Abstract
Background and purpose:
Recombinant human erythropoietin (rhEPO; Epoetin-α; PROCRIT™) has been shown to exert neuroprotective and restorative effects in a variety of CNS injury models. However, limited information is available regarding the dose levels required for these beneficial effects or the neuronal responses that may underlie them. Here we have investigated the dose-response to rhEPO and compared the effects of rhEPO with those of carbamylated rhEPO (CEPO) in a model of cerebral stroke in rats.
Experimental approach:
Rats subjected to embolic middle cerebral artery occlusion (MCAo) were treated with rhEPO or CEPO, starting at 6 h and repeated at 24 and 48 h, after MCAo. Cerebral infarct volumes were assessed at 28 days and neurological impairment at 7, 14, 21 and 28 days, post-MCAo.
Key results:
rhEPO at dose levels of 500, 1150 or 5000 IU kg−1 or CEPO at a dose level of 50 μg kg−1 significantly reduced cortical infarct volume and reduced neurologic impairment. All doses of rhEPO, but not CEPO, produced a transient increase in haematocrit, while rhEPO and CEPO substantially reduced the number of apoptotic cells and activated microglia in the ischemic boundary region.
Conclusions and implications:
These data indicate that rhEPO and CEPO have anti-inflammatory and anti-apoptotic effects, even with administration at 6 h following embolic MCAo in rats. Taken together, these actions of rhEPO and CEPO are likely to contribute to their reduction of neurologic impairment following cerebral ischemia.
Keywords: MCAo, EPO, CEPO, microglia, infarct volume, neurobehavioural outcome
Introduction
Erythropoietin (EPO) is a naturally occurring cytokine most widely recognized for its role in stimulating the maturation, differentiation and survival of haematopoietic progenitor cells (Naranda et al., 1999; Wojchowski et al., 1999). Recently, however, a more general cytoprotective role for EPO has been described. In the central nervous system (CNS), for example, expression of EPO and the EPO receptor (EPOR) is greatly increased in neurons, neuronal progenitor cells, glia and cerebrovascular endothelial cells in response to many different types of cell injury (Anagnostou et al., 1990; Masuda et al., 1994; Bernaudin et al., 1999; Marti, 2004; Wang et al., 2004; Tsai et al., 2006). Inhibition of EPO activity by administration of soluble EPOR worsens the severity of injury (Sakanaka et al., 1998), suggesting that endogenously produced EPO is directly involved in an intrinsic neuronal repair pathway. In the best-studied experimental neuronal injury paradigm, hypoxic–ischaemic brain injury, an upregulation of neuronal, endothelial and glial EPO and EPOR expression occurs following cerebral ischaemia (Lewczuk et al., 2000; Sinor and Greenberg, 2000; Siren et al., 2001; Marti, 2004; Maiese et al., 2005). Administration of exogenous recombinant human EPO (rhEPO) after focal or global cerebral ischaemia (Sadamoto et al., 1998; Sakanaka et al., 1998; Brines et al., 2004; Leist et al., 2004), augments the cytoprotective and restorative EPO response pathway leading to a substantial improvement in neurobehavioural outcome. The neuroprotective and restorative activity of exogenous EPO in rodent ischaemia models has been documented in studies published by several independent laboratories and has also translated into the human clinical setting where evidence for a clinical benefit of rhEPO in patients suffering middle cerebral artery (MCA) territory stroke has been reported (Ehrenreich et al., 2002).
Carbamylated EPO (CEPO) has been observed in patients suffering from end-stage renal disease (Mun and Golper, 2000; Park et al., 2004). CEPO does not show any binding to the classical EPOR in vitro or stimulate an haematopoietic response in vivo, but nevertheless has been shown to exert neuroprotective effects when administered following cerebral ischaemia or other types of neuronal injury (Leist et al., 2004). Although the specific cellular mechanisms responsible for the cytoprotective activity of CEPO, and the relationships between signalling pathways that mediate the beneficial effects of rhEPO and CEPO remain to be fully elucidated, the protective effects of CEPO may involve signalling through the common β-receptor (CD 131) (Brines et al., 2004).
Although the beneficial effects of exogenous rhEPO and CEPO have been demonstrated in a range of cerebral ischaemia models, investigators frequently use high doses of rhEPO (typically 5000 IU kg−1) in these studies. As this dose of rhEPO is far greater than that required to evoke haematopoietic response, there remains a need for a systematic investigation of the dose levels, treatment window and treatment interval that are required for efficacy in these models. Moreover, the levels of peripherally administered EPO or CEPO that reach brain tissue in models of cerebral ischaemia have not been reported, highlighting the need to establish a link between peripheral intravenous dose and brain levels of drug. In the present study, we examined the dose–response to EPO and CEPO in an embolic model of MCA occlusion (MCAo) stroke, using a repeated dosing protocol that parallels the dosing regimen reported to show clinical benefit in human stroke patients (Ehrenreich et al., 2002). We examined the effects of rhEPO or CEPO treatment on neurobehavioural outcome and volume of infarction, when the treatment was initiated 6 h after MCAo.
Methods
All experimental procedures were approved by the Henry Ford Hospital Committee for the Care of Experimental Animals.
Model of embolic MCAo
Male Wistar rats (The Jackson Laboratory, Bar Harbor, ME, USA) weighing 350–400 g were employed in the present study. The MCA was occluded by placement of an embolus at the origin of the MCA, as described previously (Zhang et al., 1997). This model frequently exhibits spontaneous clot lysis and reperfusion within 24–48 h following MCAo (Zhang et al., 1997; Jiang et al., 1998).
