Declaration of Helsinki and have been approved by the Department of Health and by the Royal College of Surgeons in Ireland Research Ethics Committee.
In vivo studies: radiotelemetry
Under pentobarbitone sodium (60 mg kg−1, i.p.) anaesthesia, animals were implanted with a radiotelemetric device enabling measurement of blood pressure, heart rate, core body temperature and locomotor activity (TAC50-PXT; Data Sciences International, St Paul, MN, U.S.A.). A cannula was inserted into the abdominal aorta below the renal arteries and fixed into place with tissue adhesive. The implant was then sutured to the abdominal wall. The abdominal wall and skin incision were closed with silk suturing. Motion and temperature sensors built into the device measured locomotor activity and core body temperature. Postoperative analgesia was not given. Animals were allowed to recover for at least 7 days before experiments were performed. Body weight was 303±9 g before surgery and 309±9 g (n=34) 7 days after surgery.
On experimental days, a PhysiolTel-Receiver (model RPC-1) was placed under each individual animal cage, enabling recording of the various parameters. Data signals were acquired from 30 min prior to and for 350 min after drug administration, and analysed using the Dataquest A.R.T.™ Gold Acquisition, Version 2.20. All recordings were obtained at room temperature (22.1±0.1°C).
Animals were injected subcutaneously (into the thigh area) with vehicle (distilled water, 1 ml kg−1), MDMA, MDEA or MDA (all 20 mg kg−1). In preliminary studies, it was found that MDMA (5 mg kg−1) did not produce any marked effects on blood pressure, whereas MDMA (20 mg kg−1) increased blood pressure. In some experiments, BRL 44408 (1 mg kg−1) was administered 30 min before beginning agonist administration. Each animal received a single dose of amphetamine derivative, although a small number of animals (n=6) were employed in vehicle experiments 3 days before receiving the amphetamine derivative.
Rat aorta and vas deferens
Following overdose of CO2 and exsanguination, thoracic aorta or whole vas deferens was removed from untreated rats and placed in Krebs–Henseleit solution of the following composition (mM): NaCl 119; NaHCO3 25; D-glucose 11.1; KCl 4.7; CaCl2 2.5; KH2PO4 1.2; MgSO4 1.0; EDTA 0.03; ascorbic acid 0.28. Propanolol (3 μM) was additionally present in studies of aorta. Aortic rings of 3–5 mm in length, or whole vas deferens, were attached to myograph transducers under 1 g tension in organ baths at 37°C in Krebs–Henseleit solution. After equilibration under resting tension for 30 min, tissues were contracted with KCl (40 mM) (aorta) or noradrenaline (10 μM) (vas deferens). Bathing fluid was changed every 15 min for the next hour. Concentration–response curves to MDMA, MDA and MDEA were then carried out in 0.5 log unit increments, beginning with 0.1 μM. After the response to the last dose of MDMA, MDA or MDEA (100 μM) reached a plateau, tissues were exposed to phenylephrine (10 μM) (aorta) or noradrenaline (10 μM) (vas deferens) to assess the maximum response of the vessel.
In some experiments, the antagonist actions of MDEA (100 μM) were studied in rat aorta. Following a concentration–response curve to noradrenaline, tissues were washed every 15 min for 1 h, followed by a second hour in which tissues were exposed to MDEA or vehicle. A second concentration–response curve to noradrenaline in the continuing presence of MDEA or vehicle was carried out. The shift in noradrenaline potency produced by MDEA was corrected for the shift in noradrenaline potency, which occurred in a paired vehicle experiment. Potency of MDEA was calculated as a pKB from the equation:
where [B] is the antagonist concentration (in this case, 4.0, −logM) and DR is the agonist (in this case noradrenaline) dose ratio of potencies obtained in the second and first concentration–response curves.
Rat vas deferens: nerve-mediated contractions
Epididymal portions of rat vas deferens were obtained. Tissues were attached to myograph transducers under 1 g tension in organ baths at 37°C in Krebs–Henseleit solution of the following composition: (mM): NaCl 119; NaHCO3 25; D-glucose 11.1; KCl 4.7; CaCl2 2.5; KH2PO4 1.2; MgSO4 1.0; EDTA 0.03, ascorbic acid 0.28. Tissues were placed between platinum electrodes and stimulated every 5 min with a single stimulus (0.5 ms pulses, supramaximal pulses) to produce isometric contractions, and nifedipine (10 μM) was present to block the non-noradrenergic component of the twitch. Agonists or vehicle were added cumulatively in 0.5 log unit increments at 5 min intervals. An isometric twitch was obtained following 5 min exposure to each agonist concentration, or following exposure to the vehicle.
Radioligand-binding studies
Preparation of rat kidney membranes was carried out as described in Connaughton & Docherty (1990), and preparation of rat vasa deferentia and submandibular gland membranes was as described for rat kidney. Membranes of Sf9 cells expressing human recombinant α2C-adrenoceptors were purchased from Research Biochemicals. The resultant pellets were used immediately or stored at −20°C for later use. Pellets were reconstituted in five volumes (submandibular), 10 volumes (kidney, vas deferens) or 25 volumes (Sf9 cells) of incubation buffer.
In saturation experiments, aliquots of membrane suspension were incubated with various concentrations of ([3H]prazosin (specific activity: 70–87 Ci mmole−1, New England Nuclear) or [3H]yohimbine (specific activity: 81 Ci mmole−1, Amersham) at 25°C (rat vas deferens: 0.1–20 nM; rat kidney: 0.5–30 nM; Sf9 cells: 0.2–20 nM; rat submandibular gland: 1.0–40 nM; incubation buffer: Tris-HCl 50 mM, EDTA 5 mM, pH 7.4 at 25°C). In competition studies, ([3H]prazosin (2 nM) or [3H]yohimbine (5 or 10 nM) was incubated with competing ligands in concentrations from 0.1 to 1 mM in 0.5 log unit increments for 30 min. Nonspecific binding was determined in the presence of phentolamine (10 μM). Specific binding was 70–90% of total binding at the concentration used in displacement experiments. Assays were terminated by washing with ice-cold incubation buffer, followed by rapid vacuum filtration through Whatman GF/C filters, using a Brandel Cell Harvester. Radioactivity retained on filters was determined by liquid scintillation spectroscopy.
