Abstract
Background and purpose:
Endothelial cell proliferation, migration and adhesion are necessary for the formation of new blood vessels. We reported previously that baicalein strongly inhibited proliferation of rat heart endothelial cells and here we assess effects on migration and adhesion of these cells.
Experimental approach:
Effects of baicalein on endothelial migration and adhesion were determined by in vitro wound assays and in modified Boyden chambers. Protein expression and subcellular distribution in rat heart endothelial cells were analysed by immunoblots and immunofluorescence staining.
Results:
Pretreatment with baicalein for 48 h resulted in a concentration-dependent inhibition of endothelial migration, with an IC50 of approximately 20 μ M. Adhesion assays revealed that baicalein stimulated endothelial cell adhesion to fibronectin and vitronectin, effects blocked by the synthetic peptide Arg-Gly-Asp (RGD). Moreover, treatment with a blocking antibody against integrin α5β1 drastically attenuated baicalein-mediated endothelial adhesion to fibronectin, but not to vitronectin. Furthermore, baicalein-mediated anti-migration effect and adhesion promotion could be partially reversed by the addition of 12(S)-hydroxyeicosatetraenoic acid (12(S)-HETE). Western blot analysis indicated that baicalein increased expression levels of integrin-α5β1, -αvβ3 and vinculin proteins. Immunofluorescence staining showed that baicalein induced a marked reorganization of actin stress fibres and the recruitment of vinculin and integrins to focal adhesion plaques, with consequently increased formation of focal adhesion contacts.
Conclusions and implications:
Baicalein markedly inhibited the migration and enhanced the adhesion of rat heart endothelial cells, possibly by up-regulation of the integrins (α5β1 and αvβ3) and vinculin and by promotion of actin reorganization and focal adhesion contact formation.
Keywords: adhesion, baicalein, fibronectin, integrin, migration, vinculin, vitronectin
Introduction
The root of Scutellaria baicalensis (Chinese name, Huangqin) has been used as a folk medicine in China and Japan for the treatment of chronic hepatitis, allergy, thrombotic stroke and inflammatory diseases (Huang et al., 1994a). Huangqin is known to contain numerous flavone derivatives (Sekiya et al., 1982), and their pharmacological properties have been extensively investigated. Among them, baicalein (5,6,7-trihydroxy-2-phenyl -4H-1-benzopyran-4-one) has attracted considerable attention, as it has a variety of interesting properties, including antithrombotic, anti-inflammation, anticancer, antioxidative, antimicrobial and antifibrosis activities (Hsu et al., 2001; Schuppan and Porov, 2002; Fujita et al., 2005). However, little is known about the modes of action of baicalein in these biological effects. A previous report has demonstrated that baicalein inhibits thrombin-induced production of plasminogen activator inhibitor-1 in cultured human umbilical vein endothelial cells (Kimura et al., 1997). Baicalein also acts as an inhibitor of lipoxygenase (LOX) in rat platelets and as a potent hypotensive compound (Chen et al., 1999). On the other hand, baicalein enhances vasoconstricting sensitivity to receptor-dependent agonists such as noradrenaline, phenylephrine, serotonin and vasopressin in isolated rat arteries (Huang et al., 2005). These studies suggest that baicalein may have a potential use in the treatment of cardiovascular disorders. In addition, baicalein exhibited remarkable antiproliferative effects on aortic smooth muscle cells (Nishio and Watanabe, 1997). A previous study showed that baicalein was able to inhibit the migration induced by arachidonic acid and platelet derived growth factor in bovine carotid artery smooth muscle cells (Kanayasu-Toyoda et al., 1998). Liu et al. (2003) reported that baicalein significantly inhibited the matrix metalloproteinase-2 activity and cell migration of human umbilical endothelial cells, indicating a potential role of this compound in modulation of the angiogenic process.
Angiogenesis is a complex process that involves endothelial proliferation, migration, remodelling of extracellular matrix (ECM) and neovessel organization. Angiogenesis also requires endothelial cell-to-cell, and cell-to-matrix interactions (Saunders et al., 2006). New capillary blood vessel formation, in adult life, takes place generally from pre-existing vessels, in direct response to tissue demands, by true sprouting or by splitting angiogenesis (Huang et al., 1994a). It is now known that endothelial progenitor cells mobilize from the bone marrow in response to a variety of signalling molecules and can target sites of angiogenesis in ischaemic peripheral vasculature, myocardium or induced ocular injury (Asahara et al., 1997). In healthy adults, angiogenesis does not normally occur, except during the female ovarian cycle. However, neo-angiogenesis may occur in several pathophysiological conditions, including wound healing, chronic inflammatory diseases and solid tumours (Folkman and Klagsbrun, 1987). Endothelial cell proliferation, adhesion and migration are early essential events for mediating angiogenesis. It is well documented that the induction of endothelial cell proliferation, migration and adhesion in response to numerous intracellular or extracellular stimuli is a tightly regulated process requiring the coordination of a complex set of inward and outward signals involving the ECM, the integrins and the actin cytoskeleton-associated molecules (Romer et al., 2006).
We have previously reported that baicalein exhibited a strong antiproliferative effect in rat heart endothelial cells (Hsu et al., 2001). In this study, we investigated the effect of baicalein on endothelial cell migration and adhesion. Since cell migration and adhesion are associated with regulation of actin dynamics and cell surface integrins (Rousseau et al., 1997), the intracellular cytoskeletal architecture and surface receptor integrins were also investigated. We found that baicalein upregulated the expression of the integrins, α5β1 and αvβ3, and of vinculin, which mediated intracellular signalling through interaction with fibronectin and vitronectin, and promoted the reorganization of actin fibres and increased focal contact formation and adhesion, as well as reducing migration of endothelial cells.
Methods
Cell culture
Endothelial cells were isolated from rat heart as described previously (Hsu et al., 2001). Cells were maintained in minimal essential medium with 10% foetal bovine serum, antibiotics and 50 μg ml−1 endothelial cell growth supplements. Endothelial cells were identified by their typical cobblestone appearance and CD31 (PECAM-1) immunostaining (Fu et al., 2004).
Cell migration assay
Migration assays were performed following two standard protocols- using a wound repair assay and Transwell chemotaxis chambers (Neuro Probe Inc., Cabin John, MD, USA). The mechanical injury of confluent endothelial cells and lesion repair assay were performed as described elsewhere (Ashton et al., 1999). Briefly, confluent endothelial cells were wounded by scraping with a pipette tip, denuding a strip of the monolayer. Cultures were washed twice with phosphate-buffered saline (PBS) and incubated with serum-containing medium supplemented with the baicalein or vehicle (0.1% DMSO), as indicated. The rate of wound closure was measured and photographed over 48 h. The progression of cell migration was assessed with a calibrated ocular grid (Christopher et al., 1999). The modified Boyden's chamber assay was performed by using cell culture inserts composed of a porous 8-μm membrane. Briefly, baicalein-pretreated or untreated rat heart endothelial cells were washed and trypsinized to induce cell detachment. Cells (5 × 104) were then suspended in 50 μl of serum-free medium, with or without baicalein (1, 3, 10, 30 and 100 μM), and seeded in the upper compartment. Vascular endothelial growth factor (VEGF) (10 ng ml−1) was added to the lower compartment and cell migration was determined after 2 h of incubation at 37°C in a CO2 incubator. Non-migrating cells were removed from the upper surface of the membrane with a cotton swab. The cells that had migrated to the lower side of the membrane were fixed with ethanol and stained with a Giemsa solution. Number of migrated cells was estimated by counting the stained cells in five random view fields (100 × magnification). Data are presented as the mean±s.d. of twelve replicates from three separate experiments.
