Abstract
Prior studies on receptor recycling through late endosomes and the TGN have suggested that such traffic may be largely limited to specialized proteins that reside in these organelles. We present evidence that efficient recycling along this pathway is functionally important for nonresident proteins. P-selectin, a transmembrane cell adhesion protein involved in inflammation, is sorted from recycling cell surface receptors (e.g., low density lipoprotein [LDL] receptor) in endosomes, and is transported from the cell surface to the TGN with a half-time of 20–25 min, six to seven times faster than LDL receptor. Native P-selectin colocalizes with LDL, which is efficiently transported to lysosomes, for 20 min after internalization, but a deletion mutant deficient in endosomal sorting activity rapidly separates from the LDL pathway. Thus, P-selectin is sorted from LDL receptor in early endosomes, driving P-selectin rapidly into late endosomes. P-selectin then recycles to the TGN as efficiently as other receptors. Thus, the primary effect of early endosomal sorting of P-selectin is its rapid delivery to the TGN, with rapid turnover in lysosomes a secondary effect of frequent passage through late endosomes. This endosomal sorting event provides a mechanism for efficiently recycling secretory granule membrane proteins and, more generally, for downregulating cell surface receptors.
Keywords: endocytosis, protein sorting, receptors, recycling, secretory granule
Introduction
Mammalian cells internalize a large quantity of plasma membrane-associated and soluble molecules, and sort these molecules into recycling or lysosomal pathways. A great deal of this sorting occurs in early sorting endosomes, which segregate most of the membrane lipids and proteins, and a small fraction of the soluble contents, into tubular extensions that give rise to recycling endosomes (Gruenberg and Maxfield 1995; Mellman 1996). In nonpolarized cells, the contents of recycling endosomes then return to the cell surface. Most of the soluble contents of sorting endosomes, and a small fraction of the membrane, are collected into endosomal carrier vesicles (Aniento et al. 1993; maturing endosomes; Dunn et al. 1989), which are transported to, and fuse with, late endosomes (Gruenberg and Maxfield 1995; Mellman 1996).
Virtually all of the soluble contents of late endosomes are delivered to lysosomes for degradation. However, a number of membrane proteins recycle to the TGN (Snider and Rogers 1985; Duncan and Kornfeld 1988; Jin et al. 1989; Green and Kelly 1992; Bos et al. 1995; Mallet and Maxfield 1999). This allows receptors that fail to recycle to the cell surface from early endosomes to recycle to the cell surface instead via late endosomes, the TGN, and the secretory pathway. This process increases the overall recycling efficiency for cell surface receptors significantly (Green and Kelly 1992; Fig. 1 A). Recycling from late endosomes to the TGN plays a particularly important role in the trafficking of mannose 6-phosphate receptors (MPR). The cation-independent (CI) and cation-dependent MPR carry nascent lysosomal enzymes from the TGN to late endosomes. The enzymes dissociate from the receptors at the low pH of late endosomes, and the receptors are then recycled efficiently to the TGN to mediate additional rounds of enzyme sorting (Goda and Pfeffer 1988; Lombardi et al. 1993). The high efficiency of MPR recycling from late endosomes is due at least in part to a cytoplasmic sorting determinant (Schweizer et al. 1997) recognized by a sorting protein, TIP47 (Diaz and Pfeffer 1998), in late endosomes.
Figure 1.

Trafficking of internalized LDL receptor in PC12 cells, and models for trafficking of internalized P-selectin. A, Trafficking of LDL receptor. Numbers denote the probabilities that internalized LDL receptors will enter each pathway, and are derived from the measured rates of transport to sorting endosomes (t1/2 = 3–4 min), to the TGN via late endosomes (t1/2 = 2.5–3 h), and to lysosomes (t1/2 = 20 h; Green and Kelly 1992). S.E., sorting endosome; L.E., late endosome; TGN, trans Golgi Network; Lys., lysosome; P.M., plasma membrane (recycling endosomes and endosomal carrier vesicles are not shown). B and C, Models for P-selectin traffic. The observation that P-selectin reaches lysosomes six to seven times faster (t1/2 = 3 h) than LDL receptor in PC12 cells (Green et al. 1994) can be accounted for either by sorting of P-selectin from LDL receptor only in early sorting endosomes (B), or by sorting of P-selectin from LDL receptor only in late endosomes (C). Large arrows indicate the selective step in each model. Early sorting (B) predicts rapid recycling of P-selectin through the TGN, whereas late sorting (C) precludes extensive recycling through the TGN.
A different endosomal sorting phenotype was identified through studies of P-selectin, a member of a family of cell adhesion proteins expressed in the vascular system (McEver et al. 1995). Whereas expression of the cell adhesion protein E-cadherin at the cell surface is regulated by internalization of those molecules not bound to substrates (Le et al. 1999), P-selectin is stored in secretory granules in platelets and endothelial cells. Stimulation of granule exocytosis in these cells transfers P-selectin rapidly to the plasma membrane, allowing binding of circulating neutrophils and monocytes expressing the P-selectin ligand (McEver and Cummings 1997). This interaction contributes to extravasation of the leukocytes, an early event in the inflammatory response. P-selectin is rapidly internalized at rates similar to other recycling receptors. However, in addition to cytoplasmic sorting determinants that mediate targeting to secretory granules and rapid internalization from the cell surface (Disdier et al. 1992; Setiadi et al. 1995; Modderman et al. 1998), P-selectin contains a sorting determinant that mediates sorting of internalized P-selectin away from typical recycling receptors, such as low density lipoprotein (LDL) receptor and transferrin receptor, leading to rapid delivery of P-selectin to lysosomes (Green et al. 1994; Blagoveshchenskaya et al. 1998; Straley et al. 1998). Its half-life of 2.3–3 h in transfected cell lines (Green et al. 1994; Straley et al. 1998) is several times shorter than the half-lives of the LDL receptor or transferrin receptor. This sorting event affords temporal regulation of the adhesive activity of P-selectin after stimulation of granule exocytosis.
Whereas it is clear that sorting of P-selectin from efficiently recycled receptors (e.g., LDL receptor) occurs in endosomes (Green et al. 1994), it is not possible to determine from the existing data whether this sorting occurs in early endosomes, in late endosomes, or a combination of the two. If sorting of P-selectin occurs only in early endosomes, it would reach late endosomes faster than LDL receptor, and could then recycle from late endosomes to the TGN with the same efficiency as LDL receptor (Green and Kelly 1992; Green et al. 1994; Fig. 1 B). This hypothesis predicts rapid transport of P-selectin from the cell surface through the TGN, which would enable the protein to recycle efficiently into nascent secretory granules. Indeed, internalized anti–P-selectin antibodies can reach Weibel-Palade bodies (secretory granules) in cultured endothelial cells (Subramaniam et al. 1993; Kobayashi et al. 2000), although with unknown efficiency. In this model, rapid turnover of P-selectin would be due to frequent trips through late endosomes, but with the same low probability as LDL receptor of entering lysosomes on each trip. Alternatively, if P-selectin recycles from sorting endosomes to the cell surface as efficiently as LDL receptor, the short half-life of P-selectin would require selective delivery of P-selectin from late endosomes to lysosomes, which would preclude extensive recycling to the TGN (Fig. 1 C).