The inhibition constant (Ki) for inhibition of radiolabelled ligand binding was determined from the Cheng & Prussoff Equation (1973):
where IC50 is the concentration of competing ligand that inhibits radioligand-specific binding by 50%, KD is the dissociation constant for the radioligand prazosin (rat vas deferens: 0.33±0.12 nM, n=6) or for the radioligand yohimbine (rat kidney: 8.80±0.62 nM, n=7; human recombinant α2C-adrenoceptors: 8.7±3.5 nM, n=3; rat submandibular gland: 23.7±2.0 nM, n=4) and 3H is the concentration of tritiated prazosin (2 nM) or yohimbine (5 nM: rat kidney; 10 nM: Sf9 cells and rat submandibular gland).
Drugs
BRL 44408 (2-((4,5-dihydro-1H-imidazol-2-yl)methyl)-2,3-dihydro-1-methyl-1H-isoindole; Tocris, UK); Nifedipine, phenylephrine hydrochloride, prazosin hydrochloride, propranolol hydrochloride, phentolamine (Sigma, Dublin, Ireland); MDMA, MDA and MDEA (Sigma and NIDA Drug Supply Program).
Drugs were dissolved in distilled water.
Statistics
Results are expressed as means±s.e.m. The minimum level for statistical significance was P<0.05. Area under the curve for heart rate, systolic and diastolic pressure, core body temperature and locomotor activity were calculated from the preinjection baseline using the trapezoidal method. All data were analysed and compared with the effects of vehicle using one-way ANOVA with Bonferroni multiple comparison test.
Results
Cardiovascular responses to MDMA analogues in conscious rats
Mean blood pressure
Systolic, diastolic and mean blood pressures were recorded, but except where changes in systolic or diastolic differed, mean blood pressure is discussed. The baseline value of mean blood pressure in conscious rats prior to treatment with vehicle, MDMA, MDA, MDEA and BRL 44408 were not significantly different. (Table 1). Figure 1a shows the effect of different amphetamine derivatives on mean blood pressure.
Table 1.
Baseline mean arterial pressure, heart rate, core body temperature and locomotor activity in rats prior to treatment with vehicle, amphetamine derivatives or BRL 44408 (BRL)
| Vehicle | MDMA | MDEA | MDA | BRL vehicle | BRL MDMA | |
|---|---|---|---|---|---|---|
| Mean arterial pressure (mmHg) | 105±3 | 103±2 | 99±3 | 98±1 | 107±3 | 108±2 |
| Heart rate (min−1) | 392±12 | 358±19 | 379±11 | 355±12 | 348±9 | 349±7 |
| Body temperature (°C) | 38.3±0.1 | 38.1±0.1 | 37.9±0.1* | 37.8±0.1* | 37.8±0.1 | 37.8±0.1 |
| Locomotor activity (counts min−1) | 5.4±2.4 | 1.1±0.5 | 1.2±0.5 | 1.0±0.4 | 2.7±1.6 | 6.0±3.1 |
Values are the means±s.e.m. (n=6–9).
Denotes significantly different from vehicle, P<0.05.
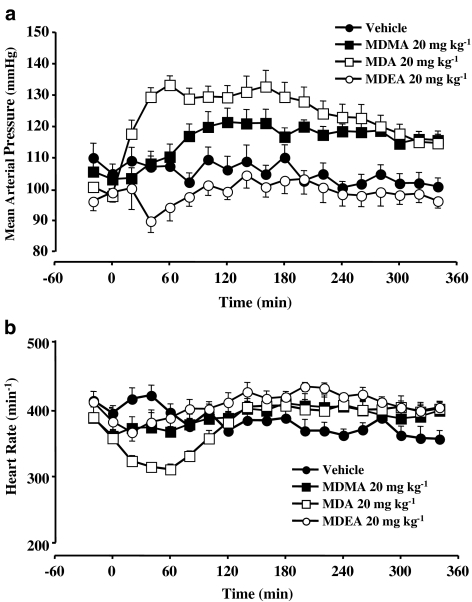
Figure 1.
(a) Mean blood pressure and (b) heart rate recordings in conscious rats before and after the administration of vehicle, MDMA (20 mg kg−1), MDA (20 mg kg−1) or MDEA (20 mg kg−1) at room temperature. Data points represent means±s.e.m. from six to nine rats.
MDMA and MDA, but not MDEA, significantly increased blood pressure, but the pattern of the pressure response for each drug was different (Figure 1a and Table 2). MDEA treatment resulted in an early drop in diastolic pressure (reaching a minimum pressure at 40 min), although this did not reach significance, followed by the return of pressure to values similar to those seen with rats treated with vehicle. MDA produced an early increase in mean blood pressure reaching a peak pressure by 40–60 min after administration. Once the peak pressure was reached, there was a gradual decline in pressure with time but the mean pressure still remained significantly higher than the vehicle group up to 300 min, although both systolic and diastolic pressure remained significantly higher for the entire recording period. MDMA caused a gradual increase in mean blood pressure reaching significance in the time frame 121–300 min (Figure 1a and Table 2), although both systolic and diastolic pressure remained significantly higher also for the time frame 121–350 min. The α2A-adrenoceptor antagonist BRL 44408 (1 mg kg−1) followed by vehicle did not significantly affect mean pressure, and BRL 44408 did not affect the time course or magnitude of the response to MDMA (see Table 2). For clarity, experiments with BRL 44408 are omitted from Figure 1.
Table 2.
Area under the curve (AUC) values for mean arterial blood pressure, at time intervals 0–120, 121–300 and 301–350 min for all treatment groups after injection
| Time interval (min) | Vehicle | MDMA | MDEA | MDA | BRL vehicle | BRL MDMA |
|---|---|---|---|---|---|---|
| 0−120 | 12,829±392 | 13,557±377 | 11,691±393 | 15,076±282* | 12,698±315 | 12,859±376 |
| 121−300 | 18,775±490 | 21,487±514* | 18,056±585 | 22,719±744* | 18,380±521 | 21,480±280* |
| 301−350 | 4933±211 | 5609±252 | 4668±166 | 5571±222 | 5046±156 | 6037±109* |
Values are mean±s.e.m. (n=6–9).
