Abstract
Endocytic vesicles undergo fission to sort ligand from receptor. Using quantitative immunofluorescence and video imaging, we provide the first in vitro reconstitution of receptor–ligand sorting in early endocytic vesicles derived from rat liver. We show that to undergo fission, presegregation vesicles must bind to microtubules (MTs) and move upon addition of ATP. Over 13% of motile vesicles elongate and are capable of fission. After fission, one vesicle continues to move, whereas the other remains stationary, resulting in their separation. On average, almost 90% receptor is found in one daughter vesicle, whereas ligand is enriched by ∼300% with respect to receptor in the other daughter vesicle. Although studies performed on polarity marked MTs showed approximately equal plus and minus end–directed motility, immunofluorescence microscopy revealed that kinesins, but not dynein, were associated with these vesicles. Motility and fission were prevented by addition of 1 mM 5′-adenylylimido-diphosphate (AMP-PNP, an inhibitor of kinesins) or incubation with kinesin antibodies, but were unaffected by addition of 5 μM vanadate (a dynein inhibitor) or dynein antibodies. These studies indicate an essential role of kinesin-based MT motility in endocytic vesicle sorting, providing a system in which factors required for endocytic vesicle processing can be identified and characterized.
Keywords: endocytosis, microtubules, kinesin, dynein, motility
Introduction
Receptor-mediated endocytosis for recycling receptors is a process in which a ligand binds to its cell surface receptor and is internalized via clathrin-coated vesicle formation (Mellman 1996; Mukherjee et al. 1997; Marsh and McMahon 1999). After uncoating, this vesicle acidifies (Forgac 1998), leading to dissociation of ligand from receptor (Harford et al. 1983). Subsequently, the endocytic vesicle undergoes a series of fissions, resulting in the eventual segregation of ligand from receptor (Wolkoff et al. 1984; Mellman 1996; Mukherjee et al. 1997). After segregation, daughter vesicles destined to recycle to the cell surface contain a majority of receptor and a reduced content of ligand. Other vesicles, which have much less receptor in proportion to the content of ligand (Geuze et al. 1984), traffic through the cell to the lysosome, where ligand is degraded (Harford et al. 1983; Wolkoff et al. 1984; Mukherjee et al. 1997). Little is known about the mechanism by which endocytic vesicles undergo fission and sort ligand from receptor, although previous studies suggested a role for microtubules (MTs) and molecular motors in this process and/or in movement of ligand-enriched late endosomes toward lysosomes (Wolkoff et al. 1984; Goltz et al. 1992; Jin and Snider 1993; Lafont et al. 1994; Satir 1994; Thatte et al. 1994; Oda et al. 1995; Hamm-Alvarez et al. 1996; Novikoff et al. 1996; Hamm-Alvarez and Sheetz 1998; Murray et al. 2000). Here, we provide the first in vitro reconstitution of the process of segregation of ligand and receptor in early endocytic vesicles. We show that it is necessary for presegregation vesicles to bind to and move along MTs to undergo fission. This results in a daughter vesicle enriched in ligand relative to receptor and a second daughter with a majority of receptor. Motility and fission are established by the addition of ATP. Although vesicle motility both before and after fission can be either toward the plus or minus end of the MT, these processes are prevented by addition of 5′-adenylylimido-diphosphate (AMP-PNP), an inhibitor of kinesins, or by incubation with kinesin antibody, but not by addition of vanadate, an inhibitor of cytoplasmic dynein, or dynein antibody. Additionally, kinesins are immunolocalized to many of the presegregation vesicles bound to MTs, whereas dynein is rarely seen in this vesicle population. Fission under these conditions evidently requires MTs and kinesin-based motors; whether other motors and related proteins are necessary is not known. However, these observations establish a well-defined system in which additional vesicle-associated and cellular factors that are required for segregation can be identified and their functional roles dissected.
Materials and Methods
Reagents
Mouse mAbs (IgM) against the dynein intermediate chain (clone 70.1) and the kinesin I heavy chain (clone IBII) were obtained from Sigma-Aldrich. Mouse mAbs (IgG) against the amino termini of the dynein intermediate chain and the kinesin I heavy chain were obtained from Chemicon International, Inc. Specificities were confirmed by immunoblot. Nonimmune mouse IgG was obtained from Sigma-Aldrich. Affinity purified rabbit IgG was prepared against a KLH-linked peptide (VNRWACERKRDITYC) corresponding to a sequence on the cytoplasmic tail of the rat asialoglycoprotein receptor (ASGPR) H2 subunit (Stockert 1995). All reagents were from Sigma-Aldrich unless otherwise noted.
Motility Assay
Texas red–labeled early endocytic vesicles were prepared from livers that were removed from male Sprague-Dawley rats (200–250 g; Taconic Farms) 5 min after i.v. injection of Texas red asialoorosomucoid (ASOR) (Murray et al. 2000). All procedures were approved by the University Animal Use Committee. After Dounce homogenization, a postnuclear supernatant was prepared and chromatographed on a Sephacryl S200 column. Vesicle-enriched peaks were pooled and subjected to centrifugation (200,000 g for 135 min) on a sucrose step gradient consisting of 1.4, 1.2, and 0.25 M sucrose. Vesicles were harvested from the 1.2/0.25 M sucrose interface and stored at −80°C until used. Details of these procedures have been published recently (Murray et al. 2000).
Motility assays were performed in a chamber consisting of two pieces of double-stick tape sandwiched between optical glass; the internal volume was ∼3 μl. The chamber was coated with an affinity-purified mixture of liver motor proteins as described previously (Murray et al. 2000). After three 15-μl washes with modified motility buffer (35 mM Pipes, 5 mM MgCl2, 1 mM EGTA, 0.5 mM EDTA, 4 mM DTT, 20 μM taxol, 2 mg/ml BSA, pH 7.4, containing an oxygen scavenging system), taxol-stabilized MTs were added and incubated for 3 min. After another three washes with motility buffer containing 5 mg/ml casein, endocytic vesicles were added to the chamber, incubated for 10 min, and washed. In some experiments, as indicated below. 0.02 mg/ml DEAE-dextran (Amersham Pharmacia Biotech), rather than motor proteins, was used to adhere MTs to glass, as described previously (Murray et al. 2000). Motility was initiated with the addition of 50 μM ATP in the absence of a regenerating system. In some experiments, as indicated below, 4 mM ATP was used.