To determine the location and consequences of endosomal sorting of P-selectin, we have measured transport of P-selectin in PC12 cells, where we previously measured transport of LDL receptor, cation-independent mannose 6-phosphate receptor (CI-MPR), the synaptic vesicle protein synaptophysin (Green and Kelly 1992), and in CHO cells, where we have characterized endosomal sorting mutants of P-selectin (Green et al. 1994). Our results strongly support the hypothesis that constitutive endosomal sorting of P-selectin from recycling receptors occurs only in early sorting endosomes, and not in late endosomes. The implications of these findings for sorting of membrane proteins in endosomes, and for trafficking of secretory granule membrane proteins, are discussed.
Materials and Methods
Cells
Ricin-resistant PC12 A1 cells and transfected clones derived from them were grown as originally described (Green and Kelly 1990), and were plated for most experiments on dishes precoated with poly-d-lysine. Secretory granule exocytosis was stimulated by adding KCl to a final concentration of 50 mM to cells in complete growth medium. CHO cells expressing P-selectin or P-selectin −ΔC1 (a deletion of the 11-amino acid residue C1 region of the cytoplasmic domain, previously denoted P-selectin ΔD762-S772; Setiadi et al. 1995; or P-selectin-C2 (Green et al. 1994) were maintained in normal or LDL-depleted medium as described (Green et al. 1994).
Expression of Human P-Selectin in PC12 A1 Cells
PC12 A1 cells in 6-cm plates were transfected with 6 μg of the plasmid pCDL-SRα containing the human P-selectin cDNA (Straley et al. 1998) and 0.1 μg pSV2neo, using the lipofection protocol of Muller et al. 1990. Cells were passaged into medium containing 0.4 mg/ml G418 (GIBCO-BRL) 2 d after transfection. Drug-resistant clones were picked, expanded, and screened for expression of P-selectin by immunofluorescence microscopy (see below) using mixed monoclonal anti–P-selectin antibodies (Straley et al. 1998).
Radioisotopes
UDP[3H]galactose was purchased from Amersham Pharmacia Biotech and from American Radiochemical Corporation. [125I]sodium iodide and ExpreSS label [35S]amino acid mixture were from New England Biolabs. [3H]glucosamine was purchased from American Radiochemical Corporation. Na35SO4 was from NEN Life Sciences.
Enzymes
Recombinant Flavobacterium meningosepticum peptide:N glycosidase F produced in E. coli (N-Glycanase) was purchased from Roche or from Glyko, Inc. Streptococcus pneumoniae β-galactosidase was purchased from Roche or from Prozyme. Bovine milk galactosyltransferase was from Sigma-Aldrich.
Antibodies
mAbs S12, G1, G5, and 2B8 recognizing P-selectin, and goat polyclonal antiserum recognizing P-selectin, were generously supplied by Rodger McEver (University of Oklahoma, Oklahoma City, OK). Antipeptide antiserum recognizing the COOH terminus of P-selectin was affinity-purified as described (Green et al. 1994). Purified antipeptide antibody was biotinylated by reaction with a 10-fold molar excess of sulfosuccinimidyl 6-(biotinamido) hexanoate (Pierce Chemical Co) for 30 min in PBS, followed by addition of a 20-fold molar excess of glycine to quench remaining reactive groups. S12 antibody was labeled with Alexa 488 (Molecular Probes) according to the manufacturer's protocol. Polyclonal rabbit antiserum was generated by a commercial service (Covance) against soluble P-selectin (Ushiyama et al. 1993), and showed specificity in immunofluorescence and immunoprecipitation experiments identical to that obtained with the mAbs. Polyclonal rabbit antibodies against rat or bovine CI-MPR were from William Brown (Cornell University, Ithaca, NY). Rabbit antiserum recognizing synaptophysin was from Regis Kelly (University of California, San Francisco, CA). Rabbit antiserum recognizing chromogranin A was from John Hutton (University of Colorado, Denver, CO). mAb H68.4 recognizing transferrin receptor was provided by Ian Trowbridge (Scripps, La Jolla, CA). Goat anti–rabbit IgG, rabbit anti–mouse IgG, Texas red-conjugated goat anti–rabbit IgG and FITC-conjugated goat anti–mouse IgG were from Cappel. Oregon green- and Texas red-conjugated deglycosylated egg avidin (Neutralite) were from Molecular Probes.
Immunofluorescence Labeling
Immunofluorescence labeling was performed as previously described (Straley et al. 1998). For screening, cells were incubated for 1 h with a mixture of ascites fluid containing mAbs S12, G5, and 2B8. For double-labeling experiments, primary antibodies recognizing chromogranin A, synaptophysin, CI-MPR, and transferrin receptor were applied, followed by the appropriate secondary antibody. After labeling endogenous proteins, cells were incubated in preimmune rabbit serum (1:50) for 15 min, and were then labeled with biotinylated anti–P-selectin COOH-terminal peptide antibody diluted 1:200 in buffer containing rabbit preimmune serum 1:50. After washing, samples were labeled with Oregon green or Texas red avidin 1:300 and washed again, with 10 μg/ml free biotin included in the last IF buffer wash. Cells were then washed three times in PBS, rinsed in distilled water, and mounted in ProLong (Molecular Probes). As a positive control for colocalization, cells were labeled only with biotinylated anti–P-selectin COOH-terminal peptide antibody, followed by a mixture of Oregon green and Texas red avidin.
Image Collection and Analysis
Immunofluorescence images were collected using a Zeiss Axioplan 2 microscope equipped with a 63× Apochromat objective lens, n.a. 1.4, a Hamamatsu C4742-95 CCD camera, in some cases fitted with a Zeiss 4× magnifying adapter (final pixel size, 0.0266 μm) and OpenLab (Improvision) software. For PC12 cells, 30 conventional images were collected at 0.2-μm intervals, in the Texas red and fluorescein channels sequentially, using an automation to drive the microscope controls. Digital deconvolution of one image near the middle of each series was performed using the OpenLab constrained iteration (confocal) algorithm, using 10–12 neighbors (20–24 images) to deconvolve each image. Grayscale matching and merging of deconvolved images was performed using Adobe Photoshop. Several cells were analyzed for each labeling condition, and representative results are presented.
Uptake of LDL and S12 Antibody
CHO cells expressing native P-selectin or P-selectin−ΔC1 were passaged onto coverslips and grown overnight. The medium was replaced with LDL-depleted medium and the cells were grown for an additional 16–18 h to increase surface expression of LDL receptor. After incubation for 10 min at 37°C in PBS containing 1 mg/ml glucose and 0.2% BSA (PBS/BSA), cells were incubated for 5 min at 37°C in PBS/BSA containing 10 μg/ml AlexaFluor 488-labeled S12 antibody and 5 μg/ml DiI-LDL (Molecular Probes). Cells were rapidly rinsed in PBS/BSA and recultured in PBS/BSA for 5–60 min. Cells were then rinsed in PBS and fixed in 3% formaldehyde; 100 mM NaPO4, pH 7.4, for 20 min at room temperature. Fixed cells were washed three times for a total of 5 min in PBS, rinsed once in distilled water, and were then mounted in FluorMount G (Fisher Scientific) containing 2.5 mg/ml n-propyl gallate (Sigma-Aldrich). Images were collected using FITC and Cy3 filter sets for Alexa 488 and DiI, respectively. Four or five fields, containing a total of 15–21 cells, were analyzed for each data point. In each field, 13 images, beginning at a focal plane 0.6–1.0 μm beneath the base of the cells, were collected at 0.2-μm intervals using a 63× objective lens (final pixel size, 0.1064 μm). Six or seven sequential images, starting from the base of the cell and comprising 80–90% of the LDL-labeled structures in each cell, were deconvolved using 2 neighbors (above and below) in each channel. The deconvolved images from each channel were merged, and transferred to Photoshop to compress the grayscales and overlay the two channels. To quantitate the fraction of LDL-labeled vesicles that contained both labels, prints were made of the red channel (LDL) images and total LDL-positive vesicles were counted. The red channel image was then compared with the merged image to identify vesicles that also contained S12 antibody, and double-labeled structures were counted. An average of 926 LDL-positive vesicles were counted for each sample, and the data are expressed as the percentage of these vesicles that also contained S12 antibody. The entire experiment was performed twice, with similar results.
