Abstract
Background and purpose:
Previous studies demonstrated that nicotine-induced endothelium-independent vasodilation is mediated by perivascular adrenergic nerves and nerves releasing calcitonin gene-related peptide (CGRPergic nerves). We characterized the nicotinic acetylcholine (ACh) receptor subtype underlying the vasodilation in response to nicotine in rat mesenteric arteries.
Experimental approach:
Rat mesenteric vascular beds without endothelium were contracted by perfusion with Krebs solution containing methoxamine and the perfusion pressure was measured with a pressure transducer.
Key results:
Perfusion of nicotine (1–100 μM) for 1 min caused a concentration-dependent decrease in perfusion pressure due to vasodilation. Perfusion of (±)-epibatidine (1–100 nM) (non-selective agonist) or (−)-cytisine (1–100 μM) (partial agonist for nicotinic β2 subtype and full agonist for nicotinic β4 subtype) induced vasodilation in a concentration-dependent manner. Vasodilation induced by nicotine, (−)-cytisine- and (±)-epibatidine was markedly attenuated by guanethidine (5 μM) and pretreatment with capsaicin (1 μM). Mecamylamine (relatively selective antagonist for α3β4 subtype), but not dihydro-β-erythroidine (selective antagonist for α4β2 subtype) or α-bungarotoxin (selective antagonist for α7 subtype), markedly inhibited nicotine-induced vasodilation. Nicotine-induced vasodilation was inhibited by methyllycaconitine at high concentrations (>1 μM), which non-selectively antagonize nicotinic receptors, while a low concentration of 10 nM, which selectively antagonizes α7 subtype, had no effect. (−)-Cytisine and (±)-epibatidine-induced vasodilation were abolished by mecamylamine
Conclusion and implications:
These results suggest that the nicotinic α3β4 receptor subtype, but not the α7 and α4β2 subtypes, is responsible for the vasodilation in rat mesenteric arteries induced by nicotine- and nicotinic ACh receptor agonists through stimulation of adrenergic and CGRPergic perivascular nerves.
Keywords: nicotine, α3β4 nicotinic receptor subtype, vasodilation, calcitonin gene-related peptide-containing nerves, adrenergic nerves, rat mesenteric resistance artery
Introduction
Nicotinic acetylcholine (ACh) receptors in the nervous system have diverse subtypes, which comprise combinations of α (α2–α9) and β (β2–β4) subunits (Sargent, 1993; Lukas et al., 1999). Heteromeric nicotinic ACh subtype receptors are composed of two α-subunits and three β-subunits. On the other hand, homomeric nicotinic ACh subtype receptors are composed of only five α-subunits. Recent studies that identified the subtype of nicotinic ACh receptors involved in physiological and pharmacological responses showed that the α3β4 receptor subtype modulates the inhibitory synaptic activity in the substantia gelatinosa of the adult rat spinal cord (Takeda et al., 2003) and is involved in the development of diarrhoea and weight loss, during opioid withdrawal (Taraschenko et al., 2005). The α7 nicotinic ACh receptor subtype, which is sensitive to α-bungarotoxin and comprises five α7 subunits, is one of the predominant nicotinic ACh receptor subtypes in the brain and mediates calcium permeability, but rapidly desensitizes (Sargent, 1993). In the porcine basilar artery, the α7 receptor subtype is involved in nicotine-induced vasodilation (Si and Lee, 2001). The α4β2 receptor subtype has been reported to be related to nicotine dependency or its withdrawal symptoms (Suemaru et al., 2002). However, in contrast to muscle nicotinic ACh receptors, little is known about the functions of neuronal nicotinic receptors.
It is widely accepted that peripheral vascular tone is mainly maintained by perivascular adrenergic nerves from which noradrenaline, neuropeptide Y and adenosine triphosphate are released when these nerves are stimulated (Lundberg et al., 1982) and that the tone is regulated by neuronal factors and hormonal factors such as endothelium-derived relaxing and constricting factors (Furchgott and Zawadzki, 1980; Yanagisawa et al., 1988). However, it has been demonstrated that the mesenteric resistance arteries are innervated not only by adrenergic nerves but also by non-adrenergic non-cholinergic nerves (Bevan and Brayden, 1987; Kawasaki et al., 1988). In previous studies, we demonstrated that nicotine induces endothelium-independent vasodilation, which is abolished by cold-storage perivascular denervation, guanethidine (an adrenergic neuron blocker), hexamethonium (a non-selective nicotinic ACh receptor antagonist), calcitonin gene-related peptide 8–37 (a CGRP receptor antagonist) and capsaicin (a CGRP depleter) (Shiraki et al., 2000). Therefore, we suggested that nicotine acts on presynaptic nicotinic ACh receptors in adrenergic nerves to release adrenergic neurotransmitters or unknown related substance(s), which then activate CGRP-containing vasodilator nerves (CGRPergic nerves) resulting in CGRP release and vasodilation (Shiraki et al., 2000). However, little is known about what subtypes of nicotinic ACh receptors are involved in the nicotine-induced vasodilation.