Image Analysis
Imaging was performed at the Analytical Imaging Facility of the Albert Einstein College of Medicine. A 60×, 1.4 numerical aperture planapo objective was used on an Olympus 1X70 inverted microscope, containing automatic excitation and emission filter wheels connected to a Photometrics charge-coupled device camera run by IPLab Spectrum software (Scanalytics) running on a Power Macintosh. IPLab Spectrum scripting software was used to collect images rapidly and to switch between fluorescence channels. Images were also recorded directly on videotape. In all experiments the microscope stage was maintained at 35°C. Videos were digitized with the use of the Scion Image (Scion Corporation) movie-making macro (1 frame/s) and saved as tiff files. Integrative density of fluorescence was determined using the density slice and wand option of Scion Image. Each quantification of fluorescence density was the average of three determinations performed on each vesicle.
Results
Preparation of Early Endocytic Vesicles
Fluorescent endocytic vesicles were prepared from rat liver that was surgically removed 5 min after intravenous injection of 3 mg of Texas red–labeled ASOR (Murray et al. 2000). This short time period was chosen so that events early in the endocytic pathway could be examined. As anticipated, ASGPR was present in >95% of the ASOR-containing vesicles, as determined by fluorescence microscopy using anti-ASGPR IgG to localize receptor (Fig. 1). This observation indicates that these ligand-containing vesicles represent a population of early (i.e., presegregation) endosomes. Partial purification of vesicles was performed to minimize nonspecific ATPase activity as described previously (Murray et al. 2000).
Figure 1.
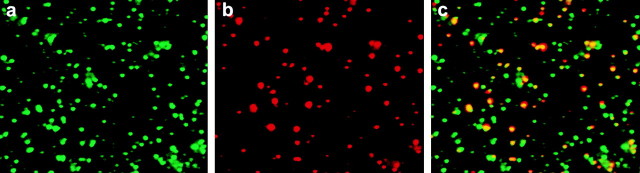
Immunolocalization of ASGPR and ASOR in isolated endocytic vesicles. A preparation enriched in endocytic vesicles was prepared from rat liver that was obtained 5 min after i.v. injection of Texas red–ASOR, as described in Materials and Methods. Vesicles were incubated at 4°C for 1 min in anti-ASGPR IgG. Cy2-labeled goat anti–rabbit IgG was added and incubation was continued for an additional 6 min. Vesicles were hydrostatically attached to a glass slide and examined by immunofluorescence microscopy. Many receptor containing vesicles were observed (a). There were fewer ligand-containing vesicles (b). A merged view (c) shows that although there were many receptor-containing vesicles devoid of ligand, virtually all ligand-containing vesicles also contained receptor, indicating that they represent a population of early endocytic vesicles.
Assay of Vesicle Motility and Fission
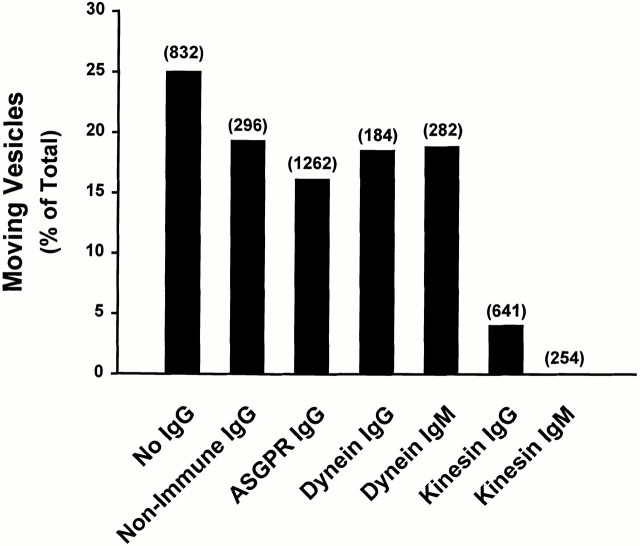
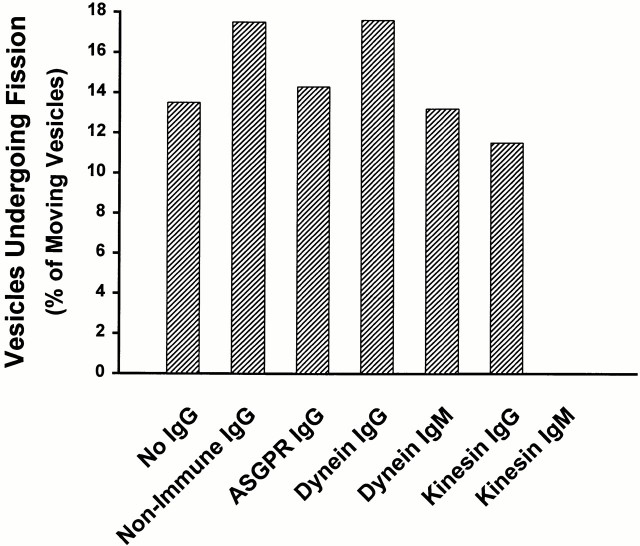
Labeled endocytic vesicles were drawn into a 3-μl microscopy chamber in which taxol-stabilized, rhodamine-labeled MTs had been attached to the glass surface after coating of the glass with an affinity-purified mixture of liver motor proteins, as described previously (Murray et al. 2000). Many vesicles bound to the MTs. Upon addition of 50 μM ATP, examination of >800 MT-attached vesicles revealed that 25% immediately became motile (Fig. 2). These vesicles moved along the MTs at ∼0.7 μm/s, as described previously (Murray et al. 2000). Vesicles that were bound to the glass surface and not to MTs did not move upon ATP addition. Of the motile vesicles, 13% underwent fission, resulting in two daughter vesicles. None of the nonmotile vesicles underwent fission. During fission, one pole of the vesicle remained attached to the MT, whereas the opposite end advanced. Fission occurred over the next 10–15 s. In companion experiments (Fig. 2), vesicles were incubated for 1 min at 4°C with affinity-purified anti-ASGPR IgG or with nonimmune IgG. Cy2-labeled secondary antibody was then added, and incubation was continued for an additional 6 min. These vesicles were then drawn into a microscopy chamber as above. IgG addition nonspecifically reduced motility slightly, but had no effect on the ability of moving vesicles to undergo fission. In vesicles to which nonimmune IgG was added, motility was reduced to 19%, but 17% of these vesicles underwent fission. Of >1,200 MT-bound, receptor-tagged vesicles that were examined, 16% moved upon addition of ATP (Fig. 2 left) and 14% of these vesicles underwent fission (Fig. 2 right). Similar to results in the absence of receptor antibody, only MT-bound vesicles moved upon ATP addition, and none of the nonmotile vesicles underwent fission. Representative frames from one of these studies are shown in Fig. 3. Before addition of ATP (0 s), the arrowhead points to a single spherical vesicle that is attached to an MT and contains ligand and receptor. Within 8 s of ATP addition, this vesicle elongates along the MT and forms a constriction in the middle. Although there is substantial ligand in both portions of this vesicle, ∼97% of receptor is present in only one (arrowhead). By 19 s, the original vesicle has clearly separated into two vesicles of near equal size, with segregation of receptor to the vesicle on the right. In this case, the receptor-containing vesicle is motile, whereas the vesicle containing essentially only ligand appears to be fixed to the MT.
