Abstract
Background and purpose:
Cisplatin drives specific types of tumour cells to apoptosis. In this study we investigate the involvement of intracellular calcium ([Ca2+]i) in triggering apoptosis in two different cell lines. As cisplatin is used for the treatment of several forms of cancer we choose HeLa-S3 and U2-OS as two examples of tumour cell lines.
Experimental approach:
Cisplatin (1nM–10μM) was applied to HeLa-S3 and U2-OS cells and [Ca2+]i measured with fluo-4, using laser scanning microscopy. Inositol-1,4,5-trisphosphate (IP3) receptors were visualized with immunostaining. Membrane conductances were measured with patch-clamp techniques. Levels of calpain and caspases were assessed by western blots and apoptotic cells were stained with Hoechst 33342 and counted.
Key results:
Cisplatin increases [Ca2+]i concentration-dependently in HeLa-S3 but not in U2-OS cells. This elevation of [Ca2+]i depended on extracellular Ca2+ but was reduced by the IP3 receptor blocker, 2-APB. This effect was not due to a Ca2+ release triggered by Ca2+ entry. Immunostaining showed IP3-receptors (type1-3) at the cellular membrane of HeLa-S3 cells, but not in U2-OS cells. Electrophysiological experiments showed an increased membrane conductance with cisplatin only when Ca2+ was present extracellularly. Increase of [Ca2+]i was related to the activation of calpain but not caspase-8 and triggered apoptosis in HeLa-S3 but not in U2-OS cells.
Conclusions and implications:
Our observations on the activation of IP3-receptors, calcium entry and apoptotic rate by cisplatin in specific carcinogenic cells might open new possibilities in the treatment of some forms of cancer.
Keywords: cisplatin, carboplatin, anticancer drugs, calcium signalling, HeLa-S3, U2-OS, calpain, apoptosis, IP3-receptor, calcium stores, [Ca2+]i
Introduction
Cisplatin (cis-diammindichloroplatin) is widely used as a potent anti-tumour agent for treating different forms of cancer, like colon, lung or cervix carcinoma. However, treatment with cisplatin is often accompanied by cytotoxic, ototoxic and nephrotoxic effects (Dehne et al., 2002; Schloffer et al., 2003; Goren, 2003; Noda et al., 2006). Owing to the impairments of kidney and hearing functions, many patients discontinue the treatment with cis-diammindichloroplatin (CDDP). Moreover, cisplatin is known to be carcinogenic and genotoxic in mammalian cells. This is a major concern because of the risk of inducing secondary malignancies. Additionally, the occurrence of cellular resistance limits the therapeutic efficiency of cisplatin (Marzano et al., 2004).
The aim of chemotherapy of human malignancies is the specific inhibition of tumour cell proliferation and/or induction of apoptosis. It has been demonstrated that intracellular calcium ([Ca2+]i) signals are involved in a number of events, including apoptotic pathways (Mandic et al., 2002; Arany et al., 2004; Jaffe, 2005). While Ca2+ could be released from the [Ca2+]i stores, as it has been demonstrated for other metal compounds, such as trimethyltin or As2O3 (Florea and Büsselberg, 2005, 2006; Florea et al., 2005a, 2005b, 2007), Ca2+ also could enter from the extracellular space through membrane channels. Recently it has been shown that the activation of inositol-1,4,5-trisphosphate (IP3) receptors, found at the cellular membrane, results in an elevation of [Ca2+]i (Dellis et al., 2006).
The modulation of [Ca2+]i influences the activation of calpain (Sharma and Rohrer, 2004), which controls cell-cycle regulation, differentiation and apoptosis (Alznauer et al., 2004). Activation of calpain from pro-calpain leads to apoptosome formation by cleavage of Bid, which in turn regulates Bax and activates caspase-3 (Mandic et al., 2002; Alznauer et al., 2004).
Cisplatin has been shown to trigger apoptotic pathways in tumour and nontumour cell models, in vitro or in vivo (Schloffer et al., 2003; Fan et al., 2005). However, details of early events leading to apoptosis after exposure to cisplatin are not yet understood. Here we have investigated the hypothesis that Ca2+ as a second messenger plays an important role in the cisplatin-induced apoptosis in HeLa-S3 cells.
Materials and methods
Cell culture
HeLa-S3 (human cervix adenocarcinoma) cells (ATCC, American-type culture collection) were cultured in Ham's F-12 medium (Sigma, Taufkirchen, Germany) supplemented with 10% foetal calf serum (Cambrex Biowhitaker, East Rutherford, NJ, USA), 100 IU ml−1 penicillin and 0.1 mg ml−1 streptomycin (Sigma). Cells were maintained at 37°C in a humidified atmosphere containing 5% CO2.
U2-OS (human osteosarcoma) cells (ATCC) were incubated in RPMI 1640 medium (GIBCO) containing 10% foetal calf serum, 100 IU ml−1 penicillin and 0.1 mg ml−1 streptomycin under the same conditions as HeLa-S3.
Confocal laser scanning microscopy
Twenty-four hours before experiments, HeLa-S3 and U2-OS cells were harvested and seeded to 7.5 cm2 ‘easy grip' petri dishes (Falcon, Franklin Lakes, NJ, USA). For experiments the culture medium was changed to Tyrode buffer (145 mM NaCl, 2.5 mM KCl, 10 mM HEPES, 10 mM glucose, pH 7.4 by 1 M NaOH, 1.8 mM CaCl2 and 1.2 mM MgCl2). In the experiments with nominal ‘no calcium' in the extracellular solution, no calcium salts were added to the solution. For measurements under ‘no calcium' conditions, the normal solution was replaced immediately before starting the experiment. Changes of [Ca2+]i as well as the changes of the fluorescence in the calcium stores were measured as described previously (Florea et al., 2005a, 2005b, 2007). Briefly, to study [Ca2+]i modulation by cisplatin, two Ca2+ sensitive dyes were used: fluo-4/AM (Molecular Probes, Karlsruhe, Germany) to observe changes of [Ca2+]i and rhod-2/AM (Sigma) to determine the changes of Ca2+ within [Ca2+]i stores. Fluorescence images were collected at room temperature every 30 s. Full-screen images were taken which allows the analysis of the [Ca2+]i rise in single cells. The cells were marked as ‘regions of interest' and the calcium concentration was calculated offline.
Cisplatin (Bristol-Meyers Squibb, Munich, Germany) or carboplatin (Sigma) in the concentration range of 1 nM–10 μM was added to the cell culture using a constant flow application system (1 ml min−1). 2-Aminoethoxydiphenyl borate (2-APB; Tocris, Bristol, UK) was dissolved in dimethylsulphoxide and used at its IC50 of 50 μM (Mignen et al., 2005). All experiments were performed at room temperature (24–26°C).