Metabolic Labeling
Metabolic labeling with [3H]glucosamine or [35S]amino acids was carried out for 1–16 h as previously described (Green and Kelly 1992; Straley et al. 1998).
Exogalactosylation
Exogalactosylation of cells was carried out as described (Green and Kelly 1992; Duncan and Kornfeld 1988), with minor modifications. Cells on polylysine-coated 15- or 10-cm tissue culture plates were labeled at 80–90% confluence, usually 1–2 d after plating. Cells were cooled to 4°C and washed twice with cold exogalactosylation buffer (Green and Kelly 1992; MEM without glucose or bicarbonate containing 0.2% BSA, 6 mM MnCl2, pH 6.8). The labeling medium contained exogalactosylation buffer with the following additions: 1.7 U/ml galactosyltransferase, and 80–100 μCi/ml UDP[3H]galactose. Cells were labeled with 3 ml/15-cm dish for 60 min. At the end of the labeling, cells were washed twice with PBS, 1 mg/ml glucose, and were then recultured in complete culture medium at 37°C. Cells were then washed twice with PBS lacking divalent cations before harvesting in PBS containing 5 mM EDTA by trituration.
Lysis and Immunoprecipitation
Cells were lysed, and proteins were immunoprecipitated as previously described (Green et al. 1994; Straley et al. 1998). For biotinylated cells, 5 mg/ml iodoacetamide was included in the lysis buffer. Immune complexes were recovered using protein A–Sepharose or protein G–Sepharose (Amersham Pharmacia Biotech), and were eluted by heating in a boiling water bath for 3 min into either 25 μl N-glycanase buffer (1% octylglucopyranoside, 0.2% SDS, 40 mM Tris, pH 8.0, 5 mM EDTA, 1% β-mercaptoethanol) or 40 μl gel sample buffer (4% SDS, 125 mM Tris pH 6.95, 10 mM EDTA, 15% sucrose, 40 mM dithiothreitol). Immunoprecipitations with each of the antibodies and cell lines used were titrated to recover 90–95% of antigen, as assessed by second round precipitations.
Analysis of N-linked Oligosaccharides after Exogalactosylation
N-linked oligosaccharides were analyzed using a minor modification (Green and Kelly 1990, Green and Kelly 1992) of the method of Duncan and Kornfeld 1988, except that β-galactosidase digestion of oligosaccharides was carried out in 50 μl 50 mM MES, pH 6.0, or 50 mM NaH2PO4, pH 6. The digested samples were analyzed by gel filtration chromatography on Sephadex G25. 350-μl fractions were counted in 4 ml ReadySafe (Beckman) scintillation fluid.
Electrophoresis, Fluorography, and Quantitation of Radioactivity in Gel Bands
Proteins were analyzed on SDS polyacrylamide gels containing 7.5% acrylamide for P-selectin, or a gradient for analysis of multiple proteins. For fluorography, gels were fixed in 30% methanol/5% acetic acid, rinsed with water and impregnated with 0.5 M sodium salicylate. The dried gels were exposed to Fuji X ray film at −70°C.
Supplemental Material
Control experiments and descriptions of the relevant methods demonstrating that P-selectin resides in functional secretory granules, is rapidly internalized, and is rapidly degraded in PC12 A1-PS cells, and evidence that binding of mAb S12 to P-selectin is stable at low pH can be found at http//:www.jcb.org/cgi/content/full/151/1/107/DC1.
Results
Immunofluorescence Localization of Antigens in PC12 A1-PS Cells
Preliminary experiments indicated that the original clones used to study P-selectin traffic in PC12 cells (Green et al. 1994) did not have sufficiently high cell surface expression levels for these experiments. Therefore, a clone of PC12 A1 cells stably expressing higher levels of P-selectin was generated and designated PC12 A1-PS. Indirect immunofluorescence labeling of PC12 A1-PS cells was performed to determine the distribution of P-selectin with respect to other relevant organelle marker proteins. Epifluorescence and confocal microscopy show extensive apparent overlap of almost all markers in these cells, so digital deconvolution was used. As a control for spatial resolution and Z-axis registration, cells were labeled with biotinylated anti–P-selectin antibody and a combination of Oregon green- and Texas red-labeled avidin. After digital deconvolution, the Oregon green label (Fig. 2 A) and the Texas red label (Fig. 2 B) show nearly identical patterns, as demonstrated in the overlay (Fig. 2 C). In double-label experiments, P-selectin (Fig. 2 D) and chromogranin A (Fig. 2 E), a soluble content marker of regulated secretory granules in neuroendocrine cells, showed significant overlap (Fig. 2 F). Many structures contained both labels, but with different relative intensities in the two channels (Fig. 2D and Fig. E). Synaptophysin was localized to punctate structures in the cell periphery (Fig. 2 H). There was little colocalization between P-selectin and synaptophysin (Fig. 2, G–I), and in areas where there was apparent overlap (Fig. 2 I), the size and shape of the structures appeared different (Fig. 2G and Fig. H). Transferrin receptor was found in peripheral structures, and in a concentration of structures in the juxtanuclear region (Fig. 2 K). P-selectin was rarely detected in structures enriched in transferrin receptor (Fig. 2, J–L). CI-MPR antibodies labeled less numerous structures with a distinct juxtanuclear accumulation (Fig. 2 N) typical of late endosomes. There was little overlap in the distributions of P-selectin and CI-MPR (Fig. 2, M–O). These experiments show that P-selectin is primarily concentrated in secretory granules in PC12 cells at steady state.
Figure 2.

Immunofluorescence localization of organelle markers in PC12 A1-PS cells. A–C, As a control for colocalization using digital deconvolution, PC12 A1-PS cells were fixed and labeled with biotinylated anti–P-selectin COOH-terminal peptide antibody, followed by a mixture of Oregon green-avidin (A) and Texas red-avidin (B). Digital deconvolution was performed on a 25 × 0.2 μm step Z-series for each label and the images were merged (C). D–O, PC12 A1-PS cells were processed for indirect immunofluorescence microscopy and labeled with antibodies recognizing chromogranin A (E and F), synaptophysin (H and I), transferrin receptor (K and L), or CI-MPR (N and O), followed by the appropriate secondary antibody. Cells were then labeled with biotinylated anti–P-selectin COOH-terminal peptide antibody, followed by labeled avidin (D, G, J, and M). For clarity, P-selectin localization (D, G, J, and M) is shown in the green channel, whereas the endogenous proteins (E, H, K, and N) are shown in the red channel of the merged images (F, I, L, and O). Colocalization appears yellow. Bar, 2 μm.
