Abstract
The early embryology of the elephant has never been studied before. We have obtained a rare series of African elephant (Loxodonta africana) embryos and fetuses ranging in weight from 0.04 to 18.5 g, estimated gestational ages 58–166 days (duration of gestation is ≈660 days). Nephrostomes, a feature of aquatic vertebrates, were found in the mesonephric kidneys at all stages of development whereas they have never been recorded in the mesonephric kidneys of other viviparous mammals. The trunk was well developed even in the earliest fetus. The testes were intra-abdominal, and there was no evidence of a gubernaculum, pampiniform plexus, processus vaginalis, or a scrotum, confirming that the elephant, like the dugong, is one of the few primary testicond mammals. The palaeontological evidence suggests that the elephant’s ancestors were aquatic, and recent immunological and molecular evidence shows an extremely close affinity between present-day elephants and the aquatic Sirenia (dugong and manatees). The evidence from our embryological study of the elephant also suggests that it evolved from an aquatic mammal.
There is now a wealth of information to show that the elephants (Proboscidea) and the sea cows (Sirenia) must share a common ancestor. The early Tethytheria, a group consisting of the Proboscidea, Sirenia, and the extinct Desmostylia, appear to have been semi-aquatic (1, 2). The Desmostylia have only been found in marine deposits and are considered to have been aquatic herbivores, feeding on marine algae and angiosperms (3). Remains of Anthracobune, probably the earliest known proboscidean ancestor from the early or early-middle Eocene (≈50 million years ago), have been recovered from sites in both Asia and Africa which were near-shore shallow water environments (2). The Moeritherium, a primitive genus of the Proboscidea (40–30 million years ago) has skeletal features highly suggestive of a semiaquatic lifestyle (1). There are similarities in dentition between the herbivorous Proboscidea and Sirenia, and they share a unique character in their enamel structure (2, 3, 4). The middle ear of the Sirenia has a perilymphatic foramen, which also has been found in the development of the fetal elephant (2). Biochemical data, such as the analysis of alpha-lens crystallin, also link the elephants and sea cows (5). They also are linked by the developmental pattern of their fetal membranes (6). Most recently, molecular studies using a mitochondrial 12s ribosomal RNA gene tree (7) and mitochondrial cytochrome b gene segments (8) have reinforced the affinity between the elephants and the Sirenia. A recent review (9) of all of the molecular studies leaves no doubt that the Proboscidea and the Sirenia must share a common aquatic ancestor. Here, we provide information on the early embryology of the African elephant, Loxodonta africana. This has provided some unexpected additional evidence in support of an aquatic ancestry. The unique development of nephrostomes in the mesonephric kidney, the intra-abdominal location of the testes, and the precocious development of the trunk could all have been adaptations to an aquatic lifestyle.
MATERIALS AND METHODS
We have been able to obtain some rare African elephant specimens consisting of one embryo (weight 0.04 g) and six fetuses (weight ranging from 0.79 to 18.5 g) that were collected from adult females shot in the Kruger National Park, South Africa, between 1993 and 1995, as part of a culling operation to reduce elephant numbers in the park. They were fixed whole in neutral buffered formalin, were photographed (Fig. 1), were measured with vernier calipers, were weighed, and then were serially sectioned at 8 μm. The crown-rump length was measured from the base of the tail to the base of the trunk, and trunk length was measured from the corner of the oral groove to the tip of the trunk. Craig’s formulae (10) were used to estimate age from body weight. The embryo was estimated to be 58 days old, and the oldest fetus was estimated to be 166 days old [duration of pregnancy is ≈660 days (11)]. For fetuses weighing <1.46 g, the formula used was t = 105w1/3 − (w1/3 + 0.193)−3 + 140; for fetuses >2.6 g, age was estimated by using the formula t = 106w1/3 + 138, where t = days and w = weight.
