Abstract
Background and Purpose:
PMX464 is a novel benzothiazole substituted cyclohexadienone reportedly targeting the thioredoxin (Trx1)/thioredoxin reductase (TrxR1) system. We have previously shown that PMX464 has enhanced hypoxic anti-proliferative effects in colorectal tumour cells, with some non-tumour cells (quiescent endothelium and fibroblasts) being relatively resistant. The current study aimed to validate the Trx1 system as a molecular target of PMX464 in tumour cells and to investigate the differential sensitivities of normal cells at the molecular level.
Experimental Approach:
Proliferation, clonogenic survival, protein expression and function, cell cycle and apoptosis assays were conducted using colorectal tumour (HT29), endothelial (HUVEC) and fibroblast (MRCV) cells treated with PMX464 under normoxic and hypoxic (1% O2) conditions.
Key Results:
Protein and enzyme assays showed that PMX464, in HT29, inhibited Trx1 function without altering expression and that inhibition correlated with decreased proliferation and survival, and was more marked under hypoxia. In contrast, although hypoxic HUVEC were sensitive, in terms of proliferation and survival, inhibition of Trx1 function was not observed. Quiescent HUVEC and MRCVs (that have undetectable Trx1 protein) were relatively resistant. The effect on HT29 cells was essentially due to cell cycle inhibition, as apoptosis was modest. Anti-proliferative effects were lost after a lag period, suggesting a reversible phenomenon.
Conclusions and Implications:
The Trx1 system is an important target in tumour cells and can be inhibited by PMX464. Quiescent HUVEC and fibroblasts are relatively resistant conferring a therapeutic benefit when targeting Trx1.
Keywords: thioredoxin, colorectal cancer, chemotherapy, angiogenesis
Introduction
Thioredoxin (Trx1) is a 12 kDa protein that contains the active catalytic sequence (W)C32GPC35(K), the Trx1 motif (Powis and Montfort, 2001). First identified in Escherichia coli (Laurent et al., 1964), Trx1 and its redox properties were extensively characterized by Luthman and Holmgren (1982). It has been shown to be upregulated in certain malignancies, especially in hypoxic regions (Hedley et al., 2004). The principal function of this system is to act as a protective cellular anti-oxidant and a regulator of the activity of transcription factors. The basic reaction catalysed by the system is as follows: thioredoxin reductase (TrxR1) and the reduced form of nicotinamide adenine dinucleotide phosphate (NADPH) recycle Trx1 from its inactive oxidized form containing a disulphide (S-S) bond to its active reduced form, containing thiol (SH) groups. The active form of Trx1, in turn, is reoxidized while providing reducing equivalents to target molecules such as ribonucleotide reductase, peroxiredoxins (that act as cellular anti-oxidants) and various transcription factors including hypoxia inducible factor-1α. Trx1 also exerts anti-apoptotic effects as its expression inhibits ASK-1 (apoptosis signal regulating kinase-1) kinase activity and subsequent ASK-1 induced apoptosis in vitro (Saitoh et al., 1998). The effect of Trx1 in the cell is thus pleiotropic and a Trx1 inhibitor may have a wide variety of effects (Powis and Montfort, 2001).
One such potential inhibitor is PMX464 (4-(benzothiazol-2-yl)-4-hydroxycyclohexa-2,5-dienone) (previously AW464; Wells et al., 2003), developed at the School of Pharmacy, University of Nottingham. National Cancer Institute (NCI) computerized pattern recognition algorithm (COMPARE) analysis, and microarray data indicate possible interactions with the Trx1/TrxR1 signalling system and resultant upregulation of TrxR1 (Bradshaw et al., 2005). Recent characterization of PMX464 in colorectal cancer cells has shown that concentrations <IC50 (0.5 μM), which are ineffective under normoxic conditions, become effective in decreasing both proliferation and angiogenic growth factor production during hypoxia (1% O2, 48 h). Although proliferating endothelial cells were sensitive, quiescent cells were resistant. Proliferating fibroblasts were also relatively resistant (Mukherjee et al., 2005). The current study aimed to investigate, at the molecular level, the Trx1 pathway as a target of PMX464 in colorectal cancer cells and examine the reasons for the different effects observed with fibroblasts and quiescent endothelial cells. Results obtained show that Trx1 is a major target of PMX464 in HT29 cells but other targets may exist in endothelial cells and fibroblasts.
Materials and methods
HUVEC isolation
Human umbilical vein endothelial cells (HUVEC) were isolated using the collagenase perfusion technique (Jaffe et al., 1973) detailed in an earlier publication (Mukherjee et al., 2005). Pooled populations (three donors) of cells for experiments were used between passage 2 and 6.
Tissue culture
The colorectal cancer cell line HT29 was, based upon previous work, chosen for this study. Two normal cell types, HUVEC and MRCV lung fibroblasts, were used for comparison. HT29 cells were used between passages 140 and 150, and MRCV between 22 and 26. Media preparation and storage were as described previously (Mukherjee et al., 2005).
Growth assays
The proliferation status of cells was assessed after treatment with drugs by counting cell numbers (Saunders et al., 1997). A total of 105 cells of each cell line were plated out on six-well tissue culture plates or 5 × 105 (for HT29, MRCV)/2.5 × 105 cells (HUVEC) were plated out in 25 cm2 tissue culture flasks. Cells were allowed to attach overnight, and then exposed to drugs for 72 h, the final 48 h of which were either under normoxic or hypoxic conditions. Hypoxia was achieved in a water-jacketed Thermoforma incubator gassed with nitrogen to achieve 1% O2.
In separate experiments, to assess protracted effects on proliferation, the drug was aspirated off after 72 h of incubation and replaced with fresh medium. Cell numbers were assessed after an additional 5 and 10 days incubation. Paclitaxel, a known cytotoxic agent, was used as a comparator and positive control.