Immunoprecipitation of P-Selectin, Synaptophysin, and CI-MPR
The selectivity of each of the antibodies was assessed by immunoprecipitation of labeled proteins. PC12 A1-PS cells were labeled metabolically with [3H]glucosamine (Fig. 3 A) or [35S]amino acids (Fig. 3 B). P-selectin, CI-MPR, and synaptophysin were sequentially immunoprecipitated from the cell lysates, and the immunoprecipitates analyzed by SDS-PAGE and fluorography. The bulk of the radioactivity in each immunoprecipitate migrated as a single band of the appropriate mobility.
Figure 3.

Immunoprecipitation of metabolically labeled proteins from PC12 A1-L14 cells. PC12 A1-PS cells were labeled with [3H]glucosamine (A) or [35S]amino acids (B) for 20 h before detergent lysis. P-selectin (PS), CI-MPR (MPR), and synaptophysin (p38) were sequentially immunoprecipitated from the lysates, resolved by electrophoresis on 7–15% SDS-polyacrylamide gels, and visualized by fluorography. Numbers indicate the position of molecular mass markers in kilodaltons. Virtually all of the radioactivity immunoprecipitated by each antibody migrated as a single band of the appropriate mobility.
Sorting of P-Selectin in PC12 A1-PS Cells
Control experiments were performed to insure that sorting of P-selectin in PC12 A1-PS cells occurred as described previously (Green et al. 1994), and can be seen at http//:www.jcb.org/cgi/content/full/151/1/107/DC1. Stimulation of granule exocytosis for two minutes increased the fraction of P-selectin accessible to biotin labeling at the cell surface, whereas this fraction decreased after four minutes of stimulation (see online supplemental Figure S1, http//:www.jcb.org/cgi/content/full/151/1/107/DC1). The transient increase in cell surface expression confirms sorting of P-selectin to functional secretory granules, and the subsequent rapid decrease suggests that internalization of granule membrane-derived P-selectin is rapid. Similar results have been obtained in primary cultures of endothelial cells (Hattori et al. 1989). Since ∼25% of the P-selectin synthesized in PC12 cells is packaged into secretory granules (Green et al. 1994), and these cells rapidly release only 15% of their granule pool upon stimulation (Lowe et al. 1988), the amount of P-selectin delivered to the cell surface by granule exocytosis was expected to be ∼4% of the total. Although it was not possible to measure internalization of this small pool specifically, we measured internalization of the total steady state cell surface P-selectin pool, which includes protein delivered to the cell surface continuously via the constitutive secretory pathway and by recycling from early endosomes (Green et al. 1994; and this study). The rate of internalization was found to be equal to the rate measured in transfected NRK fibroblasts (see online supplemental Figure S2, http//:www.jcb.org/cgi/content/ full/151/1/107/DC1). Degradation of metabolically labeled P-selectin occurred with a half-time of 3.8 h, similar to its turnover in other transfected cells (see online supplemental Figure S3, http//:www.jcb.org/cgi/content/full/151/1/107/DC1).
Rapid Transport of P-Selectin from the Cell Surface to the Golgi Apparatus
The rate of P-selectin transport from the cell surface to the TGN was measured using an assay that is a minor modification (Green and Kelly 1992) of the method of Duncan and Kornfeld 1988. PC12 A1-PS cells, which are deficient in the incorporation of galactose into glycoproteins (Green and Kelly 1990), were exogalactosylated with UDP[3H]galactose and galactosyltransferase at 4°C to label cell surface glycoproteins. After reculture at 37°C for varying times, cells were lysed, and specific glycoproteins were immunoprecipitated and digested exhaustively with N-glycosidase F to release N-linked oligosaccharide chains. Released oligosaccharides were isolated by acid precipitation of the proteins, followed by gel filtration chromatography of the supernatant, and were then digested exhaustively with β-galactosidase. The free galactose liberated by β-galactosidase was separated from intact oligosaccharides by gel filtration chromatography, and the radioactivity in the two peaks was quantitated. Only terminal galactose residues are sensitive to β-galactosidase, so additional glycosylation of these residues by any of several Golgi glycosyltransferases that act on terminal galactose (Green and Kelly 1990) renders the labeled galactose resistant to removal from the oligosaccharides. The readout of the assay is the percentage of galactose that has acquired resistance to β-galactosidase digestion, reflecting its passage through the TGN.
Previous studies of synaptophysin, CI-MPR, and LDL receptor showed that the fraction of galactose resistant to β-galactosidase reached a plateau by four hours of reculture for all three proteins (Green and Kelly 1992). The plateau levels for P-selectin were determined by reculturing the cells for four or six hours, to provide a basis for subsequent measurement of the rate of transport (Fig. 4). The fractions of galactose resistant to β-galactosidase at four hours of reculture were: P-selectin, 53 ± 11%; synaptophysin, 36 ± 3.7%; and CI-MPR, 35% (n = 2; Fig. 4), and were defined as the maximum signal for each protein for the purpose of determining rates of transport.
Figure 4.
Transport of cell surface glycoproteins to the Golgi apparatus. Cell surface glycoproteins on PC12 A1-PS cells were labeled with galactosyltransferase and UDP[3H]galactose at 4°C and recultured for the indicated intervals at 37°C. P-selectin, synaptophysin, and CI-MPR were immunoprecipitated from the cell lysates, and oligosaccharides from the immunoprecipitates were analyzed as described in the text to determine the fraction of galactose that had acquired resistance to β-galactosidase, reflecting addition of terminal sugars in the TGN, during reculture. After a lag period estimated to be ∼25–30 min, the percentage of galactose resistant to β-galactosidase on oligosaccharides from P-selectin increased with a half-time of ∼20–25 min. Each point represents the mean ± SD of three independent experiments for P-selectin and synaptophysin, and the mean of two experiments for CI-MPR, except 20-min chase (n = 1), and 6-h chase (n = 2).
Preliminary experiments indicated that P-selectin acquired resistance to β-galactosidase much more rapidly than synaptophysin and CI-MPR, so the analysis focussed on short reculture periods. For all three proteins, the fraction of galactose resistance to β-galactosidase at 20 min of chase was similar to that measured in cells that were not recultured at all (t = 0), indicating a lag period preceding any significant transport of the labeled proteins to the TGN (Fig. 4). By 40 and 60 min of chase, the signals for synaptophysin and CI-MPR had increased, but the signal for P-selectin had increased to a greater extent. The 40- and 60-min measurements were extrapolated back to estimate a lag period of ∼25–30 min before acquisition of resistance to β-galactosidase, similar to the lag period for transport from the cell surface to the TGN observed using a different assay (Snider and Rogers 1985; Jin et al. 1989).