In this study, therefore, we characterized the nicotinic ACh receptor subtype involved in nicotine-induced vasodilation in rat mesenteric arteries, using several agonists and antagonists for the nicotinic ACh receptor subtypes. In this report, we provide evidence that α3β4 nicotinic ACh receptors are responsible for the adrenergic and CGRPergic nerve-dependent vasodilation induced by nicotine in rat mesenteric resistance arteries.
Methods
Animals
Male Wistar rats weighing 250–350 g were used in the present study. All animals were given food and water ad libitum. They were housed in the Animal Research Center of Okayama University at a controlled ambient temperature of 22°C±2 with 50±10% relative humidity and a 12-h light/12-h dark cycle (lights on 0800 hours). This study was carried out in accordance with the Guidelines for Animal Experiments at Okayama University Advanced Science Research Center, Japanese Government Animal Protection and Management Law (No. 115) and Japanese Government Notification on Feeding and Safekeeping of Animals (No. 6). Every effort was made to minimize the number of animals used and their suffering. All experiments conformed to international guidelines on the ethical use of animals.
Perfusion of the mesenteric vascular beds
The animals were anesthetized with pentobarbital-Na (50 mg kg−1, intraperitoneally) and the mesenteric vascular beds were isolated and prepared for perfusion as described previously (Kawasaki and Takasaki, 1984; Kawasaki et al., 1988). The superior mesenteric artery was cannulated and flushed gently with Krebs–Ringer bicarbonate solution (Krebs solution) to eliminate blood in the vascular bed. After removal of the entire intestine and associated vascular bed, the mesenteric vascular bed was separated from the intestine by cutting close to the intestinal wall. Only the four main arterial branches from the superior mesenteric trunk running to the terminal ileum were perfused. All other branches of the superior mesenteric artery were tied off. The isolated mesenteric vascular bed was then placed in a water-jacketed organ bath maintained at 37°C and perfused with a modified (see below) Krebs solution at a constant flow rate of 5 ml min−1 with a peristaltic pump (model AC-2120, ATTO Co., Tokyo, Japan). The preparation was also superfused with the same solution at a rate of 0.5 ml min−1 to prevent drying. The Krebs solution was bubbled with a mixture of 95% O2–5% CO2 before passage through a warming coil maintained at 37°C. The modified Krebs solution had the following composition (mM): NaCl 119.0 KCl 4.7; CaCl2 2.4; MgSO4 1.2; NaHCO3 25.0; KH2PO4 1.2; disodium ethylenediaminetetraacetic acid 0.03 and dextrose 11.1 (pH 7.4). Changes in the perfusion pressure were measured with a pressure transducer (model TP-400 T, Nihon Kohden, Tokyo, Japan) and recorded using a pen recorder (model U-228, Nippon Denshi Kagaku, Tokyo, Japan).
Periarterial nerve stimulation
Periarterial nerve stimulation (PNS) was applied for 30 s using bipolar platinum ring electrodes placed around the superior mesenteric artery. Rectangular pulses of 1 ms and supramaximal voltage (50 V) were applied at 2 Hz using an electronic stimulator (model SEN 3301, Nihon Kohden).
Chemical removal of the vascular endothelium
All experiments in the present study were carried out in the preparations without vascular endothelium. After the basal perfusion pressure was allowed to stabilize, the preparations with resting tone were perfused with a 1.8 mg ml−1 solution of sodium deoxycholate in saline for 30 s to remove the vascular endothelium, as described by Takenaga et al. (1995) and Shiraki et al. (2000). Then, the preparations were rinsed with sodium deoxycholate-free Krebs solution for 60 min. After the preparations were contracted by perfusion with Krebs solution containing methoxamine (α1-adrenoceptor agonist, 2 μM), chemical removal of the endothelium was assessed by the lack of a relaxant effect after a bolus injection of 1 nmol ACh, which was injected directly into the perfusate proximal to the arterial cannula with an infusion pump (model 975, Harvard Apparatus, Holliston, MA, USA). The volume injected was 100 μl over 12 s.