Figure 2.
MT-based motility and fission of endocytic vesicles. Fluorescent early endocytic vesicles were prepared after injection of a rat with Texas red–labeled ASOR. Endocytic vesicles were flowed into a 3-μl microscopy chamber in which taxol-stabilized MTs had been attached to the glass surface. Vesicle motility was examined after addition of 50 μM ATP. In some experiments, vesicles were preincubated with rabbit anti–rat ASGPR IgG directed against the receptor cytoplasmic tail, nonimmune mouse IgG, or mouse mAbs IgG or IgM against kinesin or cytoplasmic dynein. Distribution of antibody was visualized after addition of Cy2-labeled goat anti–rabbit IgG, Cy2-labeled goat anti–mouse IgG, or FITC-labeled anti–mouse IgM, as appropriate. The bars in the left graph indicate the percentage of MT-bound vesicles that moved upon ATP addition. The total number of MT-bound vesicles that were examined in each experiment is in parentheses. The bars in the right graph indicate the percentage of moving vesicles that underwent fission.
Figure 3.
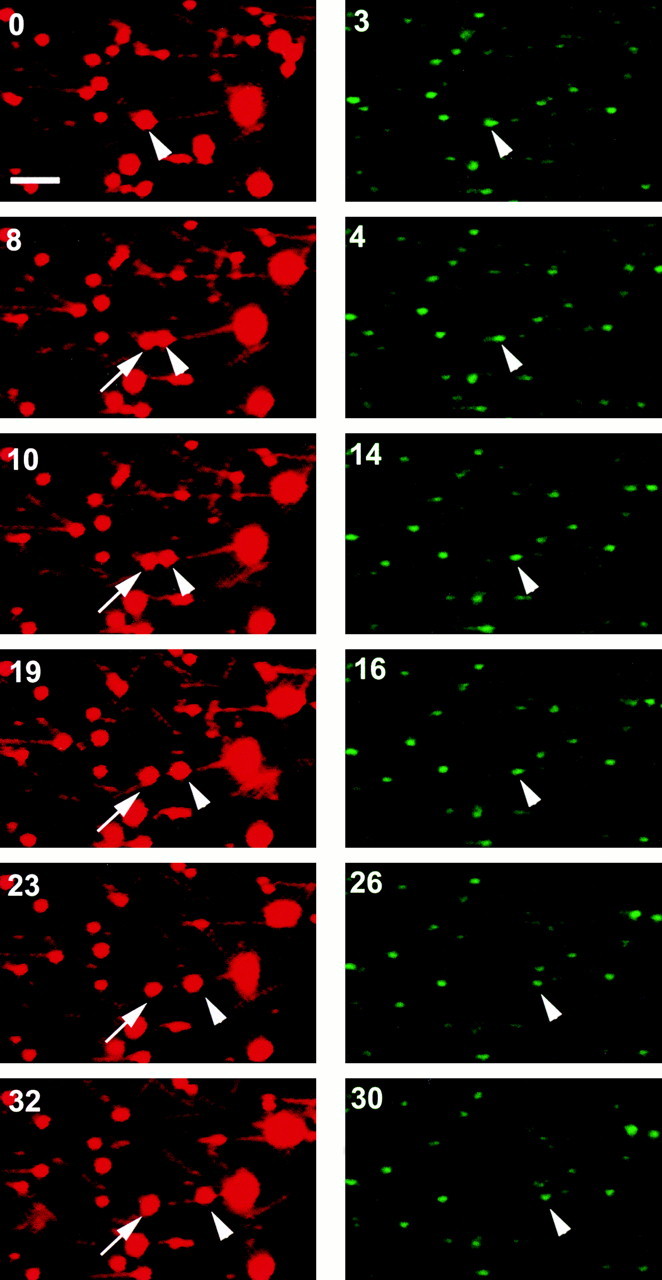
Fission of an endocytic vesicle on an MT after addition of ATP. Texas red–ASOR and rhodamine-labeled MTs are visualized in the left panels, whereas rabbit IgG against the cytoplasmic tail of the ASGPR after incubation with Cy2-labeled secondary antibody is visualized in the right panels. Time in seconds after the addition of 50 μM ATP is shown in the upper left. Before addition of ATP (0 s), the arrowhead points to a single vesicle that is attached to an MT and contains ligand and receptor. In subsequent panels, the arrow indicates the nonmotile daughter vesicle that contains only ∼3% of the original receptor fluorescence. This study corresponds to event 14 in Table . Bar, 10 μm.
