Abstract
Background and purpose:
Stonefish (Synanceia genus) are commonly found in shallow waters of the Pacific and Indian Oceans. The venom of stonefish is stored in the dorsal fine spines and contains a proteinaceous toxin, verrucotoxin (VTX). The stings produced by the spines induce intense pain, respiratory weakness, damage to the cardiovascular system, convulsions and paralysis, sometimes leading to death. Although there are many studies on VTX, the mechanism(s) underlying the VTX-mediated cardiotoxicity is not yet fully understood. The aim of this study was to investigate the modulation of ion channels in cardiac tissue by VTX.
Experimental approach:
The effects of VTX on changes in the voltage or current in guinea-pig ventricular myocytes were investigated using a patch clamp method.
Key results:
VTX (10 μg ml−1) prolonged the action potential duration by 2.5-fold. VTX increased L-type Ca2+ currents (I Ca(L)) in a concentration-dependent manner with a EC50 value of 7 μg ml−1 and a maximum increase of 3.1-fold. The non-selective β-adrenoceptor antagonist, propranolol (1 μM) and the selective β1-adrenoceptor antagonist, CGP20712A (10 μM) each abolished the effect of VTX (100 μg ml−1) on I Ca(L). Furthermore, the protein kinase A (PKA) antagonists H-89 (10 μM) and Rp-8-Br-cAMPS (30 μM) inhibited the effect of VTX on I Ca(L).
Conclusions and implications:
VTX modulates Ca2+ channel activity through the β-adrenoceptor-cAMP-PKA pathway.
Keywords: verrucotoxin, calcium channel, β-adrenoceptor, heart
Introduction
The stonefish Synanceia verrucosa belongs to the genus Synanceia, which is commonly found in shallow waters of the Pacific and Indian Oceans. The venom of the stonefish is a protein stored in the dorsal fine spines. The stings produced by the spines induce intense pain, respiratory arrest, damage to the cardiovascular system, convulsions and skeletal muscle paralysis, sometimes leading to death (Saunders, 1959; Breton et al., 2002; Khoo, 2002). Verrucotoxin (VTX), a tetrameric glycoprotein with a molecular weight of 322 kDa (Garnier et al., 1995), and a dimeric 166-kDa protein (Ueda et al., 2006) have been isolated from the venom.
Some previous studies have investigated the mechanism of action of the venom or VTX on receptors and ion channels (Sauviat et al., 1995; Garnier et al., 1997). At concentrations below 3 μg ml−1, the venom increased the peak contraction of frog atrial fibres and this effect was suppressed by the β-adrenoceptor antagonist propranolol (Sauviat et al., 1995), suggesting that VTX activates β-adrenoceptors. β-Adrenoceptor stimulation is known to activate protein kinase A (PKA) and phosphorylate Ca2+ channels, thereby increasing the intracellular Ca2+ concentration. This increased intracellular Ca2+ concentration may provide one explanation for the cardiac effects of VTX, as high concentrations of Ca2+ can lead to arrhythmia or activate Ca2+-activated proteases. Sauviat et al. (1995) also reported that crude stonefish venom induces negative inotropic and chronotropic actions at concentrations above 5 μg ml−1. The mechanism underlying the biphasic actions of the venom has not been fully elucidated. It has also been reported that VTX may activate ATP-sensitive potassium channels (Garnier et al., 1997). In addition, Breton et al. (2002) reported that VTX produces neurological disorders, including paralysis, seizures and convulsions, that are not blocked by propranolol. Thus, the cellular and molecular mechanisms of VTX toxicity, especially its cardiotoxicity towards the mammalian heart, have not yet been fully investigated. The aim of this study was to elucidate the mechanism of the VTX-mediated modulation of ion channels in guinea-pig ventricular myocytes using a patch–clamp method. The results show that VTX modulates Ca2+ channel activity through the β-adrenoceptor–cAMP–PKA pathway.