Denotes significantly different from vehicle, P<0.05.
Heart rate
Baseline values were similar between all treatment groups when compared to the vehicle group (Table 1). MDMA did not have a significant effect on heart rate. MDA elicited an initial significant bradycardia, and MDEA elicited a late tachycardia, which reached significance as compared to the effects of vehicle (Figure 1b). However, this late tachycardia to MDEA was similar to the effects seen for MDMA and MDA although the effects of the latter two compounds did not reach significance.
Effects of MDMA analogues on core body temperature
The resting body temperature values of animals from MDEA and MDA groups, but not the MDMA or BRL 44408 groups, prior to drug administration, were significantly different from the vehicle group (Table 1).
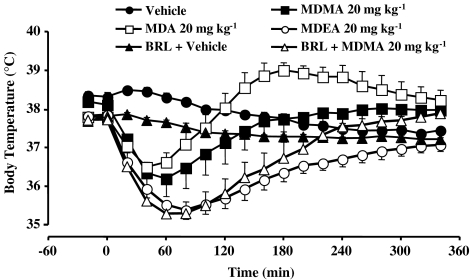
The acute effects of MDMA, MDA and MDEA on rat core body temperature are shown in Figure 2. Administration of MDMA produced hypothermia, which gradually returned to baseline levels by 300 min. The onset of hypothermia was seen as early as 10 min after the injection of MDMA and lasted for 60 min before temperature began to rise again. MDA produced a biphasic response consisting of an initial hypothermia followed by hyperthermia. The minimum core temperature was reached 50 min after the injection of MDA, and the maximum core temperature was reached 180 min after administration of the drug. MDEA caused hypothermia and after reaching a minimum core temperature between 80 and 90 min after administration, temperature began to gradually rise reaching vehicle values by the end of the recording period.
Figure 2.
Core temperature versus time responses for rats after the administration of vehicle, BRL 44408 (1 mg kg−1) and vehicle, MDMA (20 mg kg−1) alone or following BRL 44408, MDA (20 mg kg−1) or MDEA (20 mg kg−1) at room temperature. Data points represent means±s.e.m. from six to nine rats.
The α2A-adrenoceptor antagonist BRL 44408 (1 mg kg−1) significantly potentiated and prolonged the hypothermic component to the response to MDMA, so that the response to MDMA closely matched to that of MDEA up to about 300 min (Figure 2).
Effects of MDMA analogues on locomotor activity
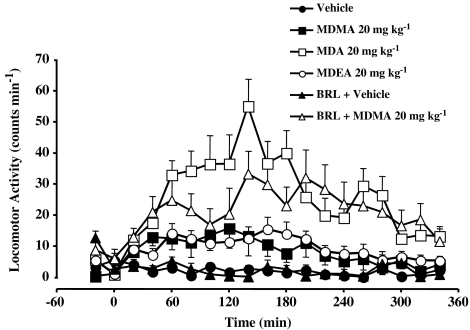
The baseline values for locomotor activity in rats prior to treatment with vehicle, MDMA, MDA, MDEA or BRL 44408 were not significantly different (Table 1). When given alone, only MDA produced a significant increase in locomotor activity as compared to the effects of vehicle (Figure 3, Table 3). However, in the presence of BRL 44408, MDMA produced a significant locomotor response (Figure 3, Table 3).
Figure 3.
Locomotor activity versus time responses for rats after the administration of vehicle, BRL 44408 (1 mg kg−1) and vehicle, MDMA (20 mg kg−1) alone or following BRL 44408, MDA (20 mg kg−1) or MDEA (20 mg kg−1) at room temperature. Data points represent means±s.e.m. from six to nine rats.
Table 3.
AUC values for locomotor activity for MDMA, MDEA and MDA, as well as BRL 44408/vehicle (BRL veh) and BRL44408/MDMA (BRL MDMA), 0–350 min after injection
| AUC | |
|---|---|
| Vehicle | 793±78 |
| MDMA | 2923±742 |
| MDEA | 3316±631 |
| MDA | 9126±876* |
| BRL veh | 771±57 |
| BRL MDMA | 7596±1970* |
Values are the means±s.e.m. (n=6–9).
Denotes significantly different from relevant vehicle, P<0.05.
In vitro studies: contractions of rat aorta and vas deferens
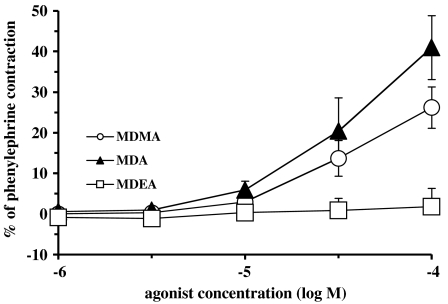
In rat aortic rings, phenylephrine (10 μM) produced a contraction of 1.15±0.08 g (n=18). In rat aortic rings, MDA and MDMA produced concentration-dependent increases in contraction which reached 41.0±7.9% (n=4) and 26.5±5.1% (n=5) of the phenylephrine contraction, respectively (Figure 4). MDEA (up to 100 μM) did not significantly affect baseline tension. MDEA (100 μM) acted as an antagonist of noradrenaline-evoked contractions, shifting noradrenaline potency without significantly affecting the maximum contraction. A pKB of 4.79±0.12 (n=4) was obtained for MDEA.
Figure 4.
Concentration contractile–response curves for presumed α1D-adrenoceptor agonist actions of MDEA, MDA and MDMA in aortae from Wistar rats. Each group consisted of five to seven rats. Values are means±s.e.m.