For determination of calcium concentrations, the META-Software (ZEISS) was used, with the equation: [Ca2+]i=Kd*(F−Fmin/Fmax−F), where Kd is the dissociation constant (taken from the data sheet for fluo-4 as provided by Molecular Probes) and F the fluo-4 intensity. The basal [Ca2+]i-concentration was considered 100 nM, as documented previously (Grynkiewicz et al., 1985; for review see Orrenius et al., 2003). All images were background substracted. [Ca2+]i concentrations obtained under control conditions were set to 100% and all other values were calculated as percentage of increase in control.
Immunostaining
HeLa-S3 and U2-OS cells were washed with Tyrode buffer and fixed with 1% paraformaldehyde. Cells were stained 1:200 with specific anti-IP3 receptor antibodies (‘anti-IP3RI–III PAb polyclonal', Calbiochem, San Diego, CA, USA; and ‘anti-IP3R-I PAb polyclonal' Alexis, Lörrach, Germany) and washed after 20 min with Tyrode buffer. Secondary antibody immunoglobulin G (IgG)-anti-rabbit fluorescein isothiocyanate used diluted 1:1000. Cells were loaded for 30 min. Coverslips were mounted on slides and pictures were collected with ZEISS 510 imaging system. Single pictures and Z-stacks were taken with ZEISS lens (63 × /1.4).
Electrophysiology
Membrane currents in HeLa-S3 cells were recorded as described by Splettstoesser et al. (2005), but electrodes were backfilled with the following solution (140 mM KCl, 4 mM MgCl2, 1 mM CaCl2·2H2O, 10 mM HEPES, 11 mM EGTA and pH 7.4 by 0.1 M NaOH). Culture medium was replaced by Tyrodes buffer.
In the experiments where the influence of the current through calcium-activated potassium channels (KCa) on cisplatin-induced changes of the membrane conductance was analysed, charybdotoxin (3.3 nM, Alomone Labs, Jerusalem, Israel) was added to the extracellular Tyrodes buffer.
Membrane potential was clamped at −80 mV. Voltage ramps from −110 to +20 mV over 1.3 s were performed and membrane currents were recorded. Currents were elicited every minute for at least 1 h. Cisplatin was applied by a bath application system, for total exchange a volume of 10 ml was flushed through the bath (volume 1 ml) with a continuous flow of 1 ml per min.
Changes of the membrane conductance at the resting membrane potential (−80 mV and at +20 mV) were analysed over the time of the experiments. Control values were set to 100% and all other values were calculated in relation to control.
Preparation of cell extracts and western blots
Cells were grown in six-well dishes, 300 000 cells ml−1 were seeded and incubated overnight. Cultures were exposed to 1 μM cisplatin and/or 50 μM 2-APB as well as to TNF-related apoptosis-inducing ligand (TRAIL; 160 ng ml−1; Calbiochem) for 15 min and 1 h. For preparation of cell lysates, the monolayer was washed with ice-cold phosphate-buffered saline (PBS), drained, lysed on the plates with 50 μl lysis buffer (300 mM NaCl, 10 mM Tris (pH 7.9), 1 mM EDTA, 0.1% NP40, 1 × protease-inhibitor-cocktail; (Roche, Mannheim, Germany)), and then scraped and left for 20 min on ice. The extract was centrifuged (2300 g, 4°C, 5 min), and the protein content was quantified using a spectrophotometer and a protein assay reagent (bovine serum albumin; Bio-Rad Laboratories, Hercules, CA, USA). Lysates were stored at −80°C until western blots were performed.
SDS-PAGE and western blotting
Sample buffer (50 mM Tris (pH 6.8), 2% SDS (sodium dodecyl sulphate), 5% β-mercaptoethanol, 0.0125% bromphenol blue, 1% glycine) was added 1:4 to the whole-cell lysate and boiled for 5 min at 95°C. A 50–75 μg weight of protein was used per well of a 7.5% SDS-PAGE (polyacrylamide gel electrophoresis). Gels were run for ∼90 min at 120 V at room temperature (running buffer: 25 mM Tris base, 190 mM glycine, 3.5 mM SDS; transfer buffer: 25 mM Tris base, 190 mM glycine, 20% methanol) and transferred for 80 min at 120 V to nitrocellulose membrane (0.2 μm pore size, Schleicher and Schuell, Dassel, Germany). A protein marker (SDS 7B, Sigma) was used as a protein size control. The efficiency of protein transfer and equal loading was confirmed by staining the nitrocellulose membrane with Ponceau S (Sigma). Membranes were blocked overnight at 4°C with 5% non-fat dry milk in TBS-T (Tris-buffered saline Tween-20) (10 mM Tris (pH 7.5), 100 mM NaCl, 0.05% Tween 20), then washed in TBS-T and incubated with the primary antibody at room temperature for 2 h with gentle shaking. Primary antibodies used were rabbit polyclonal antibody against calpain-1 (58 Kda) domain I (1:5000, Calbiochem) and goat polyclonal antibody against active caspase-8 (2 kDa large and 18 kDa small subunit) (R&D Systems, Minneapolis, MN, USA). After washing with TBS-T, the blot was incubated with horseradish peroxidase-conjugated anti-rabbit or anti-goat IgG antibody (Sigma). Immunoreactive proteins were visualised using ECL Advance Western Blotting Detection Kit (Amersham Biosciences, Uppsala, Sweden), followed by exposure to X-ray films (Agfa, Mortsel, Belgium).
Hoechst staining and scoring of apoptotic cells
Chamber slides (LabTek, Wiesbaden, Germany) were seeded with 100 000 cells, each well containing 1 ml culture medium were incubated overnight. One micromolar of cisplatin was applied for 1 h. After the incubation, cells were washed twice with PBS, fixed and treated with cold methanol (−20°C) and left overnight at −20°C. After fixation, slides were air dried and stained with 10 μM Hoechst 33342 (Molecular Probes) for 30 s and mounted with cover slips. The cells were analysed using a × 63 magnification with an Axiovert fluorescent microscope.
Trypan blue cytotoxicity test
For determination of the cell viability, treated as well as untreated cells were taken for the experiments. Non-confluent cell monolayers were exposed to 1 μM cisplatin in cell culture flasks for 1, 2, 6, 24, 48 and 72 h. All experiments were repeated twice. After the treatment with cisplatin, cells were harvested using trypsin and resuspended in culture medium, washed, centrifuged (2 min, 100 g) and resuspended again in complete culture media. Then, a small aliquot of the cell suspension (50 μl) was mixed with the same volume of 0.4% trypan blue (Sigma) solution and the sample was counted after 3 min of staining using an improved Neugebauer Chamber. The number of bright (viable) cells and blue cells (nonviable) were evaluated using a light microscope with a 20-fold magnification. After counting, the cell viability (CV) was expressed as the percentage of surviving cells compared to the total number of cells: CV=(viable cells/total number of cells)100. Cisplatin was considered to be cytotoxic when it induced a decrease of cell viability of more than 50% compared to the negative control.