The effect of the drug on quiescent as opposed to proliferating endothelial cells was elucidated as described previously (Mukherjee et al., 2005). Briefly, cells were seeded in 25 cm2 flasks at a density of 2.5 × 105 cells and cell numbers counted daily. When cells reached confluence, they were maintained for a further 2 days to confirm lag growth, media was aspirated off and replaced with either fresh media for controls or different drug dilutions of PMX464 and treated for a further 72 h.
Clonogenic survival assay
Cell survival assays were utilized to measure reproductive integrity. These assays were performed according the protocol of Liebmann et al. (1993) as detailed in an earlier publication (Mukherjee et al., 2005). Exponentially growing cells were exposed to PMX464 for 72 h either in normoxia or hypoxia (final 48 of 72 h incubation, 1% O2) following which they were trypsinized and plated out for colony formation. Incubation time was 4 weeks for HT29, 14 days for MRCV and 10 days for HUVEC. Staining and counting of colonies were performed as previously.
Western blots
Cells were treated with PMX464 as described above (Growth assays). After counting, cells were resuspended in 1% sodium dodecyl sulphate and frozen until use. Lysed cells were assayed for protein content by BCA-kit according to manufacturer's instructions, gel electrophoresed and transferred via standard blotting techniques. Blots were probed simultaneously with goat anti-human TrxR1 antibody diluted 1:5000 and with goat anti-human Trx1 antibody diluted 1:1000 followed by horseradish peroxidase (HRP)-conjugated rabbit anti-goat secondary antibody diluted 1:5000. Bands were analysed with an enhanced chemiluminescence protocol and visualized on radiographic film. Blots were again stripped and reprobed with HRP-conjugated mouse anti-human β-actin diluted 1:5000. For experiments with lysates of endothelial cells, some blots were probed for EndoPDI (endothelial-specific protein disulphide isomerase) expression with rabbit anti-human EndoPDI diluted 1:100 and HRP-goat anti-rabbit secondary antibody diluted 1:1000.
Enzyme assays
The insulin reduction assay and 5,5′-dithiobis(2-nitrobenzoate) (DTNB) assay were used to test for the functional activity of Trx1 and TrxR1, respectively. Function assessments were performed using purified Trx1 (human) and TrxR1 (rat liver) enzymes in both a cell-free enzyme system (Kunkel et al., 1997) and also with cell lysates (Holmgren and Bjornstedt, 1995).
Insulin reduction assay: testing for Trx1 activity. Cell-free system: The assay mixture for pure enzymes was modified from Kunkel et al. (1997) and contained the following in a final volume of 100 μl HE buffer (100 mM HEPES pH 7.2; 5 mM EDTA): 1 mM NADPH, 0.32 μM oxidized Trx1, 0.4 μM TrxR1, 1 mg ml−1 insulin and PMX464 (5–50 μM). All ingredients were freshly reconstituted for each experiment. The insulin levels were optimized as it is known that excess insulin keeps Trx oxidized and additional disulphides in the stored Trx protein may affect the outcome of the assay. Two negative controls were used: one comprised of a sample lacking Trx1, the other, lacking insulin. Wells with all enzymes, but no drug, were used as the positive control. After 30 min incubation at 37°C, the reaction was stopped with 150 μl stop buffer containing 6 M guanidine hydrochloride, 50 mM Tris (pH 8.0) and 10 mM DTNB. The plate was read at 405 nm. Results of the cell-free enzyme assay were compared without the pre-incubation step (all components incubated together, as described) or with the pre-incubation step (all components except the substrate insulin were incubated for 30 min at 37°C, then insulin added and re-incubated for 30 min) to examine kinetics and nature of drug-substrate binding.
Assays with cell lysates: Cell culture conditions were as described for growth assays and western blotting. After incubation with drug, cells were trypsinized, counted on a haemocytometer and sonicated in cell lysis buffer (50 mM Tris–HCl, pH 7.5 and 2 mM EDTA) for 20 min (Yamada et al., 1996). Lysates were analysed immediately or stored for future use at −80°C. A BioRad DC protein assay kit was used to determine the protein content in lysates, using standard conditions. Protein (20 μg) isolated from each condition was made up to a final volume of 70 μl in HE buffer and TrxR, insulin and NADPH (10 μl each) were added separately. Duplicate samples were used, either with or without exogenous TrxR enzyme. A cell-free enzyme assay was simultaneously conducted, using 10 μl of different concentrations of Trx1 (0, 0.4, 1.6 and 3.2 μM), to generate a standard curve. The reaction was incubated at 37°C for 30 min before addition of stop buffer. The absorbance of the sample lacking exogenous TrxR was subtracted from that of the corresponding sample containing exogenous TrxR. The difference in absorbance indicates Trx1 function. The amount of functional Trx1 was calculated from the standard curve, generated simultaneously, with pure enzymes (Holmgren and Bjornstedt, 1995).
DTNB assay: testing for TrxR1 activity. The cell-free enzyme assay for TrxR1 using DTNB as a substrate was measured according to the method adapted from Kunkel et al. (1997). The assay mixture contained the following in a final volume of 100μl HE buffer (100 mM HEPES pH 7.2; 5 mM EDTA), 1 mM NADPH, 0.4 μM TrxR1 and PMX464 (10–100 μM). Two negative controls were used: one lacking TrxR1, the other lacking DTNB. Wells, with enzymes but no drugs, were used as the positive control. The reaction was performed in 96-well plates preheated to 37°C. TrxR1 was pre-incubated for 30 min with different doses of PMX464 following which NADPH and 150 μl of DTNB substrate were added to initiate the reaction. The optical density at 405 nm was measured. The control reaction plateaued after 5 min, and therefore all experimental points were read at this time. All data points were in duplicate.