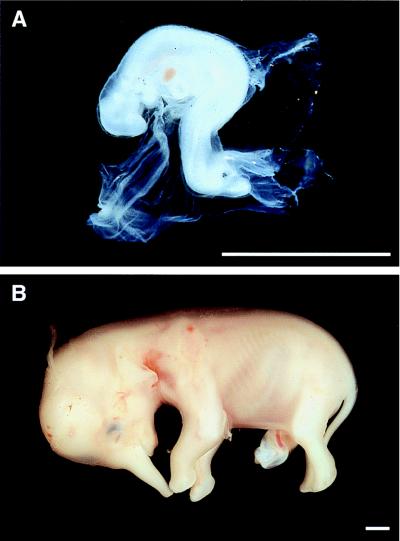
Figure 1.
The youngest and oldest African elephant embryo and fetus used for this study. (A) Embryo, estimated gestation 58 days. (B) Fetus, estimated gestation 166 days. [Bars = 5 mm.]
Several methods were used to determine the sex of the fetuses. Sections through the gonads were examined for indications of sexual differentiation. The percentage of cells containing sex chromatin masses was calculated by using the method described by Wijayanti (12). The presence of a “Y”-shaped urethral opening, characteristic of the adult male elephant, was used to sex the fetuses in which the phallus had differentiated.
RESULTS AND DISCUSSION
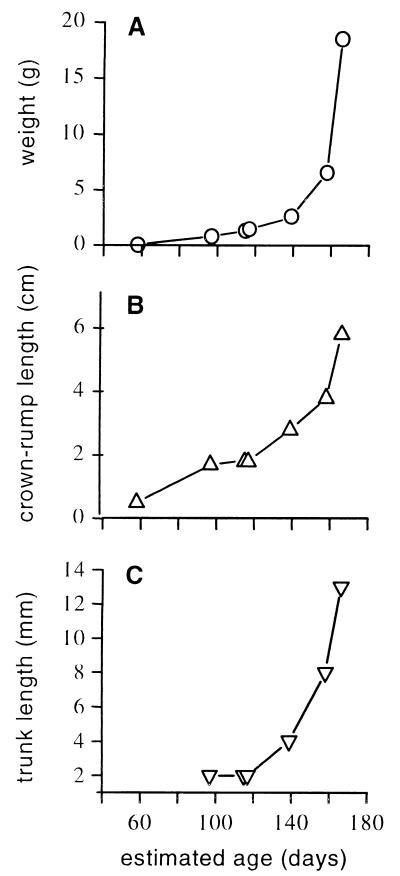
The elephant has by far the longest gestation length of any mammal. The estimated gestational ages of our specimens are shown in Fig. 2A; they ranged from 58 days (0.04 g) to 166 days (18.5 g). The crown-rump lengths relative to estimated gestational age are shown in Fig. 2B.
Figure 2.
Growth of the African elephant fetus. Fetal weight (A), crown-rump length (B), and trunk length (C) increase with estimated gestational age.
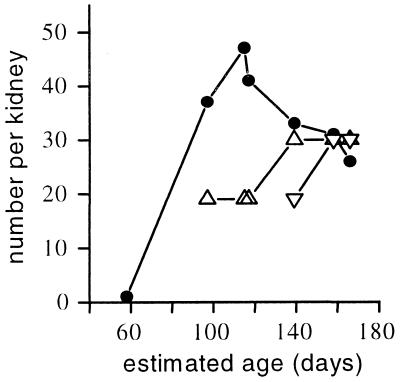
On histological examination of the serial sections, the most striking feature not seen in other mammalian embryos was the nephrostomes, which were present in the mesonephric kidneys of all of the fetuses (Fig. 3 A and B and Fig. 4). The nephrostome is a funnel-shaped ciliated duct opening on the surface of the mesonephros and connecting the coelomic cavity to the capsule of the renal glomerulus. It enables osmotic exchange between the coelomic fluid and the blood supply. In the embryo (58 days), the mesonephros was in the early stages of development. It was elongated, and S-shaped nephric tubules connected the glomeruli to the Wolffian duct. The early stages of nephrostome development could be seen as an invagination of the mesonephric surface leading toward the glomerulus. In the early fetuses (97–117 days), there was one fully developed nephrostome for each mesonephric glomerulus. In later fetuses (139 to 166 days), the pronephros had degenerated, and the mesonephros was beginning to regress as the metanephric kidney developed. The largest number of nephrostomes was found in a fetus of 115 days of age when the metanephric kidney was still relatively poorly developed, with few tubules and no glomeruli. By day 166, the mesonephros had clearly regressed and had the smallest number of nephrostomes (Fig. 4) whereas the metanephros had many tubules and glomeruli and had acquired the lobulated appearance characteristic of the adult elephant kidney.