After this lag, P-selectin oligosaccharides acquired resistance to β-galactosidase rapidly, reaching 60% of the plateau level by 60 min of chase. In contrast, synaptophysin and CI-MPR oligosaccharides had reached only 35 and 25% of maximum levels, respectively, by 60 min (Fig. 4). Since the fraction of galactose resistant to β-galactosidase exceeds the half-maximal value for P-selectin by 60 min of chase, these data provide an estimate of the half-time for transport of the P-selectin pool labeled at the cell surface. We calculated this half-time to be ∼20–25 min for P-selectin, after correcting for the 25–30 min lag. Correcting for the lag period identified in the current study, we refine the value reported previously (Green and Kelly 1992) for the half-time for transport of synaptophysin, CI-MPR, and LDL receptor from the cell surface to the TGN to 2–2.5 h, which is slower than transport of P-selectin by a factor of six to seven.
Internalized LDL and Anti–P-Selectin Antibodies Follow the Same Pathway
To determine whether P-selectin was delivered to the TGN via late endosomes or directly from early endosomes, the transport pathway of internalized P-selectin was compared with that of internalized LDL, using mAb S12 to track internalized P-selectin. This antibody dissociated from P-selectin with a half-time of 156 min at 37°C and pH 5.5, conditions typical of late endosomes (Mellman 1996; and see online supplemental Figure S4, http//:www.jcb.org/cgi/content/full/151/1/107/DC1). CHO cells expressing P-selectin, or P-selectin-ΔC1, which is deficient in endosomal sorting (Green et al. 1994), were incubated for 5 min with DiI-LDL and Alexa 488-labeled mAb S12. Cells were recultured for up to 60 min before fixation. Epifluorescence images were collected and analyzed after digital deconvolution (see Materials and Methods). In both cell lines, S12 antibody was observed in numerous punctate structures throughout the cytoplasm, and in a concentration of structures at one pole of the nucleus, visible in epifluorescence, but not in the deconvolved images (our unpublished observation). The juxtanuclear staining was not prominent after 5 min of chase, but the pattern of S12 labeling did not change or diminish significantly between 10 and 60 min of chase. At early chase times, LDL was observed in a smaller number of structures, many of which contained S12 antibody, in both native P-selectin (Fig. 5 A) and P-selectin-ΔC1 cells (Fig. 5 B). After 20 min of reculture, few LDL-labeled structures in P-selectin-ΔC1 cells contained detectable levels of S12 antibody (Fig. 5 D), whereas in cells expressing native P-selectin, double-labeled structures persisted (Fig. 5 C). After 40 min of reculture, few double-labeled structures were seen in either cell line (Fig. 5E and Fig. F). Quantitation of double labeling on deconvolved images showed that LDL separated rapidly from S12 antibody in P-selectin-ΔC1 cells, whereas little separation occurred in cells expressing native P-selectin for 20 min (Fig. 6). The clear difference between the two cell lines indicates that binding of S12 antibody does not divert P-selectin-ΔC1 to the lysosomal pathway, which can occur when using cross-linking antibodies (Mellman and Plutner 1984; Gartung et al. 1985). This result indicates that native P-selectin, unlike the efficiently recycled P-selectin-ΔC1 mutant, becomes concentrated in LDL-containing carrier vesicles (maturing endosomes) with relatively high efficiency soon after internalization, indicating selective sorting in early endosomes for efficient delivery to late endosomes en route to the TGN.
Figure 5.
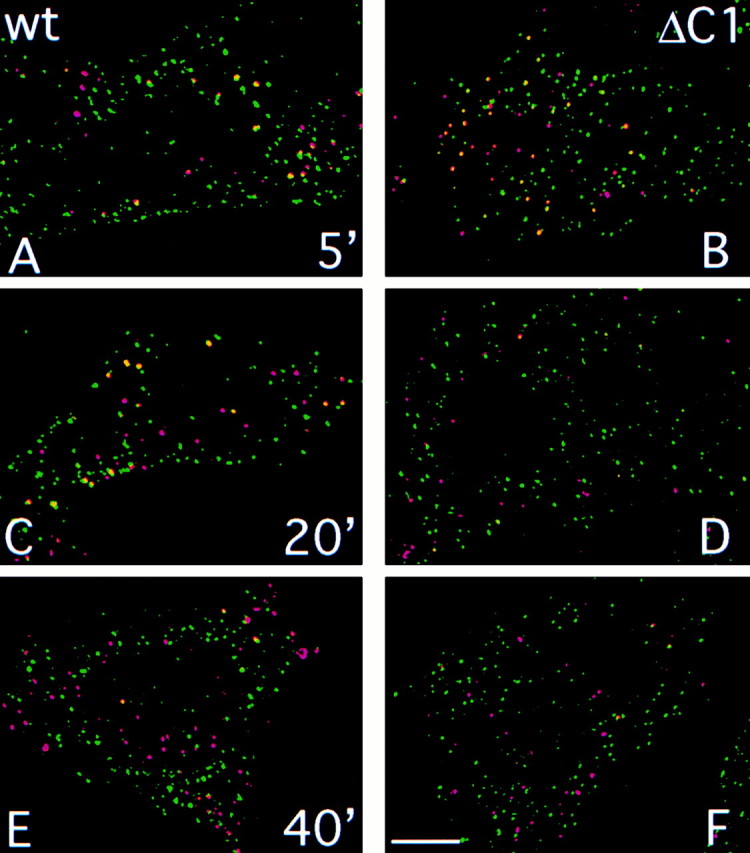
Localization of internalized Alexa488-S12 antibody and DiI-LDL. CHO cells expressing native P-selectin (A, C, and E) or P-selectin-ΔC1 (B, D, and F) were incubated in PBS/BSA containing 10 μg/ml Alexa488-S12 antibody (green channel) and 5 μg/ml DiI-LDL (red channel) for 5 min at 37°C, then in PBS/BSA for 5 min (A and B), 20 min (C and D), or 40 min (E and F). Cells were fixed and mounted for fluorescence microscopy. Stacked images were collected separately using FITC and Cy3 filters. Layers comprising 1.2–1.4-μm beginning at the base of the cells were processed by digital deconvolution and were merged (see Materials and Methods). Vesicles containing both labels appear yellow. Bar, 2 μm.
Figure 6.
Quantitation of S12 antibody and LDL colocalization. Images such as those shown in Fig. 5 were used to determine the number of LDL-containing structures that also contained S12 antibody. Images containing a total of 15–21 cells, with an average of 926 LDL-positive vesicles, were analyzed for each data point. Numbers represent the percentage of all LDL-positive vesicles that also contained S12 antibody for the entire sample. Similar results were obtained in two independent experiments.
Discussion
P-Selectin Traffic
We show here that P-selectin is sorted from long-lived recycling receptors in early sorting endosomes, and not in late endosomes: P-selectin, synaptophysin, CI-MPR, and LDL receptor are all rapidly internalized (this study, and Green and Kelly 1992), reflecting rapid delivery to sorting endosomes. Sorting into cell surface recycling or late endocytic pathways at this stage is reflected in the rates of transport from the cell surface to the TGN, since access of these proteins to the TGN recycling pathway is most likely through late endosomes (see below). Transport of P-selectin from the cell surface to the TGN is six to seven times faster than the long-lived proteins (e.g., LDL receptor), showing that the P-selectin pool in sorting endosomes in PC12 cells must enter endosomal carrier vesicles (maturing endosomes) to reach late endosomes six to seven times more efficiently than LDL receptor (Fig. 1A and Fig. B, and Fig. 7). Direct evidence that P-selectin does enter carrier vesicles efficiently is provided by the observation that internalized anti–P-selectin antibodies colocalized with internalized LDL for 20 min after internalization.
