Abstract
Background and purpose:
Diabetes mellitus is associated with a specific cardiomyopathy. We compared the cardioprotective effects of an endothelin-A receptor blocker (ETA-RB) with those of an angiotensin-converting enzyme inhibitor (ACE-I) in rats with streptozotocin (STZ)-induced diabetes.
Experimental approach:
Diabetic rats were left untreated or received either the ETA-RB atrasentan or the ACE-I ramipril (each 3 mg kg−1 per day) orally for 8 weeks. Isolated isovolumic heart function was studied during normoxia and in response to ischaemia-reperfusion. Cardiac fibrosis, tissue oxidative stress and tissue nitric oxide synthase (NOS) activity were determined.
Key results:
Basal left ventricular systolic contractility was lower in diabetic compared to nondiabetic hearts and ETA-RB or ACE-I treatment significantly antagonised the decline. Following 15 min of no-flow ischaemia, reperfusion systolic function was depressed and left-ventricular end-diastolic pressure (LVEDP) was elevated in diabetic hearts. ETA-RB or ACE-I treatment significantly improved recovery of reperfusion systolic and diastolic function, without differences between groups. Hydroxyproline (an index of tissue fibrosis) and malondialdehyde (a measure of tissue oxidative stress) were elevated at the end of reperfusion in diabetic, compared to nondiabetic hearts. Either treatment reduced hydroxyproline and malondialdehyde to control level. Constitutive NOS activity was similar in nondiabetic and diabetic hearts and unaffected by ETA-RB or ACE-I treatment.
Conclusions and implications:
These results suggest that in experimental type 1 diabetes ETA-RB is as effective as an ACE-I in ameliorating myocardial functions during normoxia and ischaemia-reperfusion. Combining the two treatments neither afforded additive effects, nor diminished any protection effect seen with either drug.
Keywords: diabetes, streptozotocin, cardiomyopathy, endothelin-1, ETA receptor blockade, ACE inhibition
Introduction
Cardiovascular complications of long-standing diabetes are important causes of morbidity and mortality in diabetic patients. Chronic hyperglycaemia impairs endothelium-dependent regulation of vascular tone, disturbs neuronal and renal function, and causes cardiomyopathy independent of atherosclerosis (Sheetz and King, 2002). For poorly understood reasons, patients with diabetes have a substantially increased risk of developing heart failure and have a markedly adverse course following myocardial infarction, with high rates of post-infarction heart failure and death (Adeghate, 2004).
Considerable evidence implicates angiotensin II in diabetic complications. In the vasculature, angiotensin II exerts vasoconstriction, stimulates pro-inflammatory and pro-thrombotic processes, induces fibrosis and remodelling and generates reactive oxygen species (ROS) with attendant destruction of nitric oxide (NO) (McFarlane et al., 2003). Angiotensin II also increases hepatic glucose production and decreases insulin sensitivity (Rao, 1996). Major clinical trials with ACE inhibitors resulted in fewer cardiovascular events and a lower rate of macrovascular complications in high-risk diabetic patients (Yusuf et al., 2000).
Recent evidence has indicated the possible importance of the endothelin (ET) system in the pathogenesis of diabetic complications. In hearts from experimental animals with chemically induced diabetes, mRNA abundance and protein expression for ET-1 and ET receptors were elevated and ET-1 receptor binding was enhanced (Hileeto et al., 2002). The increased expression of mRNA encoding for ET-1 and ET receptors was associated with myocardial cell death, focal scarring of the myocardium and increased expression of several extracellular matrix proteins. All these effects were mitigated by an ET receptor antagonist (Hileeto et al., 2002). Similarly, in diabetic animals, ET receptor blockade limited the rise in blood pressure (Witte et al., 2003) and antagonised myocardial contractile depression (Verma et al., 2001), macrovascular endothelial dysfunction and renal target organ damage (Dhein et al., 2000).
Several features of diabetic cardiomyopathy are exacerbated following even brief ischaemia, although opposite observations have also been reported (for review, see Feuvray and Lopaschuk, 1997). Recent pre-clinical and clinical evidence revealed ACE inhibitors as potent in preventing ischaemic events and in blocking several ischaemic processes in the myocardium (Pitt, 2000). The anti-ischaemic potential of ET receptor antagonists, on the other hand, in diabetic animals or diabetics with ischaemic myocardial syndromes has received little attention so far.
In the present investigation, the effects of chronic ETA receptor blockade (ETA-RB) with atrasentan on left ventricular function in hearts isolated from STZ-induced diabetic rats subjected to normoxia and ischaemia-reperfusion were investigated. As a positive control, hearts from animals treated with the ACE inhibitor (ACE-I) ramipril were chosen. In addition, we evaluated the protective potential resulting from combining the ET receptor antagonist with the ACE inhibitor, which should be useful to judge whether ET antagonism might result in therapeutic progress.
Methods
Animals and experimental groups
Studies were performed on 89 female normotensive Sprague–Dawley rats with initial body weights of 203±2 g. Eight groups of animals (n=8 per group) were studied: (1) nondiabetic vehicle-treated; (2) nondiabetic atrasentan-treated; (3) nondiabetic ramipril-treated; (4) nondiabetic atrasentan plus ramipril-treated; (5) vehicle-treated diabetic; (6) atrasentan-treated diabetic; (7) ramipril-treated diabetic; and (8) diabetic rats treated with the combination of ramipril and atrasentan. The nominal atrasentan and ramipril doses were 3 mg kg−1 per day; calculated daily doses ranged from 2.9±0.05 to 3.3±0.10 mg kg−1 per day (nondiabetic) and from 3.2±0.08 to 3.5±0.09 mg kg−1 per day (diabetic). All animals received care in accordance with the Austrian law on experimentation with laboratory animals (last amendment, 2004), which is based on the principles of laboratory animal care as adopted by the American Heart Association and the Declaration of Helsinki.
Atrasentan plasma levels
Plasma levels of atrasentan were determined at 8 weeks of dosing using a HPLC method (Bryan et al., 2001). They were 38±6 ng ml−1 (10 determinations in five nondiabetic and five diabetic animals). The adequacy of the atrasentan dose was tested in pilot experiments in vivo: i.p. injection of 2 nmol kg−1 ET-1 raised blood pressure by 52±5 mm Hg (tail cuff method; TSE, Bad Homburg, Germany), while prior treatment with ∼3 mg kg−1 atrasentan per day for 4 days completely abolished this effect (n=3). Ramipril is known to be bioavailable in rats and plasma levels were therefore not determined.
Diabetes induction
Diabetes was induced by a single i.p. injection of STZ (55 mg kg−1) dissolved in citrate buffer (0.5 ml, pH 4.5). This model is well characterized and contains a high-oxidant component and no autoimmune involvement. Nondiabetic (control) animals were injected with 0.5 ml citrate buffer alone. Animals were maintained on normal chow (1324 Forti standard diet; Altromin, Lage, Germany) and did not receive supplemental insulin injections. Diabetes was confirmed by measuring nonfasted glucose levels in blood derived from a tail vein early in the morning using OneTouch Ultra glucose test strips (Lifescan, Neckargemünd, Germany). Test drugs were dissolved in the drinking water, prepared fresh every day, and administered over 8 weeks, starting on the day after STZ administration. Animals were killed after 8 weeks and the experiments were done on the same day unless stated otherwise. Arterial blood pressure was measured in unrestrained animals via tail cuff plethysmography (TSE).