Treatment with capsaicin
In vitro depletion of CGRPergic nerves was performed according to the method described by Takenaga et al. (1995) and Shiraki et al. (2000). After the endothelium is removed, the preparation was rinsed with sodium deoxycholate-free Krebs solution for 30 min. Then, the preparation was perfused with Krebs solution containing capsaicin (1 μM) for 20 min. After the perfusion pressure was elevated by perfusion of methoxamine, successful loss of CGRPergic nerve function was confirmed by the lack of a relaxant effect for PNS (2 Hz).
Perfusion of nicotine, (−)-cytisine and (±)-epibatidine
After stabilization of the elevated perfusion pressure, the Krebs solution containing methoxamine and the final concentration of nicotine (1–100 μM), (−)-cytisine (1–100 μM) (partial agonist for nicotinic β2 subtype and full agonist for nicotinic β4 subtype) or (±)-epibatidine (1–100 nM) (non-selective nicotinic ACh receptor agonist) was perfused for 1 min. To avoid tachyphylaxis, the perfusion of nicotine or nicotinic ACh receptor agonists was carried out at 20-min intervals.
In another series of experiments, the vascular responses of nicotine, (−)-cytisine or (±)-epibatidine were examined in the presence of guanethidine (5 μM) or in preparations treated with capsaicin (1 μM).
Perfusion of nicotinic ACh receptor agonists plus nicotinic ACh receptor antagonist
The denuded preparation was perfused with Krebs solution containing methoxamine and 1 or 10 μM mecamylamine (a relatively selective antagonist for α3β4 receptor subtype), 1 or 10 μM dihydro-β-erythroidine (DHβE; a relatively selective antagonist forα4β2 receptor subtype), 100 nM α-bungarotoxin (an antagonist for α7 receptor subtype) or methyllycaconitine at 10 nM (antagonizing α7 nicotinic receptor subtype), 1 μM or 10 μM (non-selectively antagonizing nicotinic ACh receptors). Then, Krebs solution containing the final concentration of nicotine and mecamylamine, DHβE, α-bungarotoxin or methyllycaconitine was perfused for 1 min.
In another series of experiments, the vascular responses to 1 min perfusion of (−)-cytisine or (±)-epibatidine were examined in the presence of mecamylamine (1 μM).
At the end of each experiment, the preparations were perfused with 100 μM papaverine to induce complete relaxation. Vasodilator activity is expressed as the percentage of perfusion pressure at the maximum relaxation induced by papaverine.
Statistical analysis
Experimental results are presented as mean±s.e.m. Statistical analysis was performed by the Student's unpaired t-test and one-way analysis of variance followed by Tukey's test. A P-value less than 0.05 was considered significant.
Drugs
The following drugs were used: ACh chloride (Daichi-Sankyo Pharmaceutical Co., Tokyo, Japan) and methoxamine hydrochloride (Nihon Shinyaku Co., Kyoto, Japan). α-Bungarotoxin, capsaicin, (−)-cytisine, DHβE, (±)-epibatidine, mecamylamine, guanethidine sulphate, methyllycaconitine, nicotine tartrate salt, papaverine hydrochloride and sodium deoxycholate were obtained from Sigma Chemical Co. (St Louis, MO, USA). All drugs, except for sodium deoxycholate and capsaicin, were dissolved in pure water and diluted with Krebs solution containing methoxamine. Sodium deoxycholate was dissolved in 0.9% saline and capsaicin was dissolved in 50% alcohol and diluted with Krebs solution.
Results
Vascular responses to perfusion of nicotine, (±)-epibatidine and (−)-cytisine
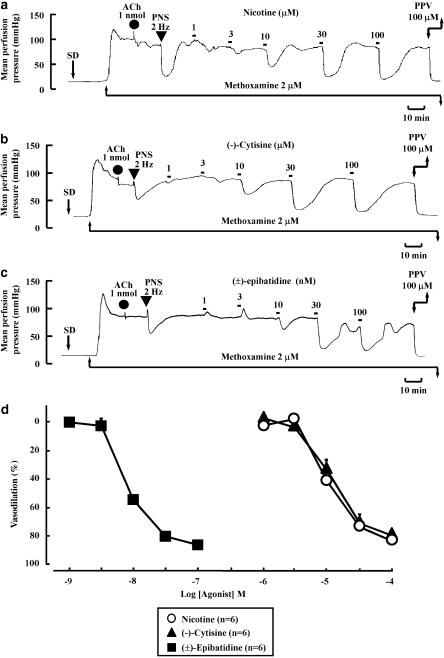
In the preparation without endothelium and with active tone produced by methoxamine (2 μM), a bolus injection of ACh (1 nmol) did not produce a rapid drop in perfusion pressure due to endothelium-dependent vasodilation (Figure 1a). PNS (2 Hz) caused a transient pressor response followed by long-lasting depressor response (Figure 1a). The initial vasoconstriction and subsequent long-lasting vasodilation induced by PNS have been shown to result from stimulation of vascular adrenergic nerves and CGRPergic nerves, respectively (Shiraki et al., 2000).