ELISA measurements of rhEPO and CEPO levels
Brain homogenate supernatant, plasma and cerebrospinal fluid (CSF) samples were used for measurement of rhEPO or CEPO levels using a human EPO Quantikine Cytokine IVD enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems, Minneapolis, MN, USA). As the antibodies used in this kit show reduced affinity for CEPO, a standard curve was generated using CEPO as the target antigen and a correction factor was calculated to adjust the ELISA results to account for the reduced antibody sensitivity. The lower limit of detection in the EPO ELISA was 2.5 mIU ml−1.
Neurological and behavioural assessment
To detect sensorimotor impairments, an array of behavioural tests including foot-fault and the modified neurological severity score (mNSS) were performed before MCAo and at 1, 3, 7, 14, 21 and 28 days after MCAo by an investigator not aware of the treatments. These tests are sensitive and reliable indices of sensorimotor impairments after ischaemic stroke and have been applied extensively in our laboratory to assess neurological outcome following MCAo in rats (Chen et al., 2003; Wang et al., 2004; Zhang et al., 2005).
Infarct volume
Rats were killed 28 days after MCAo and infarct volume was measured on seven equally spaced (2 mm) haematoxylin and eosin-stained coronal sections, which covers the entire territory supplied by the MCA (Bregma; 4.7 to −7.3 mm), including the ischaemic core, were used for each rat using a Global Lab Image analysis program (Data Translation, Marlboro, MA, USA), as described previously (Paxinos and Watson, 1986; Zhang et al., 1997). Briefly, the area of both hemispheres and the area containing the ischaemic neuronal damage (mm2) were calculated by tracing the area on the computer screen. The lesion volume (mm3) was determined by multiplying the appropriate area by the section interval thickness. To reduce errors associated with processing of tissue for histological analysis, the ischaemic volume is presented as the percentage of infarct volume of the contralateral hemisphere (indirect volume calculation).
Histology and terminal deoxynucleotidyl transferase-mediated biotinylated UTP nick end labelling
To examine the effect of EPO on microglial response, deparaffinized coronal sections were histochemically stained with peroxidase-labeled isolectin-B4 from Griffonia simplicifolia seeds (GSAI-B4-HRP; Sigma, St Louis, MO, USA; Zhang et al., 1997c). Briefly, the sections were incubated with isolectin (20 mg ml−1) in phosphate-buffered saline (PBS) containing divalent cations at room temperature for 3 h and then overnight at 4°C. Sections were then reacted with diaminobenzidine and H2O2 to generate orange-brown reaction product at sites of isolectin-B4 binding. Eight fields of view within the ischaemic boundary, defined as an area 0.35 mm away from the infarct rim, were acquired from each coronal section. Four coronal sections from Bregma (0.7 to −1.3 mm), which cover the ischaemic core were used for each rat (Paxinos and Watson, 1986; Zhang et al., 1997). Data are presented as percentage of pixels with isolectin-B4 within the field of view. To measure the number of apoptotic cells, deoxynucleotidyl transferase-mediated biotinylated UTP nick end labelling (TUNEL) was performed using the Apotosis Detection Kit (ApopTag; Chemicon International, Temecula, CA, USA) according to the manufacturer's protocol. Total number of TUNEL-positive cells in the ischaemic boundary region was counted on four coronal sections per rat. Data are presented as the number of TUNEL-positive cells.
Haematocrit
To determine each animal's haematocrit, a blood sample (100 μl) was drawn via a tail vein immediately before the initial drug dose treatment and again once per week up to 28 days after MCAo. Haematocrit was measured in microcapillary tubes using standard procedures (Readacrit Centrifuge, Clay Adams, Parsippany, NJ, USA).
Experimental protocols
To determine brain levels of EPO and CEPO: MCAo rats were treated (intravenous) with a bolus dose rhEPO of 1000, 2500, 5000 and 10 000 IU kg−1 or CEPO of 5 and 50 μg kg−1 administered 6 h after MCAo. MCAo rats treated with the same volume of saline were used as a control group. Thirty minutes after administration of rhEPO or CEPO, plasma (400–500 μl) and CSF (250–300 μl) samples were obtained via tail vein bleed or cisterna magna puncture, respectively. Immediately following blood and CSF collection, animals were perfused with ice-cold saline, and their brains were removed, separated into left and right hemispheres and stored frozen on dry ice. Brains were subsequently thawed, homogenized in 1 ml ice-cold PBS containing a protease inhibitor cocktail (Sigma-Aldrich, St Louis, MO, USA) and centrifuged at 1000 g for 15 min at 4°C to pellet nuclei and cellular debris.
To examine the dose response of EPO and CEPO on infarct volume and functional outcome, following embolization animals were randomly divided into treatment groups (n=10 per group) and were treated (intravenous, tail vein) with rhEPO at a dose level of 50, 500, 1150 or 5000 IU kg−1, CEPO at a dose of 50 μg kg−1 or vehicle (vehicle was PROCRIT™ formulation: 2.5 mg human serum albumin, 5.6 mg sodium citrate, 5.6 mg NaCl, 0.06 mg citric acid in 100 ml sterile water (pH 6.9)). Treatment was initiated 6 h after embolization. For each dose level, an intravenous bolus dose of rhEPO, CEPO or vehicle was given 6, 24 and 48 h after MCAo. The injection volume was 0.32–0.37 ml per rat based on 0.1 ml 100 g−1 animal body weight. Activity units of rhEPO can be converted to mass units based on the formula 120 IU=1 μg protein. Therefore, 500 IU kg−1=4.16 μg kg−1; 1000 IU kg−1=8.32 μg kg−1; 2500 IU kg−1=20.8 μg kg−1; 5000 IU kg−1=41.6 μg kg−1.