HbA1C determination
HbA1C was determined using a Roche-Hitachi/Tina-quant analyzer (Roche Diagnostics, Mannheim, Germany). The method is based on the immunoturbidimetric determination of the stable glucose adduct to the N-terminal group of the haemoglobin β chain.
Heart function and in vitro perfusion protocol
Heart perfusion experiments were performed as described previously (Brunner, 1997). Hearts were excised under anaesthesia, mounted in a perfusion apparatus (Hugo Sachs Elektronik/Harvard Instruments, March-Hugstetten, Germany) and retrogradely perfused with Krebs–Henseleit buffer at 10 ml min−1 per g wet weight (pH 7.4, 37°C). A fluid-filled balloon (∼150 μl) was inserted into the left ventricle and connected to a pressure transducer. The following cardiac parameters were monitored in unpaced hearts: left-ventricular developed pressure (LVDevP), left-ventricular end-diastolic pressure (LVEDP; set at 5 mm Hg at the beginning of the experiment), maximum rate of rise and fall of left-ventricular pressure (+dP/dt, −dP/dt), time-to-onset and peak ischaemic contracture, coronary perfusion pressure (an index of coronary arterial function) and heart rate (electronically derived from the pressure signal).
After equilibration (60 min; baseline) hearts were subjected to 15 min of no-flow ischaemia at 36−37°C and reperfused for 60 min at 10 ml min−1 (total duration of experiment 135 min). Subsequently hearts were weighed, frozen immediately in liquid nitrogen, stored at −70°C and later used for biochemical assays. During the equilibration and reperfusion phases, coronary effluent was collected in appropriate intervals for the determination of nitrite concentration.
Nitrite concentration
Nitrite concentration in the coronary effluent was measured by examining the conversion of 2,3-diaminonaphthalene (DAN) to its fluorescent product, 1-(H)-naphthotriazole, that is stable in alkaline solution (Gharavi and El-Kadi, 2003). Samples (100 μl) of effluent were mixed with 15 μl of freshly prepared DAN (17 μg ml−1 in 0.62 M HCl) and incubated at 37°C for 30 min in the dark. The reaction was terminated with 10 μl of NaOH (2.8 M). After 10 min the samples were centrifuged at 16 000 g for 3 min to remove any precipitate and the supernatant was used for HPLC analysis (Merck-Hitachi D-6000; Vienna, Austria). The mobile phase (53% Na2HPO4 15 mM, pH 7.5, 47% methanol) was pumped through a Lichrospher column (RP=18.5 μm) at a flow rate of 1 ml min−1. Samples were measured with a fluorescence detector (Hitachi F-1050) at 380 nm excitation and 405 nm emission. Effluent nitrite concentration was obtained from a standard curve (50 nM to 1 μM) for known concentrations of sodium nitrite. The nitrite concentration of the perfusion buffer itself in the absence of an attached heart was 177±14 nM (20 determinations). Because of the high nitrate levels in the perfusion buffer (∼7 μM), nitrate was not determined in these samples.
Hydroxyproline determinations
Frozen left ventricle (∼50 mg) was homogenized in 500 μl phosphate-buffered saline and 100 μl of the resulting homogenate were hydrolysed overnight at 110°C in 6 M HCl. The hydrolysate was dried under nitrogen and reconstituted with 1 ml acetate/citrate buffer (citric acid, 24 mM; NaOH, 85 mM; sodium acetate, 88 mM; glacial acetic acid 0.12%, pH 6.0) The hydroxyproline content was measured photometrically at 557 nm by its reaction with chloramine T, perchloric acid and p-dimethylaminobenzaldehyde using a standard curve. Hydroxyproline content was expressed as mg per g of homogenate protein as determined by the Bradford method. All details have been reported previously (Stessel and Brunner, 2004).
Cardiac oxidant status
Levels of thiobarbituric acid-reactive substance were measured as an index of lipid peroxidation. About 100 μl of ventricular homogenate (see above) was combined with 2.5 ml thiobarbituric acid from an assay kit (Oxitec, ZeptoMetrix Corporation, Buffalo, NY, USA), incubated for 1 h at 95°C and further processed as described previously (Wölkart et al., 2006). Results were expressed as malondialdehyde equivalents per mg protein determined by the Bradford method.
NOS catalytic activity
Nitric oxide synthase (NOS) activity was measured in separate hearts derived from untreated and treated diabetic rats and one control group (untreated nondiabetic rats) (n=5 per group). The hearts of these rats were not subjected to ischaemia/reperfusion. Hearts were crushed in 1 ml of homogenization buffer, the homogenate centrifuged and the supernatant was retained on ice as described in detail previously (Wölkart et al., 2006). NOS activity of the supernatant was quantitated by measuring the formation of [3H]L-citrulline from [3H]L-arginine (Schmidt and Mayer, 1999). Total specific activity was obtained by incubating supernatant in the presence of 100 μM NG-nitro-L-arginine, inducible NOS (iNOS) activity was obtained by adding 5 mM ethylenediaminetetraacetic acid (EDTA), and constitutive (eNOS+neuronal NOS [nNOS]) was obtained after blocking iNOS with 10 μM 1400 W (Garvey et al., 1997). Results were expressed as pmol [3H]L-citrulline formed min−1 (mg protein)−1.
Statistics
All values are given as mean±s.e.m. of the number of animals, tissues or assays indicated. Statistical analysis was performed using analysis of variance (ANOVA-2) with vehicle, ramipril and atrasentan treatment as factors and the parameter measured as dependent variable. If ANOVA indicated significant differences, Student's t-test for unpaired observations was performed. Probability values of P<0.05 were considered significant. Stat view (version 5.0) software on an Apple MacIntosh computer was used for analyses.
Materials
The sources of the chemicals used in these experiments were as follows: ramipril, (Sanofi-Aventis Deutschland GmbH, Frankfurt/Main, Germany); atrasentan (Abbott Pharmaceuticals Inc., Abbott Park, IL, USA); 2,3-diaminonaphthalene (DAN), perchloric acid and p-dimethylaminobenzaldehyde (Fluka, Vienna, Austria); streptozotocin and 1400 W (Sigma, Vienna, Austria); chloramine T (Riedl deHaën, Vienna, Austria). [3H]L-arginine (specific activity=1.85 × 1012 Bq mmol−1) from American Radiolabelled Chemicals Inc., St. Louis, MO, USA).
Results
General characteristics
The general characteristics of the eight different groups of rats are given in Table 1. Induction of diabetes resulted in characteristic symptoms including hyperglycaemia, increased HbA1C values and increased fluid intake when compared to age-matched nondiabetic controls. These values remained unchanged after treatment with atrasentan, ramipril or their combination. Body weight was decreased in ramipril-treated (−9%) and combination-treated rats (−24%). Heart weights were similar in all nondiabetic and diabetic groups except for the combination-treated diabetic group in which it was significantly lower (P<0.05). Blood pressure was increased in untreated diabetic animals compared to nondiabetic controls, whereas there was no difference in any of the other three groups. Heart rate declined from 390 to 320 beats min−1 after 8 weeks of diabetes. Heart rate was not affected by ETA-RB or ACE-I treatment in any group (Table 1).