Figure 1.
Typical records showing vascular responses to nicotine (a), (−)-cytisine (b) and (±)-epibatidine (c) and concentration–response curves (d) in rat perfused mesenteric vascular beds without endothelium and with active tone produced by methoxamine. Nicotine (1–100 μM), (−)-cytisine (1–100 μM) or (±)-epibatidine (1–100 nM) was perfused for 1 min at the times indicated by the filled squares. SD, perfusion of sodium deoxycholate for 30 s. ACh, bolus injection of acetylcholine (1 nmol; closed circles). PNS, periarterial nerve stimulation (2 Hz; closed inverted triangles). PPV, perfusion of 100 μM papaverine. In (d), the concentration–response results for nicotine, (−)-cytisine and (±)-epibatidine as inducers of vasodilation are shown.
In this preparation with active tone, perfusion of nicotine (1–100 μM) for 1 min concentration-dependently caused a decrease in the perfusion pressure due to vasodilation (Figures 1a and d). The maximum vasodilation (Emax) at the highest concentration was 83.2±3.8% and the effective concentration that induced 50% vasodilation (EC50) was 13.7±3.4 μM. Long-lasting vasodilation induced by nicotine returned to the pre-perfusion level within 5–15 min, as shown in Figure 1a. In some preparations, a very slight vasoconstriction by nicotine at low concentrations of 1–10 μM preceded the vasodilation, but no vasoconstriction in response to nicotine was observed at higher concentrations.
As shown in Figures 1b, c and d, perfusion of (−)-cytisine (1–100 μM) or (±)-epibatidine (1–100 nM) induced a concentration-dependent decrease in the perfusion pressure due to vasodilation. The maximum vasodilation (Emax) at the highest concentrations of (−)-cytisine and (±)-epibatidine were 79.3±3.1 and 85.2±3.0%, respectively. The EC50 for (−)-cytisine and (±)-epibatidine were 16.2±1.4 μM and 9.8±1.3 nM, respectively. In some preparations, vasoconstriction was induced by (−)-cytisine (1–10 μM) and (±)-epibatidine (1–10 nM) at low concentrations, but no vasoconstriction was observed at higher concentrations.
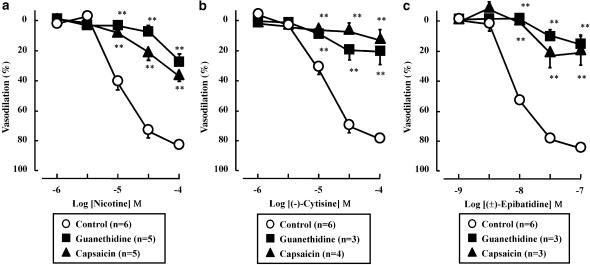
The transient vasoconstriction, but not the subsequent vasodilation, in response to PNS (2 Hz) was inhibited by guanethidine (5 μM) (data not shown). Furthermore, guanethidine (5 μM) abolished the nicotine, (−)-cytisine- or (±)-epibatidine-induced vasodilation (Figure 2).
Figure 2.
Effects of guanethidine or capsaicin on vasodilation induced by nicotine (a), (−)-cytisine (b) and (±)-epibatidine (c) in rat perfused mesenteric vascular beds. The presence of guanethidine (5 μM) or treatment with capsaicin (1 μM) blocked the vasodilator effects of all three nicotinic agonists. **P<0.01, compared with control (without treatment).
Capsaicin treatment of the endothelium-removed preparation abolished the PNS (2 Hz)-induced vasodilation, but not transient vasoconstriction (data not shown). As shown in Figure 2, vasodilation induced by nicotine, (−)-cytisine and (±)-epibatidine was markedly inhibited by treatment with capsaicin.