Cell cycle block
HT29 (5 × 105 cells) and HUVEC (2.5 × 105 cells) were plated onto 25 cm2 flasks and allowed to attach for 24 h, media aspirated and exponentially growing cells exposed to 5 ml of PMX464 (10 nM–1 μM) for either 12, 24, 48 and 72 h under normoxic or hypoxic conditions (1% O2). Cells were fixed and analysed by propidium iodide staining on an FACScan flow cytometer, using Cell Quest software.
Apoptosis assays
Cells treated as above were analysed by Annexin-V/Propidium iodide double staining. Camptothecin was used as a positive control drug for inducing apoptosis in HT29 cells (Arnould et al., 2002). Media was centrifuged at 670 g for 5 min to collect floating cells in addition to the cells attached to the flask to account for any apoptosed or necrosed cells. The Apoptest-FITC (fluorescein isothiocyanate) kit was used as per manufacturer's protocol. A 2.5 μl of AnnexinV-FITC and 2.5 μl of propidium iodide were added to every 95 μl of diluted binding buffer. Cells were washed with phosphate-buffered saline, trypsinized and 105 cells from each condition suspended in 100 μl of this solution and incubated for 10 min in the dark. Cells were immediately analysed on an FACScan flow-cytometer, using Cell Quest software.
Statistical analysis
Student's t-test was used for statistical analysis of results. A P-value of <0.05 was considered to be statistically significant. For combining errors from individual experiments when pooling data, the following formula was used:
where SDcombined is the combined standard deviation from y (number of experiments) experiments where SD1 and SD2 are the standard deviations in individual experiments and n1 and n2 are number of replicates in each experiment.
Drugs and reagents
PMX464 was a gift by Professor Malcolm Stevens at the School of Pharmacy, University of Nottingham. Paclitaxel and camptothecin were purchased from Sigma (Poole, UK). All drugs were stored in dimethyl sulphoxide (DMSO) and diluted to final concentrations in appropriate culture media for the cell line investigated (final DMSO concentration ⩽0.1%). Reagents for experiments were obtained from Sigma unless listed otherwise. HT29 and MRCV fibroblasts were obtained from ATCC (American type Culture Collection, Teddington, UK) and ECACC (European Collection of Cell Cultures, Salisbury, UK). The anti-human TrxR1 antibody was purchased from Upstate (Chandlers Ford, UK), goat anti-human Trx1 antibody from American Diagnostica (Kimbolton, UK) and rabbit anti-human EndoPDI was a gift from R Bicknell, University of Birmingham. Both HRP-goat anti-rabbit and HRP-rabbit anti-goat secondary antibodies were obtained from Dako (Ely, UK), as was the Apoptest-FITC kit. HRP-conjugated mouse anti-human β-actin was obtained from Abcam (Cambridge, UK). Chemiluminiscence and radiographic material were obtained from Amersham Biosciences (Chalfont St Giles, UK). Trx1 (human recombinant E. coli) was purchased from Calbiochem (Nottingham, UK). Reagents for media preparation were obtained as described previously (Mukherjee et al., 2005).
Results
Growth and clonogenic assays demonstrate that HT29 cells exhibit increased hypoxic sensitivity to PMX464 whereas quiescent HUVEC and fibroblasts are relatively resistant
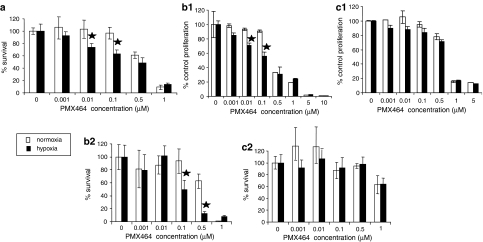
The effects of PMX464, in terms of proliferation and clonogenicity, were assessed on HT29s and two ‘normal' cell types: HUVEC and MRCV. As hypoxia has been reported to upregulate Trx1 effects were examined under both normoxic and hypoxic conditions. We have previously shown anti-proliferative effects of PMX464 on HT29 cells (Mukherjee et al., 2005). Current results indicate that such effects may be due to decreased reproductive ability of HT29 (Figure 1a). There was, as with proliferation assays (Mukherjee et al., 2005), increased sensitivity to the drug under hypoxic conditions – doses of the drug ineffective under normoxic conditions became effective under hypoxia (Figure 1a). There was also decreased proliferation and clonogenic survival of proliferating HUVEC but with little increased hypoxic sensitivity (Figures 1b1 and b2). Quiescent HUVEC were, as described previously (Mukherjee et al., 2005), resistant to PMX464. MRCV fibroblasts were also relatively resistant (Figures 1c1 and c2), but became sensitive at doses ⩾1 μM. As with proliferating endothelial cells, there was no increased sensitivity of fibroblasts under hypoxic conditions. To summarize, HT29 cells showed increased hypoxic sensitivity to PMX464; quiescent endothelial cells and fibroblasts were relatively resistant.
Figure 1.
Clonogenic survival and growth assay results. Effects of PMX464 on (a) HT29 cells, (b) HUVEC and (c) MRCVs treated with PMX464 for 72 h under normoxic and hypoxic (1% O2, final 48 h of incubation) conditions analysed by clonogenic assays (a, b2 and c2) and growth assays (b1 and c1). Results were normalized to respective controls. For clonogenic assays, mean plating efficiencies were HT29: 12±0.5% (normoxia) and 9±0.4% (hypoxia); HUVEC: 22±6.7% (normoxia) and 17.6±1.8% (hypoxia); MRCV: 17.1±1.6% (normoxia) and 14.8±2.5% (hypoxia). For proliferation assays, mean control cell numbers (× 105 cells) were HUVEC: 2.72±0.7 (normoxia) and 3.09±0.5 (hypoxia); MRCV: 4.6±0.5 (normoxia) and 4.2±0.3 (hypoxia). All experiments were repeated at least twice, with data points in duplicate (for growth assays) or triplicate (for clonogenic assays) in individual experiments and results pooled. Error bars show s.d. and ★ represents significant decrease compared to respective normoxic equivalent (P<0.05). PMX464, 4-(benzothiazol-2-yl)-4-hydroxycyclohexa-2,5-dienone; s.d., standard deviation.