Methods
Animals and cell preparation
This study was conducted in accordance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH publication no. 85–23, revised 1996). In addition, the Committee on Animal Experimentation, Kagoshima University, Japan, granted permission for the study.
Single ventricular cells were obtained from adult guinea-pig hearts using an enzymatic dissociation method (Yazawa etal., 1990). Briefly, the guinea-pigs were anaesthetized with sodium pentobarbital (50 mg kg−1, intraperitoneally) and the hearts were removed. Each heart was then mounted on a Langendorff apparatus and perfused with nominally Ca2+-free Tyrode solution containing collagenase (0.08%; Yakult, Japan) at 37°C. After 10 min of collagenase treatment, the enzyme solution was washed with a storage solution (see below). The ventricle was then cut into pieces, and the dispersed cells were maintained in the storage solution at 4°C until use.
Solutions
The compositions of the external solutions were as follows: the control Tyrode solution contained (in mM): NaCl, 135; KCl, 5.4; CaCl2, 1.8; MgCl2, 1.0; NaH2PO4, 0.33; HEPES, 10.0; glucose, 5.5; adjusted to pH 7.4 with NaOH. The Ca2+-free Tyrode solution was made by omitting CaCl2 from the standard Tyrode solution.
The storage solution contained (in mM): KOH, 70; KCl, 40; L-glutamic acid, 50; taurine, 20; KH2PO4, 20; MgCl2, 3; glucose, 10; HEPES, 10; EGTA, 0.5; adjusted to pH 7.2 with KOH.
The pipette solution for measurement of action potentials and currents contained (in mM): K-aspartate, 110; KCl, 20; MgCl2, 5; K2ATP, 5; K2CrP, 5; HEPES, 5; EGTA, 10; CaCl2, 1.43 (pCa 8); adjusted to pH 7.2 with KOH.
A stock solution of 8-bromoadenosine 3′,5′-cyclic monophosphorothioate, Rp-isomer (Rp-8-Br-cAMPS), was prepared in distilled water, stored at −20°C and added to the pipette solution at appropriate concentrations before use. A stock solution of propranolol was prepared in distilled water, stored at 4°C and dissolved in Tyrode solution before use. Stock solutions of H-89, nifedipine and [2-(3-carbamoyl-4-hydroxyphenoxy)-ethylamino]-3-[4-(1-methyl-4-trifluormethyl-2-imidazolyl)-phenoxy]-2-propanol methanesulphonate (CGP20712A) were prepared in dimethyl sulphoxide (DMSO) and diluted 1000-fold in the perfusion solution (final concentration of DMSO: 0.1%). These drugs were purchased from Sigma-Aldrich (St Louis, MO, USA).
Electrophysiological experiments
The whole-cell clamp technique used was essentially the same as that described by Hamill et al. (1981). A tight seal was established on a cell superfused with Tyrode solution, and the perfusate was then changed to the test solution. The resistance of the pipette filled with the internal solution was 2–4 MΩ. The voltage drop across the pipette resistance was electrically compensated. Data were recorded with a patch–clamp amplifier (CEZ-2300; Nihon Koden, Japan) filtered at 1 kHz, and fed to a computer at a sampling rate of 1 kHz. All experiments were performed at 20–22°C.
Separation of crude venom
Specimens of Synanceia verrucosa (2.5–3.2 kg) originating from Okinawa Island, Japan, were maintained alive in a fish tank in our laboratory. The stonefish were anaesthetized with 0.04% phenoxyethanol in seawater and then decapitated to dissect the dorsal spines at their bases. The integument sheath and tissue residing in the grooves of the spines were removed and extracted with 150 mM NaCl. After centrifugation at 3000 g for 20 min, the resulting supernatant was immediately lyophilized and stored at −80°C until use. The yield of crude venom was 18–24 mg kg−1 fish. The venom was diluted in Tris (25 mM)-glycine (192 mM) buffer, pH 8.4. The purity of VTX was evaluated by SDS-PAGE and found to be 40–50%. The freeze-dried powder was dissolved in Tyrode solution on the day of the experiments.