Statistics
Data were evaluated for normality and data were not normally distributed. The Generalized Estimating Equation (GEE) approach, considered to have less restriction on data distribution, was employed. Analysis of variance was used to study the effects of treatment, dose and time to first dose on functional recovery. An analysis was performed for each treatment group (rhEPO or CEPO) in comparison to the vehicle control group, respectively. Analysis began testing the treatment/dose by administration time interaction, followed by testing the main effect if no interaction was detected at P<0.05 level, or a pair-wise comparisons, if otherwise. All data are presented as means±s.e. Statistical significance was set at P<0.05.
Drugs
In this paper, rhEPO refers specifically to Epoetin-Alfa (PROCRIT; distributed by Ortho Biotech Product, LP, Bridgewater, NJ, USA as the finished commercial product (10 000 IU ml−1). Doses used in these studies were drawn by syringe directly from the commercial drug vial. All other reagents were obtained from standard commercial vendors except where noted otherwise.
Carbamylated rhEPO (CEPO) was prepared from 50 ml of rhEPO (2.0 mg ml−1), obtained from Ortho Biologics Inc. (Manati Puerto Rico). Stock rhEPO was diluted with 50 ml of 1 M borate buffer (pH 8.8). To this solution was added 8.1 g of potassium cyanate, recrystallized from ethanol. The reaction mixture was incubated at 37°C for 24 h and then dialysed twice against 3.5 l of water, and five additional times against 3.5 l of sodium citrate (20 mM, 0.1 M NaCl (pH 6.0)). The resulting solution was concentrated using a Centricon centrifugal concentrator to a final volume of 28 ml. The concentration was determined to be 3.47 mg ml−1 using an extinction coefficient of ɛ0.1%=1.345. This concentrated material was analysed by size exclusion high-performance liquid chromatography, polyacrylamide gel electrophoresis gel electrophoresis (4–12% gel), and surface-enhanced laser desorption ionization mass spectroscopy (SELDI-MS). The protein was de-glycosylated for mass spectrometry studies as follows: 10 μl Rapigest (Waters Corp., Milford, MA, USA) (2 mg ml−1 in PBS), 3 μl NP-40 detergent (15%), 4 μl ethanol, 4 μl each of PNGase, sialidase and O-glycanase (all from Prozyme Inc., San Leandro, CA, USA) were added to 10 μl of CEPO or an EPO control sample and incubated for 72 h at 37°C. Both de-glycosylated and glycosylated samples were analysed by SELDI-MS. The mass spectrum of de-glycosylated, carbamylated EPO showed an increase of 368 AMU over de-glycosylated EPO, corresponding to an average of 8.6 carbamoyl groups per molecule (EPO has eight lysines and a free N terminus). Trinitrobenzene sulphonic acid analysis was unable to detect any free amino groups.
Results
Intravenous rhEPO and CEPO cross the blood–brain barrier following MCAo
As the antibodies used in the ELISA assay to quantitate rhEPO in this study are specific for human EPO, endogenous rat EPO does not contribute to the observed drug levels and therefore neither CEPO nor EPO was detected in plasma or brain of rats treated with saline. High levels of rhEPO and CEPO were detected in plasma, and measurable levels of the corresponding protein were observed in CSF and brain parenchyma 30 min after an intravenous bolus dose given 6 h after MCAo (Table 1). At the lower dose levels, the levels of EPO achieved in CSF and brain parenchyma were at the low end of the sensitivity range for our ELISA assay and there was considerable variability in the data. Interestingly, there was not a statistically significant difference in rhEPO or CEPO levels in the ipsilateral (that is, MCA occluded) hemisphere versus the contralateral non-ischaemic hemisphere. Nevertheless, these data demonstrate that across a fairly wide dose range, peripherally administered rhEPO and CEPO crossed the blood–brain barrier (BBB) and entered the brain parenchyma and CSF compartments.
Table 1.
CEPO and EPO levels measured 30 min after CEPO or rhEPO administration
| Groups |
rhEPO (IU kg−1) |
CEPO (μg kg−1) |
||||
|---|---|---|---|---|---|---|
| 1000 (ng ml−1, n=3) | 2500 (ng ml−1, n=4) | 5000 (ng ml−1, n=3) | 10 000 (ng ml−1, n=3) | 5 (ng ml−1, n=3) | 50 (ng ml−1, n=3) | |
| CSF | 17.8±11.7 | 2.6±2.0 | 3.0±0.7 | 28.9±3.2 | 2.0±0.4 | 16.5±5.7 |
| Plasma | 476±313 | 329±137 | 969±60 | 11574±766 | 177±5.3 | 504±1006 |
| Ipsilateral | 0.22±0.11 | 0.20±0.18 | 1.03±0.11 | 7.37±1.09 | 0.4±0.01 | 1.2±0.2 |
| Contralateral | 0.2±0.1 | 0.1±0.04 | 0.8±0.2 | 5.8±1.4 | 0.4±0.03 | 0.7±0.04 |
Abbreviations: CEPO, carbamylated rhEPO; CSF, cerebrospinal fluid; EPO, erythropoietin; rhEPO, recombinant human erythropoietin.
Values are mean±s.e.