Data analysis
Results are presented as means±s.d. from the number of assays shown. Statistical analysis was carried out using Student's t-test (two tailed, paired or unpaired as appropriate) and values of P<0.05 was considered significant.
Results
Cisplatin induces a concentration-dependent increase in [Ca2+]i in HeLa-S3 but not in U2-OS cells
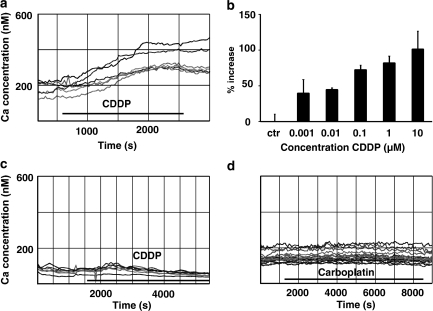
Measurements of [Ca2+]i concentration in untreated HeLa-S3 cells using Fluo-4 ranged between 120 and 220 nM (154.6±48.8 nM). With the application of cisplatin (10 μM), [Ca2+]i increased over a time interval of 15–35 min until it reached a plateau at 250–400 nM (Figure 1a). The increase in [Ca2+]i caused by cisplatin in HeLa-S3 cells was concentration dependent with a threshold below 1 nM, the lowest concentration tested (Figure 1b). Between 1 nM and 10 μM, the increase in [Ca2+]i ranged from 39.5±19.1 to 101.3±24.9%, respectively. Concentrations above 10 μM were not tested because they are clinically irrelevant. The elevation of [Ca2+]i was only slightly (<20%) reversible at any of the concentrations used. The increase in [Ca2+]i is not a general effect caused by cisplatin, since the application of 10 μM CDDP did not elevate [Ca2+]i in U2-OS cells (Figure 1c). Furthermore, the effect in HeLa-S3 cells was restricted to cisplatin since the related compound, carboplatin (up to a concentration of 10 μM), did not substantially elevate [Ca2+]i (Figure 1d).
Figure 1.
Modulation of [Ca2+]i by platinum compounds under different conditions. (a) Cisplatin (CDDP; 10 μM) increases [Ca2+]i in HeLa-S3 cells (n=47). Illustrated is the calcium rise in six cells. The basal [Ca2+]i levels ranged from 120 to 220 nM. CDDP raised [Ca2+]i over a time interval of 1000 s to a ∼two-fold higher level. The effect was only slightly reversible (less than 20%; data not shown). (b) Concentration dependency of the [Ca2+]i increase by cisplatin. Threshold concentration was below 1 nM and the [Ca2+]i was doubled with 10 μM cisplatin (1 nM: n=28; 10 nM: n=18; 100 nM: n=29; 1 μM: n=32; 10 μM: means±s.d., n=47). (c) [Ca2+]i did not increase in U2-OS cells during cisplatin (10 μM) application (n=26). (d) Carboplatin (10 μM) did not elevate [Ca2+]i in HeLa-S3 cells (n=39). [Ca2+]i, intracellular calcium.
Cisplatin-induced [Ca2+]i increase depends on extracellular Ca2+
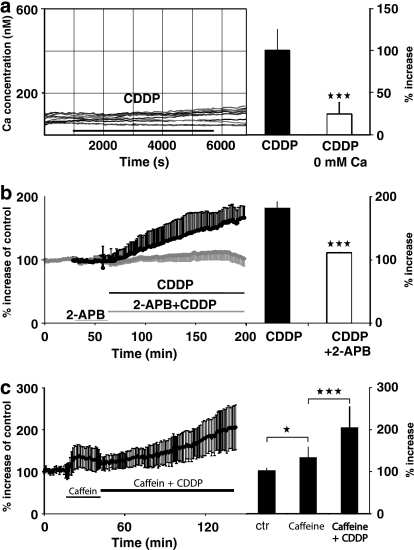
To determine the source of the increase in [Ca2+]i during the application of cisplatin, we tested whether Ca2+ is released from the internal stores or enters from the extracellular space. Ca2+ entry from the extracellular solution was examined by using an external buffer solution with no added Ca2+. In this solution, cisplatin (10 μM) applied to HeLa-S3 cells did not significantly elevate [Ca2+]i (Figure 2a). In four experiments (47 cells), in nominally Ca2+-free buffer, the [Ca2+]i rise was significantly diminished compared to experiments in a solution containing 1.8 mM Ca2+ (Figure 2a, right side; P<0.001). This is an indication that cisplatin-induced Ca2+ enter from the extracellular side. The increase in [Ca2+]i observed when no Ca2+ was added to the extracellular solution is most likely due to impurities of the substances used (for example, NaCl has an impurity of 0.002% of calcium, which could result in a total extracellular Ca2+ concentration of up to 3 μM). We were unable to ‘clamp' the extracellular calcium concentration, using 1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid) or EGTA, since the cell cultures did not survive under such conditions over the course of the experiments.
Figure 2.
Cisplatin (CDDP)-induced [Ca2+]i rise depends on extracellular calcium and IP3 receptors but not calcium release from stores. (a) Left side: in HeLa-S3 cells, [Ca2+]i did not increase when no calcium was added to the extracellular solution. Right side: averaged increase in [Ca2+]i by cisplatin (10 μM) in HeLa-S3 is highly significantly (***P<0.001; means±s.d., n=27) reduced when no calcium is added to the extracellular solution. (b) Left side: averaged time course of the [Ca2+]i concentration in HeLa-S3 during the application of cisplatin (1 μM) and with the coapplication of the IP3-receptor antagonist 2-APB (50 μM). Right side: with the coapplication of 2-APB, the [Ca2+]i elevation is highly significantly reduced (***P<0.001; means±s.d., n=41). (c) Left side: pre-application of caffeine (10 mM) did not abolish the CDDP-induced [Ca2+]i elevation (time course of averaged data; left side). Right side: summary of caffeine-induced rise in [Ca2+]i and the further CDDP induced increase (*P<0.05; ***P<0.001; means±s.d., n=19). [Ca2+]i, intracellular calcium; IP3 receptors, inositol-1,4,5-trisphosphate receptors; 2-APB, 2-aminoethoxydiphenyl borate.
To investigate whether Ca2+ was also released from [Ca2+]i stores, we blocked the activation of IP3 receptors using a specific antagonist (2-APB; 50 μM) to reduce Ca2+ release from the intracellular stores (Mignen et al., 2005). In regard to the results described above, we expected that 1 μM cisplatin, which alone caused a substantial increase in [Ca2+]i (Figure 1b), would have a similar effect when coapplied with 2-APB in the 1.8 mM Ca2+ containing external solution. Instead, 2-APB blocked the increase in [Ca2+]i caused by cisplatin (Figure 2b). The difference between the [Ca2+]i rise with and without 2-APB was highly significant (Figure 2b, right side; P<0.001).