Matrix proteins coating and cell adhesion assay
Fibronectin, collagen and laminin were coated on 12-well plates, 200 μl per well (5 μg ml−1), and incubated at 4°C overnight. Wells were washed using PBS, blocked with 200 μl 1% BSA in PBS for 2 h at 37°C, and then washed with PBS and stored at 4°C until use. The same procedure was followed for vitronectin (5 μg ml−1) except that wells were coated for 2 h at room temperature. After treatment, plates were stored at 4°C until used. Cell adhesion was estimated as described elsewhere (Kimura et al., 1997). Briefly, endothelial cells were treated without or with various concentrations of baicalein in DMEM medium, and incubated at 37°C for indicated time points. After treatment, cells were harvested with 1 mM ethylenediamine tetraacetic acid in PBS, and suspended in medium without supplements. Cell suspensions (5 × 104 cells ml−1) were added to the matrix protein-coated or uncoated wells under serum-free condition. After 2 h of incubation, non-adherent cells were removed, while the adherent cells were fixed with 2% paraformaldehyde for 20 min at room temperature. Fixed cells were stained with haematoxylin and counted. The adherent cells were expressed as a percentage of the initial seeded cells.
Western blot analysis
Endothelial cytoskeletal protein levels were examined by western blot analysis. After treatment with baicalein in the serum-containing medium for the indicated times, cells were washed and lysed with lysis buffer (20 mM Tris-HCl, pH 7.5, with 1% Triton X-100, 0.3% deoxycholate) containing proteinase inhibitors (Complete, Boehringer Mannheim, Germany), followed by centrifugation at 15 000 g for 30 min at 4°C. Supernatants were separated and used as whole-cell extracts. Protein concentration was determined by Bradford method. Equal amount of protein samples were separated on sodium dodecyl sulphate-polyacrylamide gel electrophoresis, and transferred to a polyvinylidene difluoride (PVDF) membrane. The membrane was incubated with Tween-PBS containing 5% non-fat dry milk for 2 h to block nonspecific antibody binding. Membranes were then incubated with primary antibodies overnight at 4°C, followed by horseradish peroxidase-conjugated anti-mouse (or anti-rabbit) IgG. Immunoreactive blots were detected using a chemiluminescence detection kit (ECL, Amersham Pharmacia Biotech, Buckinghamshire, UK).
Cytoimmunostaining
Cell were untreated or treated with 100 μM baicalein in serum-containing medium for 48 h, washed with PBS and fixed with 2% paraformaldehyde in PBS for 20 min, and incubated with 0.1% Triton X-100 in PBS for 20 min. Rhodamine-labelled phalloidin (100 μg ml−1) (Molecular Probes Inc., Eugene, OR, USA) was used to stain actin filaments. For integrin staining, cells were blocked with 5% bovine serum albumin in PBS for 60 min at room temperature. Cells were incubated with anti-rat-integrin antibody (1:100) for 1 h, and then detected with an fluorescein isothiocyanate (FITC)-conjugated secondary antibody. To visualize focal adhesion contacts, the cells were stained with monoclonal antibody against vinculin for 1 h, washed twice with PBS and stained with FITC-labelled anti-mouse IgG for 30 min. The slides were mounted using Vectashield (Vector Labs, Burlingame, CA, USA) diluted 1:1 in Tris buffer (pH 8.4). Images were obtained using a Leica laser scanning confocal microscope. Cell nuclei were stained using 4′6′-diamidino-2-phenylindole (DAPI).
Statistical analysis
All results are expressed as means±s.d. of 12 replicates from three independent experiments. Images shown here were obtained from at least three independent experiments with similar patterns. One-way analysis of variance (ANOVA) and Student's t-test were used to determine the level of significance for the statistical analysis of data by using the SPSS 10.0 statistical program. *P<0.05, **P<0.01 and ***P<0.001 were used to indicate statistical significance.
Materials
Baicalein, propidium iodide (PI), 4′,6′-diamidino-2-phenylindole (DAPI), collagen I, fibronectin, laminin, anti-vinculin antibody, 5(S)-hydroxyeicosatetraenoic acid (5(S)-HETE), 12(S)-hydroxyeicosatetraenoic acid (12(S)-HETE), 15(S)-hydroxyeicosatetraenoic acid (15(S)-HETE), the synthetic peptide Arg-Gly-Asp (RGD) and the synthetic peptide Ser-Asp-Gly-Arg-Gly (SDGRG) were obtained from Sigma (St Louis, MO, USA). Vitronectin was purchased from Collaborative Biomedical Products (Bedford, MA, USA). Baicalein was dissolved in dimethyl sulphoxide (DMSO) at a concentration of 100 mM and stored in sealed tubes in liquid nitrogen. The following antibodies were obtained from Transduction Laboratory (Lexington, KY, USA): anti-β-actin, anti-α-tubulin, anti-talin, anti-paxillin and anti-focal adhesion kinase (FAK). Anti-phospho-FAK (S722), anti-integrin-β5, anti-integrin-αv, and anti-α-actinin were obtained from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA). Anti-phospho-FAK (Y397) was obtained from Abcam (Abcam, Cambridge, UK). VEGF and endothelial cell growth supplement were obtained from Upstate Biotechnology (Lake Placid, NY, USA). Anti-integrin-α5, anti-integrin-α2 and anti-integrin-α5β1 antibodies were purchased from Chemicon International (Temecula, CA, USA). Anti-integrin-β3 and anti-integrin-β1 antibodies were obtained from BD Pharmingen (San Diego, CA, USA).
Results
Inhibition of endothelial cell migration by baicalein
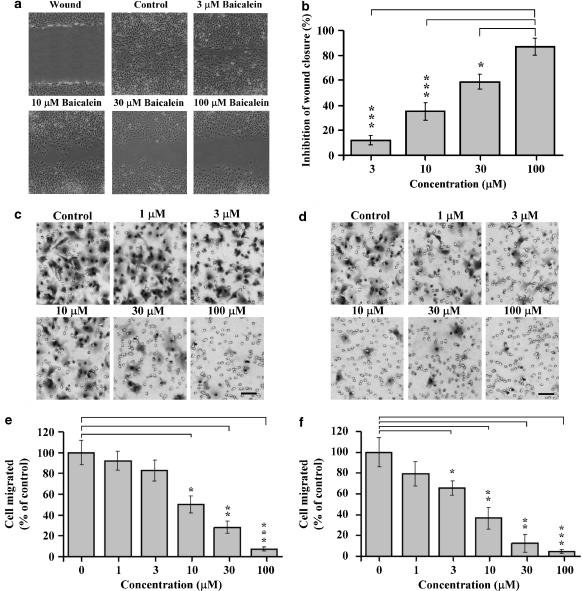
We used an in vitro mechanical wound model to assess the effect of baicalein on endothelial cell migration. Confluent, scrape-wounded rat heart endothelial cell monolayers were incubated with various concentrations of baicalein, and the rate of closure was observed for 48 h. In the scratch injury model (mechanical wound assay) of the endothelial monolayer, endothelial cell sprouting was significantly attenuated in baicalein-treated cultures but not in the control cultures, treated with vehicle. As shown in Figure 1a, cells in control plates migrated into the denuded area, almost completely covering the exposed surface after 48 h incubation (Figure 1a). In contrast, baicalein induced a concentration-dependent inhibition of the migration of endothelial cells into the denuded area (Figure 1a); in comparison with control culture. Wound closure was inhibited by a range of concentrations of baicalein (Figure 1b).