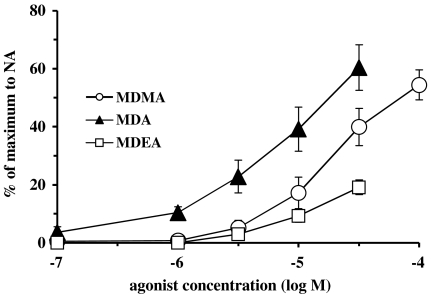
In rat whole vas deferens, noradrenaline (10 μM) produced a contraction of 1.72±0.09 g (n=12). In rat whole vas deferens, all three agonists produced contractions but the order of potency was MDA>MDMA>MDEA (Figure 5).
Figure 5.
Concentration contractile–response curves for presumed α1A-adrenoceptor agonist actions of MDEA, MDA and MDMA in whole vas deferens from Wistar rats. Each group consisted of four to five rats. Values are means±s.e.m.
Epididymal portions of rat vas deferens: nerve-mediated responses
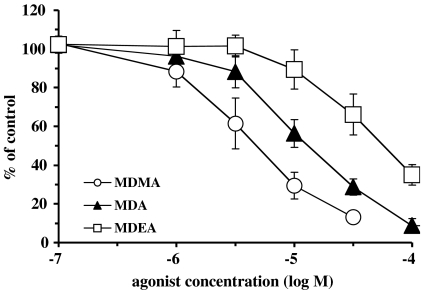
In epididymal portions of rat vas deferens, the initial biphasic twitch to a single electrical stimulus was reduced to a monophasic response by nifedipine (10 μM), which eliminates the first (non-noradrenergic) phase, leaving the second (noradrenergic) phase. Under these conditions, the twitch to a single stimulus was 0.80±0.05 g (n=68), and agonists reduced the size of the twitch in a concentration-dependent manner (Figure 6), with pD2 values (concentration producing 50% inhibition, −log M) of 5.88±0.16 (n=4), 5.46±0.11 (n=8) and 4.91±0.20 (n=4) for MDMA, MDA and MDEA, respectively (MDEA was significantly less potent than MDMA, P<0.05). The order of potency was MDMA>MDA>MDEA.
Figure 6.
Concentration inhibitory–response curves for presumed α2A-adrenoceptor agonist actions of MDEA, MDA and MDMA at inhibiting contractions to a single electrical stimulus in epididymal portions of rat vas deferens in the presence of nifedipine (10 μM). Each group consisted of four to eight rats. Values are means±s.e.m. MDMA data are taken from Lavelle et al. (1999).
Ligand-binding studies
The affinities of MDMA, MDEA and MDA for α1A-adrenoceptor ligand-binding sites were examined in competition experiments employing [3H]prazosin-labelled membrane preparations of rat vas deferens. The pKi values obtained are shown in Table 4. MDEA showed a higher affinity for α1A-adrenoceptors when compared with MDMA.
Table 4.
Affinity of MDMA, MDEA and MDA for α1A-adrenoceptor ligand-binding sites in rat vas deferens, α2A-adrenoceptor ligand-binding sites in rat submandibular gland, α2B-adrenoceptor ligand-binding sites in rat kidney and α2C-adrenoceptor ligand-binding sites expressed in sf9 cell membranes
| Drug pKi (−log M) | 1A | 2A | 2B | 2C |
|---|---|---|---|---|
| MDMAa | 4.34±0.32 | 5.31±0.14 | 5.14±0.16 | 5.11±0.05 |
| MDEA | 5.39±0.04* | 4.69±0.15* | 4.88±0.31 | 4.81±0.12 |
| MDA | 4.95±0.19 | 4.97±0.14 | 5.06±0.07 | 4.69±0.07 |
Values are the mean pKi (−log M)±s.e.m. (n=4).
P<0.05 denotes significantly different from MDMA.
MDMA data taken from Lavelle et al. (1999).
The affinities of MDMA, MDEA and MDA for α2A-, α2B- and α2C-adrenoceptor ligand-binding sites were examined in competition experiments employing [3H]yohimbine-labelled membrane preparations (Table 4). The three agents showed no clear selectivity between subtypes of α2-adrenoceptor ligand-binding sites, but MDEA showed significantly lower affinity than MDMA for the α2A-adrenoceptor ligand-binding site (Table 4).
Discussion
In this study, we have investigated the effects of administration of amphetamine derivatives on cardiovascular responses, body temperature and locomotor activity in conscious rats, and how differing actions may be explained in terms of differing affinities for α-adrenoceptors. In comparison to MDMA (and MDA), MDEA has significantly higher affinity for α1A- and lower affinity for α2A-adrenoceptors. However, despite this higher affinity for α1A-adrenoceptors, MDEA had low potency at producing contractions of rat vas deferens (predominantly α1A), suggesting low efficacy, and failed to contract rat aorta (predominantly α1D), acting as an antagonist in the present study. Hence, the potency order for α1-adrenoceptor agonism was MDA>MDMA>>MDEA. In terms of prejunctional α2A-adrenoceptor potency in rat vas deferens, the potency order agreed with the ligand-binding affinity order of MDMA>MDA>MDEA.
In anaesthetized rat and mouse, MDMA (5–20 mg kg−1) produces a biphasic pressor/depressor effect on blood pressure (McDaid & Docherty, 2001; Vandeputte & Docherty, 2002). In conscious rat, MDMA (20 mg kg−1) produced double the hyperthermia seen with 10 mg kg−1 (Colado et al., 1995), and, in our studies of conscious mice, MDMA (20 mg kg−1) produced a hyperthermia (Bexis & Docherty, 2005). Hence, a dose of 20 mg kg−1 was chosen for all three agonists to allow comparison of adrenoceptor agonist actions. Other studies of hyperthermia in rat have utilized doses of up to 40 mg kg−1 (Sprague et al., 2004). In human studies of MDMA, doses ranging between 1.75 and 4.18 mg kg−1 have been administered (see McCann et al., 1996).