Table 1.
Baseline characteristics
| Nondiabetic | Diabetic | |
|---|---|---|
| Untreated | ||
| Blood glucose (mM) | 8.1±0.4 | 30.9±1.9# |
| HbA1C (%) | 4.3±0.1 | 10.4±0.9# |
| Fluid intake (ml day−1) | 32±1.6 | 134±12.4# |
| Body weight (g) | 231±5 | 222±4 |
| Heart weight (g) | 0.98±0.02 | 0.96±0.02 |
| Heart/body weight | 0.0042±0.0001 | 0.0043±0.0001 |
| Blood pressure (mm Hg) | 110±2 | 132±5# |
| Heart rate (min−1) | 383±10 | 319±10# |
| Atrasentan-treated | ||
| Blood glucose (mM) | 8.1±0.5 | 29.7±1.4# |
| HbA1C (%) | ND | 11.1±0.8 |
| Fluid intake (ml day−1) | 33±1.9 | 118±11.6# |
| Body weight (g) | 232±7 | 214±12 |
| Heart weight (g) | 1.00±0.03 | 0.97±0.04 |
| Heart/body weight | 0.0043±0.0001 | 0.0046±0.0002 |
| Blood pressure (mm Hg) | 113±2 | 118±3* |
| Heart rate (min−1) | 391±9 | 301±7# |
| Ramipril-treated | ||
| Blood glucose (mM) | 7.6±0.3 | 31.0±1.3# |
| HbA1C (%) | ND | 10.4±1.0 |
| Fluid intake (ml day−1) | 38±2.3 | 133±13.2# |
| Body weight (g) | 221±6 | 201±4# |
| Heart weight (g) | 0.96±0.03 | 0.90±0.03 |
| Heart/body weight | 0.0044±0.0002 | 0.0045±0.0001 |
| Blood pressure (mm Hg) | 106±4 | 116±4* |
| Heart rate (min−1) | 398±9 | 301±7# |
| Combination-treated | ||
| Blood glucose (mM) | 7.9±0.6 | 32.6±0.7# |
| Fluid intake (ml day−1) | 36±1.8 | 112±9.1# |
| HbA1C (%) | ND | 10.3±0.6 |
| Body weight (g) | 228±6 | 172±11#,* |
| Heart weight (g) | 0.95±0.01 | 0.88±0.03#,* |
| Heart/body weight | 0.0042±0.0001 | 0.0052±0.0003#,* |
| Blood pressure (mm Hg) | 108±3 | 106±4* |
| Heart rate (min−1) | 380±5 | 300±7# |
Data are mean±s.e.m. derived from eight animals per group. #P<0.05 vs corresponding nondiabetic group; *P<0.05 vs diabetic untreated group. ND, not determined.
P<0.05
P<0.05
Basal myocardial function
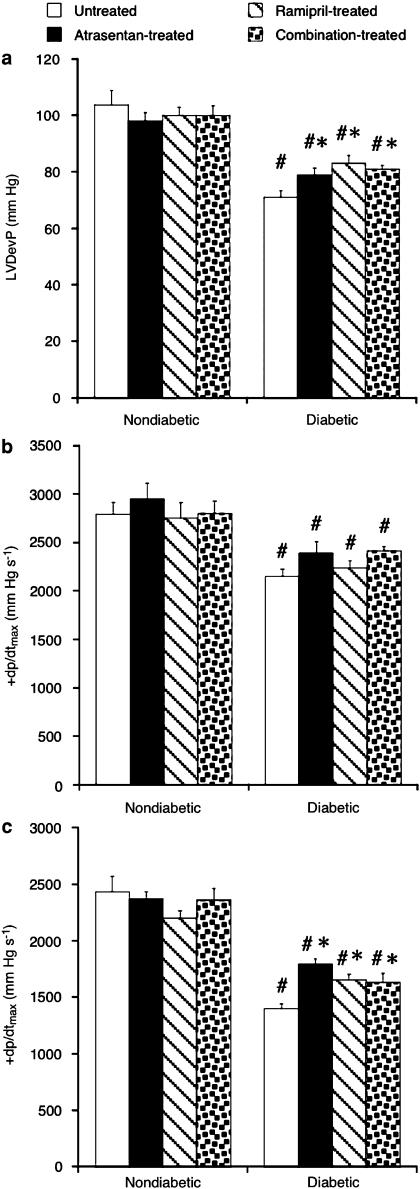
Basal left-ventricular function was measured at a fixed intraventricular balloon volume. LVDevP and +dp/dt (which characterize systolic contractility) and −dp/dt (a measure of diastolic relaxation) were similar in the four nondiabetic groups but reduced by 25% (contractility) and 43% (−dp/dt) in the diabetic state (P<0.05; Figure 1). ETA-RB, ACE-I or combination treatment partly restored LVDevP and −dp/dt but not +dp/dt. All three treatments were similarly effective (P=NS among treatments).
Figure 1.
Basal left-ventricular developed pressure (LVDevP) (a), maximum rate of systolic contraction (+dp/dtmax) (b) and maximum rate of diastolic relaxation (−dp/dtmax) (c) in nondiabetic and diabetic hearts. Animals were either left untreated or treated with atrasentan, ramipril or with both drugs. Data are mean±s.e.m. of eight hearts. #P<0.05 vs nondiabetic; *P<0.05 vs untreated diabetic.
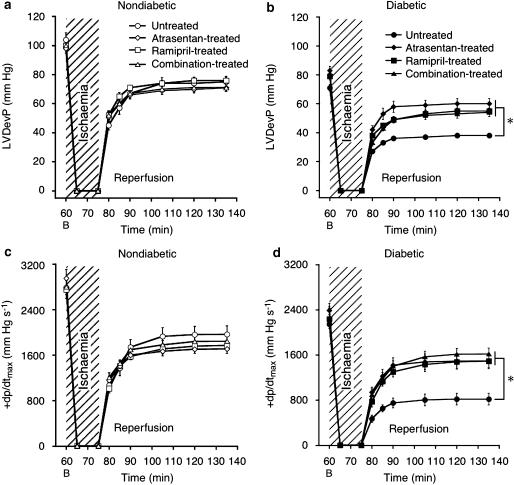
Effects of ETA-RB and ACE-I on systolic function during reperfusion
Myocardial function was assessed during 1 h of reperfusion following 15 min of ischaemia (Figure 2). In nondiabetic hearts, recovery of LVDevP and +dp/dt was ∼70% (P<0.05), irrespective of drug treatment (Figures 2a and c). In diabetic hearts, both parameters were significantly reduced compared to nondiabetic hearts and treatment of diabetic rats with atrasentan, ramipril or their combination antagonized the myocardial depression induced by the diabetic state (Figures 2b and d). Again, all three treatment protocols were similarly cardioprotective.
Figure 2.
Reperfusion contractile function. Recovery of LVDevP and +dp/dtmax were similar in nondiabetic hearts (a, c) but reduced in untreated diabetic hearts (b, d). Atrasentan, ramipril and combination treatment improved contractility. Data are mean±s.e.m. of eight hearts. *P<0.05 vs untreated diabetic (ANOVA-2). B, baseline.