Quantification of Vesicle Fission
Distribution of fluorescence was quantified in vesicles before and after fission. In nonmotile vesicles that did not undergo fission, recovery of fluorescence was essentially unchanged over time. When a group of 30 nonmotile vesicles was examined before and 10 s after ATP addition, recovery of ligand fluorescence was 105 ± 4.5% (mean ± SEM) of the initial level. In a group of 20 nonmotile vesicles labeled with fluorescent antibody to the receptor, recovery of fluorescence was 99.3 ± 1.1%. Similarly, fluorescence due to ligand or receptor in motile vesicles before fission was recovered completely in the daughter vesicles. In motile vesicles that underwent fission, as seen in Table , ligand was distributed in the daughter vesicles after each fission event. In most cases, the distribution was uneven. On average, the more fluorescent of the two vesicles contained ∼67% of the ligand in the original vesicle. In a few cases, ligand was about equally distributed. In this series of experiments, distribution of receptor was not monitored.
Table 1.
Fluorescence Density Recovery in Segregated Endocytic Vesicles in the Absence of Antibody
| Event number | Ligand vesicle 1 | Ligand vesicle 2 |
|---|---|---|
| 1 | 94.2 | 5.8 |
| 2 | 81.1 | 18.9 |
| 3 | 79.0 | 21.0 |
| 4 | 78.7 | 21.3 |
| 5 | 74.7 | 25.3 |
| 6 | 67.9 | 32.1 |
| 7 | 67.9 | 32.1 |
| 8 | 66.8 | 33.2 |
| 9 | 66.2 | 33.8 |
| 10 | 65.2 | 34.8 |
| 11 | 64.2 | 35.8 |
| 12 | 62.5 | 37.5 |
| 13 | 59.4 | 40.6 |
| 14 | 58.9 | 41.1 |
| 15 | 54.5 | 45.5 |
| 16 | 51.8 | 48.2 |
| 17 | 50.7 | 49.3 |
| Mean ± SEM | 67.3 ± 2.9 | 32.7 ± 2.9 |
Results are expressed as a percentage of the total recovered fluorescence for 17 randomly selected endocytic vesicles that underwent fission. In each row, the vesicle with the majority of ligand fluorescence was designated vesicle 1. In these experiments, receptor was not monitored.
Quantification of Segregation of Ligand and Receptor
To examine whether sorting of ligand from receptor occurred in this in vitro system, similar experiments were performed in which the receptor was tagged with a fluorescent antibody. In these experiments, in motile vesicles that underwent fission, on average ∼90% of receptor was segregated to one of the two daughter vesicles (Table ). In approximately half of these fission events, sorting of receptor into one daughter was virtually complete. The other daughter vesicle with ∼10% of the receptor contained almost 40% of the ligand (Table ).
Table 2.
Fluorescence Density Recovery in Segregated Endocytic Vesicles in the Presence of Receptor Antibody
| Daughter vesicle 1 | Daughter vesicle 2 | |||||
|---|---|---|---|---|---|---|
| Event | Ligand | Receptor | Motile | Ligand | Receptor | Motile |
| 1 | 84.4 | 100 | No | 15.6 | 0 | Yes |
| 2 | 83.2 | 100 | No | 16.8 | 0 | Yes |
| 3 | 79.9 | 100 | Yes | 20.1 | 0 | No |
| 4 | 74.4 | 100 | No | 25.6 | 0 | Yes |
| 5 | 73.8 | 100 | Yes | 26.2 | 0 | No |
| 6 | 73.7 | 100 | Yes | 26.3 | 0 | No |
| 7 | 63.8 | 100 | No | 36.2 | 0 | Yes |
| 8 | 60.1 | 100 | Yes | 39.9 | 0 | No |
| 9 | 57.9 | 100 | No | 42.1 | 0 | Yes |
| 10 | 56.4 | 100 | No | 43.6 | 0 | Yes |
| 11 | 30.5 | 100 | No | 69.5 | 0 | Yes |
| 12 | 15.6 | 100 | No | 84.4 | 0 | Yes |
| 13 | 14 | 100 | No | 86 | 0 | Yes |
| 14 | 68.3 | 96.6 | Yes | 31.7 | 3.4 | No |
| 15 | 77.7 | 93.4 | Yes | 22.3 | 6.6 | No |
| 16 | 80.7 | 93.1 | No | 19.3 | 6.9 | Yes |
| 17 | 55.5 | 92.6 | Yes | 44.5 | 7.4 | No |
| 18 | 21.5 | 88.1 | No | 78.5 | 11.9 | Yes |
| 19 | 33.8 | 84.3 | Yes | 66.2 | 15.7 | No |
| 20 | 0 | 80 | No | 100 | 20 | Yes |
| 21 | 53.7 | 77.4 | Yes | 46.3 | 22.6 | No |
| 22 | 82.8 | 72.2 | Yes | 17.2 | 27.8 | No |
| 23 | 100 | 72.1 | Yes | 0 | 27.9 | No |
| 24 | 100 | 72.1 | Yes | 0 | 27.9 | No |
| 25 | 100 | 69.4 | Yes | 0 | 30.6 | No |
| 26 | 100 | 62.1 | Yes | 0 | 37.9 | No |
| 27 | 65.9 | 60.1 | Yes | 34.1 | 39.9 | No |
| 28 | 0 | 60 | No | 100 | 40 | Yes |
| 29 | 56.8 | 55.9 | No | 43.2 | 44.1 | Yes |
| Mean ± SEM | 60.8 ± 5.6 | 87.2 ± 2.9 | 39.2 ± 5.6 | 12.8 ± 2.9 | ||
Results are expressed as a percentage of the total recovered fluorescence for 29 randomly selected endocytic vesicles that underwent fission in which ligand and receptor were simultaneously monitored. In each row, the vesicle with the majority of receptor fluorescence was designated as vesicle 1. Of the 29 fission events, 17 resulted in recovery of >90% of receptor in only 1 of the 2 daughter vesicles.