Cardiovascular responses
MDMA and MDA caused both systolic and diastolic pressure to increase. These results are consistent with human studies that have also shown increases in arterial pressure with the above compounds (Gouzoulis et al., 1993; Vollenweider et al., 1998; Hegadoren et al., 1999). MDEA had no significant effect on systolic pressure but transiently (admittedly, nonsignificantly) reduced diastolic pressure. MDMA has been shown to have actions as an agonist at both α1- and α2- adrenoceptors in addition to 5-HT-2 receptors to raise blood pressure in the anaesthetized rat (McDaid & Docherty, 2001), and in the present study, we have demonstrated that MDMA and MDA caused a dose-dependent contraction in the thoracic aorta and vas deferens. MDEA weakly contracted the rat vas deferens but failed to contract the rat aorta and was found to act as an α1D-adrenoceptor antagonist in aorta (present results). The blood pressure actions of these agents are consistent with their actions at α1-adrenoceptors: MDA more potent than MDMA as an agonist, and MDEA with low efficacy or acting as an antagonist. However, it is likely that other receptors and actions are involved in the pressor actions, and the response is likely to be as complex as the situation found in the anaesthetized rat, in which peripheral 5-HT-2 receptors are likely to be involved (McDaid & Docherty, 2001), so that this was not examined further.
In addition, the amphetamine derivatives have been shown to potentiate the contractile actions of noradrenaline (Fitzgerald & Reid, 1994; Al-Sahli et al., 2001; Cleary et al., 2002), involving competitive blockade of the noradrenaline transporter (Cleary & Docherty, 2003), and an ability to displace noradrenaline from peripheral noradrenergic nerve terminals (Fitzgerald & Reid, 1994; Lavelle et al., 1999) and the adrenal gland (O'Cain et al., 2000). MDEA is less potent in inhibiting uptake of NA in the left ventricle when compared to MDA and MDMA (Cleary & Docherty, 2003), and it may also have a postjunctional cardiac depressant action (Cleary et al., 2002). Hence, the pressor responses elicited by the amphetamine derivatives can be attributed to direct and indirect sympathomimetic actions in the periphery, and in both cases MDEA should be less active.
The major effect seen on heart rate in conscious rats was that MDA elicited an initial bradycardia. All three agents tended to increase heart rate later, but this reached significance only for MDEA. These changes may be, at least, partly baroreflex responses to changes in blood pressure, given that MDA produced the largest increase in blood pressure and MDEA produced a fall. In conscious animals and humans, MDMA has been shown to produce no change in heart rate, bradycardia (at high doses) or tachycardia (O'Cain et al., 2000; Pedersen & Blessing, 2001; Badon et al., 2002; Cole & Sumnall, 2003a). In humans, both MDA and MDEA increased heart rate (Gouzoulis et al., 1993; Hegadoren et al., 1999).
Core body temperature
In the present study, MDMA, MDEA and MDA all produced initially a hypothermic response. While the hypothermic response to MDEA was prolonged, the hypothermic response to MDMA was abolished by approximately 2 h, and the hypothermia to MDA reversed to a hyperthermia. At least with regards to MDMA, hypothermia at room temperature has been noted by other authors (Malberg & Seiden 1998; Malpass et al., 1999; Daws et al., 2000). However, hyperthermia has also been reported to MDMA both in the Dark Agouti (Colado et al., 1995; 1999) and Wistar rat (O'Loinsigh et al., 2001). MDEA has also been shown to produce a hyperthermic response both in the Dark Agouti (Colado et al., 1999) and Wistar rat (O'Loinsigh et al., 2001). The Dark Agouti lacks the equivalent enzyme to the human CYP2D6, involved in the metabolism of MDMA and MDEA (Barham et al., 1994). The discrepancies seen in the literature may be due to the different recording procedures, ambient temperature and species or strains used.
The mechanisms of the hypothermic and hyperthermic responses produced by the amphetamine derivatives are still not clear. The serotonergic, noradrenergic and dopaminergic neurotransmitter systems have all been implicated in the mediation of hypothermia. D-fenfluramine elicits a hypothermic response in rats under normal laboratory temperature (20–24°C) and this response was blocked by a serotonin reuptake inhibitor, 5-HT1a and 5-HT2c receptor and D2-receptor antagonists but not by the α2-adrenoceptor antagonist, yohimbine (Cryan et al., 2000). D-fenfluramine has been shown to induce both 5-HT and dopamine release and increase extracellular 5-HT and dopamine levels in the brain. The increase in extracellular dopamine concentration involves 5-HT receptors in addition to action at dopamine uptake sites (Cryan et al., 2000). Since it has also been shown that MDMA, MDEA and MDA increase extracellular levels of both 5-HT and dopamine in the brain, and since the increases in extracellular dopamine concentrations seen with MDMA treatment involve both dopamine uptake sites and an interaction between 5-HT and dopamine, it could be postulated that MDMA, MDEA and MDA may also produce hypothermia via the above receptors.
As with the hypothermic response, the mechanisms involved in producing a hyperthermic response are also unclear. It has been suggested that a central dopamine and 5-HT interaction is involved and that 5-HT2A and dopamine D1 receptors are involved in the mediation of hyperthermia (Sugimoto et al., 2000; 2001). In addition, cutaneous vasoconstriction has been shown to contribute, in part, to the induction of hyperthermia (Pedersen & Blessing, 2001). In the present study, MDMA did not produce hyperthermia. In this respect, this study does not replicate the human ‘rave' situation, but it is noticeable that the metabolite of MDMA, MDA, did produce hyperthermia in our study, whereas the other agents caused only hypothermia.
The role of α-adrenoceptors in the temperature responses to MDMA has not been widely studied. Clonidine, an α2-adrenoceptor agonist, induces a hypothermia by action at central α2A-adrenoceptors (Zarrindast et al, 2003). Despite this, α2A-adrenoceptor actions of MDMA are hyperthermic in the mouse (Bexis & Docherty, 2005), since α2A-adrenoceptor knockout or the selective alpha2A-adrenoceptor antagonist BRL 44408 (Young et al., 1989; see Docherty, 1998; Guimaraes & Moura, 2001) caused a hypothermia to MDMA. Hence, in the mouse, α2A-adrenoceptor activation prevents MDMA from inducing an initial hypothermic response (Bexis & Docherty, 2005). The present results in rat are similar in that BRL 44408 prolonged the hypothermic actions of MDMA, so that the effects of MDMA resembled those to MDEA, which has low α2A-adrenoceptor affinity and potency. It can be suggested that under conditions of increased extracellular levels of 5-HT, dopamine and noradrenaline produced by MDMA, concomitant activation of the α2A-adrenoceptor results in a component of the hyperthermic response. In the absence of α2A-adrenoceptors, this component of the hyperthermia is absent, and the resultant changes in levels of dopamine, 5-HT and possibly other neurotransmitters lead to the hypothermic component seen in α2A-KO mice.