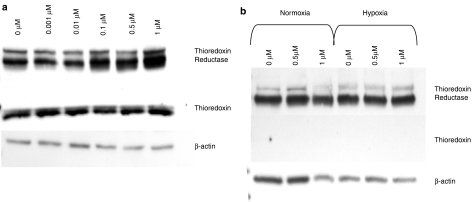
PMX464 does not affect Trx1 protein levels but increases TrxR1 levels
As NCI COMPARE analysis and microarray data indicated possible interactions with the Trx1/TrxR1 signalling system (Bradshaw et al., 2005), effects of PMX464 on Trx/TrxR1 proteins were assessed by western blot. HT29 cells express both Trx1 and TrxR1 protein (Figure 2a), whereas fibroblasts have undetectable levels of Trx1 (Figure 2b) perhaps explaining their relative resistance. There is no alteration in Trx1 protein levels in HT29 cells following drug treatment either under normoxic (Figure 2a) or hypoxic conditions. In contrast, TrxR1 is induced following drug treatment under both conditions (hypoxic data not shown). Similar results were obtained for HUVEC (not shown). The upregulation of TrxR1 is concordant with NCI microarray data showing induction of TrxR1 at the mRNA level on PMX464 treatment (Bradshaw et al., 2005). The western blot results suggested a feedback mechanism and therefore functional assays were next conducted.
Figure 2.
Western blot analysis of Trx1 and TrxR1 levels. (a) HT29 cells and (b) MRCV cells were treated with varying concentrations of PMX464 for 72 h (final 48 h either under normoxic or hypoxic [1% O2] conditions; hypoxic data not shown for HT29). Blots were probed simultaneously for TrxR1 (∼58 kDa) and Trx1 (∼12 kDa), and stripped and reprobed for β-actin (∼42 kDa) as a loading control. PMX464, 4-(benzothiazol-2-yl)-4-hydroxycyclohexa-2,5-dienone; Trx1, thioredoxin; TrxR1, thioredoxin reductase.
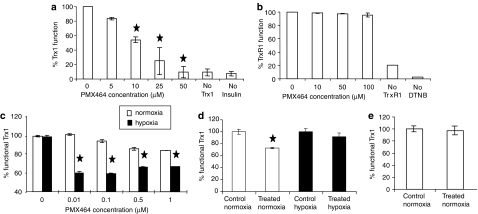
PMX464 differentially inhibits Trx1 function in tumour, endothelial and fibroblast cells
Previous investigations into the functional inhibition of Trx1 by PMX464 have only used cell-free systems (Bradshaw et al., 2005). The current study investigated inhibition of Trx1 function, by PMX464, both in cell-free systems and in cellular lysates.
PMX464 inhibits the Trx1-dependent reduction of insulin in assays with pure enzymes (Figure 3a), giving an IC50 of 10 μM that decreased, with pre-incubation, to 5 μM (not shown). This matches results from collaborating groups who report an IC50 of ∼20–25 μM (Pallis et al., 2003). In contrast to its effect on Trx1 function, PMX464 did not inhibit TrxR1 function in the DTNB assay (Figure 3b). It appears that the drug is a functional inhibitor of Trx1 with no effect upon TrxR1 function.
Figure 3.
Enzyme assay results from cell-free systems. (a) Cell-free insulin reduction assay for Trx1 function. Varying concentrations of PMX464 were incubated with Trx1, TrxR1, NADPH, and insulin for 30 min and enzyme function inhibition noted. (b) Cell-free DNTB assay for TrxR1 function. Varying concentrations of PMX464 were incubated with TrxR1 for 30 min and enzyme function inhibition noted. Results are summarized from two experiments, with each data point in duplicate. Error bars represent s.d. and ★ represents significant difference compared to untreated control (P<0.05). Enzyme assay results: cell lysate system – insulin reduction experiments for percentage of functional Trx1 per cell in (c) HT29, (d) proliferating HUVEC and (e) quiescent HUVEC treated with PMX464 (0.01–1 μM for HT29; 0.5 μM for HUVEC) for 72 h under normoxia or hypoxia (1% O2, final 48 of the 72 h drug incubation period). Functional Trx1 levels (fg cell−1) in untreated controls were as follows: (c) HT29: 2000±10 (normoxia) and 3000±12 (hypoxia); (d) proliferating HUVEC: 4200±5 (normoxia), 3200±30 (hypoxia) and (e) quiescent HUVEC: 1800±17 (normoxia), 2100±28 (hypoxia). Functional Trx1 cell−1 in MRCVs measured during the same experiments was as follows: 800±7 (normoxia) and 900±10 (hypoxia). Data has been normalized to respective controls and each data point was in duplicate in individual experiments, repeated twice. Standard deviation (s.d.) is depicted as error bars and ★ represents greater % functional inhibition under hypoxic conditions in paired sample t-test (P<0.05). NADPH, nicotinamide adenine dinucleotide phosphate reduced; PMX464, 4-(benzothiazol-2-yl)-4-hydroxycyclohexa-2,5-dienone; Trx1, thioredoxin; TrxR1, thioredoxin reductase; s.d., standard deviation.
Cellular assays, using lysates, were next conducted. As cell-free enzyme assays showed no inhibition of TrxR1 by PMX464, the cell lysate experiments examined Trx1 function only.