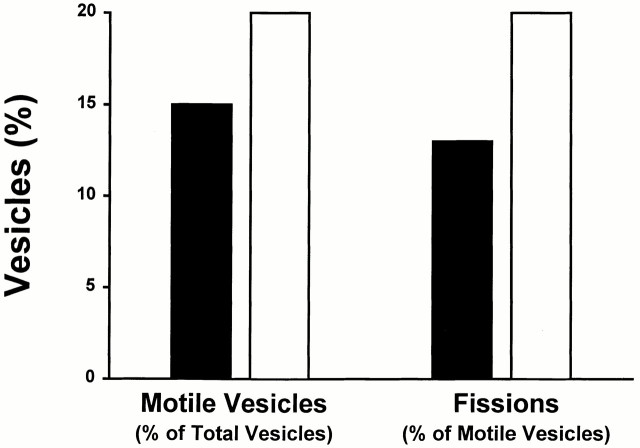
From a consideration of the geometry of vesicle–MT interaction, the possibility that two overlapping vesicles move to produce a spurious fission seems remote. To test this, we diluted the vesicle population by 50% before binding to MTs. This would be expected to reduce considerably the possibility of overlap. As seen in Table and Fig. 4, there was no reduction in motility and fission in experiments in which diluted vesicles were used.
Figure 4.
Effect of vesicle dilution on motility and fission. Vesicles were diluted by 50% before binding to MTs and addition of 50 μM ATP, to test the possibility that under more concentrated conditions, movement of two overlapping vesicles could produce a spurious fission. Black bars indicate results for 1,093 vesicles that were bound to MTs when concentrated; white bars indicate results for 169 vesicles that were bound to MTs when diluted. Motility and fission were determined as described in Materials and Methods.
Roles of Molecular Motors in Endocytic Vesicle Motility and Fission
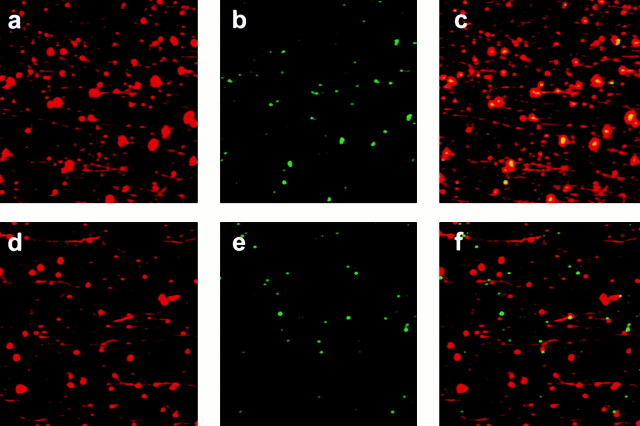
Experiments were performed to determine whether kinesins or cytoplasmic dynein were associated with endocytic vesicles. In these studies, rhodamine-labeled MTs were attached to the chamber surface using DEAE-dextran as we have described previously (Murray et al. 2000). Vesicles were incubated for 3 min at 4°C with mouse mAb IgM against kinesin or cytoplasmic dynein. FITC-labeled goat anti–mouse IgM was then added and incubation was continued for an additional 6 min. These vesicles were then drawn into a microscopy chamber as above. As seen in Fig. 5, kinesin antibody colocalized with endocytic vesicles that contained Texas red–ASOR; many of these vesicles appear to be bound to MTs. Although antibody to cytoplasmic dynein was clearly associated with vesicles, most of which were attached to MTs, few of these vesicles contained ASOR; those containing ASOR apparently colocalize with MTs (Fig. 5).
Figure 5.
Colocalization of early endocytic vesicles with kinesin, but not with cytoplasmic dynein. Two representative studies are shown in this figure in which Texas red–ASOR and rhodamine-labeled MTs are visualized (a and d). Mouse mAbs (IgM) against the kinesin heavy chain (b) and the dynein intermediate chain (e) were incubated with vesicles and visualized after addition of FITC-labeled goat anti–mouse IgM. Merged images reveal that the majority of ligand-containing vesicles are associated with kinesin (c) but not with dynein (f).
We also examined the effects of antikinesin and antidynein IgG and IgM antibodies on endocytic vesicle motility and fission (see Fig. 2). Motility and fission in the presence of either IgG or IgM antibodies to dynein were identical to controls. In contrast, in vesicles treated with IgG or IgM antibodies to kinesin, motility and fission were markedly reduced. There was no motility of vesicles that had been treated with antikinesin IgM, and only 4% of vesicles were motile when treated with antikinesin IgG compared with 19% of vesicles that had been treated with nonimmune IgG. Of the small number (26) of motile vesicles in this group, only 3 (11%) underwent fission. Although this rate of fission is similar to that seen in control studies, the actual number of fission events is markedly reduced. The finding of a small residual amount of fission suggests either that inhibition of kinesin function is incomplete in this experiment or that a nonkinesin motor is functional in this small population of vesicles.
Studies were also performed to examine the effect of vanadate, an inhibitor of cytoplasmic dynein (Kobayashi et al. 1978; Brady 1991; Vale et al. 1992; Fullerton et al. 1998), and AMP-PNP, an inhibitor of kinesins (Brady 1991; Fullerton et al. 1998), on vesicle motility and fission. Polarity-marked MTs (Murray et al. 2000) were used in these studies. In previous studies (Murray et al. 2000) performed in an MT gliding assay, we found that 1 mM AMP-PNP inhibited 80% of plus end–directed movement and only 20% of minus end–directed movement; 5 μM vanadate had the opposite effect. As seen in Table , upon addition to the microscopy chamber of 50 μM ATP in the absence of inhibitor, there were approximately equal amounts of minus and plus end–directed vesicle movements. Simultaneous addition of 50 μM ATP and 5 μM vanadate had no effect on the number or direction of motile vesicles. Vesicle fission and directional movement of daughter vesicles were also unaffected by vanadate inclusion. In contrast, simultaneous addition of 50 μM ATP and 1 mM AMP-PNP eliminated vesicle motility and fission (Table ).
Table 3.
Motility and Fission of Endocytic Vesicles on Polarity-marked MTs
| Inhibitor | No. of experiments | Total no. of vesicles | No. of moving vesicles | No. of minus end– directed vesicles | No. of plus end– directed vesicles | Total no. of fissions | No. of minus end– directed daughters | No. of plus end– directed daughters |
|---|---|---|---|---|---|---|---|---|
| None | 5 | 176 | 49 | 23 | 26 | 5 | 3 | 2 |
| Vanadate | 9 | 374 | 84 | 44 | 38 | 11 | 6 | 5 |
| AMP-PNP | 3 | 244 | 0 | 0 | 0 | 0 | 0 | 0 |
Fluorescent early endocytic vesicles were prepared after injection of a rat with Texas red–labeled ASOR. Endocytic vesicles were flowed into a 3-μl microscopy chamber in which taxol-stabilized MTs had been attached to the glass surface. Vesicle motility was examined after addition of 50 μM ATP. In some experiments, as indicated, 5 μM vanadate or 1 mM AMP-PNP was included with the ATP.
