Abstract
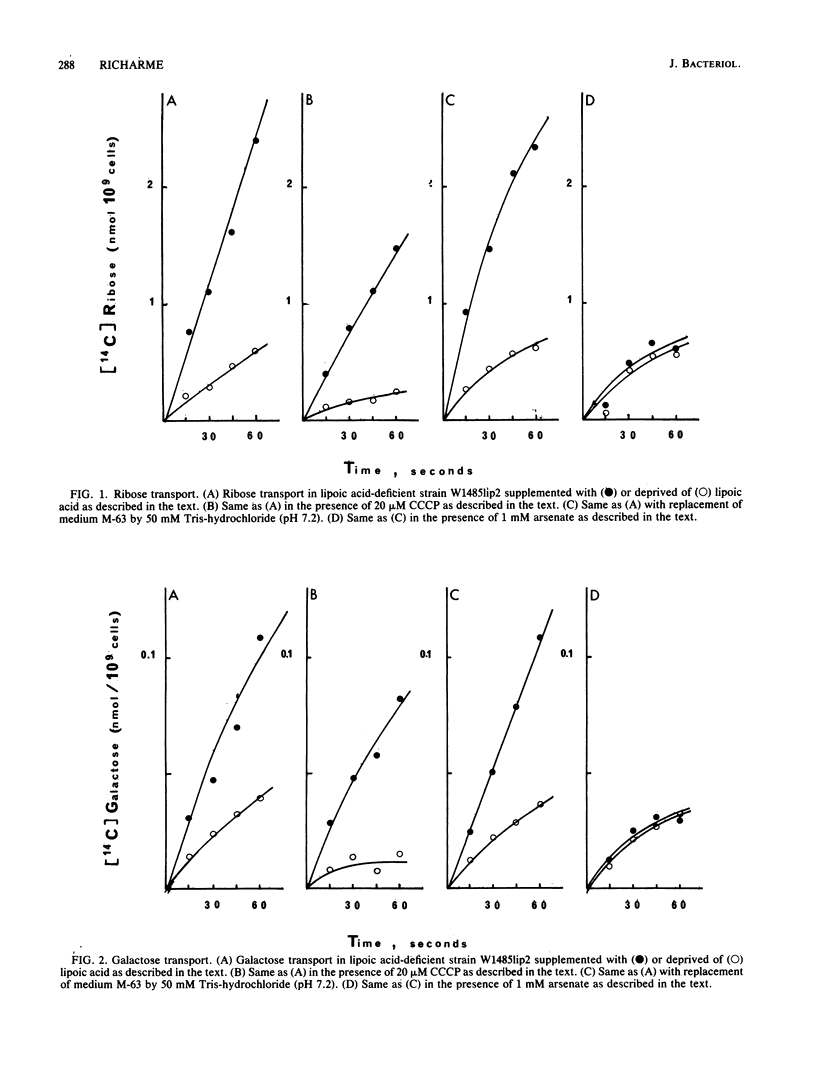
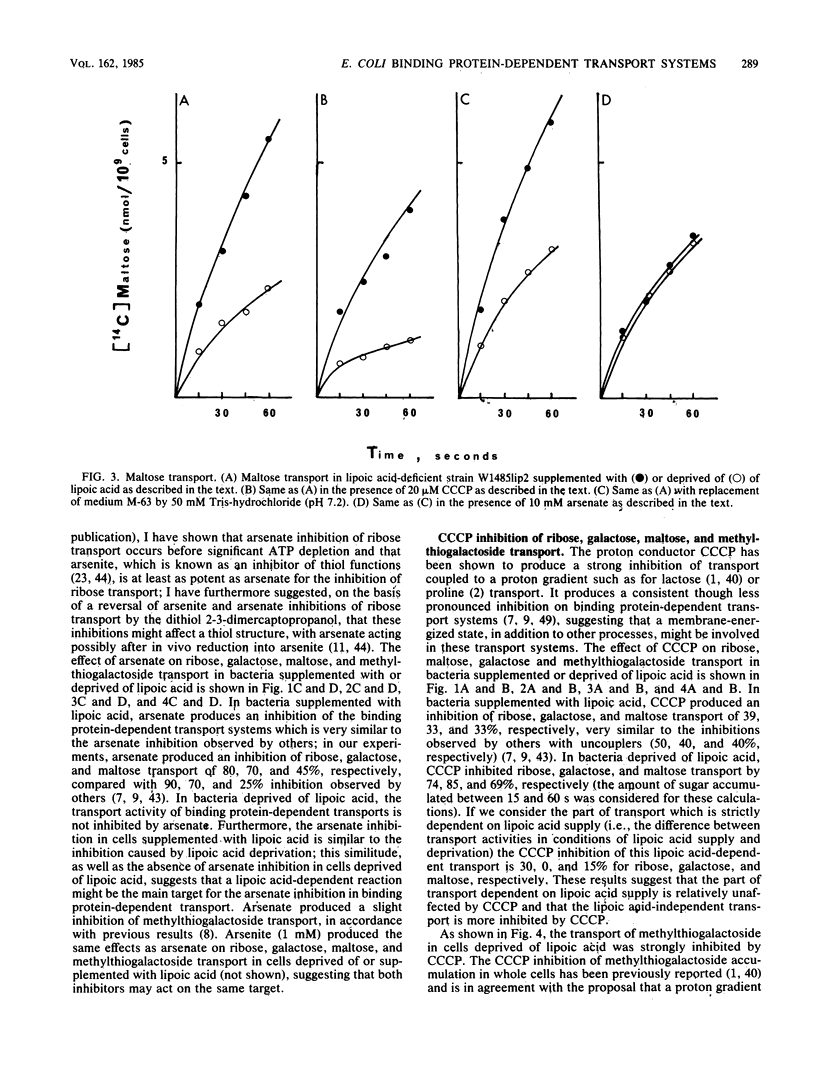
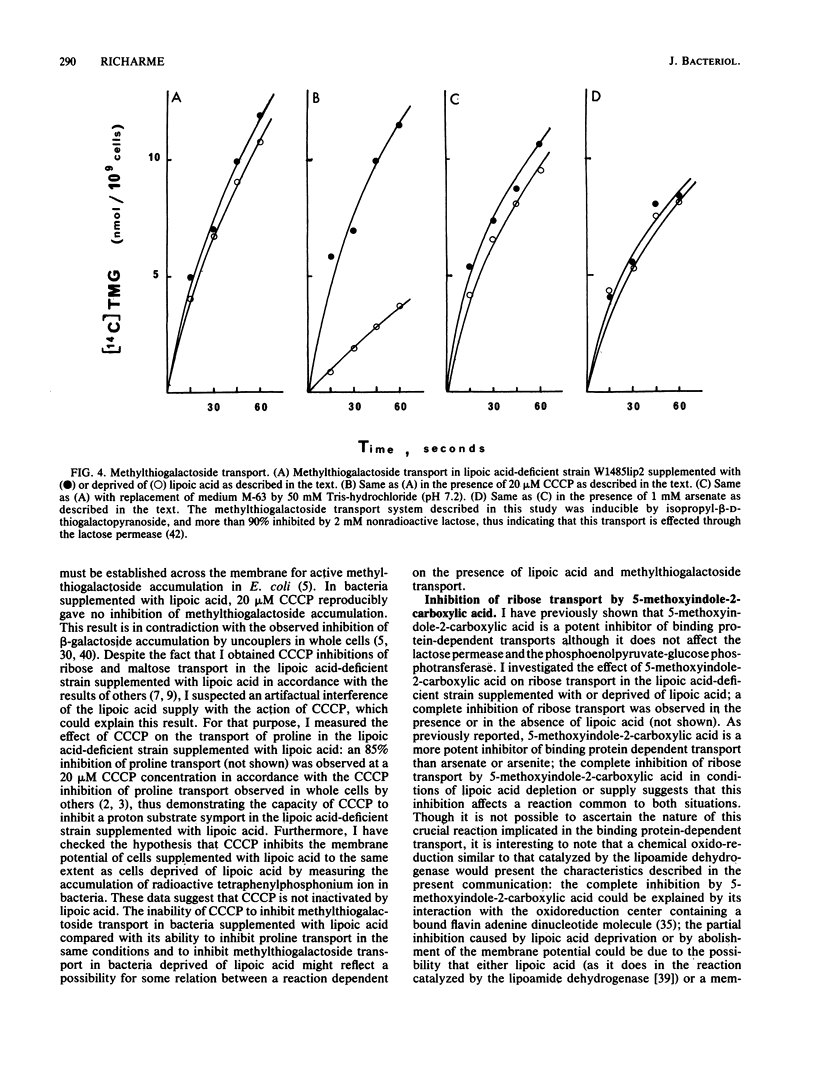
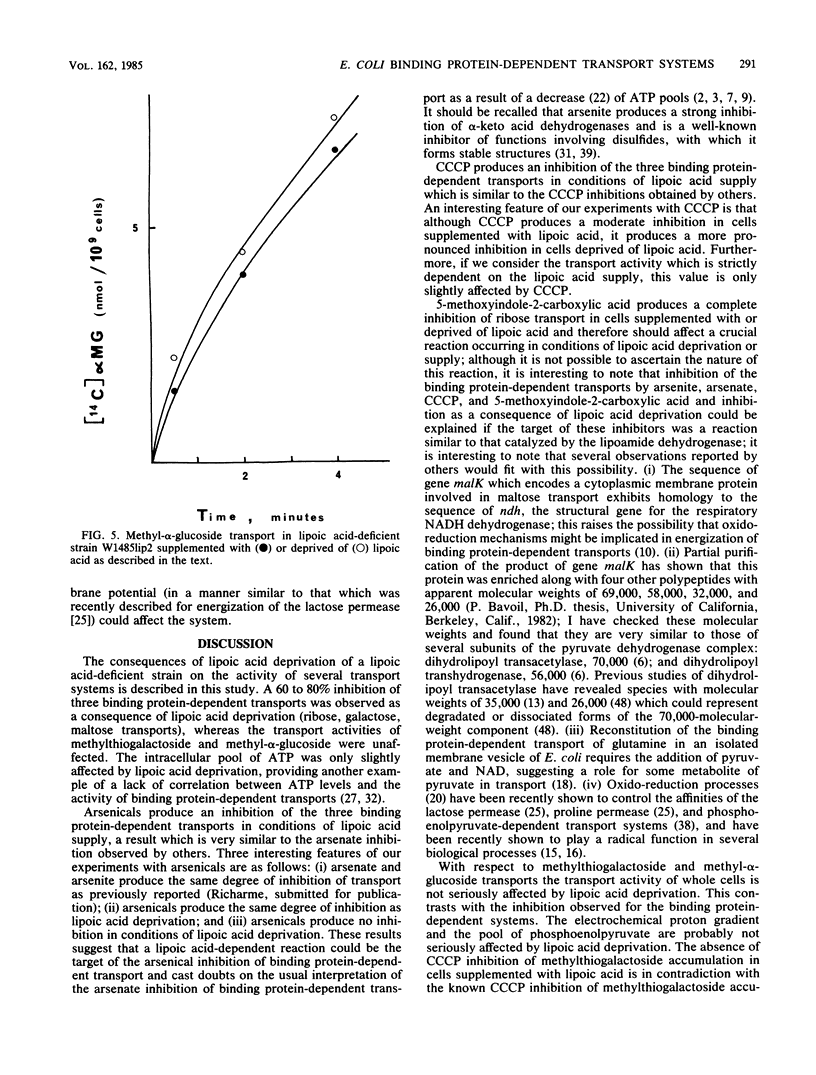
We describe the properties of the binding protein dependent-transport of ribose, galactose, and maltose and of the lactose permease, and the phosphoenolpyruvate-glucose phosphotransferase transport systems in a strain of Escherichia coli which is deficient in the synthesis of lipoic acid, a cofactor involved in alpha-keto acid dehydrogenation. Such a strain can grow in the absence of lipoic acid in minimal medium supplemented with acetate and succinate. Although the lactose permease and the phosphoenolypyruvate-glucose phosphotransferase are not affected by lipoic acid deprivation, the binding protein-dependent transports are reduced by 70% in conditions of lipoic acid deprivation when compared with their activity in conditions of lipoic acid supply. The remaining transport is not affected by arsenate but is inhibited by the uncoupler carbonylcyanide-m-chlorophenylhydrazone; however the lipoic acid-dependent transport is completely inhibited by arsenate and only weakly inhibited by carbonylcyanide-m-chlorophenylhydrazone. The known inhibitor of alpha-keto acid dehydrogenases, 5-methoxyindole-2-carboxylic acid, completely inhibits all binding protein-dependent transports whether in conditions of lipoic supply or deprivation; the results suggest a possible relation between binding protein-dependent transport and alpha-keto acid dehydrogenases and shed light on the inhibition of these transports by arsenicals and uncouplers.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnes E. M., Jr, Kaback H. R. Beta-galactoside transport in bacterial membrane preparations: energy coupling via membrane-bounded D-lactic dehydrogenase. Proc Natl Acad Sci U S A. 1970 Aug;66(4):1190–1198. doi: 10.1073/pnas.66.4.1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger E. A. Different mechanisms of energy coupling for the active transport of proline and glutamine in Escherichia coli. Proc Natl Acad Sci U S A. 1973 May;70(5):1514–1518. doi: 10.1073/pnas.70.