Statistical analysis
The results are expressed as means±s.e.m. Statistical significance was determined by Student's t-test for paired data. P-values of less than 0.05 were considered significant.
Results
Effect of VTX on action potentials
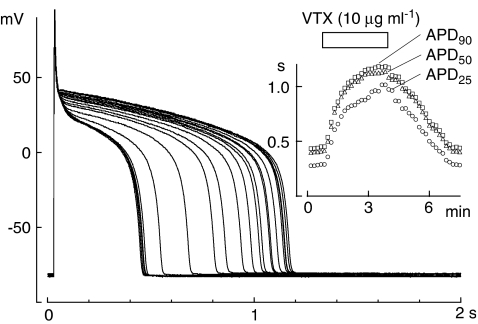
We evaluated the effect of VTX on the action potentials of guinea-pig ventricular cells. In the experiment shown in Figure 1, the action potentials were recorded under the whole-cell patch–clamp condition. VTX (10 μg ml−1) prolonged the action potential duration (APD) in a reversible manner (Figure 1, inset). The APD25, APD50 and APD90 values, representing the duration for 25, 50 and 90% repolarization, respectively, increased from 204±57 to 676±152 ms, from 305±86 to 833±187 ms and from 347±77 to 867±194 ms (P<0.05), respectively (n=4). The resting membrane potential (RMP) was not changed (from −84.0±0.8 to −82.0±0.6 mV; P<0.1, n=4). On the other hand, VTX (10 μg ml−1) had no effect on the action potential amplitude (125.0±4.5 vs 121.6±4.3 mV, n=4).
Figure 1.
Verrucotoxin (VTX)-mediated prolongation of the action potential duration (APD) in guinea-pig ventricular cells. Action potentials were elicited every 10 s using the whole-cell patch–clamp method. Superimposed traces were obtained before and during the application of 10 μg ml−1 VTX, and after wash-out of the drug. Inset: Time courses of the changes in APD25, APD50 and APD90. The time period of VTX application is shown above the graph. The ordinate in the inset figure represents the action potential duration (s).
Effect of VTX on ICa(L)
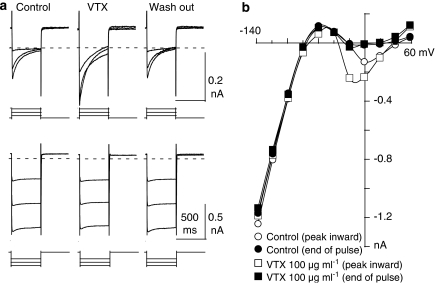
The effect of VTX in prolonging the APD suggested the possibility that VTX-enhanced L-type Ca2+ currents (ICa(L)) and/or reduced the delayed outward K+ currents underlying the action potential. Therefore, we examined the effects of VTX on ICa(L) and other currents. The pCa in the pipette was fixed at 8, since ICa(L) was reported to be modulated by the intracellular calcium concentration (Wu et al., 2001; Lindegger and Niggli, 2005). In the experiment shown in Figure 2a, superimposed currents were obtained by applying 500-ms hyperpolarizing or depolarizing pulses from −40 mV, for blocking sodium channel activity, to −20, 0 and 20 mV for the upper panel and −140, −120 and −100 mV for the lower panel at a rate of 10 s in the absence (left row), presence (middle row) or wash out (right row) of 100 μg ml−1 VTX. The current to voltage (I–V) relationships in the control sample and in the presence of 100 μg ml−1 VTX are illustrated in Figure 2b. The increased inward currents were blocked by 3 μM nifedipine, showing that the currents were ICa(L) (data not shown). VTX increased ICa(L) and shifted the negative slope region of ICa(L) in the hyperpolarizing direction. These effects of VTX were reversible and reproducible. Although VTX increased the delayed outward K+ currents at positive potentials (>40 mV), these potentials were scarcely involved in the main plateau phase for APD prolongation (Figure 1). VTX also slightly depolarized the RMP (Figure 1). To investigate the effect of VTX on the inward rectifier K+ currents (IK1) that contributed to the RMP, 100 μg ml−1 VTX, a sub-maximum concentration for ICa(L) enhancement, was applied. VTX decreased IK1 by 18.8±10.2% (P=0.06, n=4) at −40 mV, a potential close to the equilibrium potential of Cl−. This result indicates that no statistical difference in the currents was observed at that concentration. As a consequence, the subsequent experiments were mainly confined to studying the effects of VTX on ICa(L).