Our observations are in agreement with two possible explanations of the increase in [Ca2+]i caused by cisplatin: (1) an IP3 receptor mediated Ca2+ entry from the extracellular space, or (2) Ca2+ release from internal stores triggered by entry of extracellular Ca2+ (Ca2+-dependent Ca2+-release). The second possibility was addressed by depleting the calcium stores with 10 mM caffeine (Thayer et al., 1988) before cisplatin (1 μM) was coapplied (Figure 2c). With caffeine, [Ca2+]i increased significantly by 31.2±25.2% (P<0.05) and reached a steady state within 5 min. When cisplatin was added, there was a further, highly significant, increase in [Ca2+]i to 102.2±50.2% (Figure 2c) compared to 81.9±9.6% as measured when the calcium stores were not depleted previously (Figure 2c, right side; P<0.001). Therefore, Ca2+ release from the stores induced by Ca2+ entry from the extracellular space is unlikely.
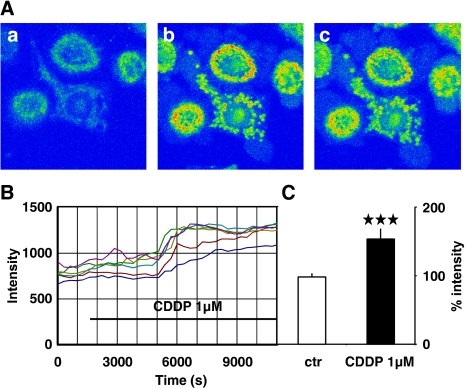
In addition, we have analysed the calcium dynamics in the calcium stores by labelling Ca2+ with the store-specific calcium sensitive dye, rhod-2. While the intracellular stores were hardly visible under control conditions (Figure 3A), the signal did not further decline after the application of cisplatin, but the calcium concentration in the stores increased about 60 min after the application of 1 μM cisplatin was started and reached a plateau about 30 min later (Figures 3Ab, c and 3B). Therefore we conclude that the cisplatin-induced increase in [Ca2+]i results in an activation of Ca2+ transport mechanisms to extrude Ca2+ from the cytosol, which will transport Ca2+ to the extracellular space as well as to the Ca2+ stores, resulting in a loading of the stores with Ca2+. Overall, the increase in the fluorescence in the stores was highly significant (Figure 3C; P<0.001).
Figure 3.
Using the store-located calcium-sensitive dye rhod-2, an increase in fluorescence was measured in the stores when cisplatin (CDDP; 1 μM) was applied. (A) Three images taken in an experiment (a) before, (b) after 80 min and (c) 140 min after cisplatin had been applied. The calcium concentration is indicated with false rainbow colours scale (blue=no calcium, green to yellow=low-to-medium calcium, red=high calcium). Clearly, during application of the anti-cancer drug, the calcium increases within the calcium stores. (B) Time course of the calcium increase in the stores measured as the total increase in the fluorescence in single cells. Note that the calcium increase in the stores occurs clearly later (the elevation begins about 60 min after the application) than the raise in the cytosolic calcium level (compare Figure 1). (C) The elevation of the fluorescence indicating the calcium rise in the stores is highly significant after cisplatin had been applied (***P<0.001; means±s.d., n=20) (for color figure see online version).
Membrane conductance of HeLa-S3 cells is increased by cisplatin since the elevation of [Ca2+]i activates KCa channels
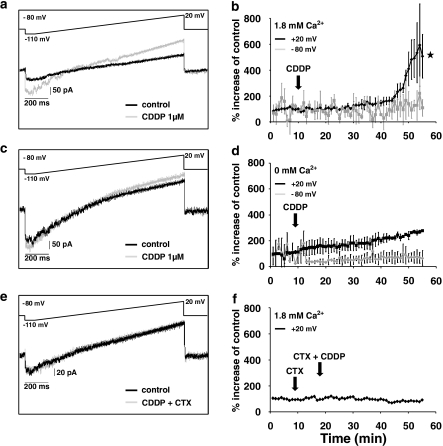
Electrophysiological experiments were performed to evaluate the possibility of an increased membrane conductance due to Ca2+ uptake in the cells via open Ca2+-selective ion channels. Total membrane currents (Figures 4a, c and d) were elicited by a voltage ramp from −110 to +20 mV over 1.3 s (10 mV per 0.1 s): before and during the application of cisplatin (1 μM). During cisplatin application in normal extracellular solution, the current was increased over the whole-voltage range without significant change in the reversal potential (control: −66.2±3.3 mV; 1 μM CDDP: −67.1±2.7 mV; P>0.5) (Figures 4a and b). The increased current at +20 mV (513.3±92.5%, P<0.05) demonstrated an increased conductance, resulting from the opening of ion channels. When the extracellular solution had no Ca2+ added, cisplatin caused only a small increase in membrane current/conductance, which did not change significantly over the whole time of the experiments (110.1±117.3%; Figure 4c, time courses in Figure 4d). These results indicate that the increased conductance triggered by cisplatin was due to the opening of calcium-activated channels. The elevated [Ca2+]i most likely opens KCa channels, as adding charybdotoxin (3.3 nM) – a blocker of the large conductance of the KCa channels – prevented the activation of the KCa channels at +20 mV (Figures 4e and f).
Figure 4.
Electrophysiological recordings of membrane currents in HeLa-S3 cells before and after the application of cisplatin (CDDP). (a) Membrane currents of HeLa-S3 cells, elicited by a voltage ramp from −110 mV to +20 mV over 130 ms, before and with cisplatin (1 μM, n=5). (b) Time course of membrane currents (black line=measured at +20 mV; grey line=measured at −80 mV)) of HeLa-S3 cells using 1.8 mM calcium in the extracellular buffer. There was a clear increase in the currents at +20 mV after ∼40 min of application. After 55 min, membrane currents elicited at +20 mV were significantly different compared to the experiments where no calcium was added (compare panel d; *P<0.05; n=5). (c and d) Same procedure as in (a) and (b) but with no Ca2+ added to the external buffer. Note that after the application of CDDP, there was only a small increase in the current (n=5) over the time of the experiment at +20 mV and no change at −80 mV. (e and f) Same procedure as in (a) and (b) but using the KCa-channel blocker charybdotoxin (CTX; 3.3 nM). The examples shown illustrate that there is no change in the membrane current when the calcium-activated potassium channels are blocked, an indication that this is the main conductance activated by the entry of Ca2+.
IP3 receptors are found at the cell membrane in HeLa-S3 but not in U2-OS cells
Because the increase in [Ca2+]i clearly depended on the extracellular calcium concentration and was prevented by the blockade of the IP3 receptor, we tested the hypothesis that IP3 receptors are expressed in the cell membrane. Dellis et al. (2006) have shown in DT40 chicken cells that most IP3 receptors are expressed in intracellular stores, but a small fraction is reliably directed to the plasma membrane. This fraction of receptors is substantially involved in the Ca2+ entry.