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger E. A., Heppel L. A. Different mechanisms of energy coupling for the shock-sensitive and shock-resistant amino acid permeases of Escherichia coli. J Biol Chem. 1974 Dec 25;249(24):7747–7755. [PubMed] [Google Scholar]
- Boos W. The galactose binding protein and its relationship to the beta-methylgalactoside permease from Escherichia coli. Eur J Biochem. 1969 Aug;10(1):66–73. doi: 10.1111/j.1432-1033.1969.tb00656.x. [DOI] [PubMed] [Google Scholar]
- Booth I. R., Mitchell W. J., Hamilton W. A. Quantitative analysis of proton-linked transport systems. The lactose permease of Escherichia coli. Biochem J. 1979 Sep 15;182(3):687–696. doi: 10.1042/bj1820687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins J. H., Reed L. J. Acyl group and electron pair relay system: a network of interacting lipoyl moieties in the pyruvate and alpha-ketoglutarate dehydrogenase complexes from Escherichia coli. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4223–4227. doi: 10.1073/pnas.74.10.4223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis S. J. Mechanism of energy coupling for transport of D-ribose in Escherichia coli. J Bacteriol. 1974 Oct;120(1):295–303. doi: 10.1128/jb.120.1.295-303.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daruwalla K. R., Paxton A. T., Henderson P. J. Energization of the transport systems for arabinose and comparison with galactose transport in Escherichia coli. Biochem J. 1981 Dec 15;200(3):611–627. doi: 10.1042/bj2000611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferenci T., Boos W., Schwartz M., Szmelcman S. Energy-coupling of the transport system of Escherichia coli dependent on maltose-binding protein. Eur J Biochem. 1977 May 2;75(1):187–193. doi: 10.1111/j.1432-1033.1977.tb11516.x. [DOI] [PubMed] [Google Scholar]