As with previous reports investigating hypoxic induction of Trx1 expression (Berggren et al., 1996), we showed that hypoxia upregulated Trx1 function 1.5-fold (P<0.005). PMX464 inhibited Trx1 function, under normoxia, at doses of 0.5 and 1 μM (∼20% inhibition) (Figure 3c). Trx1 inhibition was greater under hypoxic conditions and was observed at lower drug doses, <IC50 (Figure 3c). This inhibition, combined with the lack of effect on Trx1 protein levels and the concomitant increase in TrxR1 protein levels, suggests a functional inhibition of Trx1.
Trx1 function was also inhibited in HUVEC under normoxic conditions (Figure 3d). In contrast to HT29s, there was no hypoxic upregulation of Trx1 function in HUVEC and no inhibition from PMX464 under hypoxic conditions (Figure 3d). Trx1 function was lower in quiescent, as opposed to proliferating, endothelial cells. In contrast to proliferating HUVEC, PMX464 did not alter Trx1 function in quiescent cells (Figure 3e). These results suggest the existence of other potential targets in endothelial cells. One such target may be EndoPDI, an endothelial-specific molecule containing three Trx1 motifs. No altered expression of EndoPDI protein was observed via western blotting (not shown) but as with Trx1, a functional inhibition cannot be discounted.
For MRCV fibroblasts, as with results from western blots, insulin reduction assays indicate low levels of functional Trx1 under control conditions (not shown), possibly explaining the relative resistance of such cells. The sensitivity at high doses (⩾1 μM) suggests additional targets.
In summary, enzyme assays indicate that PMX464 inhibits Trx1 function in HT29 tumour cells and that such inhibition is enhanced by hypoxia. A beneficial therapeutic index for the drug may stem from the lack of inhibition of Trx1 function in quiescent endothelial cells and fibroblasts.
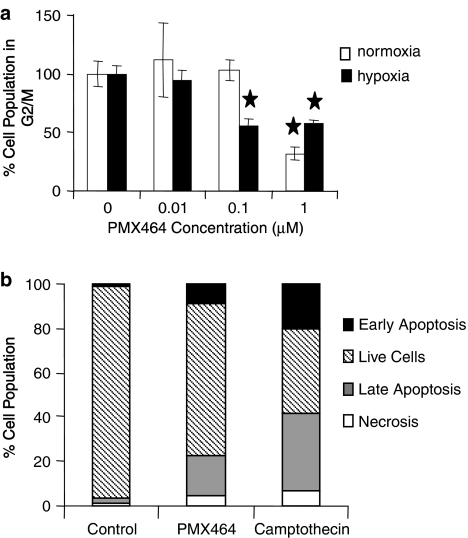
PMX 464 induces a G1/S block in HT29s with only modest apoptosis evident
Cell cycle analysis and apoptosis assays were conducted to examine the mechanism responsible for the observed decreases in proliferation and clonogenic survival. Current results, unlike previous reports that indicate G2/M activity in other colorectal cells (Bradshaw et al., 2005), show that high doses of the drug (1 μM) arrest HT29 cells in the G1/S phase of the cell cycle (Figure 4a). G1/S phase arrest is also observed at low doses (0.1 μM) under hypoxic conditions, explaining the effects of PMX464 under hypoxia in colorectal tumour cells. In contrast, no G1/S phase arrest was observed with HUVEC treated with PMX464 under similar drug exposure conditions (not shown).
Figure 4.
Cell cycle analysis and apoptosis assay results. Flow cytometry analysis of HT29 (a) cell cycle as measured by propidium iodide staining following 24 h exposure to PMX464 under normoxic and hypoxic conditions. Error bars represent s.d. and ★ represents significant difference compared to respective untreated control (P<0.05); (b) apoptosis as measured by annexin V/propidium iodide staining following 48 h exposure to 1 μM PMX464 or 20 μM camptothecin under normoxic conditions. Doses of PMX464 and camptothecin producing an anti-proliferative effect of ⩾50% at 48 h were chosen for experiments. PMX464, 4-(benzothiazol-2-yl)-4-hydroxycyclohexa-2,5-dienone; s.d., standard deviation.
PMX464 induced apoptosis in HT29 cells under both normoxic and hypoxic conditions. The apoptosis was late in onset (not prominent at 24 h) and evident only after 48 h of exposure. PMX464-induced cell death was not as prominent as with the control drug, camptothecin (Figure 4b).
In summary, the effect of PMX464 on HT29 cells is essentially due to cell cycle inhibition, as apoptosis was modest. A differential effect is again seen with HUVEC in that no cell cycle block was induced.
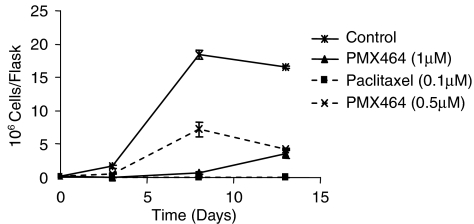
Cytostatic effects of PMX464 are reversible
Long-term effects and reversibility of drug action are often investigated by drug washout experiments (Kusaka et al., 1994). When drug was replaced, after 72 h incubation, with fresh media and growth assessed for further 10 days, 1 μM caused prolonged inhibition of proliferation (Figure 5). However, cells recover and start re-proliferation towards the end of this period. This is in contrast to paclitaxel that causes permanent growth inhibition at a dose of 0.1 μM. Cells treated with lower doses of PMX464 (0.5 μM) start re-proliferation earlier. Similar results were also obtained for HUVEC treated with PMX464 (not shown). Such results suggest that the effect of PMX464 is a combination of both reversible cytostatic and cytotoxic events.
Figure 5.
Long-term effects of PMX464 on HT29 cells. Cell numbers were assessed at days 0 and 3 after exposure to PMX464. Drug containing media was removed at day 3 and cell numbers were monitored for an additional 10 days. Paclitaxel was used as a positive control. PMX464, 4-(benzothiazol-2-yl)-4-hydroxycyclohexa-2,5-dienone.