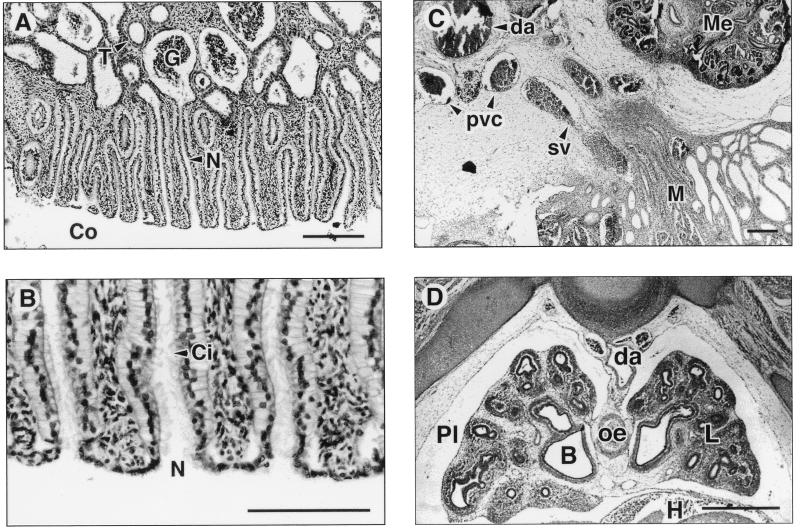
Figure 3.
Histological appearance of the mesonephric kidneys and lungs of African elephant fetuses. (A) Transverse section through the 115-day fetus showing the mesonephros with nephrostomes (N) opening into the coelom (Co) and connecting to the glomerulus (G) and associated collecting tubules (T). (B) High-power photomicrograph of one mesonephric nephrostome (N) clearly showing cilia (Ci). The cilia are characteristically angled inwards indicating the direction of flow of the filtrate. (C) Transverse section through the 139-day fetus showing mesonephros (M), metanephros (Me), dorsal aorta (da), and the posterior vena cava (pvc). The future spermatic vein (sv) leads directly from the mesonephros and the developing testis (not shown) to the posterior vena cava. (D) Transverse thoracic section through the 139-day fetus showing lung (L) and pleural cavity (Pl), heart (H) surrounded by pericardium, dorsal aorta (da), oesophagus (oe), and bronchus (B). [Bars = 0.25 mm (A), 0.16 mm (B), 0.64 mm (C), and 0.75 mm (D).]
Figure 4.
The number of nephrostomes, renal tubules, and glomeruli in the mesonephric kidney of the African elephant. Shown are Nephrostomes (●), tubules (▵), and glomeruli (▿).
Nephrostomes are a characteristic feature of the mesonephric kidneys of freshwater vertebrates such as sturgeons (13) and frogs (14–16). When vertebrates moved onto dry land, they preserved the aquatic environment of the embryo by encasing it in a fluid-filled amniotic sac, and functional nephrostomes are still present in the first stages of development of the mesonephric kidneys of egg-laying reptiles (17) and all birds (18, 19). The platypus, an egg-laying monotreme, has extensive development of nephrostomes in the mesonephros of 8-mm-long specimens, but, by the time of hatching, only remnants of the nephrostomes are present (20). The echidna, also an egg-laying monotreme, has nephrostomes in the mesonephros to a lesser degree than in the platypus (20, 21). Rudimentary nephrostomes, which are never connected with glomeruli, occur only in the pronephros of some mammals, such as the sheep (22), domestic cat (23), and common brushtail possum, Trichosurus vulpecula (24). However, nephrostomes never occur in the mesonephric kidneys of present-day viviparous mammals, nor do they develop in the definitive metanephric kidney (19, 20, 25).