- Gilson E., Nikaido H., Hofnung M. Sequence of the malK gene in E.coli K12. Nucleic Acids Res. 1982 Nov 25;10(22):7449–7458. doi: 10.1093/nar/10.22.7449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon J. J., Quastel J. H. Effects of organic arsenicals on enzyme systems. Biochem J. 1948;42(3):337–350. [PMC free article] [PubMed] [Google Scholar]
- Hanson R. L., Ray P. D., Walter P., Lardy H. A. Mode of action of hypoglycemic agents. I. Inhibition of gluconeogenesis by quinaldic acid and 5-methoxyindole-2-carboxylic acid. J Biol Chem. 1969 Aug 25;244(16):4351–4359. [PubMed] [Google Scholar]
- Henney H. R., Jr, Willms C. R., Muramatsu T., Mukherjee B. B., Reed L. J. Alpha-keto acid dehydrogenase complexes. VII. Isolation and partial characterization of the polypeptide chains in the dihydrolipoyl transacetylase of Escherichia coli. J Biol Chem. 1967 Mar 10;242(5):898–901. [PubMed] [Google Scholar]
- Herbert A. A., Guest J. R. Biochemical and genetic studies with lysine+methionine mutants of Escherichia coli: lipoic acid and alpha-ketoglutarate dehydrogenase-less mutants. J Gen Microbiol. 1968 Oct;53(3):363–381. doi: 10.1099/00221287-53-3-363. [DOI] [PubMed] [Google Scholar]
- Holmgren A. Thioredoxin catalyzes the reduction of insulin disulfides by dithiothreitol and dihydrolipoamide. J Biol Chem. 1979 Oct 10;254(19):9627–9632. [PubMed] [Google Scholar]
- Hong J. S., Hunt A. G., Masters P. S., Lieberman M. A. Requirements of acetyl phosphate for the binding protein-dependent transport systems in Escherichia coli. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1213–1217. doi: 10.1073/pnas.76.3.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt A. G., Hong J. The reconstitution of binding protein-dependent active transport of glutamine in isolated membrane vesicles from Escherichia coli. J Biol Chem. 1981 Dec 10;256(23):11988–11991. [PubMed] [Google Scholar]