Discussion
PMX464 is a novel benzothiazole-substituted heteroaromatic cyclohexadienone compound with analysis on the NCI-60 cell panel showing particular activity in colon, renal and certain breast cancer cell lines, MCF7, MDA-MB-435 and MDA-N (Wells et al., 2003). Subsequent database mining suggested Trx1 as a target. The compounds that were COMPARE positive did not include any known anti-cancer agent but showed correlation with some compounds (NSC 140377, NSC 645300) (Wells et al., 2003) that had already been identified as potential inhibitors of the Trx1/TrxR1 signalling pathway (Kunkel et al., 1997). Subsequent microarray analysis, carried out at the NCI using RNA samples isolated from drug-treated HCT116 cells, showed that, at 1 μM PMX464 for 24 h, the only gene upregulated, from the 10 000 examined, was TrxR1, indicating that this pathway played a key role in the mechanism of action of PMX464. Subsequent HPLC studies showed that all five cysteine residues of Trx1, including the cysteines at the Trx1 active site (residues 32 and 35) might bind PMX464 (Bradshaw et al., 2005).
It was shown in a previous publication (Mukherjee et al., 2005) that PMX464 decreases the proliferation of various colorectal cancer cells. Low doses of the drug, not effective under normoxic conditions, decreased proliferation under hypoxia. The current study further characterizes such effects with clonogenic assays confirming and extending the proliferation results. Such results suggest a cytotoxic mode of action of PMX464 with drug washout experiments demonstrating that, although the proliferation of cells was inhibited for a prolonged period of time after removal of PMX464, some recovery and re-proliferation was ultimately evident, that is the mode of action of PMX464 appears to be a combination of cytotoxic and reversible cytostatic events. It has been suggested, by molecular modelling, that the drug binds irreversibly with cysteine residues 32 and 35 of Trx1, thereby inhibiting enzyme activity (Bradshaw et al., 2005). Results from the current study suggest a reversible phenomenon. The relative resistance of quiescent endothelial cells and fibroblasts as compared to HT29s suggests a therapeutic advantage for PMX464.
The upregulation of TrxR1 protein with no alteration of Trx1 protein, in western blots, suggests a functional inhibition of Trx1 that then induces a positive feedback and upregulates TrxR1. Although PMX464 decreased functional Trx1 in HT29, there was a greater decrease under hypoxia, at doses of the drug not effective under normoxic conditions. Such results may reflect the critical role that Trx1 plays in cells when exposed to hypoxic conditions (Hedley et al., 2004). This pro-survival function is so vital that the slightest inhibition may have significant consequences.
Trx1 function was inhibited in proliferating normoxic HUVEC but, in contrast to colorectal cancer cells, hypoxia did not increase function, a finding that confirms other studies that indicate that most of the Trx1 in endothelial cells exists in the reduced state, with little variation under conditions of oxidative stress (Fernando et al., 1992). Interestingly, in the current study, there was no evidence of inhibition of Trx1 function in proliferating HUVEC under hypoxic conditions, suggesting that effects of PMX464 in decreasing proliferation and clonogenic survival of endothelial cells under hypoxia may be due to the existence of other targets. Proteins that contain Trx1 domains such as the protein disulphide isomerases (PDI) (Graven et al., 2002) may be examples of such targets. PDI has protective effects on endothelial cells, under both normoxic and hypoxic conditions, and a recently discovered congener of PDI, endothelial PDI (EndoPDI), that is specific for endothelial cells, is protective only during hypoxia (Sullivan et al., 2003). The three redox-active Trx1 domains in EndoPDI could be targeted by PMX464 under hypoxic conditions, explaining differential effects. There was no alteration in EndoPDI protein levels in PMX464-treated HUVEC from western analysis, but this does not rule out a functional effect of PMX464 on EndoPDI as has been observed with Trx1. The lack of functional assays for EndoPDI prohibits verification of this at present, but the effects of PMX464 on total PDI function are currently under investigation. Preliminary results show that PMX464 inhibits PDI function at a level similar to bacitracin, a known PDI inhibitor, but at IC50 values much higher than that for Trx inhibition, that is 400–600 μM as opposed to 10 μM (Zhang et al., 2006). Quiescent HUVEC have lower functional Trx1, as opposed to their proliferating counterparts, explaining their relative resistance; however, the level of functional Trx1 in quiescent HUVEC is similar to that found in HT29s suggesting that quiescent HUVEC are less reliant upon the Trx1 system. The resistance of MRCV fibroblasts to PMX464 might be explained by the very low Trx1 levels. Sensitivity to higher doses (⩾1 μM) suggests other targets.
Flow cytometry results showed that PMX464 caused a G1/S arrest for HT29 cells that was particularly evident under hypoxic conditions and at low drug concentrations. The Trx1/TrxR1 signalling pathway transfers reducing equivalents to ribonucleotide reductase involved in DNA synthesis and this may explain the PMX464-induced G1/S block and indeed other TrxR1 inhibitors, such as BBSKE, have been shown to arrest cells in the G1/S phase (Shi et al., 2003). Trx1 activity peaks in mid-S phase of the cell cycle in HTLV-1 (+) T-cell leukaemia and decreases thereafter (U-Taniguchi et al., 1995), and hence a G1/S phase block may be related to drug target availability. The observation in HT29 cells is in contrast to studies with HCT116 colorectal tumour cells, where a block was induced in the G2/M phase (Bradshaw et al., 2005). Such G2/M block is also shown to be induced by the Trx1 inhibitor imidazolyl disulphide IV-2 (Vogt et al., 2000) and could result from the inhibition of cysteine residues in tubulin by a Trx1 inhibitor. HUVEC treated with PMX464 showed no cell cycle block. Such results, as with those mentioned above, suggest differing levels of target molecules or even differing target molecules in different cell types.