Figure 2.
Effects of verrucotoxin (VTX) on Ca2+ and K+ currents. (a) Test pulses of 500 ms duration were applied every 10 s from −40 to −20 mV, 0 and 20 mV for the upper panel, and −140, −120 and −100 mV for the lower panel in the absence (left row), presence (middle row) or wash out (right row) of 100 μg ml−1 VTX. The broken lines indicate the zero current level. X axis scale bar: 500 ms. (b) Current–voltage (I–V) relationships for the same cell shown in (a) in the control experiment and in the presence of 100 μg ml−1 VTX. Open and filled symbols represent the peak inward current and current level at 500 ms after the onset of the clamp pulse, respectively.
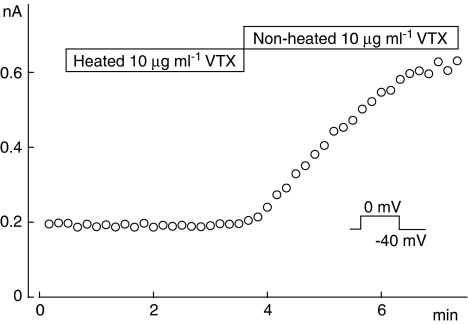
To confirm that the effects observed above were produced by a venom protein, we examined the effect of VTX after heating at 70°C for 3 min, a condition known to denature proteins, on ICa(L). As shown in Figure 3, heated VTX failed to modulate ICa(L), whereas ICa(L) was increased by non-heated VTX. On the other hand, noradrenaline (1 μM) heated under the same conditions increased ICa(L). These results were further confirmed in four other cells and strongly support the assumption that the increase in ICa(L) was produced by the venom protein VTX.
Figure 3.
Effects of heated and non-heated verrucotoxin (VTX) on L-type Ca2+ currents (ICa(L)). ICa(L) was elicited by 500 ms depolarizing pulses from −40 to 0 mV every 10 s. The current amplitude was measured as the difference between the peak of ICa(L) and the current at the end of the pulse, and plotted against the experimental time.
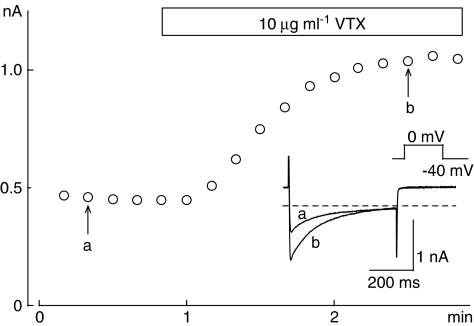
Since the enhancement of ICa(L) by VTX was notable in Figures 2 and 3, we examined the onset of the increase and inactivation time course of ICa(L) during the application of VTX. In the experiment shown in Figure 4, ICa(L) was recorded at 0 mV from a holding potential of −40 mV, with a duration of 500 ms. ICa(L) started to increase within 30 s after VTX application without changing the time course of inactivation (Figure 4, inset), consistent with the effect produced by β-adrenergic stimulation.
Figure 4.
Time course of the changes in L-type Ca2+ currents (ICa(L)) during the application of 10 μg ml−1 verrucotoxin (VTX). ICa(L) was elicited by 500 ms depolarizing pulses from −40 to 0 mV every 10 s. The current amplitude was measured as the difference between the peak of ICa(L) and the current at the end of the pulse, and plotted against the experimental time. In the inset, original current traces recorded at the times indicated on the graph (a, b) are shown.