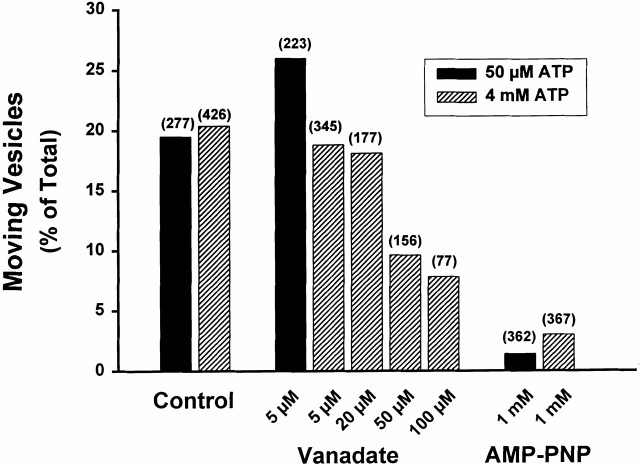
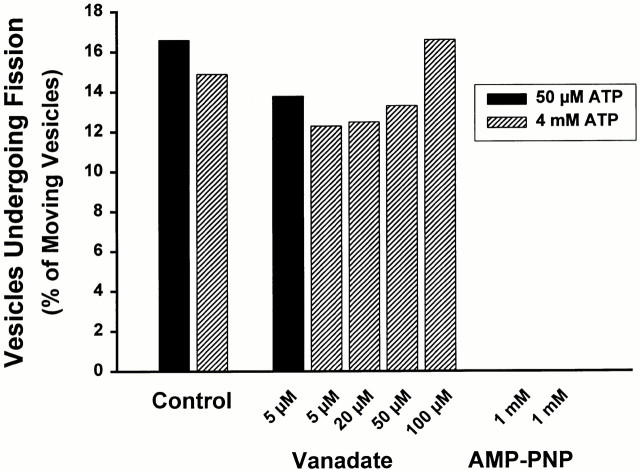
There is a possibility that proteins in the motor fraction that were used to coat the glass to which MTs were attached could bind substantial amounts of vanadate, thus falsely suggesting that it is without effect in the endosomal motility and fission assays. For this reason, a series of experiments was performed using MTs that were bound to glass via DEAE-dextran (Fig. 6). In addition, studies were performed at ATP concentrations of either 50 μM or 4 mM, in the absence of a regenerating system. As seen in Fig. 6, vanadate at concentrations as high as 20 μM in the presence of 4 mM ATP had no effect on vesicle motility or fission. Under these conditions, vanadate should preferentially inhibit dynein function. At concentrations of 50 μM or above, motility was reduced. Although the fraction of fission events in motile vesicles remained unchanged (Fig. 6 right), the absolute number of fissions was reduced in proportion to the reduction in motility. At these high concentrations, vanadate is known to inhibit many ATPases, including kinesins (Brady 1991; Vale et al. 1992). Vesicle motility was markedly reduced by 1 mM AMP-PNP even at high (4 mM) concentrations of ATP, and no fission events were seen. Under these conditions, AMP-PNP should preferentially inhibit function of kinesins.
Figure 6.
Effects of vanadate and AMP-PNP on vesicle motility and fission. MTs were bound to the glass chamber via DEAE-dextran. Studies were performed at ATP concentrations of 50 μM (black bars) or 4 mM (cross-hatched bars) in the absence of a regenerating system. The bars in the left graph indicate the percentage of MT-bound vesicles that moved upon ATP addition in the presence or absence of varied concentrations of vanadate or 1 mM AMP-PNP. The total number of MT-bound vesicles that were examined in each experiment is in parentheses. The bars in the right graph indicate the percentage of moving vesicles that underwent fission.
Discussion
These studies indicate that early endocytic vesicles have the capacity to undergo fission and receptor–ligand segregation in vitro, which is dependent on their binding to MTs and on their motility in the presence of ATP. Previous studies suggested that molecular motors might be involved in the interaction of endocytic vesicles with MTs (Wolkoff et al. 1984; Goltz et al. 1992; Jin and Snider 1993; Lafont et al. 1994; Satir 1994; Thatte et al. 1994; Oda et al. 1995; Hamm-Alvarez et al. 1996; Novikoff et al. 1996; Hamm-Alvarez and Sheetz 1998; Murray et al. 2000). However, a system for direct examination of the role of molecular motors in endocytic vesicle segregation and fission has been lacking. Here we show that early endocytic vesicles, as defined by the presence of both ligand and receptor in the same vesicle, are associated with kinesins, but not cytoplasmic dynein, and are capable of movement, receptor–ligand sorting, and fission in vitro. ATP-mediated motility of these vesicles is inhibited by antibodies to kinesins, but not by antibodies to cytoplasmic dynein. In addition, inclusion of vanadate at concentrations as high as 20 μM does not interfere with vesicle motility or fission, whereas inclusion of 1 mM AMP-PNP prevents both of these processes, even when ATP is in molar excess. When motility of vesicles was quantified on polarity-marked MTs, vesicles were seen to move approximately equally towards plus and minus ends. AMP-PNP inhibited motility in both directions, consistent with the possibility that plus and minus end–directed kinesins mediate vesicle motility and fission (Vale and Fletterick 1997; Hirokawa 1998; Goldstein and Philp 1999). Each fission event was associated with movement of only one daughter vesicle after fission along the MT. This could be an attribute peculiar to the method of preparing the endosomes.