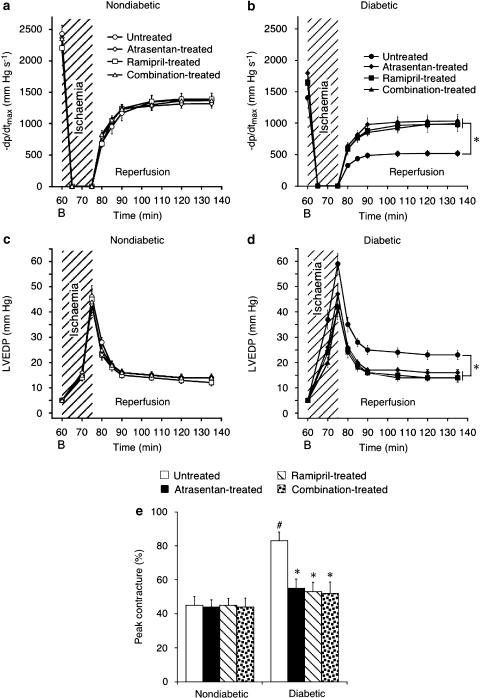
Effects of ETA-RB and ACE-I on diastolic function during ischaemia and reperfusion
As seen with systolic function, diastolic function was depressed in diabetic hearts compared to nondiabetic hearts (Figure 3). Thus, −dp/dt was ∼500 mm Hg s−1 at the end of 1 h reperfusion in untreated diabetic hearts compared to ∼1400 mm Hg s−1 in nondiabetic control hearts (P<0.05; Figures 3a and b). All three treatment protocols partly restored −dp/dt, without reaching the level in nondiabetic hearts. A similar picture emerged for LVEDP, which was increased throughout reperfusion as a result of diabetes (Figures 3c and d). To evaluate the effects of the test drugs on ischaemic function of diabetic hearts, time-to-onset of ischaemic contracture and extent of peak contracture were calculated (Brunner and Opie, 1998). As expected, contracture started earlier in diabetic hearts (∼6 min after initiation of ischaemia) than in nondiabetic controls (∼9 min) (P<0.05). However, none of the treatments affected this parameter (data not shown). Peak contracture was almost doubled in untreated diabetic hearts compared to nondiabetic controls (P<0.05), and all three treatment regimens reduced peak contracture to the level observed in nondiabetic hearts (Figure 3e).
Figure 3.
Diastolic function during ischaemia and reperfusion. –dp/dtmax and left-ventricular end-diastolic pressure (LVEDP) were similar in nondiabetic hearts (a, c) but deteriorated in untreated diabetic hearts (b, d). Peak ischaemic contracture was similar in nondiabetic hearts, and doubled in untreated diabetic hearts (e). Atrasentan, ramipril or combination treatment improved –dp/dtmax and restored LVEDP and peak contracture to nondiabetic level. Data are mean±s.e.m. of eight hearts. *P<0.05 vs untreated diabetic (ANOVA-2). B, baseline. #P<0.05 vs nondiabetic.
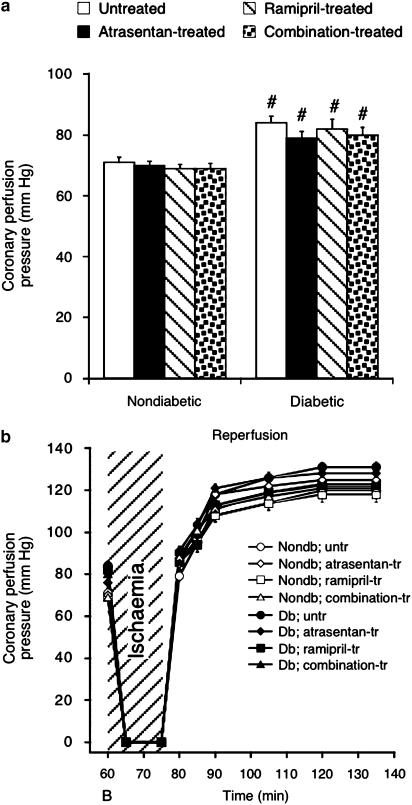
Coronary vascular function and heart rate
Figure 4 shows coronary perfusion pressure, which is a measure of coronary microvascular function in hearts perfused at constant flow. Compared to nondiabetic hearts, the pressure reading was increased in diabetic hearts, reflecting vascular dysfunction (P<0.05). However, none of the treatment regimes affected baseline perfusion pressure (Figure 4a) or the pressure registered during reperfusion (Figure 4b).
Figure 4.
Coronary perfusion pressure at baseline and during reperfusion. Basal perfusion pressure was elevated in diabetic compared to nondiabetic hearts, but unaffected by the test drugs (a). After ischaemia perfusion pressure was similarly elevated compared to baseline in all groups (b). B, baseline; Nondb, nondiabetic; Db, diabetic. Data are mean±s.e.m. of eight hearts. #P<0.05 vs nondiabetic.
The spontaneous heart rate was similar in all four nondiabetic groups both at baseline and after 1 h of reperfusion (∼300 beats min−1). In diabetic hearts, heart rate was some 40 beats min−1 lower (P<0.05), without differences between the four experimental groups.
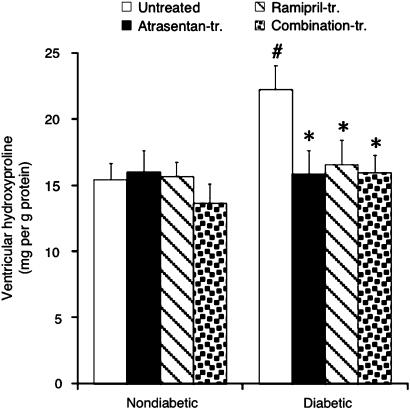
Ventricular hydroxyproline content
Although heart weight was not increased in diabetic animals (Table 1), ventricular hydroxyproline, a major component of collagen, was significantly elevated after 8 weeks of diabetes (Figure 5). Importantly, ETA-RB and ACE-I as well as the combination normalized cardiac hydroxyproline content (P=NS vs nondiabetic groups; Figure 5).
Figure 5.
Ventricular hydroxyproline content determined at the end of 1 h reperfusion. Data are mean±s.e.m. of eight hearts. #P<0.05 vs nondiabetic; *P<0.05 vs untreated diabetic.