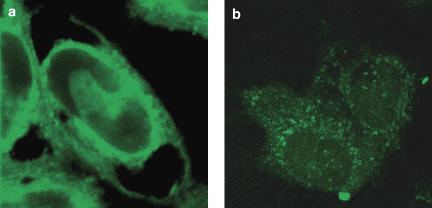
Using immunostaining and confocal fluorescence microscopy, we tested two different antibodies, one for all three subtypes of IP3 receptors (Calbiochem) and one for the type 1 subtype (Alexis), both with affinity for the cytoplasmic C-terminus side of the receptor. The staining was identical for both antibodies. The results with the staining of all three subtypes are shown in Figure 4. Immunostaining for IP3 receptors in HeLa-S3 cells resulted in fluorescence at the nuclear envelope and at the cell membrane (Figure 5a), while in U2-OS cells the immunostaining detected IP3 receptors only at the nuclei and at cell organelles (Figure 5b). Z-stacks of both cell types illustrate these findings even more clearly than two-dimensional confocal images (data provided on request). No nonspecific binding of the secondary antibody was found (data not shown).
Figure 5.
Distribution of IP3-receptor antibody in (a) HeLa-S3 cells (n=9) and (b) U2-OS cells (n=6). Notice in HeLa-S3 cells IP3 receptor distribution at the cellular membrane, the nuclear envelope and at organelles, while in U2-OS cells the receptor is present only in organelles. Staining with the secondary antibody did not reveal any nonspecific staining in any of the two cell lines (data not shown). IP3 receptor, inositol-1,4,5-trisphosphate receptor.
The cisplatin-induced elevation of [Ca2+]i activates calpain but not caspase-8
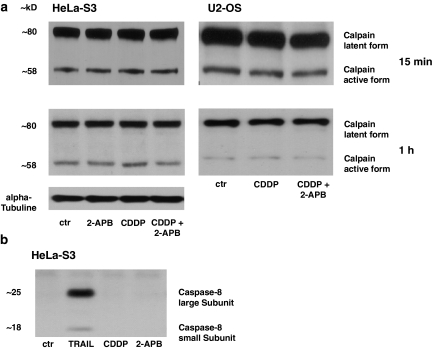
Although cisplatin induces apoptosis and increases [Ca2+]i in different cancer cell lines, the role of [Ca2+]i in the initiation of apoptosis remains unclear. Recently, in a mouse photoreceptor cell line it was demonstrated that the calcium-induced activation of calpain results in apoptosis (Sharma and Rohrer, 2004), but it is known that calpain can also be activated by cisplatin (Mandic et al., 2002). To address whether this is also an issue in our cell lines, we investigated the expression of calpain and caspase-8 after the application of cisplatin (1 μM) for 15 min and 1 h. Both substances are key proteins involved in triggering apoptotic death. We tested their expression in HeLa-S3 as well as in U2-OS cells. While there were no changes after 15 min and 1 h after application (a time at which cisplatin increases [Ca2+]i in HeLa-S3 cells but not in U2-OS cells; compare Figures 1a and 2b), cisplatin significantly activated calpain in HeLa-S3 cells, while this effects was diminished by 2-APB. In U2-OS cells, cisplatin did not increase the active form of calpain significantly. Like all caspases, caspase-8 is activated by cleavage of a single-chain zymogen into two subunits. Neither the small nor large catalytic subunit increased following cisplatin application in HeLa-S3 cells (Figure 6b). To test whether we are able to trigger an increase in caspase-8 under the conditions used, we applied TRAIL (160 ng ml−1) to our HeLa-S3 cell line and induced a clear increase in the caspase-8 (Figure 6b).
Figure 6.
Calpain, but not caspase-8, is increased in HeLa-S3 cells, while calpain is not elevated in U2-OS cells, after exposure to cisplatin (CDDP). (a) Left panel: while there was no change after 15 min of incubation, after 1 h of exposure to 1 μM cisplatin, active calpain was highly increased in HeLa-S3 cells and this increase was blocked by 50 μM 2-APB (n=13). Right panel: this effect was not observed in U2-OS cells (n=6; 1 μM cisplatinfor 15 min and 1 h of exposure). (b) Cisplatin did not increase the large or small subunit of caspase-8 in HeLa-S3 cells after 1 h of exposure, while the activation of the TRAIL receptor showed that the caspase-8 antibody was effective (n=9). 2-APB, 2-aminoethoxydiphenyl borate; TRAIL, TNF-related apoptosis-inducing ligand.
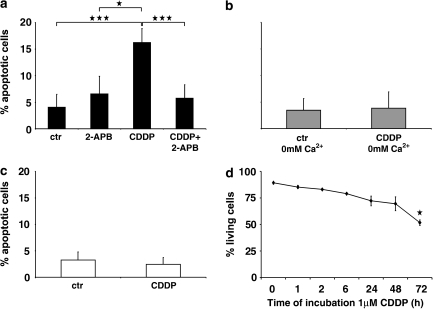
Cisplatin induces [Ca2+]i-dependent apoptosis
Exposure of HeLa-S3 cells to 1 μM cisplatin significantly reduced cell viability when the trypan blue cytotoxicity test was applied. The effect was time dependent. To further test if cell death occurred by apoptosis, Hoechst staining was carried out and nuclei with morphology typical of apoptosis were scored in relative to healthy nuclei. Counts of apoptotic nuclei showed that cisplatin increased cell death by apoptosis. The effect was reduced when the rise of [Ca2+]i was diminished either by 2-APB or a nominal calcium-free extracellular solution (Figure 7). In addition, the apoptotic rate was unchanged in U2-OS cells (Figure 7).
Figure 7.
Cisplatin (CDDP)-induced increase of apoptotic rate is reduced in HeLa-S3 but not in U2-OS cells, when no calcium is added or IP3 receptors are blocked. (a) Apoptotic rate under different conditions in normal calcium (1.8 mM) containing medium. In HeLa-S3 cells, cisplatin increased apoptotic rate (***P<0.001 compared to control) and this increase was significantly reduced by 2-APB (***P<0.001; means±s.d.). (b) Apoptotic rates with calcium-free incubation medium are not significantly changed by cisplatin. (c) Apoptotic rate in U2-OS cells incubated in normal calcium medium is also unchanged after cisplatin. (d) Cell viability test. The number of living cells is decreased significantly (*P<0.05; means±s.d.) beginning at 72 h of exposure to 1 μM cisplatin. Note time dependence of cytotoxicity. IP3 receptors, inositol-1,4,5-trisphosphate receptors; 2-APB, 2-aminoethoxydiphenyl borate.