Drug effects on haematocrit
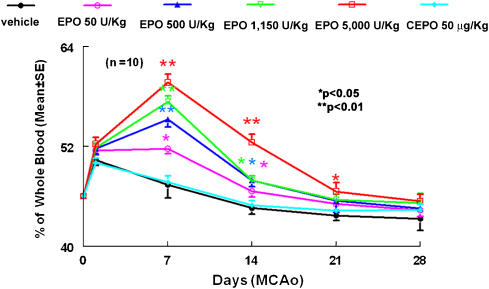
Administration of three equal intravenous bolus doses of rhEPO (50, 500, 1150 or 5000 IU kg−1) at 6, 24 and 48 h following MCAo produced significant (P<0.05) but transient rise in haematocrit, with the peak response occurring at day 14 after the initial dose (Figure 1). Thereafter, haematocrit levels decreased, approaching pre-treatment levels at day 28. Consistent with previous reports (Leist et al., 2004), administration of three equal intravenous bolus doses of CEPO (50 μg kg−1) at 6, 24 and 48 h following MCAo did not alter haematocrit at any time point.
Figure 1.
Changes in haematocrit before, during and after treatment with rhEPO and CEPO. Zero and 1 day time points represent prior to MCA occlusion and CEPO or rhEPO treatment, respectively. *P<0.05 and **P<0.01 vs the vehicle group. N=10 rats per group. CEPO, carbamylated rhEPO; MCA, middle cerebral artery; rhEPO, recombinant human erythropoietin.
Delayed (6 h) treatment with EPO or CEPO reduces infarct volume
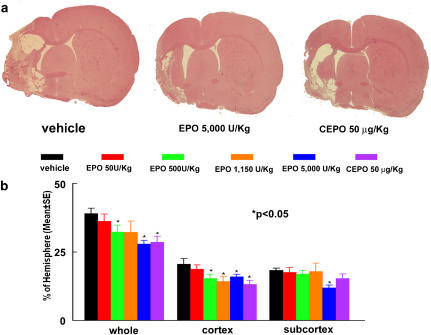
To examine the long-term neuroprotective effects of CEPO and rhEPO, ischaemic rats were treated 6 h after MCAo with different dose levels of CEPO or rhEPO and killed 28 days after MCAo. We first measured the entire infarct volume including the cortex and the subcortex. Treatment with rhEPO at doses of 500 and 5000 IU kg−1 or CEPO at a dose of 50 μg kg−1, significantly (P<0.05) reduced mean infarct volume compared with the mean infarct volume in animals treated with vehicle (Figure 2). The rhEPO 5000 IU kg−1 group exhibited 28% reduction of mean infarct volume, an effect that is indistinguishable with the outcome observed in animals treated with 50 μg kg−1 CEPO (27%). The rhEPO 500 IU kg−1 dose group showed 17% reduction of infarct volume (Figure 2). There was no effect on infarct volume in the rhEPO 50 IU kg−1 group (Figure 2).
Figure 2.
Infarct volumes 28 days after embolic MCA occlusion. Panel a shows infarction on a coronal section stained with H&E of a representative rat from vehicle, rhEPO 5000 IU kg−1 and CEPO 50 μg kg−1 groups. Panel b shows quantitative analysis revealing that delayed (6 h) treatment with CEPO or rhEPO reduced infarct volume. Infarct volumes were measured as a whole hemisphere (Whole), cortex and subcortex. N=12 rats for control, rhEPO 500 IU kg−1, and rhEPO 5000 IU kg−1 groups. N=6 rats for rhEPO 50 IU kg−1, rhEPO 1150 IU kg−1, and CEPO 50 μg kg−1 groups. *P<0.05 vs the vehicle group. CEPO, carbamylated rhEPO; H&E, haematoxylin and eosin; MCA, middle cerebral artery; rhEPO, recombinant human erythropoietin.
Administration of rhEPO or CEPO 6 h after MCAo could protect against subsequent neuronal damage in the cerebral cortex. We then separately measured infarct volume in the cortex and the subcortex. Treatment with rhEPO at doses of 500 and 1150 IU kg−1 or CEPO at a dose of 50 μg kg−1 significantly reduced cortical (26 and 30% for rhEPO 500 and 1150 IU kg−1, respectively, 36% for CEPO) but not subcortical infarct volume (Figure 2). Remarkably, rhEPO at a dose of 5000 IU kg−1 significantly reduced infarct volume in both the cortex (22%) and subcortex (36%, Figure 2).
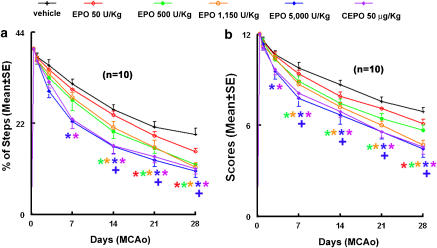
To examine whether treatment with rhEPO or CEPO improves neurobehavioural outcome, we performed a battery of behavioural tests that are sensitive to sensorimotor impairment in rodents (Li et al., 2000; Chen et al., 2003; Zhang et al., 2003, 2004, 2005; Wang et al., 2004). All rats subjected to embolic MCAo and treated with vehicle exhibited severe neurological deficits. However, rats treated 6 h after MCAo with rhEPO at doses of 500, 1150 and 5000 IU kg−1 or CEPO at a dose of 50 μg kg−1, showed significantly (P<0.05) improved neurological outcome. The separation between vehicle and treated animals began 7–21 days after MCAo, and the reduction of sensorimotor impairment versus vehicle control persisted to the end of the study, 28 days after MCAo (Figure 3). Improvement of behavioural outcome was better (P<0.01) in rats treated with 5000 IU kg−1 rhEPO when compared to rats treated with rhEPO at doses of 500 or 1150 IU kg−1 (Figure 3). Surprisingly, treatment with rhEPO 50 IU kg−1 beginning at 6 h post-MCAo also slightly but significantly improved neurological outcome when assessed 28 days after MCAo (P=0.03, Figure 3).