Figure 1.
Inhibition of endothelial migration by baicalein. Migration was assessed by (a) the mechanical wound healing assay. Rat heart endothelial cells were plated on six-well plates at a density of 3 × 105 cells per well. After 24 h incubation, confluent endothelial cells were wounded and maintained in 0, 3, 10, 30, 100 μM baicalein for another 48 h. Healing of the wound was observed under an inverted phase contrast microscope, magnification × 60. (b) Quantitation of the effect of baicalein on migration. The degree of closure was monitored using a calibrated ocular grid. The data are from four separate experiments and are expressed as area remaining as a percentage of the size of the initial wound area (mean±s.d.). Migration was also assessed in a modified Boyden chamber assay (c–f). Endothelial cells were incubated in the absence or presence of baicalein (1, 3, 10, 30 and 100 μM) for 48 h, and then vehicle-treated (0.1% DMSO) or baicalein-pretreated cells were seeded into the upper chamber at a density of 2.5 × 104 cells per chamber. In (c), VEGF was used as a chemotactic stimulus and in (d), the chemotactic experiment was performed in the absence of VEGF stimulation. Cells were incubated for 2 h at 37°C. After fixation and Giemsa staining, migrated cells were visualized under a microscope (× 200 magnification). Scale bar, 50 μm. The number of migrated cells was estimated by counting the stained cells in five random view fields with (e) VEGF as a chemotactic stimulus and (f) absence of VEGF stimulation. Data are presented as the mean±s.d. of twelve replicates from four separate experiments. Asterisks indicate *P<0.05, **P<0.01, ***P<0.001. DMSO, dimethyl sulphoxide; VEGF, vascular endothelial growth factor.
Our previous study showed that baicalein strongly inhibited the proliferation of endothelial cells (Hsu et al., 2001). Because the scratch injury assay cannot distinguish which process (proliferation or migration or both) is inhibited, we further analysed the effects of baicalein on cell migration by Boyden chamber assay. Addition of a range of concentrations of baicalein (1–100 μM) to the cells immediately before loading into the upper Boyden chamber, or pretreatment with baicalein for up to 6 h, had no effect on VEGF-stimulated endothelial migration (data not shown). However, when cells were pretreated with baicalein (1–100 μM) for 48 h and then washed with media and seeded into the upper chamber of Boyden chambers, VEGF-stimulated endothelial migration was inhibited in a concentration-dependent manner, with an IC50 of approximately 20 μM (Figures 1c and e). Pretreatment with 100 μM baicalein for 48 h almost completely abolished the endothelial migration. Baicalein also attenuated endothelial cell migration in the absence of VEGF stimulation (Figures 1d and f). These results indicate that inhibition of endothelial migration by baicalein requires long-term exposure to baicalein.
Stimulation of endothelial cell adhesion to fibronectin and vitronectin by baicalein
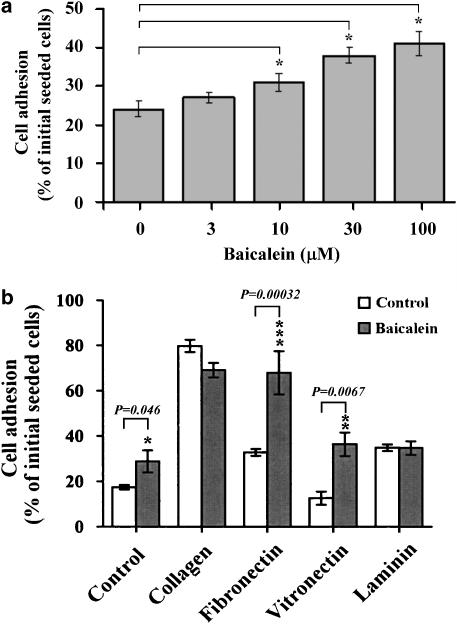
Cell migration depends on cell adhesion to the ECM (Palecek et al., 1997; Pereda et al., 1999). To address whether the effect of baicalein on endothelial cell migration could be attributed to changes in cell adhesion, endothelial cells were incubated in the absence or presence of baicalein for 48 h, and then suspended and incubated for 2 h on dishes. As summarised in Figure 2a, baicalein had stimulatory effects on the attachment of cells to the substratum of culture plates in a concentration-dependent manner. However, short-term (up to 6 h) incubation of baicalein had no influence on cell adhesion (data not shown). We next examined the effect of baicalein on the adhesion of endothelial cells to various components of the ECM. Endothelial cells were untreated or treated with 100 μM baicalein for 48 h, and then suspended and incubated for 2 h on dishes coated with collagen, laminin, fibronectin or vitronectin. As shown in Figure 2b, baicalein increased endothelial cell adhesion to fibronectin and vitronectin, compared to untreated control cultures. In contrast, treatment with baicalein had no significant effect on attachment of endothelial cells to collagen or laminin. These results indicate that baicalein strengthens cell interaction with only some matrix molecules, such as fibronectin and vitronectin.
Figure 2.
Enhancement of adhesion of endothelial cells to ECM by baicalein. (a) Treatment with various concentration of baicalein for 2 days, or (b) preincubation with or without 100 μM baicalein for 2 days, and then cells were harvested and seeded (at a density of 1 × 105 cells per well) in 12-well culture plates, coated with collagen, laminin, fibronectin, or vitronectin and allowed to adhere for 2 h. The attached endothelial cells were quantified by dye staining. Attached cells are expressed as the percentage of the seeded cells. The values are means±s.d. of 12 replicates from four separate experiments. Asterisks indicate *P<0.05, **P<0.01, ***P<0.001. ECM, extracellular matrix.
Regulation of integrins expression by baicalein
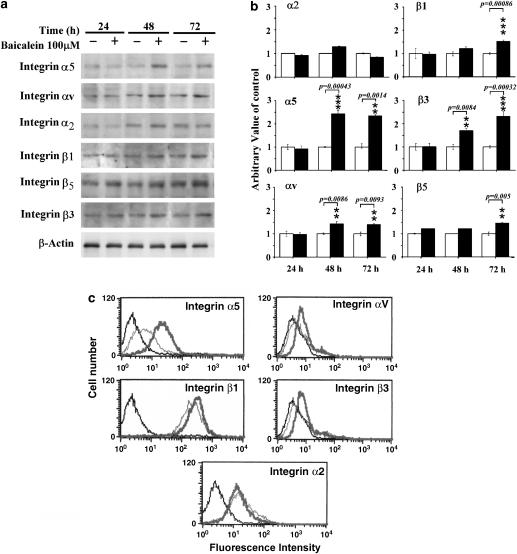
Since integrins are the major family of surface receptors for extracellular matrices, this increased adhesion by baicalein was mostly likely to be mediated by integrin molecules. It has been reported that α5β1, αvβ3 and αvβ5 integrins act as receptors for fibronectin and vitronectin, suggesting that functional expression of these integrins at the cell surface may be increased by baicalein in endothelial cells. So we next determined the effects of baicalein on the expression of various integrin subunits (including α2, α5, αv, β1, β3 and β5) by western blot analysis. Increased expression of integrin subunits αv, β3, β5, α5 and β1 proteins, but not of integrin-α2, was observed in baicalein-treated cells, compared with control culture (Figures 3a). Densitometric analysis indicated that levels of receptors on cells exposed to baicalein for 24 or 48 h were increased (Figure 3b). Data from flow cytometry assay showed that baicalein upregulated the expression levels of these integrins on the cell surface (Figure 3c). The cell surface expression level of integrin β5 could not be examined by flow cytometry, as there is commercially available antibody. These results indicate a possibility that baicalein increased endothelial cell adhesion to fibronectin and vitronectin through increased expression of integrins αvβ3, αvβ5 and α5β1 on the cell surface.