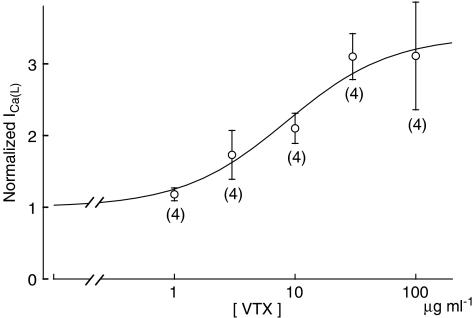
Next, we examined the concentration dependence of the effects of VTX. ICa(L) was measured upon depolarization from −40 to 0 mV at various concentrations of VTX and normalized to the values obtained in the absence of VTX. The concentration–response relationship thus obtained is shown in Figure 5. The continuous curve represents the best fit of the data using the Hill equation with a Hill coefficient of 1. The threshold concentration of VTX for increasing ICa(L) was close to 1 μg ml−1 and the maximum effect was obtained at approximately 100 μg ml−1. The normalized response of ICa(L) to 100 μg ml−1 VTX was 3.11±0.75 (n=4), and the half-maximum concentration (EC50) was 8.5 μg ml−1.
Figure 5.
Concentration-dependent effect of verrucotoxin (VTX) on L-type Ca2+ currents (ICa(L)). ICa(L) recordings were obtained by applying a 500 ms depolarizing pulse from −40 to 0 mV at various concentrations. The amplitude of ICa(L) in the presence of VTX was normalized to that of the control current. The data represent the mean values of four experiments, and the bars indicate the s.e.m. The continuous curve is the best fit of the data using the Hill equation with a Hill coefficient of 1. See text for further details.
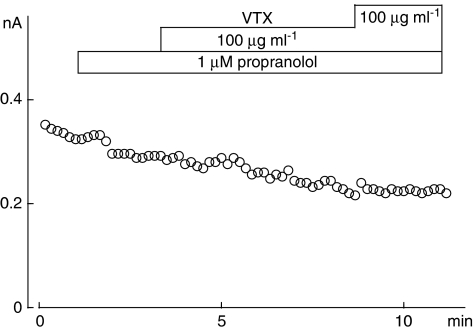
Crude stonefish venom has been reported to activate β-adrenoceptors in frog atrial fibres (Sauviat et al., 1995). To investigate whether the effect of VTX on ICa(L) is mediated by β-adrenoceptors, we examined the effect of propranolol, a β-adrenoceptor antagonist, on the enhancement of ICa(L) by VTX (Figure 6). Although ICa(L) tended to decline following application of propranolol (1 μM), no clear enhancement of ICa(L) by VTX (10 or 100 μg ml−1) was observed. β1-Adrenoceptors, but not β2-adrenoceptors, are present on guinea-pig ventricular myocytes. To clarify whether the effect of VTX on ICa(L) was mediated by β1-adrenoceptors, we examined whether the β1-adrenoceptor antagonist CGP20712A suppressed the effect of VTX on ICa(L). In the presence of CGP20712A (10 μM), VTX (10 μg ml−1) did not increase ICa(L) and the normalized current amplitude was 1.05±0.04 (n=5). These results suggest that the effect of VTX on ICa(L) was mediated by β1-adrenoceptor stimulation.
Figure 6.
Propranolol inhibited the effect of verrucotoxin (VTX) on L-type Ca2+ currents (ICa(L)). ICa(L) was elicited by 500 ms depolarizing pulses from −40 to 0 mV every 10 s. The protocol for the drug application is shown at the top.