Figure 7.
Selective and nonselective steps in post-Golgi traffic. Post-Golgi trafficking pathways between the TGN, secretory granules (S.G.), plasma membrane (P.M.), sorting endosomes (S.E.), recycling endosomes (R.E.), late endosomes (L.E.), and lysosomes (Lys.) are shown. Solid arrows indicate pathways requiring sorting information for efficient transport; proteins discussed in the text that are recognized in those pathways are named. Dashed arrows indicate nonselective sorting pathways (in nonpolarized cells). MPR appear to be transported selectively from late endosomes to the TGN, whereas this pathway is apparently nonselective for many proteins (see text). Furin and TGN38 are selectively retained in the TGN, accounting in part for their steady state concentration at that site.
P-selectin is not sorted from LDL receptor in late endosomes. P-selectin is transported to the TGN approximately eight to nine times faster than it is delivered to lysosomes in PC12 cells (20–25 min vs. 3–3.8 h), showing that P-selectin molecules entering late endosomes are eight to nine times more likely to recycle to the TGN than to go directly to lysosomes. The ratio of these two transport rates is similar for LDL receptor (t1/2 = 2–2.5 h to the TGN vs. 20 h to lysosomes; Green and Kelly 1992). The simplest explanation for these observations is that both proteins have the same sorting phenotype in late endosomes (Fig. 1A and Fig. B). Therefore, the short half-life of P-selectin is most likely not due to its selective targeting to lysosomes, as has been suggested (Blagoveshchenskaya et al. 1998), but is due to its selective delivery from sorting endosomes to late endosomes. One significant consequence of this sorting event is rapid recycling of P-selectin through the TGN. Rapid turnover of P-selectin can be considered a secondary consequence of its frequent passage through late endosomes. Consistent with this conclusion, a recent report demonstrates transport of internalized anti–P-selectin antibodies from the cell surface to Weibel-Palade bodies (secretory granules) in endothelial cells by way of lysobisphosphatidic acid-enriched late endosomes (Kobayashi et al. 2000).
P-selectin has cytoplasmic sorting determinants that mediate rapid internalization (Setiadi et al. 1995) and delivery to regulated secretory granules (Modderman et al. 1998; Blagoveshchenskaya et al. 1999), in addition to the endosomal sorting determinant (Straley et al. 1998). Disruption of any of the three sorting activities would be expected to result in a significant change in the steady state distribution of the protein. Recent mutagenesis studies (Modderman et al. 1998; Blagoveshchenskaya et al. 1999) found that alanine substitution of L768, which abrogates endosomal sorting (Straley et al. 1998), reduces the steady state concentration of P-selectin in secretory granules, and increases the fraction at the cell surface. Whereas L768 may well be required for sorting to secretory granules, the altered steady state distribution could be due to a block in endosomal sorting (Straley et al. 1998), combined with a partial impairment of internalization activity in the L768A mutant (Setiadi et al. 1995). A direct biosynthetic sorting assay, as opposed to a steady state measurement, would be required to distinguish between these possibilities.
Based on cell fractionation studies in PC12 cells transiently expressing HRP/P-selectin chimeric proteins, it has been proposed that the cytoplasmic domain of P-selectin mediates sorting to synaptic-like vesicles, with sequence dependence similar to that required for sorting into dense core secretory granules (Blagoveshchenskaya et al. 1999; Blagoveshchenskaya and Cutler 2000). The HRP/P-selectin chimera, but not synaptophysin, is rapidly depleted from a small vesicle fraction in the presence of brefeldin A (Blagoveshchenskaya et al. 1999; Blagoveshchenskaya and Cutler 2000), which was interpreted as evidence that P-selectin and synaptophysin reach the same small vesicles via two or three different pathways. However, our finding that P-selectin did not colocalize with synaptophysin in PC12 cells (Fig. 2) demonstrates that there is little or no native P-selectin in synaptic-like vesicles, even at high expression levels. Indeed, colocalization of the HRP-chimeric protein with synaptophysin has not been addressed. An alternative interpretation of the fractionation data is that the small vesicles enriched in the HRP/P-selectin chimera represent constitutive-like vesicles forming from maturing secretory granules, a pathway that can be blocked by brefeldin A treatment (De Lisle and Bansal 1996; Fernandez et al. 1997), and not synaptic-like vesicles.
P-selectin is a secretory granule membrane protein in endothelial cells (McEver et al. 1989). Selective sorting of P-selectin from early to late endosomes has a number of consequences that bear on the function of P-selectin in inflammation, and possibly more generally on the trafficking of secretory granule membrane proteins. It is likely that sorting of secretory granule proteins into granules in endothelial cells is not perfectly efficient, and/or that granule maturation may include removal of some membrane to a constitutive-like pathway (Arvan and Castle 1998). In both cases, some P-selectin would be delivered to the cell surface in the absence of a secretory stimulus (which appears to be the case, at least in cultured endothelial cells; Kobayashi et al. 2000). Rapid internalization followed by endosomal sorting would prevent any significant accumulation of P-selectin at the cell surface under these conditions (Fig. 1 A and 7). After stimulated granule exocytosis, cell surface P-selectin levels are transiently high, and mediate binding of leukocytes expressing the P-selectin ligand (McEver et al. 1995; McEver and Cummings 1997). If P-selectin recycled to the cell surface as efficiently as LDL receptor, it would be cleared from the cell surface with kinetics similar to turnover of the LDL receptor (0.5–1 d). Endosomal sorting provides a mechanism to reduce cell surface P-selectin levels significantly within a few hours, providing temporal regulation of its binding activity. Finally, endosomal sorting of P-selectin results in its rapid transport to the TGN, where it can be reincorporated into nascent secretory granules, both before and after regulated exocytosis (Subramaniam et al. 1993; Kobayashi et al. 2000). Thus, early endosomal sorting may represent a general mechanism mediating efficient recycling of granule membrane proteins to the TGN for repackaging into nascent granules.
The TGN Recycling Pathway
Recently, it has been shown that TGN38 (Ghosh et al. 1998) and Shiga toxin B chain (Mallard et al. 1998), are rapidly transported to the TGN from sorting and/or recycling endosomes, and not via late endosomes (Fig. 7). In contrast, the TGN resident protein furin follows the LDL pathway shortly after internalization, indicating transport to maturing and/or late endosomes, then rapidly accumulates in the TGN (Mallet and Maxfield 1999). Our results show that P-selectin, like furin, recycles to the TGN via late endosomes and not from sorting or recycling endosomes. It should be noted that P-selectin, like furin (Mallet and Maxfield 1999), is not readily detected in CI-MPR–enriched late endosomes (Green et al. 1994; Straley et al. 1998, and this study), despite the fact that P-selectin is rapidly delivered to lysosomes, presumably after passing through late endosomes. We interpret this to mean that exit of P-selectin from CI-MPR–enriched late endosomes is rapid, precluding accumulation of the protein to levels that are detectable by morphological means. As noted above, internalized anti–P-selectin antibodies pass through late endosomes en route to recycling to Weibel-Palade bodies (secretory granules) in endothelial cells (Kobayashi et al. 2000). One important implication of our study of P-selectin is that selective transport of membrane proteins from early endosomes to late endosomes, resulting in rapid delivery to the TGN, is not specific to TGN resident proteins. Rather, this may represent a more general sorting pathway used by many different types of proteins.