Apoptosis induced by PMX464 in colorectal cancer cells was modest in comparison to that caused by camptothecin. It was late in onset, being evident earliest at the 48 h time-point. Early onset apoptosis in leukaemia cells (after 8 h) and HCT116 cells (after 12 h) treated with PMX464 has been reported by Pallis et al. (2003), Bradshaw et al. (2005) and Chew et al. (2006). Trx1 controls apoptosis by regulating ASK-1 kinase activity and hence a Trx1 inhibitor would be expected to produce more dramatic apoptosis. Apoptosis was also not marked in endothelial cells even after 72 h drug treatment. As with effects upon the cell cycle, such differing results with respect to the mode of cell death may reflect differing levels of target molecules in differing populations.
The efficacy of low doses of the drug under hypoxic conditions and the mixed cytotoxic/cytostatic mechanism makes PMX464 a candidate for combination therapy. Only low doses may have to reach the hypoxic tumour cells to cause significant Trx1 inhibition, cytostasis and cytotoxicity and also indirect anti-angiogenic effects (re-decreased vascular endothelial growth factor production) (Mukherjee et al., 2005). To avoid tumour cell repopulation during the break between successive cycles, as suggested by washout experiments, repeated administration of PMX464 may be warranted. Such effects need further verification both in vitro and in vivo.
In conclusion, our study suggests that the effect of PMX464 in HT29 colorectal tumour cells is due to functional Trx1 inhibition inducing a cell cycle block in the G1/S phase and subsequent cellular toxicity. Although the direct anti-proliferative effects in endothelial cells under normoxia may be explained by effects on Trx1 function, lack of functional effects on Trx1 under hypoxia and quiescence and a lack of cell cycle arrest suggest the existence of other targets and mechanisms of action. This is also supported by results with fibroblasts that express low levels of functional Trx1 and are only sensitive to PMX464 at high doses. Such targets could be closely related redox proteins that contain Trx1 motifs, for example the PDIs, the glutaredoxins and calcium binding proteins, and require further characterization. Other potential targets are currently being investigated to further delineate the mechanism of PMX464 activity.
Acknowledgments
We thank Andrew Westwell, Welsh School of Pharmacy, Cardiff University, Cardiff UK and Tracey Bradshaw and Malcolm Stevens, Centre for Bio-Molecular Sciences, School of Pharmacy, University of Nottingham, University Park, Nottingham, NG7 2RD, UK, for supplying PMX464 and critical appraisal of the manuscript. We also thank Roy Bicknell, University of Birmingham, Centre for Cardiovascular Sciences, Cancer Research UK Angiogenesis Group, Division of Immunity and Infection, University of Birmingham Medical School, Birmingham, UK, for supplying EndoPDI antibody.
Abbreviations
- ASK-1
apoptosis signal regulating kinase-1
- COMPARE
computerized pattern recognition algorithm
- DMSO
dimethyl sulphoxide
- DTNB
5,5′-dithiobis(2-nitrobenzoate)
- EndoPDI
endothelial-specific protein disulphide isomerase
- FITC
fluorescein isothiocyanate
- HRP
horseradish peroxidase
- HUVEC
human umbilical vein endothelial cells
- NADPH
nicotinamide adenine dinucleotide phosphate reduced
- NCI
National Cancer Institute
- PMX464
4-(benzothiazol-2-yl)-4-hydroxycyclohexa-2,5-dienone
- Trx1
thioredoxin
- TrxR1
thioredoxin reductase
Conflict of interest
The authors state no conflict of interest.
References
- Arnould S, Guichard S, Hennebelle I, Cassar G, Bugat R, Canal P. Contribution of apoptosis in the cytotoxicity of the oxaliplatin–irinotecan combination in the HT29 human colon adenocarcinoma cell line. Biochem Pharmacol. 2002;64:1215–1226. doi: 10.1016/s0006-2952(02)01291-1. [DOI] [PubMed] [Google Scholar]
- Berggren M, Gallegos A, Gasdaska JR, Gasdaska PY, Warneke J, Powis G. Thioredoxin and thioredoxin reductase gene expression in human tumors and cell lines, and the effects of serum stimulation and hypoxia. Anticancer Res. 1996;16:3459–3466. [PubMed] [Google Scholar]
- Bradshaw TD, Matthews CS, Cookson J, Chew EH, Shah M, Bailey K, et al. Elucidation of thioredoxin as a molecular target for antitumor quinols. Cancer Res. 2005;65:3911–3919. doi: 10.1158/0008-5472.CAN-04-4141. [DOI] [PubMed] [Google Scholar]
- Chew EH, Matthews CS, Zhang J, McCarroll AJ, Hagen T, Stevens MF, et al. Antitumor quinols: role of glutathione in modulating quinol-induced apoptosis and identification of putative cellular protein targets. Biochem Biophys Res Commun. 2006;346:242–251. doi: 10.1016/j.bbrc.2006.05.106. [DOI] [PubMed] [Google Scholar]
- Fernando MR, Nanri H, Yoshitake S, Nagata-Kuno K, Minakami S. Thioredoxin regenerates proteins inactivated by oxidative stress in endothelial cells. Eur J Biochem. 1992;209:917–922. doi: 10.1111/j.1432-1033.1992.tb17363.x. [DOI] [PubMed] [Google Scholar]