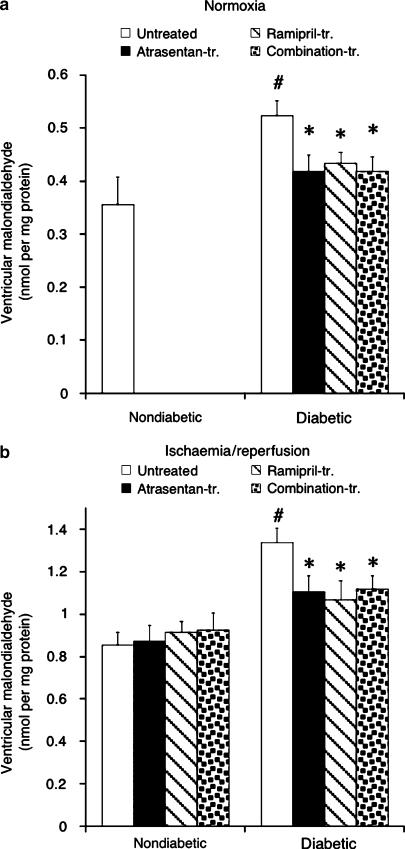
Oxidant status
As diabetes is known to be associated with increased generation of ROS (Cohen, 1993), we determined ventricular malondialdehyde content, which reflects oxidant-induced lipid peroxidation reactions. A qualitatively similar picture emerged for normoxic hearts (Figure 6a) and hearts reperfused after a period of no-flow ischaemia (Figure 6b). Malondialdehyde content was similar in all nondiabetic groups (determined for reperfused myocardium), higher in the untreated diabetic group (∼20%; P<0.05) and significantly reduced (−17%) in all three drug-treated groups (P=NS vs nondiabetic hearts).
Figure 6.
Ventricular malondialdehyde content determined in normoxic hearts (a) and hearts subjected to ischaemia/reperfusion (b). Data are mean±s.e.m. of eight hearts. #P<0.05 vs nondiabetic; *P<0.05 vs untreated diabetic.
Cardiac NOS activity and post-ischaemic nitrite formation
Cardiac catalytic NOS activity was determined in diabetic groups and in the untreated nondiabetic group after 8 weeks (Table 2). Total NOS activity was indistinguishable in untreated diabetic and nondiabetic hearts and neither atrasentan nor ramipril affected enzyme activity. All NOS activity was Ca2+-dependent and unaffected by the iNOS-selective inhibitor 1400 W, indicating that iNOS activity was not detectable in these samples (Table 2).
Table 2.
Cardiac NOS activities
| Nondiabetic untreated | Diabetic untreated | Diabetic atrasentan-treated | Diabetic ramipril-treated | Diabetic combination-treated | |
|---|---|---|---|---|---|
| Total activity | 1.22±0.090 | 1.03±0.092 | 1.08±0.095 | 1.20±0.031 | 1.20±0.027 |
| 1400 W (10 μM) | 1.23±0.093 | 1.07±0.094 | 1.04±0.063 | 1.22±0.043 | 1.18±0.023 |
| EDTA (5 mM) | −0.02±0.020 | −0.01±0.026 | −0.03±0.014 | 0.03±0.013 | −0.02±0.042 |
Abbreviation: EDTA, ethylenediaminetetraacetic acid.
Enzyme activity is given as pmol [3H]L-citrulline formed min−1 (mg protein)−1. Data are mean±s.e.m. (n=5 per group). Activities obtained in the presence of 1400 W were not significantly different from total activity (P=NS).
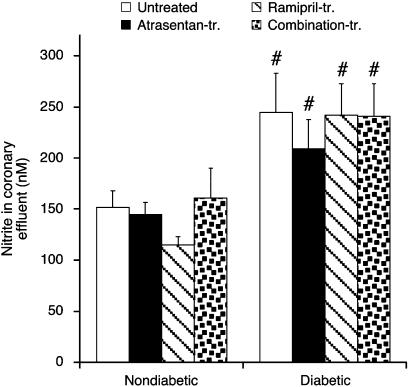
Nitrite concentration was taken as an index of cardiac NO abundance and determined in coronary effluent from isolated perfused hearts at baseline and after ischaemia (Figure 7). Nitrite was below detection limit at baseline and amounted to ∼150 nM in untreated nondiabetic hearts and ∼250 nM in untreated diabetic hearts within the initial 15 s of reperfusion (P<0.05). Neither atrasentan nor ramipril affected cardiac nitrite release after ischaemia (P=NS vs untreated diabetic hearts).
Figure 7.
Nitrite concentration in coronary effluent measured during the initial 15 s of reperfusion. Data are mean±s.e.m. of eight hearts. #P<0.05 vs nondiabetic.
Discussion
The main findings of this study were: (1) diabetic hearts exhibited reduced baseline systolic contractility, slower diastolic relaxation and exacerbated post-ischaemic contractile depression, compared to nondiabetic hearts. All functions were significantly improved by ETA-RB or ACE-I treatment over 8 weeks, (2) Both treatments normalized ventricular fibrosis and oxidant load, whereas ventricular NOS activity was unaffected by either drug regime, (3) ETA-RB or ACE-I treatment were equally effective and combining the treatments provided no additional benefit.
Test drugs
ETA receptors were antagonized with atrasentan, a highly potent (KI=0.034 nM) and selective (1800-fold) ETA receptor antagonist (Wessale et al., 2002), which has been extensively studied experimentally in different models of heart disease and has been developed until recently for the treatment of hormone-refractory prostate cancer. Ramipril is safe and effective in the treatment of hypertension and heart failure and improves kidney function in diabetic patients independent of blood pressure reduction (Doggrell, 2001). The ramipril dose was 3 mg kg−1 per day, as used previously in studies with STZ-diabetic rats (Tschöpe et al., 2003). Atrasentan also was used at 3 mg kg−1 per day, which is a maximally effective dose in the rat as we have shown in pilot experiments (see Methods on Atrasentan plasma levels).
Baseline dysfunction
As observed by several other groups (Dhein et al., 2000; Gross et al., 2004), diabetic rats had a significantly higher arterial blood pressure than nondiabetic controls. The increase in blood pressure could be related to the stimulatory effect of hyperglycaemia on the RAS (Fiordaliso et al., 2000) or generation of oxygen radicals that may limit the availability and vasodilatory action of NO (Hink et al., 2001). Both ramipril and atrasentan lowered blood pressure in our study, presumably by peripheral arterial vasodilation resulting in reduced in vivo afterload and subsequent myocardial remodelling processes. These chronic effects are expected to facilitate systolic contraction and diastolic relaxation. However, reduced blood pressure per se was probably not decisive because the functional benefit in the combination-treated diabetic group was no greater than in the atrasentan or ramipril group. The β-adrenergic, inotropic, responsiveness of the heart is known to be compromised in STZ-induced diabetes (Smith et al., 1997; Cheng et al., 2004). Impaired sympathetic response may in large measure explain the reduced basal LVDevP and −dp/dt observed in our diabetic rat hearts. In support of this, hearts from animals treated with atrasentan or ramipril over 8 weeks were three times more responsive to exogenous norepinephrine than control hearts (unpublished data). Furthermore, ACE-I treatment is expected to raise bradykinin tissue levels with resulting stimulation of myocardial eNOS activity and NO formation (McFarlane et al., 2003). At low levels, myocardial NO enforces systolic contraction by inhibiting the degradation of cAMP through cGMP-dependent phosphodiesterase (Kojda et al., 1996) and in particular facilitates diastolic relaxation (Shah and MacCarthy, 2000). Whether ETA-RBs similarly activate eNOS by diverting endogenous ET-1 to endothelial ETB receptors, which are linked to NOS activation is not known. A negative inotropic effect of NO derived from inducible NOS (iNOS) as observed in patients with heart failure or transplant recipients (Paulus et al., 1997) is unlikely in the diabetic rats investigated here, as iNOS activity was undetectable (Table 2).