Several previous investigations indicated that segregation of ligand from the ASGPR is incomplete, and that ∼20% of internalized ligand returns with the receptor back to the cell surface (diacytosis) (Samuelson et al. 1988; Chang and Chang 1989; Stockert et al. 1995). However, here, substantial amounts of ligand remained associated with receptor in one daughter vesicle after fission (Table ). We recently demonstrated that movement of early endocytic vesicles along MTs is oscillatory, with frequent stops and changes in direction (Murray et al. 2000). This could facilitate the distribution of these vesicles throughout the cytoplasm and may aid in the separation of ligand from receptor by allowing receptor-enriched daughter vesicles to undergo subsequent rounds of fission. Specifically, a more complete segregation of ligand from receptor, as occurs in vivo before recycling, could be achieved by an iterative process whereby the receptor-enriched daughter vesicles undergo multiple rounds of fission, as has been suggested by Maxfield and colleagues (Dunn et al. 1989; Dunn and Maxfield 1992; Mukherjee et al. 1997). Consistent with our in vitro data, each event would generate a new complementary daughter vesicle containing ∼40% of the ligand of the parent vesicle with little receptor. Eventually, this vesicle would move toward and fuse with lysosomes where ligand would be degraded.
This in vitro system has been optimized to study components that are required for processing of endocytic vesicles. Nevertheless, the percentage of MT-attached vesicles that become motile in our studies is somewhat less than might be predicted, which suggests that critical factors required for reconstitution of motility may be lost or that conditions of reconstitution are still suboptimal (Sheetz 1999). An important component of this system is the use of a liver-derived endocytic vesicle preparation that is depleted of contaminating ATPase activity (Murray et al. 2000). This has permitted reconstitution of vesicle motility and sorting at ATP concentrations as low as 50 μM, as used here. Although these low levels of ATP suggest that this nucleotide would not normally be limiting or regulatory in these processes, it is possible that higher levels are needed in vivo due to competition for ATP with other cellular reactions, as we have hypothesized previously (Murray et al. 2000). Our earlier studies suggested that late endocytic vesicles are associated with cytoplasmic dynein as they traffic to the lysosome along MTs. Another recent publication suggests a role for dynein in movement of late endocytic vesicles and in the transition of early to late endocytic vesicles, but not in early endosomal vesicle trafficking or receptor recycling (Valetti et al. 1999). When proteins were examined in endosomal populations that were isolated from rat liver, it was found that early and recycling endosomes were enriched in kinesin and dynein, whereas late endosomes were enriched in dynein only (Pol et al. 1997). It should be noted that none of these studies preclude functional association of other proteins and multiple motors with endocytic vesicles as well. The immunomicroscopic technology described here may allow determination of a point in which motility of endocytic vesicles undergoes a switch from kinesin to dynein dependency and should provide a means to identify and characterize other proteins that may be involved in vesicle movement and processing.
Acknowledgments
The authors thank Dr. Toshikazu Hamasaki and Mr. Michael Cammer for their helpful comments and suggestions.
This work was supported by National Institutes of Health grants DK41918 and DK41296.
Footnotes
Abbreviations used in this paper: AMP-PNP, 5′-adenylylimido-diphosphate; ASGPR, asialoglycoprotein receptor; ASOR, asialoorosomucoid; MT, microtubule.
References
- Brady S.T. Molecular motors in the nervous system. Neuron. 1991;7:521–533. doi: 10.1016/0896-6273(91)90365-7. [DOI] [PubMed] [Google Scholar]
- Chang T.M., Chang C.H. Diacytosis of asialoglycoprotein in isolated hepatocytes is dependent on the structure of ligand and cellular distribution of the receptors. Biochim. Biophys. Acta. 1989;1014:229–234. doi: 10.1016/0167-4889(89)90217-6. [DOI] [PubMed] [Google Scholar]
- Dunn K.W., Maxfield F.R. Delivery of ligands from sorting endosomes to late endosomes occurs by maturation of sorting endosomes. J. Cell Biol. 1992;117:301–310. doi: 10.1083/jcb.117.2.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn K.W., McGraw T.E., Maxfield F.R. Iterative fractionation of recycling receptors from lysosomally destined ligands in an early sorting endosome. J. Cell Biol. 1989;109:3303–3314. doi: 10.1083/jcb.109.6.3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forgac M. Structure, function and regulation of the vacuolar (H+)-ATPases. FEBS Lett. 1998;440:258–263. doi: 10.1016/s0014-5793(98)01425-2. [DOI] [PubMed] [Google Scholar]
- Fullerton A.T., Bau M.-Y., Conrad P.A., Bloom G.S. In vitro reconstitution of microtubule plus end-directed, GTPγS-sensitive motility of Golgi membranes. Mol. Biol. Cell. 1998;9:2699–2714. doi: 10.1091/mbc.9.10.2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geuze H.J., Slot J.W., Strous G.J.A.M., Peppard J., von Figura K., Hasilik A., Schwartz A.L. Intracellular receptor sorting during endocytosiscomparative immunoelectron microscopy of multiple receptors in rat liver. Cell. 1984;37:195–204. doi: 10.1016/0092-8674(84)90315-5. [DOI] [PubMed] [Google Scholar]
- Goldstein L.S.B., Philp A.V. The road less traveledemerging principles of kinesin motor utilization. Annu. Rev. Cell Dev. Biol. 1999;15:141–183. doi: 10.1146/annurev.cellbio.15.1.141. [DOI] [PubMed] [Google Scholar]