Several lines of evidence indicate that recycling of CI-MPR through the TGN also occurs primarily (or exclusively) from late endosomes, and not directly from early endosomes (Goda and Pfeffer 1988; Green and Kelly 1992; Lombardi et al. 1993; Diaz and Pfeffer 1998). The available evidence strongly suggests that LDL and transferrin receptors, which undergo transport from the cell surface to the TGN at the same rate as CI-MPR (see Green and Kelly 1992), also follow the same pathway. These receptors reach the TGN after internalization at rates far slower than either TGN38 (Ghosh et al. 1998), which is targeted from sorting/recycling endosomes, or furin and P-selectin, which recycle via late endosomes (Mallet and Maxfield 1999; Kobayashi et al. 2000; and this study). The slow rates of transport to the TGN for LDL and transferrin receptors are readily explained by inefficient, nonselective transport (mis-sorting) from sorting to late endosomes, followed by recycling from late endosomes to the TGN with the same efficiency observed for P-selectin (Fig. 1 A). In contrast, direct transport of these molecules from sorting/recycling endosomes to the TGN, bypassing late endosomes, is difficult to reconcile with their relative rates of transport to the TGN and to lysosomes.
The Function of Early Endosomal Sorting
The observation that endosomal sorting of P-selectin occurs in several cell types that do not normally express it (Green et al. 1994; Blagoveshchenskaya et al. 1998; Straley et al. 1998) suggests that the endosomal sorting machinery recognizing P-selectin is ubiquitous, and serves more general functions in the endocytic pathway than recycling of granule membrane proteins. These may include endosomal sorting of resident lysosomal membrane proteins that pass through the cell surface. More generally, this sorting activity may function to limit exposure of certain membrane proteins at the cell surface, either to minimize activities that are not useful or are detrimental when expressed on the surface (e.g., furin), or to provide critical temporal regulation of the activity, as we propose for P-selectin. In addition, sorting of receptors that undergo ligand-dependent degradation, such as epidermal growth factor receptor, is controlled both at the level of internalization and by endosomal sorting (French et al. 1994; Kornilova et al. 1996). Downregulation of growth factor receptors may also be mediated in part by the same endosomal sorting mechanism that recognizes P-selectin.
Supplemental Material
Acknowledgments
We thank Rodger McEver (University of Oklahoma, OK) for generously supplying soluble P-selectin and monoclonal and goat polyclonal antibodies recognizing P-selectin, and for many helpful discussions; Ken Dunn (Indiana University, Indianapolis, IN) for advice on the LDL uptake experiments; William Brown (Cornell University, Ithaca NY), John Hutton (University of Colorado, Denver), and Regis Kelly (University of California, San Francisco, CA) for gifts of antisera, and Brandy Daugherty, Rodger McEver, David Castle, and Judy White for comments on the manuscript.
This work was supported by grants RPG96-019-03-CSM from the American Cancer Society and MCB-9904433 from the National Science Foundation.
Footnotes
The online version of this article contains supplemental material.
Abbreviations used in this paper: CI, cation-independent; LDL, low density lipoprotein; MPR, mannose 6-phosphate receptor.
References
- Aniento F., Emans N., Griffiths G., Gruenberg J. Cytoplasmic dynein-dependent vesicular transport from early to late endosomes. J. Cell Biol. 1993;123:1373–1387. doi: 10.1083/jcb.123.6.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvan P., Castle D. Sorting and storage during secretory granule biogenesislooking backward and looking forward. Biochem. J. 1998;332:593–610. doi: 10.1042/bj3320593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blagoveshchenskaya A.D., Cutler D.F. Sorting to synaptic-like microvesicles from early and late endosomes requires overlapping but not identical targeting signals. Mol. Biol. Cell. 2000;11:1801–1814. doi: 10.1091/mbc.11.5.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blagoveshchenskaya A.D., Norcott J.P., Cutler D.F. Lysosomal targeting of P-selectin is mediated by a novel sequence within its cytoplasmic domain. J. Biol. Chem. 1998;273:2729–2737. doi: 10.1074/jbc.273.5.2729. [DOI] [PubMed] [Google Scholar]
- Blagoveshchenskaya A.D., Hewitt E.W., Cutler D.F. A complex web of signal-dependent trafficking underlies the triorganellar distribution of P-selectin in neuroendocrine PC12 cells. J. Cell Biol. 1999;145:1419–1433. doi: 10.1083/jcb.145.7.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos C.R., Shank S.L., Snider M.D. Role of clathrin-coated vesicles in glycoprotein transport from the cell surface to the Golgi complex. J. Biol. Chem. 1995;270:665–671. doi: 10.1074/jbc.270.2.665. [DOI] [PubMed] [Google Scholar]
- De Lisle R.C., Bansal R. Brefeldin A inhibits the constitutive-like secretion of a sulfated protein in pancreatic acinar cells. Eur. J. Cell Biol. 1996;71:62–71. [PubMed] [Google Scholar]
- Diaz E., Pfeffer S.R. TIP47a cargo selection device for mannose 6-phosphate receptor trafficking. Cell. 1998;93:433–443. doi: 10.1016/s0092-8674(00)81171-x. [DOI] [PubMed] [Google Scholar]
- Disdier M., Morrissey J.H., Fugate R.D., Bainton D.F., McEver R.P. Cytoplasmic domain of P-selectin (CD62) contains the signal for sorting into the regulated secretory pathway. Mol. Biol. Cell. 1992;3:309–321. doi: 10.1091/mbc.3.3.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan J.R., Kornfeld S. Intracelluar movement of two mannose 6-phosphate receptorsreturn to the Golgi apparatus. J. Cell Biol. 1988;106:617–628. doi: 10.1083/jcb.106.3.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn K.W., McGraw T.E., Maxfield F.R. Iterative fractionation of recycling receptors from lysosomally destined ligands in and early sorting endosome. J. Cell Biol. 1989;109:3303–3314. doi: 10.1083/jcb.109.6.3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez C.J., Haugwitz M., Eaton B., Moore H.P. Distinct molecular events during secretory granule biogenesis revealed by sensitivities to brefeldin A. Mol. Biol. Cell. 1997;8:2171–2185. doi: 10.1091/mbc.8.11.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French A.R., Sudlow G.P., Wiley H.S., Lauffenburger D.A. Postendocytic trafficking of epidermal growth factor-receptor complexes is mediated through saturable and specific endosomal interactions. J. Biol. Chem. 1994;269:15749–15755. [PubMed] [Google Scholar]
- Gartung C., Braulke T., Hasilik A., von Figura K. Internalization of blocking antibodies against mannose 6-phosphate specific receptors. EMBO (Eur. Mol. Biol. Organ.) J. 1985;4:1725–1730. doi: 10.1002/j.1460-2075.1985.tb03842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh R.N., Mallet W.G., Soe T.T., McGraw T.E., Maxfield F.R. An endocytosed TGN38 chimeric protein is delivered to the TGN after trafficking through the endocytic recycling compartment in CHO cells. J. Cell. Biol. 1998;142:923–936. doi: 10.1083/jcb.142.4.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goda Y., Pfeffer S.R. Selective recycling of the mannose 6-phosphate/IGF-II receptor to the trans Golgi network in vitro. Cell. 1988;55:309–320. doi: 10.1016/0092-8674(88)90054-2. [DOI] [PubMed] [Google Scholar]