Response to brief ischaemia
We observed a clear functional depression in diabetic hearts subjected to brief ischaemia as evident from raised peak contracture, shorter time-to-onset of contracture and higher LVEDP. Both ETA-RB and ACE-I treatment largely normalised ischaemic function, so that LVEDP was no longer different from that observed in nondiabetic hearts. There is considerable evidence for de novo synthesis of ET-1 by cardiomyocytes subjected to ischaemia in vivo or even after short periods of oxygen deprivation in vitro (Brunner et al., 2006). The additionally generated cardiac ET-1 exerts a pro-ischaemic effect, since ETA receptor antagonists are highly protective during ischaemia and reperfusion (Brunner and Opie, 1998). The protective effect is in all likelihood NO-dependent, because inhibiting NOS has been shown to abolish the protective effect of ETA receptor antagonism, at least in isolated rat hearts (Gourine et al., 2001). Although not specifically determined in the present study, NO may similarly account for improved ischaemic heart function in diabetic animals treated with ETA-RB. This is supported by a previous report of increased cGMP formation in diabetic mouse hearts subjected to brief ischaemia (Pozo-Navas et al., 2006). On the other hand, nitrite formation during ischaemia was not augmented in the present study, possibly due to acidotic and/or enzymatic reduction of nitrite to NO during ischaemia (Webb et al., 2004).
Protection of function during reperfusion
Contractile dysfunction after a period of ischaemia is a recognized phenomenon in normal hearts. The underlying mechanisms include Ca2+ overload, production of ROS (especially during early reperfusion) and changes in myofilament Ca2+ sensitivity (Opie, 1989). An important novel finding of the present study was the considerable improvement of reperfusion systolic and diastolic function after treating diabetic rats with ETA-RB for 8 weeks before the ischaemic period. The improved recovery of systolic and diastolic function was paralleled by a significantly lower hydroxyproline and malondialdehyde content of reperfused ventricular muscle. Hydroxyproline is a major component of collagen, which together with other proteins forms the extracellular matrix lattice. The reduced accumulation of hydroxyproline strongly suggests that ETA-RB treatment inhibited the well-known cardiovascular growth-promoting and remodelling effects of ET-1 (Kirchengast and Münter, 1999). In contrast to reduced hydroxyproline content, heart wet weight was not changed following drug treatment. The reason for this discrepancy is not clear at present. Increased cardiomyocyte apoptosis is unlikely to have occurred, because inotropic function was improved, not worsened. Additional histochemical data are necessary to clarify this point. ROS formation was approximately doubled in reperfused hearts compared to hearts perfused in normoxia, as evident from the respective malondialdehyde levels (Figures 6a and b). The reduced formation of malondialdehyde following ETA receptor blockade indicates a lower rate of lipid peroxidation, induced by ROS such as superoxide anions and hydroxyl radicals. The important role of myocardial oxidants is also evident from the reduced post-hypoxic diastolic dysfunction of STZ-induced diabetic hearts treated with the ROS scavenger thiourea (El-Omar et al., 2003). Whether vascular ROS formed during early reperfusion are involved in myocardial ischaemic or reperfusion contractile dysfunction, is not known. In diabetic blood vessels, angiotensin II stimulates NAD(P)H oxidase resulting in the formation of superoxide anion, an action antagonised by ETA-RB (Kanie and Kamata, 2002). In the present study, vascular endothelial reperfusion function (coronary perfusion pressure) was not different between untreated, ETA-RB-treated and ACE-I- treated hearts, implying that vascular ROS may not be causal to the post-ischaemic rise in coronary perfusion pressure. By implication, coronary ROS generation is unlikely to contribute to the myocardial impairments during reperfusion observed in untreated diabetic hearts. Taken together, the comparable functional improvements and antioxidative and antifibrotic actions found after blocking ETA receptors or limiting angiotensin II formation suggest that the ET system plays an important role in diabetic cardiac complications, equal to that of the RAS system.
Blood HbA1c values
In addition to the reactive oxygen intermediate pathway, hyperglycaemia stimulates the advanced glycation end-product pathway, which leads to the formation of glucotoxins that cause cellular dysfunction and damage (Sheetz and King, 2002). A general measure of glucose-induced macromolecular damage is the HbA1c level observed in experimental and clinical diabetes. As observed by others (Gross et al., 2004), HbA1c was doubled in our untreated diabetic rats compared to nondiabetic controls. In line with unaltered plasma glucose levels, neither ETA-RB nor ACE-I treatment affected HbA1c, indicating that the improvement in myocardial function was independent of advanced glycation end-product formation. This interpretation leaves aside the possibility of carboxymethyllysine formation via combined nonenzymatic glycation and oxidation reactions triggered by ROS.
Angiotensin II–ET interactions
A remarkable feature of the present study was the cardioprotective equivalence of ETA-RB and ACE-I treatment on cardiac function as well as hydroxyproline and lipid peroxidation. This may partly depend on interactions between the renin–angiotensin system and the ET system. Angiotensin II is a potent in vivo stimulant of ET-1 formation via increased protein expression and enhanced ET converting enzyme activity (Barton et al., 1997). A similar mechanism appears to be important in diabetic myocardium as well (Gross et al., 2004). On the other hand, ACE inhibitors may increase tissue NO via bradykinin, which would be expected to downregulate ET-1 generation. Results from human umbilical cord endothelial cells suggest an additional mechanism at the level of NADPH oxidase. In these studies ramipril reduced the expression of p22phox and gp91phox, two essential subunits of the enzyme (Otto et al., 2006). On the other hand, ET-1 also induces NADPH oxidase to produce ROS from endothelial and smooth muscle cells (Duerrschmidt et al., 2000). Taken together, the beneficial effects of ACE-I and ETA-RB treatment may also partly be due to reduced expression and activation of cardiomyocyte NADPH oxidase, resulting in a reduced tissue load of deleterious ROS. Therefore, the cardioprotective effects of ACE-I treatment in the present study may in part be due to a reduced ET-1 activity.
Combined effects
In the present study, combining both test drugs resulted in benefit no greater than that observed with the ACE-I or the ETA-RB alone. This was not unexpected, because both drugs exerted quantitatively similar effects on cellular alterations manifested as increased ROS activity and tissue fibrosis. In fact, given a sequential action of angiotensin II and ET-1 in cardiac myocytes (Brunner and Kukovetz, 1996), combined inhibition of the renin–angiotensin and ET systems would not be expected to result in a greater myocardial protection than observed with either inhibitor alone, unless the drug doses were not maximally effective. Hence, the drug doses used here were probably maximally effective, precluding further augmentation of protection.
Conclusion
The present study clearly shows that ETA receptor antagonism is effective in alleviating myocardial dysfunction in an established model of insulinopenic diabetes associated with chronic hyperglycaemia. However, the favourable observations may partly be linked to the metabolic specificities of this model. Therefore, investigations in models of type II diabetes generally featuring only mild increases in glucose, but high insulin plasma levels, are also necessary. Eventually, clinical studies are warranted to test whether diabetic patients might benefit from ETA receptor antagonism, in which case they could become an alternative to ACE inhibitors (at least when also used as the potent anti-hypertensives they seem to be).