Figure 3.
Regulation of integrin expression by baicalein. (a) Immunoblot analysis with cell extracts obtained from untreated or baicalein-treated cultures for indicated time points, was performed using anti-integrin subunit specific antibodies. β-actin was used as an internal loading control. (b) Densitometric analysis of the expression of integrins (from three independent experiments). The arbitrary value of control was presented as 1; values shown are means±s.d. (c) Flow cytometric analysis. After treatment, cells were harvested and surface expression of the integrin subunits was examined using specific antibodies, and expression was analysed by flow cytometry. Histograms are composites with the expression of integrins on control cultures in green, baicalein-treated cultures in red and negative controls in grey. Asterisks indicate *P<0.05, **P<0.01, ***P<0.001.
Suppression of baicalein-enhanced cell adhesion by RGD peptide and integrin α5β1 blocking antibody
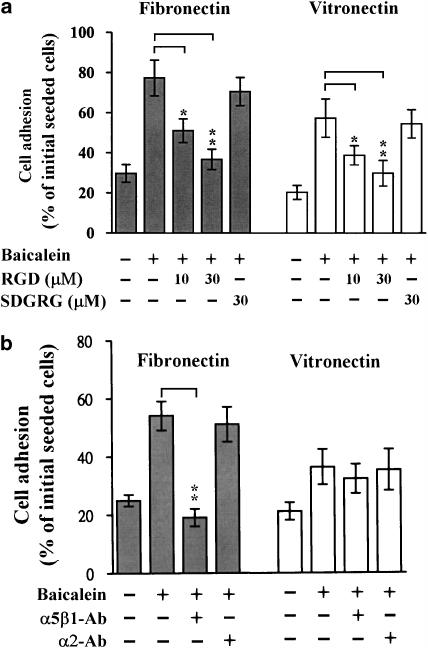
To determine if the increased expression of these integrin subunits was actually involved in the enhancement of cell adhesion to extracellular matrices, we examined cell adhesion to fibronectin and vitronectin in the presence or absence of the synthetic tripeptide RGD. This peptide was originally found to be responsible for the fibronectin or vitronectin cell attachment activity (Dejana et al., 1987). When endothelial cell suspensions were plated on dishes coated with fibronectin or vitronectin, many of the adherent cells did not spread and remained round. After treatment with baicalein, the number of adherent cells was increased and most of the adherent cells spread with extension of lamellipodia. As shown in Figure 4a, baicalein-enhanced endothelial adhesion to fibronectin and vitronectin was significantly suppressed by RGD peptide, but not by the control peptide SDGRG. In addition, treatment with a blocking antibody to integrin α5β1 (which is a well known fibronectin receptor) almost completely blocked the baicalein-enhanced endothelial cell adhesion to fibronectin but not to vitronectin (Figure 4b). However, anti-integrin-α2 antibody did not affect baicalein-induced cell adhesion. These findings suggest that baicalein-induced integrin upregulation might play an essential role in the increased adhesion.
Figure 4.
RGD peptide and integrin antibody blocked baicalein-mediated adhesion to fibronectin and vitronectin. After preincubation with 100 μM baicalein for 2 days, cells were seeded at a density of 1 × 105 cells per well, (a) in the absence or presence of RGD (or SDGRG), or (b) without or with antibodies against integrins. Cells were then allowed to adhere to plates coated with fibronectin or vitronectin; the attached endothelial cells were quantified by dye staining. Attached cells are expressed as the percentage of the seeded cells. The values are means±s.d. of 12 replicates from four separate experiments. Asterisks indicate significance *P<0.05, **P<0.01. RGD, the synthetic peptide Arg-Gly-Asp; SDGRG, the synthetic peptide Ser-Asp-Gly-Arg-Gly.
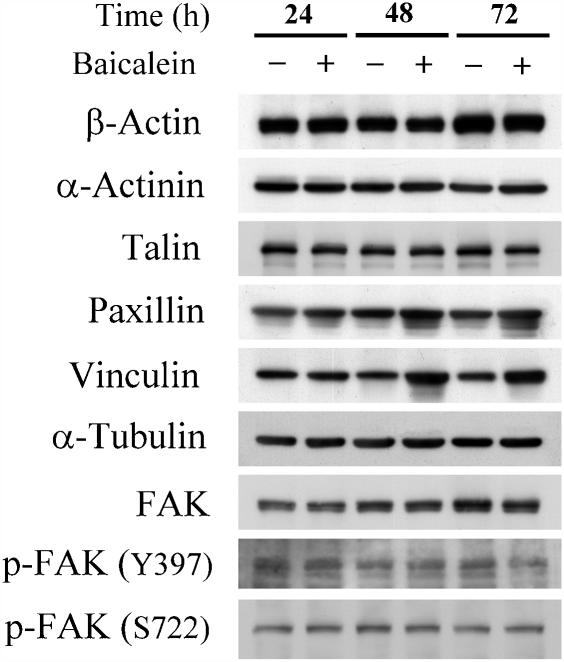
Regulation of cytoskeletal proteins by baicalein
In many cell types, the sites of cell–matrix adhesion are called focal contacts (Hynes 2002; Tsuruta and Jones, 2003). Within each focal contact, cell surface receptors of the integrin family members interact with ECM molecules outside the cell and with the cytoskeletal system in the cytoplasm (Hynes, 2002; Tsuruta and Jones, 2003). Thus, we examined the expression of cytoskeletal molecules in baicalein-treated cells by western blot analysis, using anti-actin, anti-α-actinin, anti-talin, anti-paxillin, anti-vinculin, anti-tubulin, anti-FAK, anti-phospho-FAK (Y397) and -phospho-FAK (S722)-specific antibodies. As shown in Figure 5, baicalein treatment increased the expression level only of vinculin protein, whereas the levels of actin, α-actinin, FAK, -phospho-FAK (Y397), -phospho-FAK (S722), paxillin, talin and tubulin were not affected by this treatment (Figure 5).
Figure 5.
Regulation of cytoskeleton protein expression. Endothelial cells were treated with 100 μM baicalein for 24, 48 and 72 h. After incubation, cell lysates were extracted. Western blot analysis was performed using anti-β-actin, anti-α-actinin, anti-talin, anti-paxillin, anti-vinculin, anti-α-tubulin, anti-FAK, anti-phospho-FAK (Y397) and anti-phospho-FAK (S722)-specific antibodies.