- Iida A., Harayama S., Iino T., Hazelbauer G. L. Molecular cloning and characterization of genes required for ribose transport and utilization in Escherichia coli K-12. J Bacteriol. 1984 May;158(2):674–682. doi: 10.1128/jb.158.2.674-682.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUNDIG W., GHOSH S., ROSEMAN S. PHOSPHATE BOUND TO HISTIDINE IN A PROTEIN AS AN INTERMEDIATE IN A NOVEL PHOSPHO-TRANSFERASE SYSTEM. Proc Natl Acad Sci U S A. 1964 Oct;52:1067–1074. doi: 10.1073/pnas.52.4.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaback H. R., Barnes E. M., Jr Mechanisms of active transport in isolated membrane vesicles. II. The mechanism of energy coupling between D-lactic dehydrogenase and beta-galactoside transport in membrane preparations from Escherichia coli. J Biol Chem. 1971 Sep 10;246(17):5523–5531. [PubMed] [Google Scholar]
- Kellermann O., Szmelcman S. Active transport of maltose in Escherichia coli K12. Involvement of a "periplasmic" maltose binding protein. Eur J Biochem. 1974 Aug 15;47(1):139–149. doi: 10.1111/j.1432-1033.1974.tb03677.x. [DOI] [PubMed] [Google Scholar]
- Klein W. L., Boyer P. D. Energization of active transport by Escherichia coli. J Biol Chem. 1972 Nov 25;247(22):7257–7265. [PubMed] [Google Scholar]
- Kobayashi H., Kin E., Anraku Y. Transport of sugars and amino acids in bacteria. X. Sources of energy and energy coupling reactions of the active transport systems for isoleucine and proline in E. coli. J Biochem. 1974 Aug;76(2):251–261. doi: 10.1093/oxfordjournals.jbchem.a130567. [DOI] [PubMed] [Google Scholar]
- Konings W. N., Robillard G. T. Physical mechanism for regulation of proton solute symport in Escherichia coli. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5480–5484. doi: 10.1073/pnas.79.18.5480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman M. A., Hong J. S. Energization of osmotic shock-sensitive transport systems in Escherichia coli requires more than ATP. Arch Biochem Biophys. 1976 Jan;172(1):312–315. doi: 10.1016/0003-9861(76)90080-1. [DOI] [PubMed] [Google Scholar]