Figure 3.
The effects of CEPO and rhEPO on neurological function. Delayed (6 h) treatment with CEPO or rhEPO improves neurological function measured by foot-fault test (a) and mNSS (b) compared with the vehicle group. *P<0.05 vs the vehicle group and +P<0.05 vs rhEPO 500 and 1150 IU kg−1 groups. N=10 rats per group. CEPO, carbamylated rhEPO; mNSS, modified neurological severity score; rhEPO, recombinant human erythropoietin.
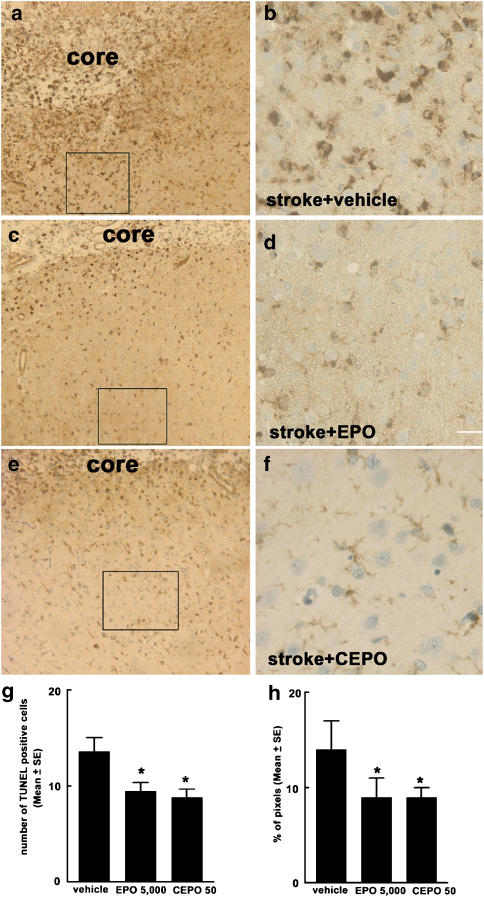
Delayed treatment with rhEPO or CEPO reduces apoptosis and microglial activation
Previous studies have reported that acute treatment with rhEPO prevents neuronal apoptosis and reduces activation of inflammatory cells within the CNS (Siren et al., 2001; Ghezzi and Brines, 2004). To examine whether delayed treatment with rhEPO reduced apoptosis and microglial activation, we measured the number of apoptotic cells and activated microglia in the ischaemic boundary region 28 days following MCAo (Zhang et al., 1997c). Treatment of rhEPO at a dose of 5000 IU kg−1 or CEPO (50 μg kg−1) significantly reduced the number of TUNEL-positive cells (31% for rhEPO and 35% for CEPO) and activated microglial cells (36% for rhEPO and CEPO) compared with the number in the vehicle group 28 days after MCA occlusion (Figure 4).
Figure 4.
The effect of CEPO and EPO on apoptosis and microglial responses. Panels a–f are images of activated microglial cells identified by IB4-positive cells in the cortical boundary region from representative rats treated with vehicle (a and b), rhEPO 5000 IU kg−1 (c and d) or CEPO 50 μg kg−1 (e and f). Panels b, d and f are high magnification images from the box area in panels a, c and e, respectively. (g and h) Quantitative data of TUNEL-positive cells and activated microglial cells, respectively, in the ischaemic boundary region. Core in the panels a, c and e indicates the ischaemic core. Bar=80 μm for panels a, c and e; Bar=20 μm for panels b, d, and f. CEPO, carbamylated rhEPO; EPO, erythropoietin; rhEPO, recombinant human erythropoietin; TUNEL, deoxynucleotidyl transferase-mediated biotinylated UTP nick end labelling model. *P<0.05 vs the vehicle group.
Discussion
The results of the present study demonstrated that rhEPO at doses of 500, 1150 and 5000 IU kg−1 or CEPO at 50 μg kg−1, administered 6 h following embolic MCAo significantly reduced infarct volume and improved neurological outcome compared with rats treated with vehicle. Moreover, our results indicate that measurable concentrations of rhEPO and CEPO are achieved in CSF and brain parenchyma following peripheral intravenous bolus dosing and that the concentrations achieved at the higher dose levels are consistent with those required for activity using in vitro models of neuronal injury. In these models, 1 IU ml−1 EPO (1 IU ml−1 EPO=8 ng ml−1 EPO) protected P19 cells from injury induced by serum withdrawal (Siren et al., 2001).
The neuroprotective effects of rhEPO have been demonstrated in several experimental models of stroke (Siren et al., 2001; Grasso et al., 2004; Leist et al., 2004; Villa et al., 2006). In a model of transient cortical ischaemia, rhEPO at doses of 500 to 5000 IU kg−1 has been shown to reduce infarct volume (Brines et al., 2000, 2004; Siren et al., 2001). In the present study, we used a model of embolic MCAo that mimics malignant MCA infarction and severe neurological impairment of human stroke (Hacke et al., 1996; Zhang et al., 1997; Carmichael, 2005). Our data show that delayed (6 h) treatment with rhEPO at doses of 500, 1150 and 5000 but not 50 IU kg−1 significantly reduced infarct volume 28 days after stroke. rhEPO at a dose of 5000 IU kg−1 was more effective in reducing the entire infarct volume (28%) compared with a dose of 500 IU kg−1 (17%). These data suggest that delayed treatment with rhEPO is effective even for malignant stroke and that the neuroprotective effects of EPO are dose-dependent.