Discussion
In clinical practice, cisplatin is the first-choice chemotherapy for epithelial malignancies and the standard treatment for cervical cancer treatment (Boulikas and Vougiouka, 2004). Extensive work on cisplatin cytotoxicity in tumour cells has been carried out, but very little is known how cisplatin interacts with calcium homeostasis in tumour cells. Recently, Kawai et al. (2006) have shown that in a nontumour model (renal cells), relatively high concentrations of cisplatin (250–750 μM) induced a rise in [Ca2+]i. In our work we investigated the effect of clinically relevant concentrations of cisplatin on calcium homeostasis of different tumour cells and we have correlated these effects with apoptotic cell death. Low concentrations of cisplatin (0.001–10 μM) increased [Ca2+]i in HeLa-S3 but not in U2-OS cells. [Ca2+]i rise was concentration dependent and mediated by IP3 receptors. Overall, [Ca2+]i increase in HeLa cells was associated with cisplatin-induced apoptotic death with the involvement of calpain. Our results are summarised in Figure 8. Our findings show that cisplatin has a higher activity on some specific cell types, in this case to tumour cells (see also Pai and Sodhi, 1992). These authors have also shown selectivity of cisplatin for certain cell lines (mononuclear cells from human peripheral blood).
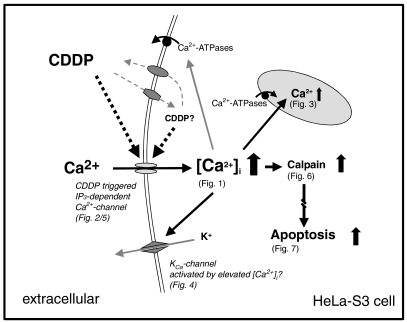
Figure 8.
An interlocking hypothesis of the interaction of cisplatin with HeLa-S3 cells, which combines the results found by our different approaches, is illustrated in this scheme. Cisplatin might be able to enter and leave the cell by transporters. Intracellular and/or extracellular cisplatin (or its metabolites) increase [Ca2+]i concentration dependently by IP3 receptor-controlled calcium channels that are located at the cellular membrane. When extracellular calcium enters the cytosol, [Ca2+]i increases after 20–30 min, which activates extruding transport mechanisms (Ca-ATPases), resulting in a consequent Ca2+ increase in the calcium stores after about 60 min and most likely by the activation of mechanisms that transport Ca2+ to the extracellular side. When the amount of Ca2+ entering the cell and the extruding mechanisms are balanced, the [Ca2+]i does not further increase (plateau phase) but the cytosolic concentration is higher than under control conditions. An elevated [Ca2+]i activates the calcium-dependent calpain (elevated after 1 h), a key protein for triggering the apoptotic pathway. When Ca2+ enter through the IP3-dependent calcium channels is blocked by the IP3 receptor antagonist 2-APB, the [Ca2+]i does not increase nor are the apoptotic pathways triggered. [Ca2+]i, intracellular calcium; IP3 receptor, inositol-1,4,5-trisphosphate receptor.
Platinum complexes are probably highly specific as cisplatin but not carboplatin was able to increase [Ca2+]i in the same HeLa-S3 cell line. This finding is compatible with earlier work (Koivusalo et al., 2002; Carland et al., 2005) showing that, in cervix carcinoma cells, different platinum compounds have different cytotoxic potency: cisplatin>oxaliplatin>carboplatin. Differences were also described in comparison to transplatin (Dalla Via et al., 1998), but only limited work was carried out in tumour cell models. Different effects on [Ca2+]i in two human lung adenocarcinoma cell lines (A549, cisplatin sensitive and A549/DDP, cisplatin resistant) were documented by Liang and Huang (2000). These authors found that in the cisplatin-resistant cell line, [Ca2+]i increased less and faster than in the sensitive cell line.
In our experiments, the increase in [Ca2+]i depended on the presence of extracellular Ca2+, while the intracellular Ca2+ stores were filled when cisplatin was applied. These observations differ from those of Kawai et al. (2006). These authors found that neither the extracellular calcium chelator EGTA nor the calcium channel antagonist nicardipine inhibited the rise of [Ca2+]i, and therefore concluded that cisplatin depleted the calcium stores. However, the cisplatin concentrations these authors used are not physiological (up to 750 μM) and therefore an additional depletion of the intracellular stores cannot be excluded. Furthermore, Tomaszewski and Büsselberg (2007) demonstrated that currents through voltage-activated calcium channels are reduced by cisplatin, but the effective concentration was higher (IC50 of peak current: 23.9±4.5 μM) than in the experiments presented in this study.
While the increased [Ca2+]i depended on the presence of extracellular Ca2+, a block of IP3 receptors with 2-APB abolished this effect in our experiments. A connection between [Ca2+]i rise and IP3 receptors (type 1) has been demonstrated in cisplatin-resistant bladder cancer cells, where this IP3 receptor was markedly reduced, as was [Ca2+]i, compared to a parental nontumour cell line (Tsunoda et al., 2005). A lower expression of IP3 receptors using small-interfering RNA, in parental cells, prevented apoptosis and resulted in decreased sensitivity to cisplatin. However, overexpression of IP3 receptors in cisplatin-resistant cells induced apoptosis and increased sensitivity to cisplatin. These results suggest that cisplatin-induced downregulation of IP3 receptor expression is closely associated with the acquisition of cisplatin resistance in bladder cancer cells (Tsunoda et al., 2005).
Unfortunately, these results neither indicate the location of the IP3 receptors (at the cellular membrane or at the calcium stores) nor their site of action (IP3 receptors located at the calcium stores might influence the opening of membrane channels by an intracellular pathway). To distinguish a possible effect of cisplatin on Ca2+ release from the stores, from extracellular Ca2+ entry, calcium stores were depleted by caffeine (Thayer et al., 1988) before cisplatin was given additionally. The identical increase in [Ca2+]i after the application of cisplatin, compared to the experiments where the calcium stores were not depleted before, is another indication that cisplatin-induced elevation of [Ca2+]i did not depend on an IP3 receptor-mediated Ca2+ release from the stores. In addition, the significant increase in Ca2+ in the stores as shown with the store-specific dye rhod-2 provides strong evidence that the majority of the calcium stores were not depleted. Furthermore, our immunostaining experiments for different types of IP3 receptors showed a clear staining at the cellular membrane of HeLa-S3 but not in U2-OS cells, an indication that this receptor complex could directly modify calcium entry from the extracellular space.
Our results also indicate an increase in membrane conductance after the application of cisplatin but only when Ca2+ is present in the extracellular solution, another hint of Ca2+ entry. It is most likely that the Ca2+ entering the cell secondarily activates other membrane ion channels such as a calcium-dependent potassium channel (KCa) (North and Tokimasa, 1987). This conclusion is underlined by the fact that a block of the large conductance KCa channel by charybdotoxin did not change the membrane conductance even when CDDP was applied for more than 1 h. Since a remaining ‘leak' current and the KCa-channel current might be relatively large compared to the expected Ca2+ entry, no change of the reversal potential was observed. Our findings are also in agreement with inside-out patch experiments of Negulyaev et al. (1993). They describe calcium permeable channels in HeLa cells, whose activities were increased when they applied IP3 (5–10 μM) to the inner membrane surface. While they did not vary the extracellular calcium concentration, the cytosolic calcium concentration (0.01 and 10 μM) did not influence the channel conductance.