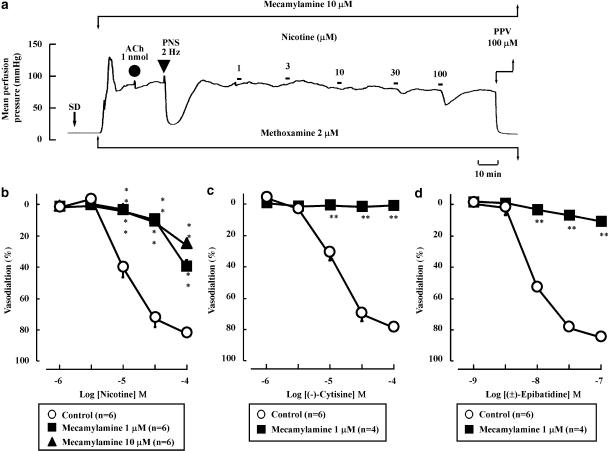
Effects of nicotinic ACh receptor antagonists on nicotine-induced vasodilation
As shown in Figures 3a and b, the nicotine-induced vasodilation was markedly inhibited by mecamylamine (1 or 10 μM). Additionally, mecamylamine (1 μM) abolished the vasodilation induced by (−)-cytisine- or (±)-epibatidine (Figures 3c and d), as observed for nicotine perfusion.
Figure 3.
Effects of mecamylamine on vasodilation induced by nicotine (a and b), (−)-cytisine (c) and (±)-epibatidine (d) in rat perfused mesenteric vascular beds. Mecamylamine at concentrations of 1 or 10 μM blocked the vasodilator effects of all three nicotinic agonists. **P<0.01, compared with control (without antagonist).
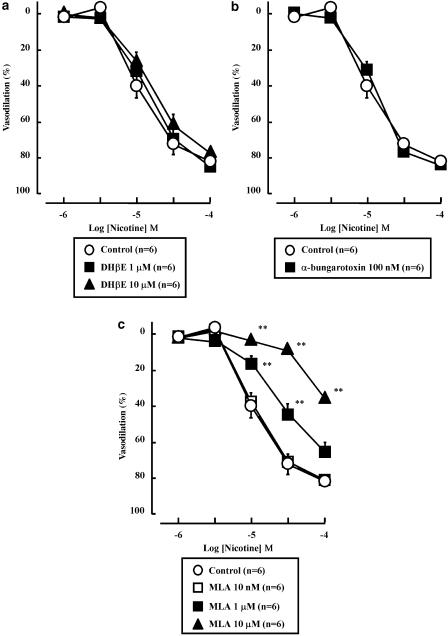
In contrast, the nicotine-induced vasodilation was not affected by DHβE (1 or 10 μM) (Figure 4a) or α-bungarotoxin (100 nM) (Figure 4b). As shown in Figures 4c and a, low concentration (10 nM) of methyllycaconitine did not affect the nicotine-induced vasodilation. However, high concentrations (1 or 10 μM) of methyllycaconitine significantly inhibited the vasodilation in response to nicotine.
Figure 4.
Effects of dihydro-beta-erythroidine (DHβE; 1 and 10 μM) (a), α-bungarotoxin (100 nM) (b) and methyllycaconitine (MLA; 10 nM, 1 and 10 μM) (c) on the nicotine-induced vasodilation in rat perfused mesenteric vascular beds. Here, DHβE (a), α-bungarotoxin (b) and MLA (c) did not affect vasodilation induced by nicotine. Note however that, at high concentrations, MLA did show some antagonism of the nicotine effect. **P<0.01, compared with control (without antagonist).
Discussion and conclusion
Our previous report showed that nicotine caused an endothelium-independent vasodilation in mesenteric resistance arteries, which was inhibited by hexamethonium, a non-selective neuronal nicotinic receptor antagonist, suggesting that nicotinic ACh receptors are responsible for the vasodilation (Shiraki et al., 2000). Additionally, this study obtained evidence that the nicotine-induced vasodilation is neurogenic and sensitive to adrenergic neuron blockers, capsaicin and CGRP (8–37). Furthermore, another earlier study of ours demonstrated that the nicotine-induced vasodilation was abolished by the vanilloid receptor antagonist capsazepine (Eguchi et al., 2004). From these findings, we hypothesized that nicotine stimulates nicotinic ACh receptors located on adrenergic nerves to release adrenergic neurotransmitter(s), which then activate vanilloid receptors located in adjacent CGRPergic nerves, thereby releasing CGRP and causing CGRP-induced vasodilation. The present findings that perfusion of nicotine in rat denuded mesenteric arteries with active tone caused endothelium-independent vasodilation and that guanethidine and capsaicin blocked the vasodilation confirm that the vasodilation depends on adrenergic and on nerves releasing CGRP (CGRPergic nerves).