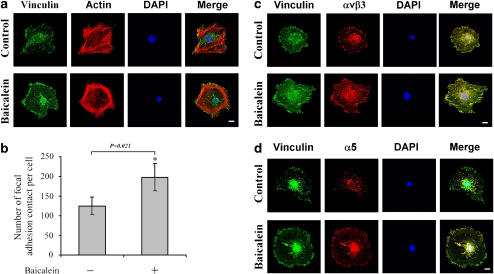
Reorganization of microfilaments and promotion of focal adhesion contact formation by baicalein
To characterize the effects of baicalein on the microfilament organization and focal adhesion contact formation, fluorescence cytostaining using rhodamine-labelled phalloidin and immunostaining of integrins and vinculin were investigated by laser scanner confocal microscopy. As illustrated in Figure 6a, staining with rhodamine-labelled phalloidin for cytoskeletal architecture showed extensive stress fibre formation in untreated endothelial cells that was in a random orientation throughout the monolayer; moreover, vinculin-specific immunofluorescence was found in fine fibrillar streaks in untreated cells and associated with the ends of actin microfilaments around the periphery of each cell and at the ends of centrally located stress fibres. However, exposure to baicalein drastically altered the arrangement of F-actin within the monolayer (Figure 6a), by inducing it to concentrate under the plasma membrane. The streaks of vinculin appeared to be longer and thicker after baicalein treatment (Figure 6a).
Figure 6.
Modifications of actin filament and focal adhesion contacts by baicalein. Rat heart endothelial cells were incubated for 2 days with or without 100 μM of baicalein. (a) To visualize focal adhesion contacts, the cells were stained with antibody against vinculin for 30 min, washed twice and stained with FITC-labelled anti-mouse IgG for 30 min. Rhodamine-labelled phalloidin was used to stain actin filaments. (b) Number of focal adhesion contacts was quantified by counting microscopic images from 100 cells after immunofluorescence staining with an antibody against vinculin. (c) Cells were double stained by antibodies against integrin-αv and vinculin, or (d) integrin-α5 and vinculin. Microphotographs were taken by a Leica confocal microscopy, at the original × 600 magnification. *P<0.05. Scale bar, 5 μm. FITC, fluorescein isothiocyanate.
The formation of stress fibres occurred simultaneously with the appearance of vinculin streaks at their terminals, in correspondence with adhesion sites (focal adhesion contacts) (Davies et al., 2001). To quantify changes in the number of intensely stained focal adhesions, 100 cells for each treatment were imaged at random and analysed by image analysis software. Results showed that the number of focal adhesion contacts per cell markedly increased in baicalein-treated cells compared to untreated cells (Figure 6b). This result is consistent with the increased cellular adhesion described above.
It is well documented that integrins are present in focal contacts in cells. Next, we carried out double labelled immunofluorescent experiments to examine the distribution of vinculin compared to integrin-αvβ3, αvβ5 and integrin-α5β1. In untreated endothelial cells, integrin αv and α5 staining was generally restricted to distinct spots (Figures 6c and d). When endothelial cells were exposed to baicalein, integrin staining was detected throughout the cell (localized to the cell body as well as the periphery). However, in both untreated and baicalein-treated endothelial cells, αv (Figure 6c) and α5 (Figure 6d) appeared to colocalize with vinculin. The expressed levels of vinculin, αv and α5 present in focal adhesions in endothelial cells exposed to baicalein appeared to increase. These data indicate that the initial baicalein-induced formation of focal adhesion might be due to the increase in the cellular levels of integrins (especially αvβ3, αvβ5 and α5β1) and vinculin expression.
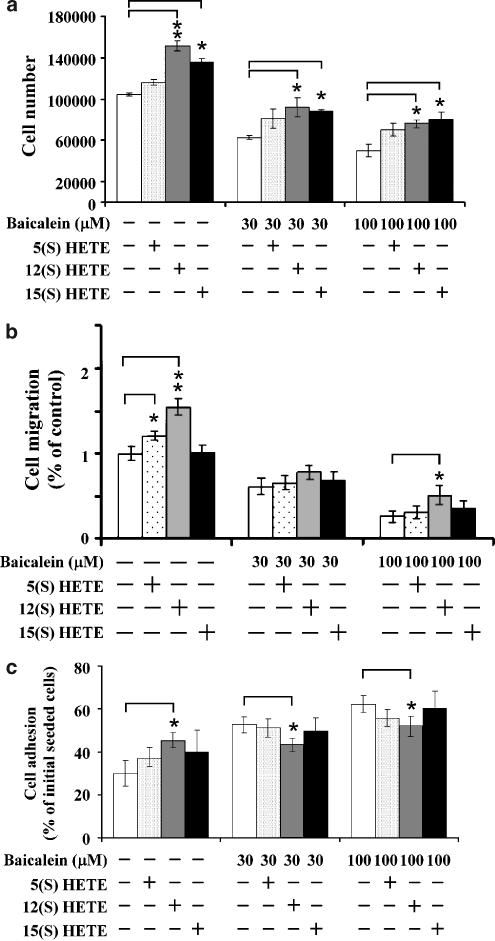
Effects of LOX products on baicalein-mediated endothelial proliferation, adhesion and migration
It has been reported that baicalein is a potent inhibitor of 5-, 12-, and 15-lipooxygenase (Cho et al., 1991; Sadik et al., 2003). To address whether the LOX metabolic pathway was involved in these baicalein-mediated endothelial responses, cells were exposed to baicalein in the absence or presence of exogenously added LOX metabolites, 5(S)-HETE (5-LOX product), 12(S)-HETE (12-LOX product), and 15(S)-HETE (15-LOX product). Modulation of endothelial cell proliferation, adhesion and migration by these HETEs was then assessed. Over a range of concentrations (10 nM–1 μM) the HETEs exhibited concentration-related effects on the baicalein-mediated biological responses (data not shown). We chose to use 100 nM as the test concentration for our further studies. At this concentration, treatment with each HETE alone significantly increased endothelial cell proliferation, adhesion and migration. However, exogenous 5(S)-HETE, 12(S)-HETE, and 15(S)-HETE could partially reverse the inhibitory effects of baicalein on endothelial proliferation (Figure 7a) and migration (Figure 7b), and slightly suppress baicalein-induced endothelial adhesion (Figure 7c).
Figure 7.
The effect of 5(S)-HETE, 12(S)-HETE, and 15(S)-HETE on baicalein-mediated endothelial growth, adhesion and migration. Endothelial cells were pretreated with each HETE (100 nM) for up to 1 h, and then treated with 100 μM baicalein for indicated time points. After treatment, (a) cell proliferation, (b) migration and (c) adhesion were determined as described in the text. Values shown are means±s.d. Asterisks indicate *P<0.05, **P<0.01, ***P<0.001. 5(S)-HETE, 5(S)-hydroxyeicosatetraenoic acid; 12(S)-HETE, 12(S)-hydroxyeicosatetraenoic acid; 15(S)-HETE, 15(S)-hydroxyeicosatetraenoic acid.