Our findings also show that [Ca2+]i rises because Ca2+ is entering through the IP3-receptor channel complex, since we were able to show the IP3 receptor is located at the cell membrane of HeLa-S3 cells. This conclusion is fully confirmed by Dellis et al. (2006) in DT40 chicken cells. They also demonstrated a fraction of IP3 receptors at the cellular membrane and a calcium entry after activation of these receptors (Dellis et al., 2006).
We suggest that the cisplatin-induced elevation of [Ca2+]i is required for activation of calpain and the induction of apoptosis. Schloffer et al. (2003) showed in HeLa cells that a 3 h treatment with cisplatin triggers cell death by apoptosis (with chromatin fragmentation, strong cytoplasmic accumulation of cytochrome C and an activation of caspase-9). This result was confirmed by Boehning et al. (2003) who in addition found the binding of cytochrome c to the IP3 receptor amplified a calcium-dependent apoptosis. Here we show that a concentration of 1 μM cisplatin induces time-dependent cell death with clear nuclear morphology of apoptosis.
Several authors have considered that DNA damage is the main cause of the anti-proliferative effect of cisplatin (Mandic et al., 2003; Jeyapalan et al., 2005). These authors also discussed different apoptotic, cisplatin-triggered, pathways in inner ear hair cells: (1) reactive oxygen species and free radicals; (2) activation of caspases; and (3) activation of calpain. In our study, we have shown that cisplatin is able to elevate activated calpain, a protease with involvement in apoptosis. Mandic et al. (2002) have shown in human melanoma cells that cisplatin-induced calpain activation occurs early in apoptosis with the maximum activity of CDDP-induced calpain activity between 3 and 5 h. The same group also emphasises that calpain-mediated Bid cleavage is important in drug-induced apoptosis (Mandic et al., 2003). They have also demonstrated that calpain activation may be associated with endoplasmic reticulum (ER) stress, suggesting that the ER is a cytosolic target of cisplatin.
To summarise, in this study we found a cisplatin-induced rise in [Ca2+]i in HeLa-S3 cells that depends on both extracellular calcium and IP3 receptors. This [Ca2+]i increase occurred within 30 min and triggered the activation of calpain (after 1 h), but not of caspase-8. Therefore, an IP3 receptor-dependent calcium influx triggered by CDDP results in the activation of calpain and possibly ends with programmed cell death. This is at least one possible signal pathway required for cisplatin-induced apoptosis.
Our results suggest that the efficiency of cisplatin treatment could be enhanced if [Ca2+]i could be increased pharmacologically, possibly by an independent activation of IP3 receptors.
Acknowledgments
We thank Dr W Michael King for critical reading and valuable comments, Dr Stilla Freede for support in performing and analysing western blots and Dr Sinclair Cleveland for fitting the concentration dependence of CDDP to the [Ca2+]i increase.
Abbreviations
- ATCC
American-type culture collection
- 2-APB
2-aminoethoxydiphenyl borate
- [Ca2+]i
intracellular calcium
- CTX
charybdotoxin
- ER
endoplasmic reticulum
- IP3 receptor
inositol-1,4,5-trisphosphate receptor
- KCa channels
calcium-activated potassium channels
- PBS
phosphate-buffered saline
- SDS
sodium dodecyl sulphate
- TBS-T
Tris-buffered saline Tween-20
- TRAIL
TNF-related apoptosis-inducing ligand
Conflict of interest
The authors state no conflict of interest.
References
- Alznauer F, Conus S, Cavalli A, Folkers G, Simon HU. Calpain-1 regulates Bax and subsequent Smac-dependent caspase-3 activation in neutrophil apoptosis. J Biol Chem. 2004;279:5947–5957. doi: 10.1074/jbc.M308576200. [DOI] [PubMed] [Google Scholar]
- Arany I, Megyesi JK, Kaneto H, Price PM, Safirstein RL. Cisplatin-induced cell death is EGFR/src/ERK signaling dependent in mouse proximal tubule cells. Am J Physiol Renal Physiol. 2004;287:543–549. doi: 10.1152/ajprenal.00112.2004. [DOI] [PubMed] [Google Scholar]
- Boehning D, Patterson RL, Sedaghat L, Glebova NO, Kurosaki T, Snyder SH. Cytochrome c binds to inositol(1,4,5)trisphosphate receptors, amplifying calcium-dependent apoptosis. Nat Cell Biol. 2003;5:1051–1061. doi: 10.1038/ncb1063. [DOI] [PubMed] [Google Scholar]
- Boulikas T, Vougiouka M. Recent clinical trials using cisplatin, carboplatin and their combination chemotherapy drugs. Oncol Rep. 2004;11:559–595. [PubMed] [Google Scholar]
- Carland M, Tan KJ, White JM, Stephenson J, Murray V, Denny WA, et al. Syntheses, crystal structure and cytotoxicity of diamine platinum(II) complexes containing maltol. J Inorg Biochem. 2005;99:1738–1743. doi: 10.1016/j.jinorgbio.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Dalla Via L, Di Noto V, Vidali M, Scomazzon F, Ni D, Deana R. Action of antitumoral platinum complexes on in vitro platelet functions. Chem Biol Interact. 1998;110:203–220. doi: 10.1016/s0009-2797(98)00014-3. [DOI] [PubMed] [Google Scholar]
- Dehne N, Lautermann J, Petrat F, Rauen U, de Groot H. Cisplatin ototoxicity: involvement of iron and enhanced formation of superoxide anion radicals. Toxicol Appl Pharmacol. 2002;174:27–34. doi: 10.1006/taap.2001.9171. [DOI] [PubMed] [Google Scholar]
- Dellis O, Dedos SG, Tovey SC, Taufig-Ur-Rahman M, Dubel SJ, Taylor CW. Ca2+ entry through plasma membrane IP3 receptors. Science. 2006;14:229–233. doi: 10.1126/science.1125203. [DOI] [PubMed] [Google Scholar]
- Fan QL, Zou WY, Song LH, Wie W. Synergistic antitumor activity of TRAIL combined with chemotherapeutic agents in A549 cell lines in vitro and in vivo. Cancer Chemother Pharmacol. 2005;55:189–196. doi: 10.1007/s00280-004-0867-1. [DOI] [PubMed] [Google Scholar]
- Florea AM, Büsselberg D. Toxic effects of metals: modulation of intracellular calcium homeostasis. Mat Wiss u Werkstofftech. 2005;12:757–760. [Google Scholar]
- Florea AM, Büsselberg D. Occurence, use and potential toxic effects of metals and metal compounds. Biometals. 2006;19:419–427. doi: 10.1007/s10534-005-4451-x. [DOI] [PubMed] [Google Scholar]