The prolonged persistence of well developed nephrostomes in the mesonephros of the elephant fetus for at least 2 months could be a result of the very slow rate of embryonic and fetal growth, so that structures that only appear transiently in other mammals remain for much longer in the elephant. Unfortunately, there is no other mammal with a comparably long gestation length, but, even in the rhinoceros, Diceros bicornis [gestation length is ≈16 months (11)], there are no nephrostomes in the fetal mesonephros (26), nor are they present in the mesonephros of the humpback whale fetus, Megaptera novaeangliae (27) [gestation length is ≈11 months (11)]. An alternative possibility is that the nephrostomes in the elephant’s mesonephric kidney are a plesiomorphic character associated with its aquatic ancestry.
Another uncommon feature of the elephant is the intra-abdominal location of its testes, known since the time of Aristotle (28). The early differentiation of the urogenital system in the four male African elephant fetuses followed the normal mammalian pattern, except that the testes remained intra-abdominal at the ventromedial aspect of the kidney and never descended into a scrotum. During organogenesis, the testes initially developed from the genital ridge located at the medial aspect of the mesonephros, but, as the metanephric kidney assumed a more cranial location, the testes assumed their definitive position adjacent to the metanephros (Fig. 5). In scrotal mammals, the testes and associated mesonephric ducts descend and are guided through the inguinal canal to the scrotum by the gubernaculum. At no stage of fetal development in the elephant was there any sign of a gubernaculum, processus vaginalis, inguinal canal, or scrotum. In scrotal mammals, the highly coiled spermatic artery is surrounded by a convoluted venous pampiniform plexus draining the testis, which cools the arterial blood supply to the testis, but, in the adult elephant, this structure is notably absent (29, 30). We found that, in the elephant fetuses, the testicular artery ran a direct course from the renal artery into the testis and the testicular vein ran straight into the posterior vena cava with no sign of a pampiniform plexus (Fig. 3C and Fig. 5). If elephants were secondary testiconda, whose testes had once been scrotal, the pampiniform plexus would probably have been retained, as it is in the seals and whales (31). Thus, we agree with the earlier conclusions of Weber (32) that the elephant, like the dugong (33), is a primary testicond mammal that shows no evidence of prior testicular descent.
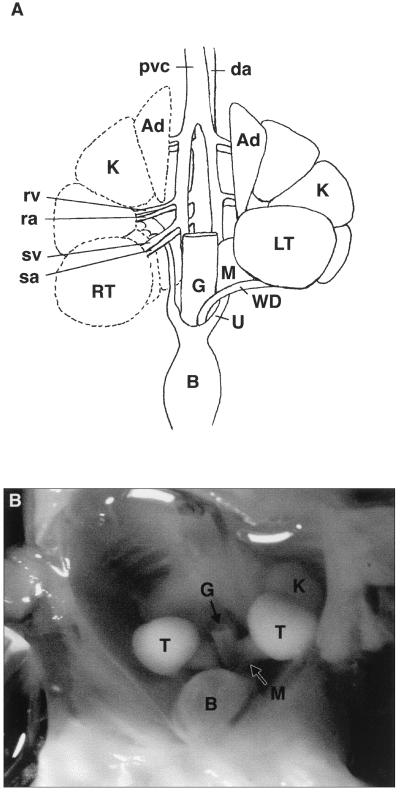
Figure 5.
Diagram and photograph of the ventral view of a dissected 166-day African elephant fetus. (A) This shows the short, straight spermatic vein (sv) and spermatic artery (sa). The right testis (RT) and Wolffian duct are displaced caudally (indicated by dotted outline) to show the course of the renal vein (rv) and the renal artery (ra) supplying the metanephric kidney (K) and the ureter (U). The right mesonephros has been omitted for clarity. The gut (G) has been removed, and the bladder (B) has been reflected caudally. Note that the adrenal glands (Ad) are large. The metanephric kidney is lobular as in the adult. The mesonephros (M) is regressing. (B) The large intra-abdominal testes (T) on the ventral aspect of the lobular metanephric kidney (K) are evident. The regressing mesonephros (M) is just visible. The gut (G) has been removed, and the bladder (B) has been reflected caudally.