- Green S.A., Kelly R.B. Endocytic membrane traffic to the Golgi apparatus in a regulated secretory cell line. J. Biol. Chem. 1990;265:21269–21278. [PubMed] [Google Scholar]
- Green S.A., Kelly R.B. Low density lipoprotein receptor and cation-independent mannose 6-phosphate receptor are transported from the cell surface to the Golgi apparatus at equal rates in PC12 cells. J. Cell Biol. 1992;117:47–55. doi: 10.1083/jcb.117.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green S.A., Setiadi H., McEver R.P., Kelly R.B. The cytoplasmic domain of P-selectin contains a sorting determinant that mediates rapid degradation in lysosomes. J. Cell Biol. 1994;124:435–448. doi: 10.1083/jcb.124.4.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenberg J., Maxfield F. Membrane transport in the endocytic pathway. Curr. Opin. Cell Biol. 1995;7:552–563. doi: 10.1016/0955-0674(95)80013-1. [DOI] [PubMed] [Google Scholar]
- Hattori R., Hamilton K.K., Fugate R.D., McEver R.P., Sims P.J. Stimulated secretion of endothelial von Willebrand factor is accompanied by rapid redistribution to the cell surface of the intracellular granule membrane protein GMP-140. J. Biol. Chem. 1989;264:7768–7771. [PubMed] [Google Scholar]
- Jin M., Sahagian G.G., Snider M.D. Transport of surface mannose 6-phosphate receptor to the Golgi complex in cultured human cells. J. Biol. Chem. 1989;264:7675–7680. [PubMed] [Google Scholar]
- Kobayashi T., Vischer U.M., Rosnoblet C., Lebrand C., Lindsay M., Parton R.G., Kruithof E., Gruenberg J. The tetraspanin CD63/lamp3 cycles between endocytic and secretory compartments in human endothelial cells. Mol. Biol. Cell. 2000;11:1829–1843. doi: 10.1091/mbc.11.5.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornilova E., Sorkina T., Beguinot L., Sorkin A. Lysosomal targeting of epidermal growth factor receptors via a kinase-dependent pathway is mediated by the receptor carboxyl-terminal residues 1022–1123. J. Biol. Chem. 1996;271:30340–30346. doi: 10.1074/jbc.271.48.30340. [DOI] [PubMed] [Google Scholar]
- Le T.L., Yap A.S., Stow J.L. Recycling of E-cadherina potential mechanism for regulating cadherin dynamics. J. Cell Biol. 1999;146:219–232. [PMC free article] [PubMed] [Google Scholar]
- Lombardi D., Soldati T., Riederer M.A., Goda Y., Zerial M., Pfeffer S.R. Rab9 functions in transport between late endosomes and the trans Golgi network. EMBO (Eur. Mol. Biol. Organ.)J. 1993;12:677–682. doi: 10.1002/j.1460-2075.1993.tb05701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe A.W., Maddedu L., Kelly R.B. Endocrine secretory granules and neuronal synaptic vesicles have three integral membrane proteins in common. J. Cell Biol. 1988;106:51–59. doi: 10.1083/jcb.106.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallard F., Antony C., Tenza D., Salamero J., Goud B., Johannes L. Direct pathway from early/recycling endosomes to the Golgi apparatus revealed through the study of Shiga toxin B-fragment transport. J. Cell Biol. 1998;143:973–990. doi: 10.1083/jcb.143.4.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallet W.G., Maxfield F.R. Chimeric forms of furin and TGN38 are transported from the plasma membrane to the trans-Golgi network via distinct endosomal pathways. J. Cell Biol. 1999;146:345–359. doi: 10.1083/jcb.146.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEver R.P., Cummings R.D. Perspectives seriescell adhesion in vascular biology. Role of PSGL-1 binding to selectins in leukocyte recruitment. J. Clin. Invest. 1997;100:485–491. doi: 10.1172/JCI119556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEver R.P., Beckstead J.H., Moore K.L., Marshall-Carlson L., Bainton D.F. GMP-140, a platelet alpha-granule membrane protein, is also synthesized by vascular endothelial cells and is localized in Weibel-Palade bodies. J. Clin. Invest. 1989;84:92–99. doi: 10.1172/JCI114175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEver R.P., Moore K.L., Cummings R.D. Leukocyte trafficking mediated by selectin-carbohydrate interactions. J. Biol. Chem. 1995;270:11025–11028. doi: 10.1074/jbc.270.19.11025. [DOI] [PubMed] [Google Scholar]
- Mellman I. Endocytosis and molecular sorting. Annu. Rev. Cell. Dev. Biol. 1996;12:575–625. doi: 10.1146/annurev.cellbio.12.1.575. [DOI] [PubMed] [Google Scholar]
- Mellman I., Plutner H. Internalization and degradation of macrophage Fc receptors bound to polyvalent immune complexes. J. Cell Biol. 1984;98:1170–1177. doi: 10.1083/jcb.98.4.1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modderman P.W., Beuling E.A., Govers L.A.T., Calafat J., Janssen H., von dem Borne A.E., Sonnenberg A. Determinants in the cytoplasmic domain of P-selectin required for sorting to secretory granules. Biochem. J. 1998;336:153–161. doi: 10.1042/bj3360153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller S.R., Sullivan P.D., Clegg D.O., Feinstein S.C. Efficient transfection and expression of heterologous genes in PC12 cells. DNA Cell Biol. 1990;9:221–229. doi: 10.1089/dna.1990.9.221. [DOI] [PubMed] [Google Scholar]
- Schweizer A., Kornfeld S., Rohrer J. Proper sorting of the cation-dependent mannose 6-phosphate receptor in endosomes depends on a pair of aromatic amino acids in its cytoplasmic tail. Proc. Natl. Acad. Sci. USA. 1997;94:14471–14476. doi: 10.1073/pnas.94.26.14471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setiadi H., Disdier M., Green S.A., Canfield W.M., McEver R.P. Residues throughout the cytoplasmic domain affect the internalization efficiency of P-selectin. J. Biol. Chem. 1995;270:26818–26826. doi: 10.1074/jbc.270.45.26818. [DOI] [PubMed] [Google Scholar]
- Snider M.D., Rogers O. Intracellular movement of cell surface receptors after endocytosisresialylation of asialo-transferrin receptor in human erythroleukemia cells. J. Cell Biol. 1985;100:826–834. doi: 10.1083/jcb.100.3.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straley K.S., Daugherty B.L., Aeder S.E., Hockenson A.L., Kim K., Green S.A. An atypical sorting determinant in the cytoplasmic domain of P-selectin mediates endosomal sorting. Mol. Biol. Cell. 1998;9:1683–1694. doi: 10.1091/mbc.9.7.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramaniam M., Koedam J.A., Wagner D.D. Divergent fates of P- and E-selectins after their expression on the plasma membrane. Mol. Biol. Cell. 1993;4:791–801. doi: 10.1091/mbc.4.8.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ushiyama S., Laue T.M., Moore K.L., Erickson H.P., McEver R.P. Structural and functional characterization of monomeric soluble P-selectin and comparison with membrane P-selectin. J. Biol. Chem. 1993;268:15229–15237. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.