- Pavlasova E., Harold F. M. Energy coupling in the transport of beta-galactosides by Escherichia coli: effect of proton conductors. J Bacteriol. 1969 Apr;98(1):198–204. doi: 10.1128/jb.98.1.198-204.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters R. A., Sinclair H. M., Thompson R. H. An analysis of the inhibition of pyruvate oxidation by arsenicals in relation to the enzyme theory of vesication. Biochem J. 1946;40(4):516–524. [PMC free article] [PubMed] [Google Scholar]
- Plate C. A. Requirement for membrane potential in active transport of glutamine by Escherichia coli. J Bacteriol. 1979 Jan;137(1):221–225. doi: 10.1128/jb.137.1.221-225.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plate C. A., Suit J. L., Jetten A. M., Luria S. E. Effects of colicin K on a mutant of Escherichia coli deficient in Ca 2+, Mg 2+-activated adenosine triphosphatase. J Biol Chem. 1974 Oct 10;249(19):6138–6143. [PubMed] [Google Scholar]
- REISS O. K., HELLERMAN L. Pyruvate utilization in heart sarcosomes; inhibition by an arsenoso compound and reactivation by lipoic acid. J Biol Chem. 1958 Mar;231(1):557–569. [PubMed] [Google Scholar]
- Ramos S., Kaback H. R. The relationship between the electrochemical proton gradient and active transport in Escherichia coli membrane vesicles. Biochemistry. 1977 Mar 8;16(5):854–859. doi: 10.1021/bi00624a007. [DOI] [PubMed] [Google Scholar]
- Reed J., Lardy H. A. Mode of action of hypoglycemic agents. 3. Studies on 5-methoxy indole-2-carboxylic acid and quinaldic acid. J Biol Chem. 1970 Oct 25;245(20):5297–5303. [PubMed] [Google Scholar]
- Robillard G. T., Konings W. N. Physical mechanism for regulation of phosphoenolpyruvate-dependent glucose transport activity in Escherichia coli. Biochemistry. 1981 Aug 18;20(17):5025–5032. doi: 10.1021/bi00520a032. [DOI] [PubMed] [Google Scholar]
- SANADI D. R., LANGLEY M., WHITE F. alpha-Ketoglutaric dehydrogenase. VII. The role of thioctic acid. J Biol Chem. 1959 Jan;234(1):183–187. [PubMed] [Google Scholar]