Locomotor activity
All three amphetamine derivatives tended to cause an increase in locomotor activity, but this reached significance only for MDA. Evidence suggests that MDMA-induced hypermotility involves activation of multiple 5-HT receptors and an interaction of dopamine and 5-HT (Bankson & Cunningham, 2001; Cole & Sumnall, 2003b), which may also be the case with MDA. However, the α2A-adrenoceptor antagonist BRL 44408 increased the locomotor actions of MDMA so that they became significantly different from the effects of vehicle. There is evidence that α2A-adrenoceptors mediate inhibition of locomotion (Lahdesmaki et al., 2003), and the current results suggest that locomotor actions of MDMA are indeed inhibited by α2A-adrenoceptor agonist actions.
Actions of MDA
One of the major findings of this study is that MDA has overall more marked cardiovascular, temperature and locomotor actions than the other agents examined. MDA, as well as being a drug of abuse in its own right, is a metabolite both of MDMA (de la Torre et al., 2000; Cole & Sumnall, 2003b) and MDEA (Ensslin et al., 1996). Plasma half-lives of around 2.5 h are reported for MDMA in rats, with the major metabolic product being MDA (see Cole & Sumnall, 2003b).
Conclusion
The present study has demonstrated that MDMA, MDA and MDEA alter cardiovascular function, thermoregulation and locomotor activity at least partly by α-adrenoceptor actions. Blood pressure actions of the three amphetamine derivatives may be, at least, partly due to α1-adrenoceptor agonism or antagonism. There is an α2A-adrenoceptor agonist component to prevent an increase in locomotor activity. The reversal of the hypothermic actions are, at least, partly due to α2A-adrenoceptor agonism since the hypothermic response was more prolonged with MDEA which exhibits low α2A-adrenoceptor potency, and effects of MDMA after α2A-adrenoceptor antagonism were similar to those of MDEA.
Acknowledgments
This study was supported by the Health Research Board (Ireland). MDMA, MDEA and MDA were generously supplied under the NIDA Drug Supply Program.
Abbreviations
- ANOVA
analysis of variance
- AUC
area under the curve
- BRL44408
2-((4,5-dihydro-1H-imidazol-2-yl)methyl)-2,3-dihydro-1-methyl-1H-isoindole
- DR
dose ratio
- 5HT
5-hydroxytryptamine
- i.p.
intraperitoneal
- KO
knockout
- MDA
3,4-methylenedioxyamphetamine
- MDEA
N-ethyl-3,4-methylenedioxyamphetamine
- MDMA
3,4-methylenedioxymethamphetamine
- NA
noradrenaline
- s.e.m.
standard error of the mean
- veh
vehicle
References
- AL-SAHLI W., AHMAD H., KHERADMAND F., CONNOLLY C., DOCHERTY J.R. Effects of methylenedioxymethamphetamine on noradrenaline-evoked contractions of rat right ventricle and small mesenteric artery. Eur. J. Pharmacol. 2001;422:169–174. doi: 10.1016/s0014-2999(01)01070-6. [DOI] [PubMed] [Google Scholar]
- ARRUE A., GOMEZ F.M., GIRALT M.T. Effects of 3,4-methylenedioxymethamphetamine (‘Ecstasy') on the jaw-opening reflex and on the alpha-adrenoceptors which regulate this reflex in the anesthetized rat. Eur. J. Oral. Sci. 2004;112:127–133. doi: 10.1111/j.1600-0722.2004.00114.x. [DOI] [PubMed] [Google Scholar]
- BADON L.A., HICKS A., LORD K., OGDEN B.A., MELEG-SMITH S., VARNER K.J. Changes in the cardiovascular responsiveness and cardiotoxicity elicited during binge administration of ecstasy. J. Pharmacol. Exp. Ther. 2002;302:898–907. doi: 10.1124/jpet.302.3.898. [DOI] [PubMed] [Google Scholar]
- BANKSON M.G., CUNNINGHAM K.A. 3,4-Methylenedioxymethamphetamine (MDMA) as a unique model of serotonin receptor function and serotonin–dopamine interactions. J. Pharmacol. Exp. Ther. 2001;297:846–852. [PubMed] [Google Scholar]
- BARHAM H.M., LENNARD M.S., TUCKER G.T. An evaluation of cytochrome P450 isoform activities in the female dark agouti (DA) rat: relevance to its use as a model of the CYP2D6 poor metaboliser phenotype. Biochem. Pharmacol. 1994;47:1295–1307. doi: 10.1016/0006-2952(94)90327-1. [DOI] [PubMed] [Google Scholar]
- BEXIS S., DOCHERTY J.R. Role of alpha2A-adrenoceptors in the effects of MDMA on body temperature in the mouse. Br. J. Pharmacol. 2005;146:1–6. doi: 10.1038/sj.bjp.0706320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOULES M., WARRINGTON L., FAUQ A., MCCORMICK D., RICHELSON E. A novel neurotensin analog blocks cocaine- and D-amphetamine-induced hyperactivity. Eur. J. Pharmacol. 2001;426:73–76. doi: 10.1016/s0014-2999(01)01197-9. [DOI] [PubMed] [Google Scholar]
- CHENG Y.C., PRUSSOFF W.H. Relationship between the inhibition constant (Ki) and the concentration of inhibitor which causes 50 per cent inhibition (IC50) of an enzymatic reaction. Biochem. Pharmacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- CLEARY L., BUBER R., DOCHERTY J.R. Effects of amphetamine derivatives and cathinone on noradrenaline-evoked contractions of rat right ventricle. Eur. J. Pharmacol. 2002;451:303–308. doi: 10.1016/s0014-2999(02)02305-1. [DOI] [PubMed] [Google Scholar]