- Florea AM, Dopp E, Büsselberg D. Elevated Ca2+i transients induced by trimethyltin chloride in HeLa cells: types and levels of response. Cell Calcium. 2005a;37:251–258. doi: 10.1016/j.ceca.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Florea AM, Splettstoesser F, Büsselberg D. Arsenic trioxide (As2O3) induced calcium signals and cytotoxicity in two human cell lines: SY-5Y neuroblastoma and 293 embryonic kidney (HEK) Toxicol Appl Pharmacol. 2007;220:292–301. doi: 10.1016/j.taap.2007.01.022. [DOI] [PubMed] [Google Scholar]
- Florea AM, Splettstoesser F, Dopp E, Rettenmeier AW, Büsselberg D. Modulation of intracellular calcium homeostasis by trimethyltin chloride in human tumour cells: neuroblastoma SY5Y and cervix adenocarcinoma HeLa-S3. Toxicology. 2005b;216:1–8. doi: 10.1016/j.tox.2005.05.029. [DOI] [PubMed] [Google Scholar]
- Goren MP. Cisplatin nephrotoxicity affects magnesium and calcium metabolism. Med Pediatr Oncol. 2003;41:186–189. doi: 10.1002/mpo.10335. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;25:3440–3450. [PubMed] [Google Scholar]
- Jaffe LF. A calcium-based theory of carcinogenesis. Adv Cancer Res. 2005;94:231–263. doi: 10.1016/S0065-230X(05)94006-2. [DOI] [PubMed] [Google Scholar]
- Jeyapalan JC, Saretzki G, Leake A, Tilby MJ, von Zglinicki T. Tumour-cell apoptosis after cisplatin treatment is not telomere dependent. Int J Cancer. 2005;118:2727–2734. doi: 10.1002/ijc.21675. [DOI] [PubMed] [Google Scholar]
- Kawai Y, Nakao T, Kunimura N, Kohda Y, Gemba M. Relationship of intracellular calcium and oxygen radicals to cisplatin-related renal cell inhury. J Pharmacol Sci. 2006;100:65–72. doi: 10.1254/jphs.fp0050661. [DOI] [PubMed] [Google Scholar]
- Koivusalo R, Krausz E, Ruotsalainen P, Helenius H, Hietanen S. Chemoradiation of cervical cancer cells: targeting human papillomavirus E6 and p53 leads to either augmented or attenuated apoptosis depending on the platinum carrier ligand. Cancer Res. 2002;62:7364–7371. [PubMed] [Google Scholar]
- Liang X, Huang Y. Intracellular free calcium concentration and cisplatin resistance in human lung adenocarcinoma A549 cells. Biosci Rep. 2000;20:129–138. doi: 10.1023/a:1005530501137. [DOI] [PubMed] [Google Scholar]
- Mandic A, Hansson J, Linder S, Shoshan MC. Cisplatin induces endoplasmic reticulum stress and nucleus-independent apoptotic signalling. J Biol Chem. 2003;278:9100–9106. doi: 10.1074/jbc.M210284200. [DOI] [PubMed] [Google Scholar]
- Mandic A, Viktorson K, Strandberg L, Heiden T, Hansson J, Linder S, et al. Calpain-mediated Bid cleavage and calpain-independent Bak modulation: two separate pathways in cisplatin-induced apoptosis. Mol Cell Biol. 2002;22:3003–3013. doi: 10.1128/MCB.22.9.3003-3013.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzano C, Bettio F, Baccichetti F, Trevisan A, Giovagnini L, Fregona D. Antitumor activity of a new platinum(II) complex with low nephrotoxicity and genotoxicity. Chem Biol Interact. 2004;148:37–48. doi: 10.1016/j.cbi.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Mignen O, Brink C, Enfissi A, Nadkarni A, Shuttleworth TJ, Giovannucci DR, et al. Carboxyamidotriazole-induced inhibition of mitochondrial calcium import blocks capacitative calcium entry and cell proliferation in HEK-293 cells. J Cell Sci. 2005;118:5615–5623. doi: 10.1242/jcs.02663. [DOI] [PubMed] [Google Scholar]
- Negulyaev YuA, Savokhina GA, Vedernikova EA. Calcium-permeable channels in HeLa cells. Gen Physiol Biophys. 1993;12:19–25. [PubMed] [Google Scholar]
- Noda K, Ohashi Y, Sugimori H, Ozaki M, Niibe H, Ogita S, et al. Phase III double-blind randomized trial of radiation therapy for stage IIIb cervical cancer in combination with low- or high-dose Z-100: treatment with immunomodulator, more is not better. Gynecol Oncol. 2006;101:455–463. doi: 10.1016/j.ygyno.2005.11.006. [DOI] [PubMed] [Google Scholar]
- North RA, Tokimasa T. Persistent calcium-sensitive potassium current and the resting properties of guinea-pig myenteric neurones. J Physiol. 1987;386:333–353. doi: 10.1113/jphysiol.1987.sp016537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orrenius S, Zhivotovsky B, Nicotera P. Regulation of cell death: the calcium-apoptosis link. Nat Rev Mol Cell Biol. 2003;4:552–565. doi: 10.1038/nrm1150. [DOI] [PubMed] [Google Scholar]
- Pai K, Sodhi A. Studies on the intracellular Ca2+, protein kinase activity, and ATP contents of cisplatin- and rIFN-Y-treated non-adherent mononuclear cells. Biochem Int. 1992;26:1017–1024. [PubMed] [Google Scholar]
- Schloffer D, Horky M, Kotala V, Wesierska-Gadek J. Induction of cell cycle arrest and apoptosis in human cervix carcinoma cells during therapy by cisplatin. Cancer Detect Prev. 2003;27:481–493. doi: 10.1016/j.cdp.2003.07.002. [DOI] [PubMed] [Google Scholar]
- Sharma AK, Rohrer B. Calcium-induced calpain mediates apoptosis via caspase-3 in a mouse photoreceptor cell line. J Biol Chem. 2004;279:35564–35572. doi: 10.1074/jbc.M401037200. [DOI] [PubMed] [Google Scholar]
- Splettstoesser F, Bonnet U, Wiemann M, Bingmann D, Büsselberg D. Modulation of voltage-gated channel currents by harmaline and harmane. Br J Pharmacol. 2005;144:52–58. doi: 10.1038/sj.bjp.0706024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer SA, Hirning LD, Miller RJ. The role of caffeine-sensitive calcium stores in the regulation of the intracellular free calcium concentration in rat sympathetic neurons in vitro. Mol Pharmacol. 1988;34:664–673. [PubMed] [Google Scholar]
- Tomaszewski A, Büsselberg D. Cisplatin modulates voltage gated channel currents of dorsal root ganglion neurons of rats. Neurotoxicology. 2007;28:49–58. doi: 10.1016/j.neuro.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Tsunoda T, Koga H, Yokomizo A, Tatsugami K, Eto M, Inokuchi J, et al. Inositol 1,4,5-trisphosphate (IP3) receptor type1 (IP3R1) modulates the acquisition of cisplatin resistance in bladder cancer cell lines. Oncogene. 2005;24:1396–1402. doi: 10.1038/sj.onc.1208313. [DOI] [PubMed] [Google Scholar]