It is well known that neuronal nicotinic ACh receptors in the peripheral nervous system mainly exist in autonomic ganglia (Rust et al., 1994). However, neuronal nicotine ACh receptors have been shown to be distributed in autonomic nerve endings in various tissues, such as rabbit musculus sphincter pupillae (Hisayama et al., 1988) and porcine basilar arteries (Si and Lee, 2001). In the rat mesenteric arteries used in this study, postganglionic adrenergic nerves innervate the adventitia of the artery, but no autonomic ganglia are distributed in these vessels. Since nicotine-induced vasodilation was blocked by hexamethonium (Shiraki et al., 2000), it is likely that site of action of nicotine in the mesenteric artery is nicotinic ACh receptors on autonomic nerves or autonomic nerve endings.
(±)-Epibatidine, an alkaloid isolated from the venom of a frog (Epipedobates tricolor) (Spande et al., 1992), is a potent but non-selective agonist of nicotinic ACh receptors, which acts as a full agonist of α4β2, α3β2, α3β4, α7 and α8 nicotinic ACh receptors (Gerzanich et al., 1995) and of ganglionic nicotinic ACh receptors (Fisher et al., 1994; Sacaan et al., 1996). In the present study, (±)-epibatidine at a 1000-fold lower concentration than nicotine induced potent vasodilation of rat mesenteric arteries. The (±)-epibatidine-induced vasodilation was blunted by guanethidine and capsaicin, supporting the notion that nicotinic ACh receptors mediate adrenergic and CGRPergic nerve-dependent vasodilation.
An analogue of nicotine, (−)-cytisine, is an agonist for neuronal nicotinic ACh receptors (Luetje and Patrick, 1991; Chavez-Noriega et al., 1997). (−)-Cytisine is a partial agonist of nicotinic ACh receptors containing β2 subunits, but a full agonist of nicotinic ACh receptors containing β4 subunits (Papke and Heinemann, 1994). In the present study, (−)-cytisine concentration-dependently induced vasodilation to the same extent as nicotine in rat mesenteric arteries. Therefore, (−)-cytisine acts as a full agonist in the rat mesenteric artery, suggesting that nicotinic ACh receptors in rat mesenteric arteries contain the β4 subunit. Additionally, the (−)-cytisine-induced vasodilation was blunted by guanethidine and capsaicin, suggesting that the vasodilation depends on adrenergic and CGRPergic nerves.
Si and Lee (2001) reported that nicotine-induced vasodilation in the porcine basilar artery, which is mediated by nitric oxide-containing nerves, is inhibited by the selective α7 subtype antagonist, α-bungarotoxin. However, in the present study, α-bungarotoxin did not affect nicotine-induced vasodilation in rat mesenteric arteries, suggesting that the α7 subtype is not responsible for the vasodilation. Reconstitution studies using Xenopus oocytes have shown that the antagonistic effect of methyllycaconitine on nicotinic ACh receptors is highly selective for the α7 subtype at low concentrations (IC50=0.1 nM), but high concentrations also antagonize the α3β4 subtype (IC50=1 μM) (Lopez et al., 1998). In the present study, the nicotine-induced vasodilation in rat mesenteric arteries was inhibited by high concentrations (1–10 μM), but not by a low concentration (10 nM) of methyllycaconitine. Additionally, mecamylamine, a relatively selective antagonist of α3β4 nicotinic ACh receptors (Yokotani et al., 2000; Papke et al., 2001), blunted the nicotine-, (−)-cytisine- or (±)-epibatidine-induced vasodilation. These findings strongly suggest that α7 nicotinic ACh receptors are not involved in nicotine-induced vasodilation of rat mesenteric arteries, but that α3β4 nicotinic ACh receptors are responsible for the nicotine-induced vasodilation.
α4β2 nicotinic ACh receptors have been shown to be highly expressed in many brain regions, including the thalamus (Lena and Changeux, 1997) and inhibitory neurons of the cerebral cortex (Alkondon et al., 2000). In these regions, nicotinic agonist-mediated responses are blocked by the selective α4β2 subtype antagonist DHβE. However, in the present study, DHβE (1–10 μM) did not antagonize the nicotine-induced vasodilation. Therefore, it is unlikely that the α4β2 nicotinic ACh receptors are involved in nicotine-induced vasodilation of the rat mesenteric arteries.
In conclusion, the present results suggest that α3β4 nicotinic ACh receptors are present on perivascular adrenergic nerve endings in rat mesenteric arteries and are the main subtype of nicotinic ACh receptors that function to mediate the nicotine- or nicotinic agonist-induced vasodilation. This result further supports our hypothesis that nicotine acts on presynaptic nicotinic receptors causing them to release adrenergic neurotransmitters or unknown related substance(s), which activate CGRPergic nerves, resulting in CGRP release and vasodilation.