There are two major differences between those earlier studies and the present work. First, earlier studies used either a permanent focal MCAo model in which there is little or no reperfusion of the ischaemic tissue or a model of cortical infarction in which a distal branch of the MCA was occluded (Sadamoto et al., 1998; Brines et al., 2000; Leist et al., 2004). The embolic MCAo model used here frequently exhibits spontaneous clot lysis and reperfusion within 24–48 h following MCAo, which closely mimics human stroke (Zhang et al., 1997; Jiang et al., 1998). Second, and perhaps most importantly, earlier reports demonstrating activity of rhEPO in rodent stroke models used a single intravenous bolus drug infusion. In the present study, we used a multiple-dose paradigm in which three equal doses of drug were administered, with the initial dose given 6 h and additional doses given 24 and 48 h after the initial dose. This dosing paradigm was used to match as closely as possible the dosing paradigm used in a small but positive clinical study using rhEPO and stroke (Ehrenreich et al., 2002).
As expected, all doses of rhEPO used in the present study produced a significant but transient elevation in haematocrit. This effect on haematocrit is consistent with the effects produced by other haematopoietic agents studied in preclinical stroke models (Belayev et al., 2005). Consistent with earlier reports, CEPO which does not bind to the classical EPOR did not elevate haematocrit (Leist et al., 2004). The present study extends previous findings by demonstrating that, as for rhEPO, delayed (6 h) treatment with CEPO significantly reduced infarct volume and improved functional outcome 28 days after embolic MCAo. Although the cellular mechanisms responsible for the neuroprotective effects of CEPO have not been fully elucidated, CEPO may evoke a protective response by signalling through the common β-receptor subunit, perhaps in a heteromeric complex with the EPOR (Brines et al., 2004). Here, we showed that CEPO and rhEPO, administered intravenously, crossed the BBB, consistent with previous reports (Brines et al., 2000; Juul et al., 2004; Leist et al., 2004).
The present study showed that the neuroprotective effect of delayed treatment with CEPO and rhEPO was primarily localized to the cerebral cortex. In this model of embolic stroke, we demonstrated previously that impairment of cerebral microvascular circulation in the cortex develops within 6 h after the onset of MCAo and the majority of ischaemic damage to neurons in the cortex are reversible (Garcia et al., 1993; Zhang et al., 2001). Thus, this 6 h window in the cortex could provide an access for rhEPO and CEPO to reach cerebral microvessels and pass the BBB rescuing potentially viable neurons in the ischaemic boundary region. Antiapoptotic and anti-inflammatory effects have been proposed as likely mechanisms contributing to the neuroprotective activity of rhEPO and CEPO (Siren et al., 2001; Agnello et al., 2002; Brines et al., 2004; Maiese et al., 2004). We found that delayed treatment with rhEPO and CEPO substantially reduced apoptosis and production of activated microglial cells in the ischaemic boundary region 28 days after the onset of MCAo. However, it remains to be determined whether these beneficial effects of rhEPO and CEPO are generated either by direct or by indirect anti-inflammatory and antiapoptotic effects.
In summary, delayed (6 h) treatment with CEPO and rhEPO reduces infarct volume and improves functional outcome following embolic MCAo. The long treatment window described here, coupled with the use of a multiple dose paradigm, suggests a viable therapeutic window for the use of these agents in the treatment of human stroke.
Acknowledgments
This work was supported by NINDS Grants nos. PO1 NS23393, PO1 NS42345, RO1NS43324 and RO1HL 64766 and by the Johnson & Johnson Stroke Management Group, Warren, NJ, USA.
Abbreviations
- CEPO
carbamylated EPO
- EPOR
EPO receptor
- MCAo
middle cerebral artery occlusion
- rhEPO
recombinant human erythropoietin
Conflict of interest
At the time this work was completed, K Rhodes and M Renzi were employees of Johnson & Johnson Pharmaceutical Research and Development, LLC. G Heavner and C Pool were employees of Centocor Inc., a Johnson & Johnson company. These employees do not derive any financial gain from the publication of this manuscript and hence there is no inherent conflict of interest.