- CLEARY L., DOCHERTY J.R. Actions of amphetamine derivatives and cathinone at the noradrenaline transporter. Eur. J. Pharmacol. 2003;476:31–34. doi: 10.1016/s0014-2999(03)02173-3. [DOI] [PubMed] [Google Scholar]
- COLADO M.I., GRANADOS R., O'SHEA E., ESTEBAN B., GREEN R. The acute effects in rats of 3,4-methylenedioxyethamphetamine (MDEA, ‘Eve') on body temperature and long term degeneration of 5HT neurones in brain: a comparison with MDMA (‘Ecstasy') Pharmacol. Toxicol. 1999;84:261–266. doi: 10.1111/j.1600-0773.1999.tb01492.x. [DOI] [PubMed] [Google Scholar]
- COLADO M.I., WILLIAMS J.L., GREEN R. The hyperthermic and neurotoxic effects of ‘Ecstasy' (MDMA) and 3,4-methylenedioxyamphetamine (MDA) in the Dark Agouti (DA) rat, a model of the CYP2D6 poor metabolizer phenotype. Br. J. Pharmacol. 1995;115:1281–1289. doi: 10.1111/j.1476-5381.1995.tb15037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COLE J.C., SUMNALL H.R. Altered states: the clinical effects of Ecstasy. Pharmacol. Ther. 2003a;98:35–58. doi: 10.1016/s0163-7258(03)00003-2. [DOI] [PubMed] [Google Scholar]
- COLE J.C., SUMNALL H.R. The pre-clinical behavioural pharmacology of 3,4-methylenedioxymethamphetamine (MDMA) Neurosci. Biobehav. Rev. 2003b;27:199–217. doi: 10.1016/s0149-7634(03)00031-9. [DOI] [PubMed] [Google Scholar]
- CONNAUGHTON S., DOCHERTY J.R. Functional evidence for heterogeneity of peripheral prejunctional alpha2-adrenoceptors. Br. J. Pharmacol. 1990;101:285–290. doi: 10.1111/j.1476-5381.1990.tb12702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRYAN J.F., HARKIN A., NAUGHTON M., KELLY J.P., LEONARD B.E. Characterization of D-fenfluramine-induced hypothermia: evidence for multiple sites of action. Eur. J. Pharmacol. 2000;390:275–285. doi: 10.1016/s0014-2999(00)00012-1. [DOI] [PubMed] [Google Scholar]
- DAWS L.C., IRVINE R.J., CALLAGHAN P.D., TOOP N.P., WHITE J.M., BOCHNER F. Differential behavioral and neurochemical effects of para-methoxyamphetamine and 3,4-methylenedioxymethamphetamine in the rat. Prog. Neuro-Psychopharmacol. Bio. Psychiat. 2000;24:955–977. doi: 10.1016/s0278-5846(00)00113-5. [DOI] [PubMed] [Google Scholar]
- DE LA TORRE R., FARRE M., ORTUNO J., MAS M., BRENNEISEN R., ROSET P.N., SEGURA J., CAMI J. Non-linear pharmacokinetics of MDMA (‘ecstasy') in humans. Br. J. Clin. Pharmacol. 2000;49:104–109. doi: 10.1046/j.1365-2125.2000.00121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOCHERTY J.R. Subtypes of functional alpha1- and alpha2-adrenoceptors. Eur. J. Pharmacol. 1998;361:1–15. doi: 10.1016/s0014-2999(98)00682-7. [DOI] [PubMed] [Google Scholar]
- ENSSLIN H.K., MAURER H.H., GOUZOULIS E., HERMLE L., KOVAR K.A. Metabolism of racemic 3,4-methylenedioxyethylamphetamine in humans. Isolation, identification, quantification, and synthesis of urinary metabolites. Drug Metab. Dispos. 1996;24:813–820. [PubMed] [Google Scholar]
- FITZGERALD J.L., REID J.J. Sympathomimetic actions of methylenedioxymethamphetamine in rat and rabbit isolated cardiovascular tissues. J. Pharm. Pharmacol. 1994;46:826–832. doi: 10.1111/j.2042-7158.1994.tb03738.x. [DOI] [PubMed] [Google Scholar]
- FOLTIN R.W., SCHUSTER C.R. Behavioral tolerance and cross-tolerance to dl-cathinone and d-amphetamine in rats. J. Pharmacol. Exp. Ther. 1982;222:126–131. [PubMed] [Google Scholar]
- GHURAN A., VAN DER WIEKEN L.R., NOLAN J. Cardiovascular complications of recreational drugs. Br. Med. J. 2001;323:464–466. doi: 10.1136/bmj.323.7311.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOLD L.H., KOOB G.K., GEYER M.A. Stimulant and hallucinogenic behavioural profiles of 3,4-methylenedioxymethamphetamine and N-ethyl-3,4-methylenedioxyamphetamine in rats. J. Pharmacol. Exp. Ther. 1988;247:547–555. [PubMed] [Google Scholar]
- GOUZOULIS E., VON BARDELEBEN U., RUPP A., KOVAR K.-A., HERMLE L. Neuroendocrine and cardiovascular effects of MDE in healthy volunteers. Neuropsychopharmacology. 1993;8:187–193. doi: 10.1038/npp.1993.20. [DOI] [PubMed] [Google Scholar]
- GUIMARAES S., MOURA D. Vascular adrenoceptors: an update. Pharmacol. Rev. 2001;53:319–356. [PubMed] [Google Scholar]
- HAYNER G.N., MCKINNEY H. MDMA, the dark side of Ecstasy. J. Psychoactive Drugs. 1986;18:341–347. doi: 10.1080/02791072.1986.10472367. [DOI] [PubMed] [Google Scholar]
- HEGADOREN K.M., BAKER G.B., BOURIN M. 3,4-Methylenedioxy analogues of amphetamine: defining the risk to humans. Neurosci. Behav. Rev. 1999;23:539–553. doi: 10.1016/s0149-7634(98)00046-3. [DOI] [PubMed] [Google Scholar]