Acknowledgments
This study was supported by the Austrian Research Fund (FWF), project 17339 (to FB). Atrasentan was provided by Dr T Opgenorth (Abbott Pharmaceuticals Inc., Abbott Park, IL, USA) and ramipril by Dr W Linz (Sanofi-Aventis Deutschland GmbH, Frankfurt/Main, Germany).
Abbreviations
- ACE-I
angiotensin-converting enzyme inhibitor
- DAN
2,3-diaminonaphthalene
- +dP/dt and −dP/dt
maximum rate of rise and fall of left-ventricular pressure
- eNOS
endothelial NOS
- ET-1
endothelin-1
- ETA-RB
endothelin-A receptor blocker
- iNOS
inducible NOS
- LVDevP
left-ventricular developed pressure
- LVEDP
left-ventricular end-diastolic pressure
- nNOS
neuronal NOS
- NOS
nitric oxide synthase
- ROS
reactive oxygen species
- STZ
streptozotocin
- TBARS
thiobarbituric acid-reactive substance
Conflict of interest
The authors state no conflict of interest.
References
- Adeghate E. Molecular and cellular basis of the aetiology and management of diabetic cardiomyopathy: a short review. Mol Cell Biochem. 2004;261:187–191. doi: 10.1023/b:mcbi.0000028755.86521.11. [DOI] [PubMed] [Google Scholar]
- Barton M, Shaw S, d'Uscio LV, Moreau P, Lüscher TF. Angiotensin II increases vascular and renal endothelin-1 and functional endothelin converting enzyme activity in vivo: role of ETA receptors for endothelin regulation. Biochem Biophys Res Commun. 1997;238:861–865. doi: 10.1006/bbrc.1997.7394. [DOI] [PubMed] [Google Scholar]
- Brunner F. Interaction of nitric oxide and endothelin-1 in ischemia/reperfusion injury of rat heart. J Mol Cell Cardiol. 1997;29:2363–2374. doi: 10.1006/jmcc.1997.0470. [DOI] [PubMed] [Google Scholar]
- Brunner F, Brás-Silva C, Cerdeira AS, Leite-Moreira AF. Cardiovascular endothelins: essential regulators of cardiovascular homeostasis. Pharmacol Ther. 2006;111:508–531. doi: 10.1016/j.pharmthera.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Brunner F, Kukovetz WR. Postischemic antiarrhythmic effects of angiotensin-converting enzyme inhibitors: role of suppression of endogenous endothelin secretion. Circulation. 1996;94:1752–1761. doi: 10.1161/01.cir.94.7.1752. [DOI] [PubMed] [Google Scholar]
- Brunner F, Opie LH. Role of endothelin-A receptors in ischemic contracture and reperfusion injury. Circulation. 1998;97:391–398. doi: 10.1161/01.cir.97.4.391. [DOI] [PubMed] [Google Scholar]
- Bryan PD, Sapochak LB, Tames MM, Padley RJ, El-Shourbagy TA. Determination of atrasentan by high performance liquid chromatography with fluorescence detection in human plasma. Biomed Chromatog. 2001;15:525–533. doi: 10.1002/bmc.106. [DOI] [PubMed] [Google Scholar]
- Cheng X, Cheng XS, Kuo K-H, Pang CCY. Inhibition of iNOS augments cardiovascular action of noradrenaline in streptozotocin-induced diabetes. Cardiovasc Res. 2004;64:298–307. doi: 10.1016/j.cardiores.2004.06.023. [DOI] [PubMed] [Google Scholar]
- Cohen RA. Dysfunction of vascular endothelium in diabetes mellitus. Circulation. 1993;87 (Suppl V):V-67–V-76. [Google Scholar]
- Dhein S, Hochreuther S, Aus dem Spring C, Bollig K, Hufnagel C, Raschack M. Long-term effects of the endothelinA receptor antagonist LU 135252 and the angiotensin-converting enzyme inhibitor trandolapril on diabetic angiopathy and nephropathy in a chronic type I diabetes mellitus rat model. J Pharmacol Exp Ther. 2000;293:351–359. [PubMed] [Google Scholar]
- Doggrell SA. Is ramipril the pril for diabetes and kidney disease. Drugs Today. 2001;37:321–331. doi: 10.1358/dot.2001.37.5.627954. [DOI] [PubMed] [Google Scholar]
- Duerrschmidt N, Wippich N, Goettsch W, Broemme H-J, Morawietz H. Endothelin-1 induces NAD(P)H oxidase in human endothelial cells. Biochem Biophys Res Commun. 2000;269:713–717. doi: 10.1006/bbrc.2000.2354. [DOI] [PubMed] [Google Scholar]
- El-Omar MM, Lord R, Draper NJ, Shah AM. Role of nitric oxide in posthypoxic contractile dysfunction of diabetic cardiomyopathy. Eur J Heart Fail. 2003;5:229–239. doi: 10.1016/s1388-9842(03)00010-2. [DOI] [PubMed] [Google Scholar]
- Feuvray D, Lopaschuk GD. Controversies on the sensitivity of the diabetic heart to ischemic injury: the sensitivity of the diabetic heart to ischemic injury is decreased. Cardiovasc Res. 1997;34:113–120. doi: 10.1016/s0008-6363(97)00037-0. [DOI] [PubMed] [Google Scholar]
- Fiordaliso F, Li B, Latini R, Sonnenblick EH, Anversa P, Leri A, et al. Myocyte death in streptozotocin-induced diabetes in rats is angiotensin II- dependent. Lab Invest. 2000;80:513–527. doi: 10.1038/labinvest.3780057. [DOI] [PubMed] [Google Scholar]
- Garvey EP, Oplinger JA, Furfine ES, Kiff RJ, Laszlo F, Whittle BJR, et al. 1400W is a slow, tight binding, and highly selective inhibitor of inducible nitric-oxide synthase in vitro and in vivo. J Biol Chem. 1997;272:4959–4963. doi: 10.1074/jbc.272.8.4959. [DOI] [PubMed] [Google Scholar]
- Gharavi N, El-Kadi AOS. Measurement of nitric oxide in murine hepatoma Hepa1c1c7 cells by reversed phase HPLC with fluorescence detection. J Pharm Pharmaceut Sci. 2003;6:302–307. [PubMed] [Google Scholar]
- Gourine AV, Gonon AT, Pernow J. Involvement of nitric oxide in cardioprotective effect of endothelin receptor antagonist during ischemia-reperfusion. Am J Physiol. 2001;280:H1105–H1112. doi: 10.1152/ajpheart.2001.280.3.H1105. [DOI] [PubMed] [Google Scholar]
- Gross M-L, Heiss N, Weckbach M, Hansen A, El-Shakmak A, Szabo A, et al. ACE-inhibition is superior to endothelin A receptor blockade in preventing abnormal capillary supply and fibrosis of the heart in experimental diabetes. Diabetologia. 2004;47:316–324. doi: 10.1007/s00125-003-1309-z. [DOI] [PubMed] [Google Scholar]
- Hileeto D, Cukiernik M, Mukherjee S, Evans T, Barbin Y, Downey D, et al. Contributions of endothelin-1 and sodium hydrogen exchanger-1 in the diabetic myocardium. Diabetes Metab Res Rev. 2002;18:386–394. doi: 10.1002/dmrr.322. [DOI] [PubMed] [Google Scholar]