The β1-adrenergic cascade has also been reported to involve cAMP-dependent PKA activation and phosphorylation of a substrate protein (Endoh, 2006). Therefore, we examined whether VTX was able to increase ICa(L) in the presence of H-89, a PKA inhibitor. The increased ICa(L) mediated by VTX (8 μg ml−1; approximately the EC50 value) was markedly suppressed by H-89 (10 μM). The normalized current amplitude was 1.08±0.06 (n=4). Since H-89 was reported to have a β-adrenoceptor antagonistic effect (Penn et al., 1999), we also examined the effect of Rp-8-Br-cAMPS, another PKA inhibitor without an adrenoceptor antagonistic effect. The increased ICa(L) mediated by VTX (8 μg ml−1) was markedly suppressed by Rp-8-Br-cAMPS (30 μM). The normalized current amplitude was 1.05±0.01 (n=4). These results suggest that the effect of VTX on ICa(L) was mediated by PKA stimulation via β1-adrenoceptors.
Discussion
The major findings of the present study are as follows: (1) VTX causes prolongation of the APD, with no change in the RMP, in guinea-pig ventricular myocytes; (2) VTX increases ICa(L) in a concentration-dependent manner; and (3) the effect of VTX on ICa(L) is prevented by β-adrenoceptor antagonists or PKA inhibitors. Taken together, these results suggest that VTX modulation of ion channels is mediated by the β-adrenoceptor–cAMP–PKA pathway.
Garnier et al. (1996) reported that stonefish venom contains noradrenaline, and it was therefore possible that residual noradrenaline in our venom may have modulated the ion channels in our myocytes. However, we were able to exclude this possibility because the effect of the venom on ICa(L) was inactivated by moderate heating at 70°C for 3 min, which is known to denature proteins, whereas noradrenaline heated under the same conditions actually increased ICa(L).
Stonefish venom is able to induce positive inotropic and chronotropic effects at concentrations below 3 μg ml−1 and has a negative inotropic effect and induces contractures at concentrations above 5 μg ml−1 (Sauviat et al., 1995). These results suggest that the venom has several characteristics for modulating cardiac functions (for example, receptor modulation or direct actions on channels). However, the ionic basis of these actions of the venom was not extensively investigated in previous studies (Sauviat et al., 1995; Garnier et al., 1996). In the current study, we examined the effects of VTX on ion channels under the whole-cell clamp condition in single myocytes. We found that ICa(L) is modulated by VTX through activation of β-adrenoceptors. There are several similarities between VTX and β-adrenoceptor agonists in their electrophysiological effects on cardiac ion channels: (1) VTX increased ICa(L) in a concentration-dependent manner up to threefold, similar to the findings for isoproterenol-induced increases in ICa (Kameyama et al., 1985) and IK (Yazawa and Kameyama, 1990); (2) the concentration–response relationship for VTX (Figure 5) showed a slope factor of ∼1, similar to that for isoproterenol vs ICa (Kameyama et al., 1985); and (3) the current–voltage relationship for ICa(L) was shifted by VTX in the negative direction (Figure 2), is similar to the finding for isoproterenol vs ICa(L) (Findlay, 2002). In addition, the effect of VTX on these currents was blocked by the β1-adrenoceptor antagonist CGP20712A and PKA inhibitors H-89 and Rp-8-Br-cAMPS. These results are consistent with the hypothesis that VTX acts as a β1-adrenoceptor agonist and modulates ICa(L) through activation of the cAMP–PKA pathway.
As VTX elicits contractures, it may have some additional actions, because extensive β-adrenergic stimulation does not generally induce contractures in cardiac muscle, due to the activation of SERCA2a. It has also been reported that VTX may activate the ATP-sensitive potassium channels in frog atrial muscle (Garnier et al., 1997). Although we did not observe such an effect, we cannot rule out this possibility, because the pipette solution was supplemented with ATP and the ATP-sensitive potassium channels were therefore continuously blocked. Thus, further studies are needed to elucidate the effects of VTX on other ion channels and transporters.
There are many toxins that act through receptor-mediated stimulation. For example, Crotalus durissus terrificus snake venom was reported to induce anti-nociception mediated by opioid receptors (Giorgi et al., 1993). More recently, this snake venom was shown to activate ATP-sensitive potassium channels by way of opioid receptors (Picolo et al., 2003). Thus, these studies suggest that some macromolecules derived from natural resources have the ability to stimulate G protein-coupled receptors.