Acknowledgments
This work was supported by a grant from the Smoking Research Foundation and in part by a Grant-in-Aid for Scientific Research (KAKENHI) (No 13672389) from the Ministry of Education, Science and Technology of Japan. This paper is dedicated to the memory of Ms Hinako Shiraki-Yamauchi, who was first to work on the nicotine-induced vasodilation and passed away suddenly in November 2006.
Abbreviations
- ACh
acetylcholine
- CGRP
calcitonin gene-related peptide
- DHβE
dihydro-beta-erythroidine
- EC50
50% effective concentration
- PNS
periarterial nerve stimulation
Conflict of interest
The authors state no conflict of interest.
References
- Alkondon M, Pereira EF, Eisenberg HM, Albuquerque EX. Nicotinic receptor activation in human cerebral cortical interneurons: a mechanism for inhibition and disinhibition of neuronal networks. J Neurosci. 2000;20:66–75. doi: 10.1523/JNEUROSCI.20-01-00066.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan JA, Brayden JE. Nonadrenergic neural vasodilator mechanisms. Circ Res. 1987;60:309–326. doi: 10.1161/01.res.60.3.309. [DOI] [PubMed] [Google Scholar]
- Chavez-Noriega LE, Crona JH, Washburn MS, Urrutia A, Elliott KJ, Johnson EC. Pharmacological characterization of recombinant human neuronal nicotinic acetylcholine receptors hα2β2, hα2β4, hα3β2, hα3β4, hα4β2, hα4β4 and hα7 expressed in Xenopus oocytes. J Pharmacol Exp Ther. 1997;280:346–356. [PubMed] [Google Scholar]
- Eguchi S, Tezuka S, Hobara N, Akiyama S, Kurosaki Y, Kawasaki H. Vanilloid receptors mediate adrenergic nerve- and CGRP-containing nerve-dependent vasodilation induced by nicotine in rat mesenteric resistance arteries. Br J Pharmacol. 2004;142:1137–1146. doi: 10.1038/sj.bjp.0705773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher M, Huangfu D, Shen TY, Guyenet PG. Epibatidine, an alkaloid from the poison frog Epipedobates tricolor, is a powerful ganglionic depolarizing agent. J Pharmacol Exp Ther. 1994;270:702–707. [PubMed] [Google Scholar]
- Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Gerzanich V, Peng X, Wang F, Wells G, Anand R, Fletcher S, et al. Comparative pharmacology of epibatidine: a potent agonist for neuronal nicotinic acetylcholine receptors. Mol Pharmacol. 1995;48:774–782. [PubMed] [Google Scholar]
- Hisayama T, Shinkai M, Takayanagi I, Morimoto S, Ishida K. Mechanism of action of nicotine in isolated iris sphincter preparations of rabbit. Br J Pharmacol. 1988;95:459–464. doi: 10.1111/j.1476-5381.1988.tb11666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki H, Takasaki K. Vasoconstrictor response induced by 5-hydroxytryptamine released from vascular adrenergic nerves by periarterial nerve stimulation. J Pharmacol Exp Ther. 1984;299:816–822. [PubMed] [Google Scholar]
- Kawasaki H, Takasaki K, Saito S, Goto K. Calcitonin gene-related peptide acts as a novel vasodilator neurotransmitter in mesenteric resistance vessels of the rat. Nature. 1988;335:54–56. doi: 10.1038/335164a0. [DOI] [PubMed] [Google Scholar]
- Lena C, Changeux JP. Role of Ca2+ ions in nicotinic facilitation of GABA release in mouse thalamus. J Neurosci. 1997;17:576–585. doi: 10.1523/JNEUROSCI.17-02-00576.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez MG, Montiel C, Herrero CJ, Garcia-Palomero E, Mayorgas I, Hernandez-Guijo JM, et al. Unmasking the functions of the chromaffin cell alpha7 nicotinic receptor by using short pulses of acetylcholine and selective blockers. Proc Natl Acad Sci USA. 1998;95:14184–14189. doi: 10.1073/pnas.95.24.14184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luetje CW, Patrick J. Both α- and β-subunits contribute to the agonist sensitivity of neuronal nicotinic acetylcholine receptors. J Neurosci. 1991;11:837–845. doi: 10.1523/JNEUROSCI.11-03-00837.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukas RJ, Changeux JP, Le Novere N, Albuquerque EX, Balfour DJ, Berg DK, et al. Inernational Union of Pharmacology. XX. Current status of the nomenclature for nicotinic acetylcholine receptors and their subunits. Pharmacol Rev. 1999;51:397–401. [PubMed] [Google Scholar]