- Hink U, Li H, Mollnau H, Oelze M, Matheis E, Hartmann M, et al. Mechanisms underlying endothelial dysfunction in diabetes mellitus. Circ Res. 2001;88:e14–e22. doi: 10.1161/01.res.88.2.e14. [DOI] [PubMed] [Google Scholar]
- Kanie N, Kamata K. Effects of chronic administration of the novel endothelin antagonist J-104132 on endothelial dysfunction in streptozotocin-induced diabetic rat. Br J Pharmacol. 2002;135:1935–1942. doi: 10.1038/sj.bjp.0704659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchengast M, Münter K. Endothelin-1 and endothelin receptor antagonists in cardiovascular remodeling. Proc Soc Exp Biol Med. 1999;221:312–324. doi: 10.1046/j.1525-1373.1999.d01-88.x. [DOI] [PubMed] [Google Scholar]
- Kojda G, Kottenberg K, Nix P, Schlüter KD, Piper HM, Noack E. Low increase in cGMP induced by organic nitrates and nitrovasodilators improves contractile response of rat ventricular myocytes. Circ Res. 1996;78:91–101. doi: 10.1161/01.res.78.1.91. [DOI] [PubMed] [Google Scholar]
- McFarlane SI, Kumar A, Sowers JR. Mechanisms by which angiotensin-converting enzyme inhibitors prevent diabetes and cardiovascular disease. Am J Cardiol. 2003;91 (Suppl):30H–37H. doi: 10.1016/s0002-9149(03)00432-6. [DOI] [PubMed] [Google Scholar]
- Opie LH. Reperfusion injury and its pharmacological modification. Circulation. 1989;80:1049–1062. doi: 10.1161/01.cir.80.4.1049. [DOI] [PubMed] [Google Scholar]
- Otto A, Fontaine J, Berkenboom G. Ramipril treatment protects against nitrate-induced oxidative stress in eNOS-/- mice: an implication of the NADPH oxidase pathway. J Cardiovasc Pharmacol. 2006;48:842–849. doi: 10.1097/01.fjc.0000238587.68239.52. [DOI] [PubMed] [Google Scholar]
- Paulus WJ, Kästner S, Pujadas P, Shah AM, Drexler H, Vanderheyden M. Left ventricular contractile effects of inducible nitric oxide synthase in the human allograft. Circulation. 1997;96:3436–3442. doi: 10.1161/01.cir.96.10.3436. [DOI] [PubMed] [Google Scholar]
- Pitt B. The anti-ischemic potential of angiotensin-converting enzyme inhibition: insights from the heart outcomes prevention evaluation trial. Clin Cardiol. 2000;23 (Suppl 4):IV9–IV14. doi: 10.1002/clc.4960230704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozo-Navas B, Stessel H, Wölkart G, Brunner F. Role of myocardial nitric oxide in diabetic ischemia-reperfusion dysfunction: studies in mice with myocyte-specific overexpression of endothelial nitric-oxide synthase. J Pharmacol Exp Ther. 2006;319:729–738. doi: 10.1124/jpet.106.107854. [DOI] [PubMed] [Google Scholar]
- Rao RH. Pressor doses of angiotensin II increase hepatic glucose output and decrease insulin sensitivity in rats. J Endocrinol. 1996;148:311–318. doi: 10.1677/joe.0.1480311. [DOI] [PubMed] [Google Scholar]
- Schmidt K, Mayer B.Assay of tissue activity of nitric oxide synthase Current Protocols in Toxicology 1999John Wiley & Sons, Inc: New York; 10.2.1–10.2.13.In: Maines M, Costa L, Reed D, Sassa S (eds). [DOI] [PubMed] [Google Scholar]
- Shah AM, MacCarthy PA. Paracrine and autocrine effects of nitric oxide on myocardial function. Pharmacol Ther. 2000;86:49–86. doi: 10.1016/s0163-7258(99)00072-8. [DOI] [PubMed] [Google Scholar]
- Sheetz MJ, King GL. Molecular understanding of hyperglycemia's adverse effects for diabetic complications. JAMA. 2002;288:2579–2588. doi: 10.1001/jama.288.20.2579. [DOI] [PubMed] [Google Scholar]
- Smith JM, Paulson DJ, Romano FD. Inhibition of nitric oxide synthase by L-NAME improves ventricular performance in streptozotocin-diabetic rats. J Mol Cell Cardiol. 1997;29:2393–2402. doi: 10.1006/jmcc.1997.0474. [DOI] [PubMed] [Google Scholar]
- Stessel H, Brunner F. Effect of endothelin antagonism on contractility, intracellular calcium regulation and calcium regulatory protein expression in right ventricular hypertrophy of the rat. Pharmacol Tox. 2004;94:37–45. [PubMed] [Google Scholar]
- Tschöpe C, Seidl U, Reinecke A, Riester U, Graf K, Schulteiss H-P, et al. Kinins are involved in the antiproteinuric effect of angiotensin-converting enzyme inhibition in experimental diabetic nephropathy. Int Immunopharmacol. 2003;3:335–344. doi: 10.1016/S1567-5769(02)00273-4. [DOI] [PubMed] [Google Scholar]
- Verma S, Arikawa E, McNeill JH. Long-term endothelin receptor blockade improves cardiovascular function in diabetes. Am J Hypertens. 2001;14:679–687. doi: 10.1016/s0895-7061(01)01302-4. [DOI] [PubMed] [Google Scholar]
- Webb A, Bond R, McLean P, Uppal R, Benjamin N, Ahluwalia A. Reduction of nitrite to nitric oxide during ischemia protects against myocardial ischemia-reperfusion damage. PNAS. 2004;101:13683–13688. doi: 10.1073/pnas.0402927101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessale JL, Adler AL, Novosad EI, Calzadilla SV, Dayton BD, Marsh KC, et al. Pharmacology of endothelin receptor antagonists: ABT-627, ABT-546, A-182086 and A-192621; ex vivo and in vivo studies. Clin Sci. 2002;103 (Suppl. 48):112S–117S. doi: 10.1042/CS103S112S. [DOI] [PubMed] [Google Scholar]
- Witte K, Reitenbach I, Stolpe K, Schilling L, Kirchengast M, Lemmer B. Effects of the endothelin A receptor antagonist darusentan on blood pressure and vascular contractility in type 2 diabetic Goto-Kakizaki rats. J Cardiovasc Pharmacol. 2003;41:890–896. doi: 10.1097/00005344-200306000-00009. [DOI] [PubMed] [Google Scholar]
- Wölkart G, Stessel H, Saad Z, Kirchengast M, Brunner F. Cardioprotective effects of atrasentan, an endothelin-A receptor antagonist, but not of nitric oxide in diabetic mice with myocyte-specific overexpression of endothelial nitric oxide synthase. Br J Pharmacol. 2006;148:671–681. doi: 10.1038/sj.bjp.0706772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med. 2000;342:145–153. doi: 10.1056/NEJM200001203420301. [DOI] [PubMed] [Google Scholar]