Discussion
Baicalein is a relatively selective 12-LOX inhibitor that also possesses many LOX-unrelated effects such as blocking calcium mobilization (Nyby et al., 1996) and acts as an antioxidant (Hanasaki et al., 1994). Our previous study demonstrated that baicalein markedly inhibited proliferation of rat heart endothelial cells (Hsu et al., 2001). In the present study, we show that pretreatment of rat heart endothelial cells with baicalein for 48 h significantly enhanced cell adhesion while suppressing cell migration. Short-term baicalein treatment (up to 6 h) of rat endothelial cells, however, had no effect on migration and adhesion, suggesting that longer exposure to baicalein is essential for its stimulation of adhesion and its antimigratory actions. Our observations also indicate that baicalein exhibits slight effects on quiescent endothelial cells, but has significant effect on endothelial cells activated by serum or VEGF. A previous study demonstrated that phenylephrine-induced cell proliferation and migration was inhibited by baicalein in vascular smooth muscle cells. This report suggests that 12-LOX may be involved in the proliferation and motility of vascular smooth muscle cells, because inhibition of smooth muscle cell proliferation and migration by baicalein could be reversed by exogenous 12-HETE (Nishio and Watanabe, 1997). Consistent with this report, our findings indicate that the endogenous 12-LOX pathway may be partly coupled to the regulation of endothelial proliferation, adhesion and migration induced by baicalein, because a combination treatment with 12-HETE (the 12-LOX product) slightly but significantly reversed the baicalein-mediated proliferation and migration inhibition and adhesion promotion (Figure 7). These results suggest that the LOX-independent pathway is involved in baicalein-mediated biological effects in endothelial cells. At this time, the precise mechanism whereby the LOX pathway is coupled to the baicalein-mediated actions in rat heart endothelial cells is still obscure and further studies will be needed to determine the underlying mechanism.
Our observations further demonstrated that baicalein stimulated endothelial cell adhesion to fibronectin and vitronectin and that these adherent cells eventually undergo spreading, reorganization of stress fibres and formation of adhesion plaques. Qualitatively and quantitatively, the pattern of microfilament organization and number of focal adhesion contacts are higher in baicalein-treated cells than untreated control cells. This phenomenon is of particular relevance, since our study provides evidence that baicalein upregulated the expression of endothelial cell surface receptors, the integrins α5β1, αvβ3 and αvβ5. Baicalein also promoted the interaction of plasma membrane with the ECM components, fibronectin and vitronectin, via these specific receptors. This interaction triggered a cascade of events leading to organization of adhesion plaques with which vinculin and other proteins are associated (Burridge and Connell, 1983b; Geiger et al., 1985; Dejana et al., 1987). In the Boyden chamber system, baicalein inhibited migration of endothelial cells.
Endothelial cells adhere to the ECM through a set of cell surface receptors and, in most cases, these receptors belong to the integrin superfamily and are composed of α and β subunits in heterodimer complexes (Zeng et al., 2006). Among the integrins, which are cell surface receptors for the ECM, the α5 integrin subunit recognizes only fibronectin as its ligand and forms a α5β1 heterodimer. The β3 or β5 subunit heterodimerizes with the αv integrin subunit and binds von Willebrand factor, thrombospondin, fibrinogen, fibronectin, as well as vitronectin (Hynes, 2002). Previous reports demonstrated that the α5β1 integrin plays a more important role in interactions with fibronectin, than other integrins (Bauer et al., 1992). It is well documented that the ability of fibronectin to bind to cells and to promote adhesion can be accounted for by the tripeptide RGD located in the cell attachment domain of fibronectin (Pierschbacher and Ruoslahti, 1984). This sequence is also present in several other ECM proteins including vitronectin (Liu, 2006). Peptides containing the RGD sequence are known to inhibit the attachment of various cell lines to fibronectin and vitronectin (Pierschbacher and Ruoslahti, 1984). We showed here that baicalein upregulated the expression of integrin α5β1, αvβ3 and αvβ5 and promoted endothelial cell adhesion to fibronectin and vitronectin. Moreover, this baicalein-induced adhesion event was significantly blocked in the presence of the RGD peptides or a blocking antibody to α5β1. Conversely, the control peptide SDGRG or antibodies against α2 integrin, did not inhibit adhesion to fibronectin or vitronectin after baicalein treatment. These observations suggest that baicalein-induced adhesion is mainly mediated by upregulation of α5β1 and αvβ3, αvβ5 integrins.
Endothelial cell migration is an important aspect of the angiogenic process, and therefore much research is focused on the intracellular mechanisms that control endothelial cell motility. Substantial amounts of data implicate both ECM–integrin and cytoskeletal interactions as critical mediators of this process. Cell migration is an integrated process requiring both adhesion to, and detachment from, the surrounding ECM (Lauffenburger and Horwitz, 1996). However, the relationship between strength of adhesion to the ECM and potential for migration seems to be complex. In this study, we found that a decrease in cell migration occurred concomitantly with increased cell adhesion in baicalein-treated endothelial cells. Thus, a causal relation between these two cellular responses may exist. The interactions of cells with fibronectin have been reported to influence or control different processes regulating the behaviour of cells, including cell migration, invasion, survival and proliferation (Akiyama et al., 1995). Mauro et al. (1999) reported that reduced growth and enhanced attachment to fibronectin in MCF-7 cells overexpressing SHC-(SH2 homology and collagen homology protein) were associated with significantly reduced cell migration. This result is consistent with our observations that baicalein-mediated suppression of endothelial migration was accompanied by inhibition of cell proliferation and promotion of cell adhesion to fibronectin. A possible mechanism for baicalein-mediated migration inhibition is provided by the fact that baicalein strongly stimulated adhesion of endothelial cells to the ECM substrates, fibronectin and vitronectin, by increasing integrin expression. Integrins have been recognized not only as the dominant family of cell adhesion molecules that mediate attachment to ECM (Hynes, 2002), but also have been credited as signalling receptors essential for cell migration (Giancotti and Ruoslahti, 1999). With low integrin expression, migration was relatively slow, because weakly attached cells do not generate enough traction to move significantly. An optimal rate of cell migration is achieved with increasing adhesion. With further cell attachment, however, cells again display impaired motility, presumably due to the inability to cycle between adherent and non-adherent states.
Endothelial adhesion and migration requires organized actin stress fibres and focal adhesion contacts (Thiagarajan et al., 1996) and the stimulation of endothelial migration by VEGF is associated with the formation of actin stress fibres and focal adhesion contacts (Rousseau et al., 1997). Disassembly of focal adhesion mediated by growth factors precedes cell migration and reduces cell–substratum adhesion (Nobes and Hall, 1999). At focal adhesion sites, actin filaments are bound to transmembrane receptors of the integrin family, through a complex of structural ‘plaque' proteins including vinculin, talin and α-actinin (Hynes, 2002). It has been reported that vinculin plays an important role in the maintenance of the focal adhesion contact and adherence junctions (Rudiger, 1998), whose alterations accompany the modulation of motility. Previous reports demonstrated that vinculin is important for the linkage of integrins to the cytoskeleton (Ezzell et al., 1997) and vinculin may promote cell spreading by stabilizing focal adhesions and transferring mechanical stresses that drive cytoskeletal remodelling (Ezzell et al., 1997). Increasing vinculin expression in 3T3 cells also increased formation of focal contacts and stress fibres, enhanced cell spreading, and reduced cell motility (Ezzell et al., 1997). In contrast, reduction of vinculin protein with an antisense vinculin RNA in 3T3 cells resulted in poor spreading and increased motility (Coll et al., 1995; Volberg et al., 1995; Ezzell et al., 1997). The present study revealed that baicalein increased the levels of vinculin and integrins (αvβ3, αvβ5 and α5β1) proteins and the immunoreactivity of these molecules in endothelial cells, indicating that baicalein stimulates the assembly of multimolecular focal adhesion complexes. The effects of baicalein treatment on endothelial cell adhesion might reflect this reorganization of focal adhesion. It appears that increase of vinculin by baicalein could promote focal adhesion contact formation as well as cell adhesion and reduce cell migration. Thus the upregulation of vinculin expression may be one of the mechanisms by which baicalein acts as an inhibitor of cell migration in rat heart endothelial cells.