References
- Agnello D, Bigini P, Villa P, Mennini T, Cerami A, Brines ML, et al. Erythropoietin exerts an anti-inflammatory effect on the CNS in a model of experimental autoimmune encephalomyelitis. Brain Res. 2002;952:128–134. doi: 10.1016/s0006-8993(02)03239-0. [DOI] [PubMed] [Google Scholar]
- Anagnostou A, Lee ES, Kessimian N, Levinson R, Steiner M. Erythropoietin has a mitogenic and positive chemotactic effect on endothelial cells. Proc Natl Acad Sci USA. 1990;87:5978–5982. doi: 10.1073/pnas.87.15.5978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belayev L, Khoutorova L, Zhao W, Vigdorchik A, Belayev A, Busto R, et al. Neuroprotective effect of darbepoetin alfa, a novel recombinant erythropoietic protein, in focal cerebral ischemia in rats. Stroke. 2005;36:1071–1076. doi: 10.1161/01.STR.0000160753.36093.da. [DOI] [PubMed] [Google Scholar]
- Bernaudin M, Marti HH, Roussel S, Divoux D, Nouvelot A, MacKenzie ET, et al. A potential role for erythropoietin in focal permanent cerebral ischemia in mice. J Cereb Blood Flow Metab. 1999;19:643–651. doi: 10.1097/00004647-199906000-00007. [DOI] [PubMed] [Google Scholar]
- Brines M, Grasso G, Fiordaliso F, Sfacteria A, Ghezzi P, Fratelli M, et al. Erythropoietin mediates tissue protection through an erythropoietin and common beta-subunit heteroreceptor. Proc Natl Acad Sci USA. 2004;101:14907–14912. doi: 10.1073/pnas.0406491101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brines ML, Ghezzi P, Keenan S, Agnello D, de Lanerolle NC, Cerami C, et al. Erythropoietin crosses the blood–brain barrier to protect against experimental brain injury. Proc Natl Acad Sci USA. 2000;97:10526–10531. doi: 10.1073/pnas.97.19.10526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael ST. Rodent models of focal stroke: size, mechanism, and purpose. NeuroRx. 2005;2:396–409. doi: 10.1602/neurorx.2.3.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Zhang ZG, Li Y, Wang Y, Wang L, Jiang H, et al. Statins induce angiogenesis, neurogenesis, and synaptogenesis after stroke. Ann Neurol. 2003;53:743–751. doi: 10.1002/ana.10555. [DOI] [PubMed] [Google Scholar]
- Ehrenreich H, Hasselblatt M, Dembowski C, Cepek L, Lewczuk P, Stiefel M, et al. Erythropoietin therapy for acute stroke is both safe and beneficial. Mol Med. 2002;8:495–505. [PMC free article] [PubMed] [Google Scholar]
- Garcia JH, Yoshida Y, Chen H, Li Y, Zhang ZG, Lian J, et al. Progression from ischemic injury to infarct following middle cerebral artery occlusion in the rat. Am J Pathol. 1993;42:623–635. [PMC free article] [PubMed] [Google Scholar]
- Ghezzi P, Brines M. Erythropoietin as an antiapoptotic, tissue-protective cytokine. Cell Death Differ. 2004;11 (Suppl 1):S37–S44. doi: 10.1038/sj.cdd.4401450. [DOI] [PubMed] [Google Scholar]
- Grasso G, Sfacteria A, Cerami A, Brines M. Erythropoietin as a tissue-protective cytokine in brain injury: what do we know and where do we go. Neuroscientist. 2004;10:93–98. doi: 10.1177/1073858403259187. [DOI] [PubMed] [Google Scholar]
- Hacke W, Schwab S, Horn M, Spranger M, De Georgia M, von Kummer R. Malignant' middle cerebral artery territory infarction: clinical course and prognostic signs. Arch Neurol. 1996;53:309–315. doi: 10.1001/archneur.1996.00550040037012. [DOI] [PubMed] [Google Scholar]
- Jiang Q, Zhang RL, Zhang ZG, Ewing JR, Divine GW, Chopp M. Diffusion-, T2-, and perfusion-weighted nuclear magnetic resonance imaging of middle cerebral artery embolic stroke and recombinant tissue plasminogen activator intervention in the rat. J Cereb Blood Flow Metab. 1998;18:758–767. doi: 10.1097/00004647-199807000-00007. [DOI] [PubMed] [Google Scholar]
- Juul SE, McPherson RJ, Farrell FX, Jolliffe L, Ness DJ, Gleason CA. Erytropoietin concentrations in cerebrospinal fluid of nonhuman primates and fetal sheep following high-dose recombinant erythropoietin. Biol Neonate. 2004;85:138–144. doi: 10.1159/000074970. [DOI] [PubMed] [Google Scholar]
- Leist M, Ghezzi P, Grasso G, Bianchi R, Villa P, Fratelli M, et al. Derivatives of erythropoietin that are tissue protective but not erythropoietic. Science. 2004;305:239–242. doi: 10.1126/science.1098313. [DOI] [PubMed] [Google Scholar]
- Lewczuk P, Hasselblatt M, Kamrowski-Kruck H, Heyer A, Unzicker C, Siren AL, et al. Survival of hippocampal neurons in culture upon hypoxia: effect of erythropoietin. Neuroreport. 2000;11:3485–3488. doi: 10.1097/00001756-200011090-00017. [DOI] [PubMed] [Google Scholar]
- Li Y, Chopp M, Chen J, Wang L, Gautam SC, Zhang Z. Intrastriatal transplantation of bone marrow nonhematopoietic cells improves functional recovery after stroke in adult mice. J Cereb Blood Flow Metab. 2000;20:1311–1319. doi: 10.1097/00004647-200009000-00006. [DOI] [PubMed] [Google Scholar]
- Maiese K, Li F, Chong ZZ. New avenues of exploration for erythropoietin. JAMA. 2005;293:90–95. doi: 10.1001/jama.293.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiese K, Li F, Chong ZZ. Erythropoietin in the brain: can the promise to protect be fulfilled. Trends Pharmacol Sci. 2004;25:577–583. doi: 10.1016/j.tips.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Marti HH. Erythropoietin and the hypoxic brain. J Exp Biol. 2004;207:3233–3242. doi: 10.1242/jeb.01049. [DOI] [PubMed] [Google Scholar]