- Goltz J.S., Wolkoff A.W., Novikoff P.M., Stockert R.J., Satir P. A role for microtubules in sorting endocytic vesicles in rat hepatocytes. Proc. Natl. Acad. Sci. USA. 1992;89:7026–7030. doi: 10.1073/pnas.89.15.7026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamm-Alvarez S.F., Sheetz M.P. Microtubule-dependent vesicle transportmodulation of channel and transporter activity in liver and kidney. Physiol. Rev. 1998;78:1109–1129. doi: 10.1152/physrev.1998.78.4.1109. [DOI] [PubMed] [Google Scholar]
- Hamm-Alvarez S.F., Wei X., Berndt N., Runnegar M. Protein phosphatases independently regulate vesicle movement and microtubule subpopulations in hepatocytes. Am. J. Physiol. 1996;271:C929–C943. doi: 10.1152/ajpcell.1996.271.3.C929. [DOI] [PubMed] [Google Scholar]
- Harford J., Wolkoff A.W., Ashwell G., Klausner R.D. Monensin inhibits intracellular dissociation of asialoglycoproteins from their receptor. J. Cell Biol. 1983;96:1824–1828. doi: 10.1083/jcb.96.6.1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirokawa N. Kinesin and dynein superfamily proteins and the mechanism of organelle transport. Science. 1998;279:519–526. doi: 10.1126/science.279.5350.519. [DOI] [PubMed] [Google Scholar]
- Jin M., Snider M.D. Role of microtubules in transferrin receptor transport from the cell surface to endosomes and the Golgi complex. J. Biol. Chem. 1993;268:18390–18397. [PubMed] [Google Scholar]
- Kobayashi T., Martensen T., Nath J., Flavin M. Inhibition of dynein ATPase by vanadate, and its possible use as a probe for the role of dynein in cytoplasmic motility. Biochem. Biophys. Res. Commun. 1978;81:1313–1318. doi: 10.1016/0006-291x(78)91279-2. [DOI] [PubMed] [Google Scholar]
- Lafont F., Burkhardt J.K., Simons K. Involvement of microtubule motors in basolateral and apical transport in kidney cells. Nature. 1994;372:801–803. doi: 10.1038/372801a0. [DOI] [PubMed] [Google Scholar]
- Marsh M., McMahon R.T. The structural era of endocytosis. Science. 1999;285:215–220. doi: 10.1126/science.285.5425.215. [DOI] [PubMed] [Google Scholar]
- Mellman I. Endocytosis and molecular sorting. Annu. Rev. Cell Dev. Biol. 1996;12:575–625. doi: 10.1146/annurev.cellbio.12.1.575. [DOI] [PubMed] [Google Scholar]
- Mukherjee S., Ghosh R.N., Maxfield F.R. Endocytosis. Physiol. Rev. 1997;77:759–803. doi: 10.1152/physrev.1997.77.3.759. [DOI] [PubMed] [Google Scholar]
- Murray J.W., Bananis E., Wolkoff A.W. Reconstitution of ATP-dependent movement of endocytic vesicles along microtubules in vitroan oscillatory bidirectional process. Mol. Biol. Cell. 2000;11:419–433. doi: 10.1091/mbc.11.2.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novikoff P.M., Cammer M., Tao L., Oda H., Stockert R.J., Wolkoff A.W., Satir P. Three-dimensional organization of rat hepatocyte cytoskeletonrelation to the asialoglycoprotein endocytosis pathway. J. Cell Sci. 1996;109:21–32. doi: 10.1242/jcs.109.1.21. [DOI] [PubMed] [Google Scholar]
- Oda H., Stockert R.J., Collins C., Wang H., Novikoff P.M., Satir P., Wolkoff A.W. Interaction of the microtubule cytoskeleton with endocytic vesicles and cytoplasmic dynein in cultured rat hepatocytes. J. Biol. Chem. 1995;270:15242–15249. doi: 10.1074/jbc.270.25.15242. [DOI] [PubMed] [Google Scholar]
- Pol A., Ortega D., Enrich C. Identification of cytoskeleton-associated proteins in isolated rat liver endosomes. Biochem. J. 1997;327:741–746. doi: 10.1042/bj3270741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelson A.C., Stockert R.J., Novikoff A.B., Novikoff P.M., Saez J.S., Spray D.C., Wolkoff A.W. Influence of cytosolic pH on receptor-mediated endocytosis of asialoorosomucoid. Am. J. Physiol. 1988;254:C829–C838. doi: 10.1152/ajpcell.1988.254.6.C829. [DOI] [PubMed] [Google Scholar]
- Satir P. Motor molecules of the cytoskeleton. Possible functions in the hepatocyte. In: Arias I.M., Boyer J.L., Fausto N., Jakoby W.B., Schachter D.A., Shafritz D.A., editors. The LiverBiology and Pathobiology. Raven Press; New York: 1994. pp. 45–52. [Google Scholar]
- Sheetz M.P. Motor and cargo interactions. Eur. J. Biochem. 1999;262:19–25. doi: 10.1046/j.1432-1327.1999.00340.x. [DOI] [PubMed] [Google Scholar]
- Stockert R.J. The asialoglycoprotein receptorrelationships between structure, function, and expression. Physiol. Rev. 1995;75:591–609. doi: 10.1152/physrev.1995.75.3.591. [DOI] [PubMed] [Google Scholar]
- Stockert R.J., Potvin B., Tao L., Stanley P., Wolkoff A.W. Human hepatoma cell mutant defective in cell surface protein trafficking. J. Biol. Chem. 1995;270:16107–16113. doi: 10.1074/jbc.270.27.16107. [DOI] [PubMed] [Google Scholar]
- Thatte H.S., Bridges K.R., Golan D.E. Microtubule inhibitors differentially affect translational movement, cell surface expression, and endocytosis of transferrin receptors in K562 cells. J. Cell. Physiol. 1994;160:345–357. doi: 10.1002/jcp.1041600216. [DOI] [PubMed] [Google Scholar]
- Vale R.D., Fletterick R.J. The design plan of kinesin motors. Annu. Rev. Cell Dev. Biol. 1997;13:745–777. doi: 10.1146/annurev.cellbio.13.1.745. [DOI] [PubMed] [Google Scholar]
- Vale R.D., Malik F., Brown D. Directional instability of microtubule transport in the presence of kinesin and dynein, two opposite polarity motor proteins. J. Cell Biol. 1992;119:1589–1596. doi: 10.1083/jcb.119.6.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valetti C., Wetzel D., Schrader M., Hasbani M.J., Gill S.R., Kreis T.E., Schroer T.A. Role of dynactin in endocytic trafficeffects of dynamitin overexpression and colocalization with CLIP-170. Mol. Biol. Cell. 1999;10:4107–4120. doi: 10.1091/mbc.10.12.4107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolkoff A.W., Klausner R.D., Ashwell G., Harford J. Intracellular segregation of asialoglycoproteins and their receptora prelysosomal event subsequent to dissociation of the ligand–receptor complex. J. Cell Biol. 1984;98:375–381. doi: 10.1083/jcb.98.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]