- KALIVAS P.W., DUFFY P., WHITE S.R. MDMA elicits behavioral and neurochemical sensitization in rats. Neuropsychopharmacology. 1998;18:469–479. doi: 10.1016/S0893-133X(97)00195-4. [DOI] [PubMed] [Google Scholar]
- LAHDESMAKI J., SALLINEN J., MACDONALD E., SIRVIO J., SCHEININ M. Alpha2-adrenergic drug effects on brain monoamines, locomotion, and body temperature are largely abolished in mice lacking the alpha2A-adrenoceptor subtype. Neuropharmacology. 2003;44:882–892. doi: 10.1016/s0028-3908(03)00080-7. [DOI] [PubMed] [Google Scholar]
- LAVELLE A., HONNER V., DOCHERTY J.R. Investigation of the prejunctional α2-adrenoceptor mediated actions of MDMA in rat atrium and vas deferens. Br. J. Pharmacol. 1999;128:975–980. doi: 10.1038/sj.bjp.0702875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MALBERG J.E., SEIDEN L.S. Small changes in ambient temperature cause large changes in 3,4-methylenedioxymethamphetamine (MDMA)-induced serotonin neurotoxicity and core body temperature in the rat. J. Neurosci. 1998;18:5086–5094. doi: 10.1523/JNEUROSCI.18-13-05086.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MALPASS A., WHITE J.M., IRVINE R.J., SOMOGYI A.A., BOCHNER F. Acute toxicity of 3,4-methylenedioxymethamphetamine (MDMA) in Sprague–Dawley and dark Agouti rats. Pharmacol. Biochem. Behav. 1999;64:29–34. doi: 10.1016/s0091-3057(99)00116-1. [DOI] [PubMed] [Google Scholar]
- MCCANN U.D., SLATE S.O., RICAURTE G.A. Adverse reactions with 3,4-methylenedioxymethamphetamine (MDMA; ‘Ecstasy') Drug Safety. 1996;15:107–115. doi: 10.2165/00002018-199615020-00003. [DOI] [PubMed] [Google Scholar]
- MCDAID J., DOCHERTY J.R. Vascular actions of MDMA involve α1 and α2-adrenoceptors in the anaesthetized rat. Br. J. Pharmacol. 2001;133:429–437. doi: 10.1038/sj.bjp.0704094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'CAIN P.A., HLETKO S.B., OGDEN B.A., VARNER K.J. Cardiovascular and sympathetic responses and reflex changes elicited by MDMA. Physiol. Behav. 2000;70:141–148. doi: 10.1016/s0031-9384(00)00235-3. [DOI] [PubMed] [Google Scholar]
- O'LOINSIGH E.D., BOLAND G., KELLY J.P., O'BOYLE K.M. Behavioural, hyperthermic and neurotoxic effects of 3,4-methylenedioxymethamphetamine anologues in the Wistar rat. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2001;25:621–638. doi: 10.1016/s0278-5846(00)00179-2. [DOI] [PubMed] [Google Scholar]
- PEDERSEN N.P., BLESSING W.W. Cutaneous vasoconstriction contributes to hyperthermia induced by 3,4-methylenedioxymethamphetamine (Ecstasy) in conscious rabbits. J. Neurosci. 2001;21:8648–8654. doi: 10.1523/JNEUROSCI.21-21-08648.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPRAGUE J.E., BRUTCHER R.E., MILLS E.M., CADEN D., RUSYNIAK D.E. Attenuation of 3,4-methylenedioxymethamphetamine (MDMA, Ecstasy)-induced rhabdomyolysis with α1- plus β3-adrenoceptor antagonists. Br. J. Pharmacol. 2004;142:667–670. doi: 10.1038/sj.bjp.0705823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STUERENBURG H.J., PETERSEN K., BAUMER T., ROSENKRANZ M., BUHMANN C., THOMASIUS R. Plasma concentrations of 5-HT, 5-HIAA, norepinephrine, epinephrine and dopamine in ecstasy users. Neuro. Endocrinol. Lett. 2002;23:259–261. [PubMed] [Google Scholar]
- SUGIMOTO Y., OHKURA M., INOUE K., YAMADA J. Involvement of the 5-HT(2) receptor in hyperthermia induced by p-chloroamphetamine, a serotonin-releasing drug in mice. Eur. J. Pharmacol. 2000;403:225–228. doi: 10.1016/s0014-2999(00)00585-9. [DOI] [PubMed] [Google Scholar]
- SUGIMOTO Y., OHKURA M., INOUE K., YAMADA J. Involvement of serotonergic and dopaminergic mechanisms in hyperthermia induced by a serotonin-releasing drug, p-chloroamphetamine in mice. Eur. J. Pharmacol. 2001;430:265–268. doi: 10.1016/s0014-2999(01)01386-3. [DOI] [PubMed] [Google Scholar]
- VANDEPUTTE C., DOCHERTY J.R. Vascular actions of 3,4-methylenedioxymethamphetamine in alpha2A/D-adrenocepor knockout mice. Eur. J. Pharmacol. 2002;457:45–49. doi: 10.1016/s0014-2999(02)02661-4. [DOI] [PubMed] [Google Scholar]
- VOLLENWEIDER F.X., GAMMA A., LIECHTI M., HUBER T. Psychological and cardiovascular effects and short-term sequelae of MDMA (‘Ecstasy') in MDMA-naïve healthy volunteers. Neuropsychopharmacology. 1998;19:241–251. doi: 10.1016/S0893-133X(98)00013-X. [DOI] [PubMed] [Google Scholar]
- YOUNG P., BERGE J., CHAPMAN H., CAWTHORNE M.A. Novel alpha 2-adrenoceptor antagonists show selectivity for alpha 2A- and alpha 2B-adrenoceptor subtypes. Eur. J. Pharmacol. 1989;168:381–386. doi: 10.1016/0014-2999(89)90801-7. [DOI] [PubMed] [Google Scholar]
- ZARRINDAST M.-R., SADEGHI S., SAHEBGHARANI M. Influence of α-adrenoceptor agonists and antagonists on imipramine-induced hypothermia in mice. Pharmacol. Toxicol. 2003;93:48–53. doi: 10.1034/j.1600-0773.2003.930107.x. [DOI] [PubMed] [Google Scholar]