- Masuda S, Okano M, Yamagishi K, Nagao M, Ueda M, Sasaki R. A novel site of erythropoietin production. Oxygen-dependent production in cultured rat astrocytes. J Biol Chem. 1994;269:19488–19493. [PubMed] [Google Scholar]
- Mun KC, Golper TA. Impaired biological activity of erythropoietin by cyanate carbamylation. Blood Purif. 2000;18:13–17. doi: 10.1159/000014403. [DOI] [PubMed] [Google Scholar]
- Naranda T, Wong K, Kaufman RI, Goldstein A, Olsson L. Activation of erythropoietin receptor in the absence of hormone by a peptide that binds to a domain different from the hormone binding site. Proc Natl Acad Sci USA. 1999;96:7569–7574. doi: 10.1073/pnas.96.13.7569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park KD, Mun KC, Chang EJ, Park SB, Kim HC. Inhibition of erythropoietin activity by cyanate. Scand J Urol Nephrol. 2004;38:69–72. doi: 10.1080/00365590310006291. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates 1986Academic Press Inc.: New York, NY; viii2nd edn.p [Google Scholar]
- Sadamoto Y, Igase K, Sakanaka M, Sato K, Otsuka H, Sakaki S, et al. Erythropoietin prevents place navigation disability and cortical infarction in rats with permanent occlusion of the middle cerebral artery. Biochem Biophys Res Commun. 1998;253:26–32. doi: 10.1006/bbrc.1998.9748. [DOI] [PubMed] [Google Scholar]
- Sakanaka M, Wen TC, Matsuda S, Masuda S, Morishita E, Nagao M, et al. In vivo evidence that erythropoietin protects neurons from ischemic damage. Proc Natl Acad Sci USA. 1998;95:4635–4640. doi: 10.1073/pnas.95.8.4635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinor AD, Greenberg DA. Erythropoietin protects cultured cortical neurons, but not astroglia, from hypoxia and AMPA toxicity. Neurosci Lett. 2000;290:213–215. doi: 10.1016/s0304-3940(00)01361-6. [DOI] [PubMed] [Google Scholar]
- Siren AL, Fratelli M, Brines M, Goemans C, Casagrande S, Lewczuk P, et al. Erythropoietin prevents neuronal apoptosis after cerebral ischemia and metabolic stress. Proc Natl Acad Sci USA. 2001;98:4044–4049. doi: 10.1073/pnas.051606598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai PT, Ohab JJ, Kertesz N, Groszer M, Matter C, Gao J, et al. A critical role of erythropoietin receptor in neurogenesis and post-stroke recovery. J Neurosci. 2006;26:1269–1274. doi: 10.1523/JNEUROSCI.4480-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villa P, van Beek J, Larsen AK, Gerwien J, Christensen S, Cerami A, et al. Reduced functional deficits, neuroinflammation, and secondary tissue damage after treatment of stroke by nonerythropoietic erythropoietin derivatives. J Cereb Blood Flow Metab. 2006;27:552–563. doi: 10.1038/sj.jcbfm.9600370. [DOI] [PubMed] [Google Scholar]
- Wang L, Zhang Z, Wang Y, Zhang R, Chopp M. Treatment of stroke with erythropoietin enhances neurogenesis and angiogenesis and improves neurological function in rats. Stroke. 2004;35:1732–1737. doi: 10.1161/01.STR.0000132196.49028.a4. [DOI] [PubMed] [Google Scholar]
- Wojchowski DM, Gregory RC, Miller CP, Pandit AK, Pircher TJ. Signal transduction in the erythropoietin receptor system. Exp Cell Res. 1999;253:143–156. doi: 10.1006/excr.1999.4673. [DOI] [PubMed] [Google Scholar]
- Zhang L, Zhang ZG, Ding GL, Jiang Q, Liu X, Meng H, et al. Multitargeted effects of statin-enhanced thrombolytic therapy for stroke with recombinant human tissue-type plasminogen activator in the rat. Circulation. 2005;112:3486–3494. doi: 10.1161/CIRCULATIONAHA.104.516757. [DOI] [PubMed] [Google Scholar]
- Zhang L, Zhang ZG, Zhang C, Zhang RL, Chopp M. Intravenous administration of a GPIIb/IIIa receptor antagonist extends the therapeutic window of intra-arterial tenecteplase-tissue plasminogen activator in a rat stroke model. Stroke. 2004;35:2890–2895. doi: 10.1161/01.STR.0000147963.68238.da. [DOI] [PubMed] [Google Scholar]
- Zhang L, Zhang ZG, Zhang R, Morris D, Lu M, Coller BS, et al. Adjuvant treatment with a glycoprotein IIb/IIIa receptor inhibitor increases the therapeutic window for low-dose tissue plasminogen activator administration in a rat model of embolic stroke. Circulation. 2003;107:2837–2843. doi: 10.1161/01.CIR.0000068374.57764.EB. [DOI] [PubMed] [Google Scholar]
- Zhang RL, Chopp M, Zhang ZG, Jiang Q, Ewing JR. A rat model of focal embolic cerebral ischemia. Brain Res. 1997;766:83–92. doi: 10.1016/s0006-8993(97)00580-5. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Chopp M, Powers C. Temporal profile of microglial response following transient (2 h) middle cerebral artery occlusion. Brain Res. 1997c;744:189–198. doi: 10.1016/S0006-8993(96)01085-2. [DOI] [PubMed] [Google Scholar]
- Zhang ZG, Zhang L, Tsang W, Goussev A, Powers C, Ho K, et al. Dynamic platelet accumulation at the site of the occluded middle cerebral artery and in downstream microvessels is associated with loss of microvascular integrity after embolic middle cerebral artery occlusion. Brain Res. 2001;912:181–194. doi: 10.1016/s0006-8993(01)02735-4. [DOI] [PubMed] [Google Scholar]