- Schairer H. U., Haddock B. A. -Galactoside accumulation in a Mg 2+ -,Ca 2+ -activated ATPase deficient mutant of E.coli. Biochem Biophys Res Commun. 1972 Aug 7;48(3):544–551. doi: 10.1016/0006-291x(72)90382-8. [DOI] [PubMed] [Google Scholar]
- Singh A. P., Bragg P. D. Energetics of galactose, proline, and glutamine transport in a cytochrome-deficient mutant of Salmonella typhimurium. J Supramol Struct. 1977;6(3):389–398. doi: 10.1002/jss.400060312. [DOI] [PubMed] [Google Scholar]
- Singh A. P., Bragg P. D. Lack of involvement of lipoic acid in membrane-associated energy transduction in Escherichia coli. Biochem Biophys Res Commun. 1978 Mar 15;81(1):161–167. doi: 10.1016/0006-291x(78)91644-3. [DOI] [PubMed] [Google Scholar]
- Vorisek J., Kepes A. Galactose transport in Escherichia coli and the galactose-binding protein. Eur J Biochem. 1972 Jul 24;28(3):364–372. doi: 10.1111/j.1432-1033.1972.tb01922.x. [DOI] [PubMed] [Google Scholar]
- West I. C., Mitchell P. Stoicheiometry of lactose-H+ symport across the plasma membrane of Escherichia coli. Biochem J. 1973 Mar;132(3):587–592. doi: 10.1042/bj1320587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West I., Mitchell P. Proton-coupled beta-galactoside translocation in non-metabolizing Escherichia coli. J Bioenerg. 1972 Aug;3(5):445–462. doi: 10.1007/BF01516082. [DOI] [PubMed] [Google Scholar]
- Willis R. C., Furlong C. E. Purification and properties of a ribose-binding protein from Escherichia coli. J Biol Chem. 1974 Nov 10;249(21):6926–6929. [PubMed] [Google Scholar]
- Wilson D. B. Source of energy for the Escherichia coli galactose transport systems induced by galactose. J Bacteriol. 1974 Nov;120(2):866–871. doi: 10.1128/jb.120.2.866-871.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilberstein D., Schuldiner S., Padan E. Proton electrochemical gradient in Escherichia coli cells and its relation to active transport of lactose. Biochemistry. 1979 Feb 20;18(4):669–673. doi: 10.1021/bi00571a018. [DOI] [PubMed] [Google Scholar]