When the terrestrial artiodactyl ancestors of the seals and whales entered the water 60 million years ago (34), they presumably had scrotal testes, which were subsequently withdrawn back into the inguinal canal or abdominal cavity to prevent them from getting too cold, as even a brief period of extreme testicular cooling can render an animal permanently sterile (35). Thus, the seals and whales are classified as secondary testiconda (32).
The trunk was evident even in the earliest of the fetuses studied (Fig. 2C), coinciding with the first appearance of the nephrostomes in the mesonephros. The trunk might have first evolved as an adaptation to an aquatic environment. For example, it could have been used as a snorkel, as it is to this day when elephants swim in deep water (36). A normal pleural cavity was present in all of the fetuses (Fig. 3D). However, it is known that the parietal and visceral pleura of the elephant fuse just before birth, so that newborn and adult elephants have no pleural cavity (37). This adaptation, thought to be a protection against the high negative intrathoracic pressures achieved when water has to be sucked up the trunk as a prelude to drinking, would only have been necessary in a terrestrial mammal.
The molecular evidence from DNA sequences is consistent with all of the earlier morphological, immunological, and paleontological data showing that the Proboscidea and Sirenia unquestionably share a common ancestry (1–9). Whether present-day embryonic structures reflect past evolutionary history is still hotly debated. Although few would now support Ernst Haeckel’s extreme view that “ontogeny recapitulates phylogeny” (38), many would agree with Ernst Mayr, who concludes that embryological development does provide some clues to evolutionary history and that even a “hard-nosed look at the facts does not lead me to a different and superior interpretation” (39). For example, we accept without question the ontogenetic significance of the cranial nerves, the branchial arches, and the bones of the middle ear, so it seems reasonable to extend the argument to the renal, reproductive, and respiratory tracts. Given such an assumption, our embryological data strongly suggest that the mesonephric kidney, the testis, the trunk, and the lungs of the elephant all originally were adapted to its aquatic environment and that some of these unusual anatomical adaptations have persisted in present-day terrestrial elephants.
Acknowledgments
We are most grateful to Ian Whyte (Kruger National Park, South Africa) for collecting the elephant embryos and fetuses used in this study. We thank Drs. M. E. Griffiths and J. A. W. Kirsch for helpful discussions, Dr. G. Shaw for help with the figures, and David Paul and Bruce Abaloz for excellent photographic and histological assistance.
References
- 1.Janus C M. TREE. 1988;3:291–297. [Google Scholar]
- 2.Shoshani T, Tassy P. The Proboscidea. New York: Oxford Univ. Press; 1996. p. 472. [Google Scholar]
- 3.Domning D P, Ray C E, McKenna M C. Smithson Contrib Paleontol. 1986;59:1–56. [Google Scholar]
- 4.de Jong W W. In: Macromolecular Sequences in Systematic and Evolutionary Biology. Goodman M E, editor. New York: Plenum; 1982. pp. 75–144. [Google Scholar]
- 5.Fischer M S, Tassey P. In: Mammal Phylogeny. Szalay F S, Novacek M J, McKenna M C, editors. New York: Springer; 1993. pp. 217–234. [Google Scholar]
- 6.Novacek M J, Wyss A R. Cladistics. 1986;2:257–287. doi: 10.1111/j.1096-0031.1986.tb00463.x. [DOI] [PubMed] [Google Scholar]