In conclusion, this study provides the first insights into the mechanism by which the LOX inhibitor baicalein enhances the adhesion, particularly to fibronectin and vitronectin, and inhibits the migration, of rat heart endothelial cells in vitro. These effects are, in part, due to baicalein upregulating the expression of integrin molecules (α5β1, αvβ3 and αvβ5) and vinculin. These molecules are critical modulators of the organization of actin fibres and formation of focal adhesion contacts, processes that regulate endothelial cell adhesion, migration and proliferation. Our observations indicate that baicalein might be a useful tool to modulate physiological (or pathological) behaviour of endothelial cells.
Acknowledgments
This study was supported by grants TCVGH-953104C, and TCVGH-943102B from Taichung Veterans General Hospital, Taiwan, ROC.
Abbreviations
- PI
propidium iodide
- DAPI
4′6′-diamidino-2-phenylindole
- 5(S)-HETE
5(S)-hydroxyeicosatetraenoic acid
- 12(S)-HETE
12(S)-hydroxyeicosatetraenoic acid
- 15(S)-HETE
15(S)-hydroxyeicosatetraenoic acid
- RGD
the synthetic peptide Arg-Gly-Asp
- SDGRG
the synthetic peptide Ser-Asp-Gly-Arg-Gly
- DMSO
dimethyl sulphoxide
- VEGF
vascular endothelial growth factor
- PBS
phosphate-buffered saline
Conflict of interest
The authors state no conflict of interest.
References
- Akiyama SK, Olden K, Yamada KM. Fibronectin and integrins in invasion and metastasis. Cancer Metastasis Rev. 1995;14:173–189. doi: 10.1007/BF00690290. [DOI] [PubMed] [Google Scholar]
- Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- Ashton AW, Yokota R, John G, Zhao S, Suadicani SO, Spray DC, et al. Inhibition of endothelial cell migration, intercellular communication, and vascular tube formation by thromboxane A(2) J Biol Chem. 1999;274:35562–35570. doi: 10.1074/jbc.274.50.35562. [DOI] [PubMed] [Google Scholar]
- Bauer JS, Schreiner CL, Giancotti FG, Ruoslahti E, Juliano RL. Motility of fibronectin receptor-deficient cells on fibronectin and vitronectin: collaborative interactions among integrins. J Cell Biol. 1992;116:477–487. doi: 10.1083/jcb.116.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burridge K, Connell L. Talin: a cytoskeletal component concentrated in adhesion plaques and other sites of actin–membrane interaction. Cell Motil. 1983b;3:405–417. doi: 10.1002/cm.970030509. [DOI] [PubMed] [Google Scholar]
- Chen ZY, Su YL, Lau CW, Law WI, Huang Y. Endothelium-dependent contraction and direct relaxation induced by baicalein in rat mesenteric artery. Eur J Pharmacol. 1999;374:41–47. doi: 10.1016/s0014-2999(99)00291-5. [DOI] [PubMed] [Google Scholar]
- Cho H, Ueda M, Tamaoka M, Hamaguchi M, Aisaka K, Kiso Y, et al. Novel caffeic acid derivatives: extremely potent inhibitors of 12-lipoxygenase. J Med Chem. 1991;34:1503–1505. doi: 10.1021/jm00108a039. [DOI] [PubMed] [Google Scholar]
- Christopher RA, Judge SR, Vincent PA, Higgins PJ, McKeown-Longo PJ. The amino-terminal matrix assembly domain of fibronectin stabilizes cell shape and prevents cell cycle progression. J Cell Sci. 1999;112 Part 19:3225–3235. doi: 10.1242/jcs.112.19.3225. [DOI] [PubMed] [Google Scholar]
- Coll JL, Ben-Ze'ev A, Ezzell RM, Rodriguez Fernandez JL, Baribault H, Oshima RG, et al. Targeted disruption of vinculin genes in F9 and embryonic stem cells changes cell morphology, adhesion, and locomotion. Proc Natl Acad Sci USA. 1995;92:9161–9165. doi: 10.1073/pnas.92.20.9161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies CdeL, Melder RJ, Munn LL, Mouta-Carreira C, Jain RK, Boucher Y. Decorin inhibits endothelial migration and tube-like structure formation: role of thrombospondin-1. Microvasc Res. 2001;62:26–42. doi: 10.1006/mvre.2001.2311. [DOI] [PubMed] [Google Scholar]
- Dejana E, Colella S, Languino LR, Balconi G, Corbascio GC, Marchisio PC. Fibrinogen induces adhesion, spreading, and microfilament organization of human endothelial cells in vitro. J Cell Biol. 1987;104:1403–1411. doi: 10.1083/jcb.104.5.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezzell RM, Goldmann WH, Wang N, Parasharama N, Ingber DE. Vinculin promotes cell spreading by mechanically coupling integrins to the cytoskeleton. Exp Cell Res. 1997;231:14–26. doi: 10.1006/excr.1996.3451. [DOI] [PubMed] [Google Scholar]
- Folkman J, Klagsbrun M. Angiogenic factors. Science. 1987;235:442–447. doi: 10.1126/science.2432664. [DOI] [PubMed] [Google Scholar]
- Fu YC, Chi CS, Yin SC, Hwang B, Chiu YT, Hsu SL. Norepinephrine induces apoptosis in neonatal rat endothelial cells via down-regulation of Bcl-2 and activation of beta-adrenergic and caspase-2 pathways. Cardiovasc Res. 2004;61:143–151. doi: 10.1016/j.cardiores.2003.10.014. [DOI] [PubMed] [Google Scholar]
- Fujita M, Shiota S, Kuroda T, Hatano T, Yoshida T, Mizushima T, et al. Remarkable synergies between baicalein and tetracycline, and baicalein and beta-lactams against methicillin-resistant Staphylococcus aureus. Microbiol Immunol. 2005;49:391–396. doi: 10.1111/j.1348-0421.2005.tb03732.x. [DOI] [PubMed] [Google Scholar]
- Geiger B, Volk T, Volberg T. Molecular heterogeneity of adherens junctions. J Cell Biol. 1985;101:1523–1531. doi: 10.1083/jcb.101.4.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giancotti FG, Ruoslahti E. Integrin signaling. Science. 1999;285:1028–1032. doi: 10.1126/science.285.5430.1028. [DOI] [PubMed] [Google Scholar]
- Hanasaki Y, Ogawa S, Fukui S. The correlation between active oxygens scavenging and antioxidative effects of flavonoids. Free Radic Biol Med. 1994;16:845–850. doi: 10.1016/0891-5849(94)90202-x. [DOI] [PubMed] [Google Scholar]
- Hsu SL, Hsieh YC, Hsieh WC, Chou CJ. Baicalein induces a dual growth arrest by modulating multiple cell cycle regulatory molecules. Eur J Pharmacol. 2001;425:165–171. doi: 10.1016/s0014-2999(01)01144-x. [DOI] [PubMed] [Google Scholar]
- Huang HC, Hsieh LM, Chen HW, Lin YS, Chen JS. Effects of baicalein and esculetin on transduction signals and growth factors expression in T-lymphoid leukemia cells. Eur J Pharmacol. 1994a;268:73–78. doi: 10.1016/0922-4106(94)90121-x. [DOI] [PubMed] [Google Scholar]