- Lundberg MJ, Terenius L, Hokfelt T, Martling RC, Tatemoto K, Mutt V, et al. Neuropeptide Y (NPY)-like immunoreactivity in peripheral noradrenergic neurons and effects of NPY on sympathetic function. Acta Physiol Scand. 1982;116:477–480. doi: 10.1111/j.1748-1716.1982.tb07171.x. [DOI] [PubMed] [Google Scholar]
- Papke RL, Heinemann SF. Partial agonist properties of cytisine on neuronal nicotinic receptors containing the β2 subunit. Mol Pharmacol. 1994;45:142–149. [PubMed] [Google Scholar]
- Papke RL, Sanberg PR, Shytle RD. Analysis of mecamylamine stereoisomers on human nicotinic receptor subtypes. J Pharmacol Exp Ther. 2001;297:646–656. [PubMed] [Google Scholar]
- Rust G, Burgunder JM, Lauterburg TE, Cachelin AB. Expression of neuronal nicotinic acetylcholine receptor subunit genes in the rat autonomic nervous system. Eur J Neurosci. 1994;6:478–485. doi: 10.1111/j.1460-9568.1994.tb00290.x. [DOI] [PubMed] [Google Scholar]
- Sacaan AI, Menzaghi F, Dunlop JL, Correa LD, Whelan KT, Lloyd GK. Epibatidine: a nicotinic acetylcholine receptor agonist releases monoaminergic neurotransmitters: in vitro and in vivo evidence in rats. J Pharmacol Exp Ther. 1996;276:509–515. [PubMed] [Google Scholar]
- Sargent PB. The diversity of neuronal nicotinic acetylcholine receptors. Annu Rev Neurosci. 1993;16:403–443. doi: 10.1146/annurev.ne.16.030193.002155. [DOI] [PubMed] [Google Scholar]
- Shiraki H, Kawasaki H, Tezuka S, Nakatsuma A, Kurosaki U. Endogenous calcitonin gene-related peptide (CGRP) mediates adrenergic dependent vasodilation induced by nicotine in mesenteric resistance arteries of the rat. Br J Pharmacol. 2000;130:1083–1091. doi: 10.1038/sj.bjp.0703376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Si LM, Lee TJF. Presynaptic α7-nicotinic acetylcholine receptors mediate nicotine-induced nitric oxidergic neurogenic vasodilation in porcine basilar arteries. J Pharmacol Exp Ther. 2001;298:122–128. [PubMed] [Google Scholar]
- Spande TF, Garraffo HM, Yeh HJ, Pu QL, Pannell LK, Daly JW. A new class of alkaloids from a dendrobatid poison frog; a structure for alkaloid 251F. J Nat Prod. 1992;55:707–722. doi: 10.1021/np50084a002. [DOI] [PubMed] [Google Scholar]
- Suemaru K, Araki H, Gomita Y. Involvement of neuronal nicotinic receptor in psychiatric disorders. Nippon Yakurigaku Zasshi. 2002;119:295–300. doi: 10.1254/fpj.119.295. [DOI] [PubMed] [Google Scholar]
- Takeda D, Nakatsuka T, Papke R, Gu JG. Modulation of inhibitory synaptic activity by a non-α4β2, non-α7 subtype of nicotinic receptors in the substantia gelatinosa of adult rat spinal cord. Pain. 2003;101:13–23. doi: 10.1016/s0304-3959(02)00074-x. [DOI] [PubMed] [Google Scholar]
- Takenaga M, Kawasaki H, Wada A, Eto T. Calcitonin gene-related peptide mediates acetylcholine-induced endothelium-independent vasodilation in mesenteric resistance blood vessels of the rat. Circ Res. 1995;76:935–941. doi: 10.1161/01.res.76.6.935. [DOI] [PubMed] [Google Scholar]
- Taraschenko OD, Panchal V, Maisonneuve IM, Glick SD. Is antagonism of alpha3beta4 nicotinic receptors a strategy to reduce morphine dependence. Eur J Pharmacol. 2005;513:207–218. doi: 10.1016/j.ejphar.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Yanagisawa M, Kurihara H, Kimura S, Tomobe Y, Kobayashi M, Mitsui Y, et al. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988;332:411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]
- Yokotani K, Wang M, Okada S, Murakami Y, Hirata M. Characterization of nicotinic acetylcholine receptor-mediated noradrenaline release from the isolated rat stomach. Eur J Pharmacol. 2000;402:223–229. doi: 10.1016/s0014-2999(00)00533-1. [DOI] [PubMed] [Google Scholar]