- 7.Springer M S, Kirsch J A W. J Mammal Evol. 1993;1:149–166. [Google Scholar]
- 8.Ozawa T, Hayashi S, Mikhelson V M. J Mol Evol. 1997;44:406–413. doi: 10.1007/pl00006160. [DOI] [PubMed] [Google Scholar]
- 9.de Jong W W. TREE. 1998;13:270–274. doi: 10.1016/s0169-5347(98)01335-4. [DOI] [PubMed] [Google Scholar]
- 10.Craig G C. S Afr J Sci. 1984;80:512–516. [Google Scholar]
- 11.Hayssen V, van Tienhoven A, van Tienhoven A. Asdell’s Patterns of Mammalian Reproduction. Ithaca, NY: Comstock; 1993. p. 1023. [Google Scholar]
- 12.Wijayanti G. M.S. thesis. Clayton, Victoria, Australia: Monash Univ.; 1994. [Google Scholar]
- 13.Weichert C K. Anatomy of the Chordates. New York: McGraw–Hill; 1970. p. 814. [Google Scholar]
- 14.Gray P. Q J Micro Sci. 1930;73:507–546. [Google Scholar]
- 15.Gray P. Q J Micro Sci. 1932;75:425–465. [Google Scholar]
- 16.Gray P. Q J Micro Sci. 1936;78:445–473. [Google Scholar]
- 17.Wiedersheim R. Arch mikr Anat. 1890;36:410–468. [Google Scholar]
- 18.Patten B M. Early Embryology of the Chick. Toronto: Blakiston; 1951. p. 244. [Google Scholar]
- 19.Balinsky B I. An Introduction to Embryology. Philadelphia: Saunders; 1975. p. 648. [Google Scholar]
- 20.van der Shoot P. Anat Rec. 1996;244:386–401. doi: 10.1002/(SICI)1097-0185(199603)244:3<386::AID-AR10>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 21.Keibel F. In: Zoologische Forschungreisen in Australien und dem Malayischen Archipel. Semon R, editor. Vol. 3. Jena, Germany: Fischer; 1904. pp. 51–206. [Google Scholar]
- 22.Davies J, Davies D V. Proc Zool Soc London. 1950;120:73–93. [Google Scholar]
- 23.Fraser E A. J Anat. 1920;54:287–304. [PMC free article] [PubMed] [Google Scholar]
- 24.Buchanan G, Fraser E A. J Anat. 1918;53:35–100. [PMC free article] [PubMed] [Google Scholar]
- 25.Larsen W J. Human Embryology. New York: Churchhill Livingstone; 1993. p. 479. [Google Scholar]
- 26.Davies J. Proc Zool Soc London. 1952;122:593–613. [Google Scholar]
- 27.Stump C W, Robins J P, Garde M L. Aust J Mar Freshwater Res. 1960;11:365–386. [Google Scholar]
- 28.Barnes J, editor. The Complete Works of Aristotle. Vol. 1. Princeton: Princeton Univ. Press; 1984. p. 796. [Google Scholar]
- 29.Short R V, Mann T, Hay M. J Reprod Fertil. 1967;13:517–536. doi: 10.1530/jrf.0.0130517. [DOI] [PubMed] [Google Scholar]
- 30.Glover T D. J Reprod Fertil. 1973;35:45–53. doi: 10.1530/jrf.0.0350045. [DOI] [PubMed] [Google Scholar]
- 31.Rommel S A, Pabst D A, McLellan W A, Mead J G, Potter C W. Anat Rec. 1992;232:150–156. doi: 10.1002/ar.1092320117. [DOI] [PubMed] [Google Scholar]
- 32.Weber M. Die Saugetiere. Jena, Germany: Fischer; 1928. [Google Scholar]
- 33.Marsh H, Heinsohn G E, Glover T D. Aust J Zool. 1984;32:721–742. [Google Scholar]
- 34.Shimamura M, Yasue H, Ohshima K, Abe H, Kato H, Kishiro T, Goto M, Munechika I, Okada N. Nature (London) 1997;388:666–670. doi: 10.1038/41759. [DOI] [PubMed] [Google Scholar]
- 35.Young G P H, Goldstein M, Phillips D M, Sundaram K, Gunsalus G L, Bardin C W. Endocrinology. 1988;122:1074–1082. doi: 10.1210/endo-122-3-1074. [DOI] [PubMed] [Google Scholar]
- 36.Spinage C A. Elephants. London: Poyser; 1994. p. 319. [Google Scholar]
- 37.Short R V. New Sci. 1962;16:570–572. [Google Scholar]
- 38.Raff R A. The Shape of Life. Chicago: Univ. of Chicago Press; 1996. p. 520. [Google Scholar]
- 39.Mayr E. This is Biology. Cambridge, MA: Harvard Univ. Press; 1997. p. 327. [Google Scholar]