- Huang Y, Tsang SY, Yao X, Chen ZY. Biological properties of baicalein in cardiovascular system. Curr Drug Targets Cardiovasc Haematol Disord. 2005;5:177–184. doi: 10.2174/1568006043586206. [DOI] [PubMed] [Google Scholar]
- Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- Kanayasu-Toyoda T, Morita I, Murota S. Arachidonic acid pretreatment enhances smooth muscle cell migration via increased Ca2+ influx. Prostaglandins Leukot Essent Fatty Acids. 1998;58:25–31. doi: 10.1016/s0952-3278(98)90126-0. [DOI] [PubMed] [Google Scholar]
- Kimura Y, Yokoi K, Matsushita N, Okuda H. Effects of flavonoids isolated from scutellariae radix on the production of tissue-type plasminogen activator and plasminogen activator inhibitor-1 induced by thrombin and thrombin receptor agonist peptide in cultured human umbilical vein endothelial cells. J Pharm Pharmacol. 1997;49:816–822. doi: 10.1111/j.2042-7158.1997.tb06119.x. [DOI] [PubMed] [Google Scholar]
- Lauffenburger DA, Horwitz AF. Cell migration: a physically integrated molecular process. Cell. 1996;84:359–369. doi: 10.1016/s0092-8674(00)81280-5. [DOI] [PubMed] [Google Scholar]
- Liu JJ, Huang TS, Cheng WF, Lu FJ. Baicalein and baicalin are potent inhibitors of angiogenesis: inhibition of endothelial cell proliferation, migration and differentiation. Int J Cancer. 2003;106:559–565. doi: 10.1002/ijc.11267. [DOI] [PubMed] [Google Scholar]
- Liu S. Radiolabeled multimeric cyclic RGD peptides as integrin alphavbeta3 targeted radiotracers for tumor imaging. Mol Pharm. 2006;3:472–487. doi: 10.1021/mp060049x. [DOI] [PubMed] [Google Scholar]
- Mauro L, Sisci D, Bartucci M, Salerno M, Kim J, Tam T, et al. SHC-alpha5beta1 integrin interactions regulate breast cancer cell adhesion and motility. Exp Cell Res. 1999;252:439–448. doi: 10.1006/excr.1999.4639. [DOI] [PubMed] [Google Scholar]
- Nishio E, Watanabe Y. Role of the lipoxygenase pathway in phenylephrine-induced vascular smooth muscle cell proliferation and migration. Eur J Pharmacol. 1997;336:267–273. doi: 10.1016/s0014-2999(97)01259-4. [DOI] [PubMed] [Google Scholar]
- Nobes CD, Hall A. Rho GTPases control polarity, protrusion, and adhesion during cell movement. J Cell Biol. 1999;144:1235–1244. doi: 10.1083/jcb.144.6.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyby MD, Sasaki M, Ideguchi Y, Wynne HE, Hori MT, Berger ME, et al. Platelet lipoxygenase inhibitors attenuate thrombin- and thromboxane mimetic-induced intracellular calcium mobilization and platelet aggregation. J Pharmacol Exp Ther. 1996;278:503–509. [PubMed] [Google Scholar]
- Palecek SP, Loftus JC, Ginsberg MH, Lauffenburger DA, Horwitz AF. Integrin–ligand binding properties govern cell migration speed through cell–substratum adhesiveness. Nature. 1997;385:537–540. doi: 10.1038/385537a0. [DOI] [PubMed] [Google Scholar]
- Pereda MP, Hopfner U, Pagotto U, Renner U, Uhl E, Arzt E, et al. Retinoic acid stimulates meningioma cell adhesion to the extracellular matrix and inhibits invasion. Br J Cancer. 1999;81:381–386. doi: 10.1038/sj.bjc.6690705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierschbacher MD, Ruoslahti E. Cell attachment activity of fibronectin can be duplicated by small synthetic fragments of the molecule. Nature. 1984;309:30–33. doi: 10.1038/309030a0. [DOI] [PubMed] [Google Scholar]
- Romer LH, Birukov KG, Garcia JG. Focal adhesions: paradigm for a signaling nexus. Circ Res. 2006;98:606–616. doi: 10.1161/01.RES.0000207408.31270.db. [DOI] [PubMed] [Google Scholar]
- Rousseau S, Houle F, Landry J, Huot J. p38 MAP kinase activation by vascular endothelial growth factor mediates actin reorganization and cell migration in human endothelial cells. Oncogene. 1997;15:2169–2177. doi: 10.1038/sj.onc.1201380. [DOI] [PubMed] [Google Scholar]
- Rudiger M. Vinculin and alpha-catenin: shared and unique functions in adherens junctions. Bioessays. 1998;20:733–740. doi: 10.1002/(SICI)1521-1878(199809)20:9<733::AID-BIES6>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Sadik CD, Sies H, Schewe T. Inhibition of 15-lipoxygenases by flavonoids: structure–activity relations and mode of action. Biochem Pharmacol. 2003;65:773–781. doi: 10.1016/s0006-2952(02)01621-0. [DOI] [PubMed] [Google Scholar]
- Saunders WB, Bohnsack BL, Faske JB, Anthis NJ, Bayless KJ, Hirschi KK, et al. Coregulation of vascular tube stabilization by endothelial cell TIMP-2 and pericyte TIMP-3. J Cell Biol. 2006;175:179–191. doi: 10.1083/jcb.200603176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuppan D, Porov Y. Hepatic fibrosis: from bench to bedside. J Gastroenterol Hepatol. 2002;17 Suppl 3:S300–S305. doi: 10.1046/j.1440-1746.17.s3.18.x. [DOI] [PubMed] [Google Scholar]
- Sekiya K, Okuda H. Selective inhibition of platelet lipoxygenase by baicalein. Biochem Biophys Res Commun. 1982;105:1090–1095. doi: 10.1016/0006-291x(82)91081-6. [DOI] [PubMed] [Google Scholar]
- Thiagarajan P, Le A, Snuggs MB, VanWinkle B. The role of carboxy-terminal glycosaminoglycan-binding domain of vitronectin in cytoskeletal organization and migration of endothelial cells. Cell Adhes Commun. 1996;4:317–325. doi: 10.3109/15419069609010775. [DOI] [PubMed] [Google Scholar]
- Tsuruta D, Jones JC. The vimentin cytoskeleton regulates focal contact size and adhesion of endothelial cells subjected to shear stress. J Cell Sci. 2003;116:4977–4984. doi: 10.1242/jcs.00823. [DOI] [PubMed] [Google Scholar]
- Volberg T, Geiger B, Kam Z, Pankov R, Simcha I, Sabanay H, et al. Focal adhesion formation by F9 embryonal carcinoma cells after vinculin gene disruption. J Cell Sci. 1995;108 Part 6:2253–2260. doi: 10.1242/jcs.108.6.2253. [DOI] [PubMed] [Google Scholar]
- Zeng ZZ, Jia Y, Hahn NJ, Markwart SM, Rockwood KF, Livant DL. Role of focal adhesion kinase and phosphatidylinositol 3′-kinase in integrin fibronectin receptor-mediated, matrix metalloproteinase-1-dependent invasion by metastatic prostate cancer cells. Cancer Res. 2006;66:8091–8099. doi: 10.1158/0008-5472.CAN-05-4400. [DOI] [PubMed] [Google Scholar]