VTX is a 322-kDa glycoprotein composed of two α subunits (83 kDa) and two β subunits (78 kDa). The cDNA for the VTX β-subunit has been inferred from the presence of an identical N-terminal sequence and 96% homology to a partial sequence (506 amino acids) of the stonustoxin β-subunit from Synanceia horrida, which also has cardiovascular effects (Ghadessy et al., 1994). Recently, neoVTX, a dimeric 166-kDa protein, was purified from Synanceia verrucosa and its character differs considerably from that of VTX. The sequence identity between the neoVTX β-subunit and the previously reported VTX β-subunit is 90% (Ueda et al., 2006). The effect of neoVTX on cardiac function has not yet been identified. From our present findings, we suggest that a macromolecule within stonefish venom, such as VTX or neoVTX, can act as a β1-adrenoceptor agonist.
In the present study, VTX was found to activate ICa(L) through β1-adrenoceptor activation. To the best of our knowledge, this is the first report to describe that VTX may act as a β-adrenoceptor agonist through the cAMP–PKA pathway. Unfortunately, the amino-acid sequences of the α-subunit and the binding region to β-adrenoceptors have not yet been clarified and future studies should address these interesting issues.
Acknowledgments
This study was supported by research grants from the Japan Society for the Promotion of Science (to M Kameyama), the Uehara Memorial Foundation, the Japan Heart Foundation (to K Yazawa) and the Kodama Memorial Foundation (to K Yazawa and J-W Wang).
Abbreviations
- APD
action potential duration
- CGP20712A
[2-(3-carbamoyl-4-hydroxyphenoxy)-ethylamino]-3-[4-(1-methyl-4-trifluormethyl-2-imidazolyl)-phenoxy]-2-propanol methanesulphonate
- DMSO
dimethyl sulphoxide
- H-89
N-[2-(p-bromocinnamylamino)ethyl]-5-isoquinolinesulphonamide
- RMP
resting membrane potential
- VTX
verrucotoxin
Conflict of interest
The authors state no conflict of interest.
References
- Breton P, Delamanche I, Buee J, Goudey-Perriere F, Perriere C. Evidence for a neurotoxic activity in crude venom of the stonefish (Synanceia verrucosa) J Nat Toxins. 2002;11:305–313. [PubMed] [Google Scholar]
- Endoh M. Signal transduction and Ca2+ signaling in intact myocardium. J Pharmacol Sci. 2006;100:525–537. doi: 10.1254/jphs.cpj06009x. [DOI] [PubMed] [Google Scholar]
- Findlay I. β-Adrenergic and muscarinic agonists modulate inactivation of L-type Ca2+ channel currents in guinea-pig ventricular myocytes. J Physiol. 2002;545:375–388. doi: 10.1113/jphysiol.2002.028605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnier P, Goudey-Perriere F, Breton P, Dewulf C, Petek F, Perriere C. Enzymatic properties of the stonefish (Synanceia verrucosa Bloch and Schneider, 1801) venom and purification of a lethal, hypotensive and cytolytic factor. Toxicon. 1995;33:143–155. doi: 10.1016/0041-0101(94)00151-w. [DOI] [PubMed] [Google Scholar]
- Garnier P, Grosclaude JM, Goudey-Perriere F, Gervat V, Gayral P, Jacquot C, et al. Presence of norepinephrine and other biogenic amines in stonefish venom. J Chromatogr B Biomed Appl. 1996;685:364–369. doi: 10.1016/s0378-4347(96)00203-4. [DOI] [PubMed] [Google Scholar]
- Garnier P, Sauviat MP, Goudey-Perriere F, Perriere C. Cardiotoxicity of verrucotoxin, a protein isolated from the venom of Synanceia verrucosa. Toxicon. 1997;35:47–55. doi: 10.1016/s0041-0101(96)00075-x. [DOI] [PubMed] [Google Scholar]