- Graven KK, Molvar C, Roncarati JS, Klahn BD, Lowrey S, Farber HW. Identification of protein disulfide isomerase as an endothelial hypoxic stress protein. Am J Physiol Lung Cell Mol Physiol. 2002;282:L996–L1003. doi: 10.1152/ajplung.00359.2001. [DOI] [PubMed] [Google Scholar]
- Hedley D, Pintilie M, Woo J, Nicklee T, Morrison A, Birle D, et al. Up-regulation of the redox mediators thioredoxin and apurinic/apyrimidinic excision (APE)/Ref-1 in hypoxic microregions of invasive cervical carcinomas, mapped using multispectral, wide-field fluorescence image analysis. Am J Pathol. 2004;164:557–565. doi: 10.1016/S0002-9440(10)63145-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren A, Bjornstedt M. Thioredoxin and thioredoxin reductase. Methods Enzymol. 1995;252:199–208. doi: 10.1016/0076-6879(95)52023-6. [DOI] [PubMed] [Google Scholar]
- Jaffe EA, Nachman RL, Becker CG, Minick CR. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973;52:2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel MW, Kirkpatrick DL, Johnson JI, Powis G. Cell line-directed screening assay for inhibitors of thioredoxin reductase signalling as potential anti-cancer drugs. Anticancer Drug Des. 1997;12:659–670. [PubMed] [Google Scholar]
- Kusaka M, Sudo K, Matsutani E, Kozai Y, Marui S, Fujita T, et al. Cytostatic inhibition of endothelial cell growth by the angiogenesis inhibitor TNP-470 (AGM-1470) Br J Cancer. 1994;69:212–216. doi: 10.1038/bjc.1994.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent TC, Moore EC, Reichard P. Enzymatic synthesis of deoxyribonucleotides IV. Isolation and characterization of thioredoxin, the hydrogen donor from Escherichia coli B. J Biol Chem. 1964;239:3436–3444. [PubMed] [Google Scholar]
- Liebmann JE, Cook JA, Lipschultz C, Teague D, Fisher J, Mitchell JB. Cytotoxic studies of paclitaxel (Taxol) in human tumour cell lines. Br J Cancer. 1993;68:1104–1109. doi: 10.1038/bjc.1993.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luthman M, Holmgren A. Rat liver thioredoxin and thioredoxin reductase: purification and characterization. Biochemistry. 1982;21:6628–6633. doi: 10.1021/bi00269a003. [DOI] [PubMed] [Google Scholar]
- Mukherjee A, Westwell AD, Bradshaw TD, Stevens MF, Carmichael J, Martin SG. Cytotoxic and antiangiogenic activity of AW464 (NSC 706704), a novel thioredoxin inhibitor: an in vitro study. Br J Cancer. 2005;92:350–358. doi: 10.1038/sj.bjc.6602338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallis M, Bradshaw TD, Westwell AD, Grundy M, Stevens MF, Russell N. Induction of apoptosis without redox catastrophe by thioredoxin-inhibitory compounds. Biochem Pharmacol. 2003;66:1695–1705. doi: 10.1016/s0006-2952(03)00471-4. [DOI] [PubMed] [Google Scholar]
- Powis G, Montfort WR. Properties and biological activities of thioredoxins. Annu Rev Biophys Biomol Struct. 2001;30:421–455. doi: 10.1146/annurev.biophys.30.1.421. [DOI] [PubMed] [Google Scholar]
- Saitoh M, Nishitoh H, Fujii M, Takeda K, Tobiume K, Sawada Y, et al. Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. EMBO J. 1998;17:2596–2606. doi: 10.1093/emboj/17.9.2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders DE, Lawrence WD, Christensen C, Wappler NL, Ruan H, Deppe G. Paclitaxel-induced apoptosis in MCF-7 breast-cancer cells. Int J Cancer. 1997;70:214–220. doi: 10.1002/(sici)1097-0215(19970117)70:2<214::aid-ijc13>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Shi C, Yu L, Yang F, Yan J, Zeng H. A novel organoselenium compound induces cell cycle arrest and apoptosis in prostate cancer cell lines. Biochem Biophys Res Commun. 2003;309:578–583. doi: 10.1016/j.bbrc.2003.08.032. [DOI] [PubMed] [Google Scholar]
- Sullivan DC, Huminiecki L, Moore JW, Boyle JJ, Poulsom R, Creamer D, et al. EndoPDI, a novel protein-disulfide isomerase-like protein that is preferentially expressed in endothelial cells acts as a stress survival factor. J Biol Chem. 2003;278:47079–47088. doi: 10.1074/jbc.M308124200. [DOI] [PubMed] [Google Scholar]
- U-Taniguchi Y, Furuke K, Masutani H, Nakamura H, Yodoi J. Cell cycle inhibition of HTLV-I transformed T cell lines by retinoic acid: the possible therapeutic use of thioredoxin reductase inhibitors. Oncol Res. 1995;7:183–189. [PubMed] [Google Scholar]
- Vogt A, Tamura K, Watson S, Lazo JS. Antitumor imidazolyl disulfide IV-2 causes irreversible G(2)/M cell cycle arrest without hyperphosphorylation of cyclin-dependent kinase Cdk1. J Pharmacol Exp Ther. 2000;294:1070–1075. [PubMed] [Google Scholar]
- Wells G, Berry JM, Bradshaw TD, Burger AM, Seaton A, Wang B, et al. 4-Substituted 4-hydroxycyclohexa-2,5-dien-1-ones with selective activities against colon and renal cancer cell lines. J Med Chem. 2003;46:532–541. doi: 10.1021/jm020984y. [DOI] [PubMed] [Google Scholar]
- Yamada M, Tomida A, Yoshikawa H, Taketani Y, Tsuruo T. Increased expression of thioredoxin/adult T-cell leukemia-derived factor in cisplatin-resistant human cancer cell lines. Clin Cancer Res. 1996;2:427–432. [PubMed] [Google Scholar]
- Zhang L, Evans H, Huber K, Stevens MFG, Bradshaw T, Westwell A, et al. In vitro evaluation of potential thioredoxin inhibitors in breast cancer and endothelial cells Eur J Cancer (Suppl) 20064101(323) [Google Scholar]