- Ghadessy FJ, Jeyaseelan K, Chung MC, Khoo HE, Yuen R. A genomic region encoding stonefish (Synanceja horrida) stonustoxin beta-subunit contains an intron. Toxicon. 1994;32:1684–1688. doi: 10.1016/0041-0101(94)90329-8. [DOI] [PubMed] [Google Scholar]
- Giorgi R, Bernardi MM, Cury Y. Analgesic effect evoked by low molecular weight substances extracted from Crotalus durissus terrificus venom. Toxicon. 1993;31:1257–1265. doi: 10.1016/0041-0101(93)90399-4. [DOI] [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Kameyama M, Hofmann F, Trautwein W. On the mechanism of β-adrenergic regulation of the Ca channel in the guinea-pig heart. Pflugers Arch. 1985;405:285–293. doi: 10.1007/BF00582573. [DOI] [PubMed] [Google Scholar]
- Khoo HE. Bioactive proteins from stonefish venom. Clin Exp Pharmacol Physiol. 2002;29:802–806. doi: 10.1046/j.1440-1681.2002.03727.x. [DOI] [PubMed] [Google Scholar]
- Lindegger N, Niggli E. Paradoxical SR Ca2+ release in guinea-pig cardiac myocytes after ß-adrenergic stimulation revealed by two-photon photolysis of caged Ca2+ J Physiol. 2005;565:801–813. doi: 10.1113/jphysiol.2005.084376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penn RB, Parent JL, Pronin AN, Panettieri Jr RA, Benovic JL. Pharmacological inhibition of protein kinases in intact cells: antagonism of beta adrenergic receptor ligand binding by H-89 reveals limitations of usefulness. J Pharmacol Exp Ther. 1999;288:428–437. [PubMed] [Google Scholar]
- Picolo G, Cassola AC, Cury Y. Activation of peripheral ATP-sensitive K+ channels mediates the antinociceptive effect of Crotalus durissus terrificus snake venom. Eur J Pharmacol. 2003;469:57–64. doi: 10.1016/s0014-2999(03)01676-5. [DOI] [PubMed] [Google Scholar]
- Saunders PR. Venom of the stonefish Synancejaverrucosa. Science. 1959;129:272–274. doi: 10.1126/science.129.3344.272-a. [DOI] [PubMed] [Google Scholar]
- Sauviat MP, Garnier P, Goudey-Perriere F, Perriere C. Does crude venom of the stonefish (Synanceia verrucosa) activate beta-adrenoceptors in the frog heart muscle. Toxicon. 1995;33:1207–1213. doi: 10.1016/0041-0101(95)00049-r. [DOI] [PubMed] [Google Scholar]
- Ueda A, Suzuki M, Honma T, Naga H, Nagashima Y, Shiomi K. Purification, properties and cDNA cloning of neoverrucotoxin (neoVTX), a hemolytic lethal factor from the stonefish Synanceia verrucosa venom. Biochim Biophys Acta. 2006;1760:1713–1722. doi: 10.1016/j.bbagen.2006.08.017. [DOI] [PubMed] [Google Scholar]
- Wu Y, Colbran RJ, Anderson ME. Calmodulin kinase is a molecular switch for cardiac excitation-contraction coupling. Proc Natl Acad Sci USA. 2001;98:2877–2881. doi: 10.1073/pnas.051449198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazawa K, Kaibara M, Ohara M, Kameyama M. An improved method for isolating cardiac myocytes useful for patch-clamp studies. Jpn J Physiol. 1990;40:157–163. doi: 10.2170/jjphysiol.40.157. [DOI] [PubMed] [Google Scholar]
- Yazawa K, Kameyama M. Mechanism of receptor-mediated modulation of the delayed outward potassium current in guinea-pig ventricular myocytes. J Physiol. 1990;421:135–150. doi: 10.1113/jphysiol.1990.sp017937. [DOI] [PMC free article] [PubMed] [Google Scholar]