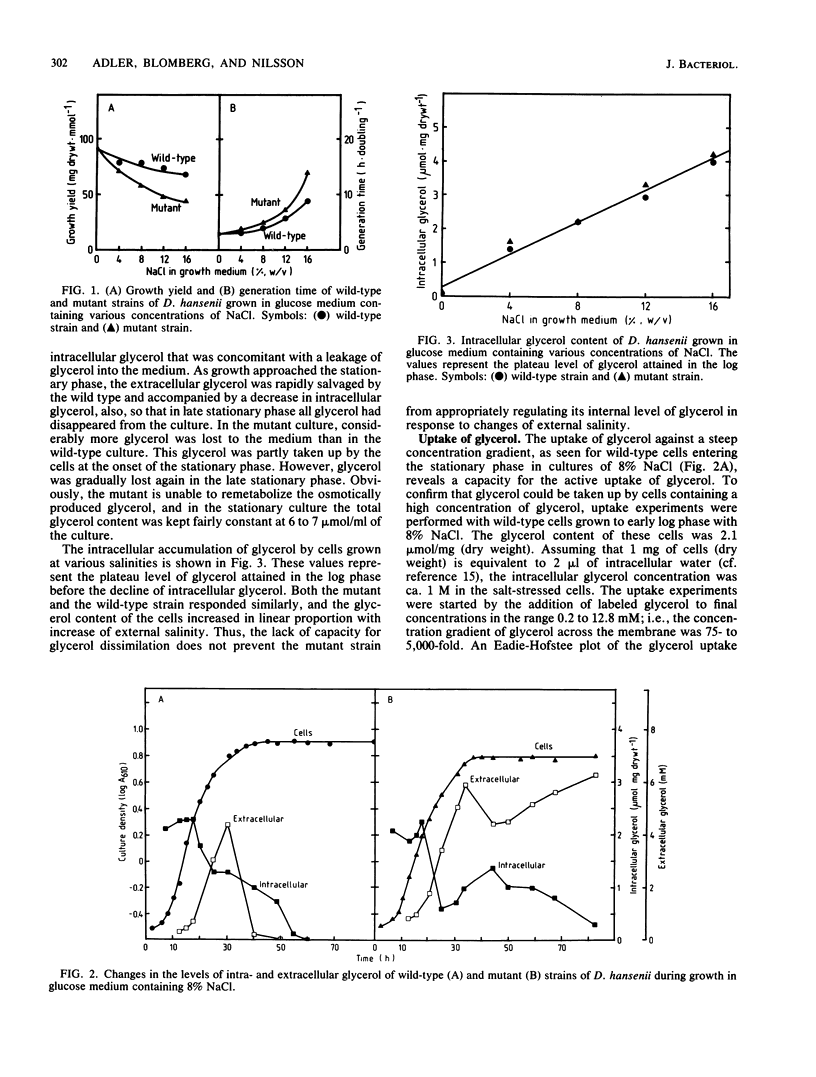
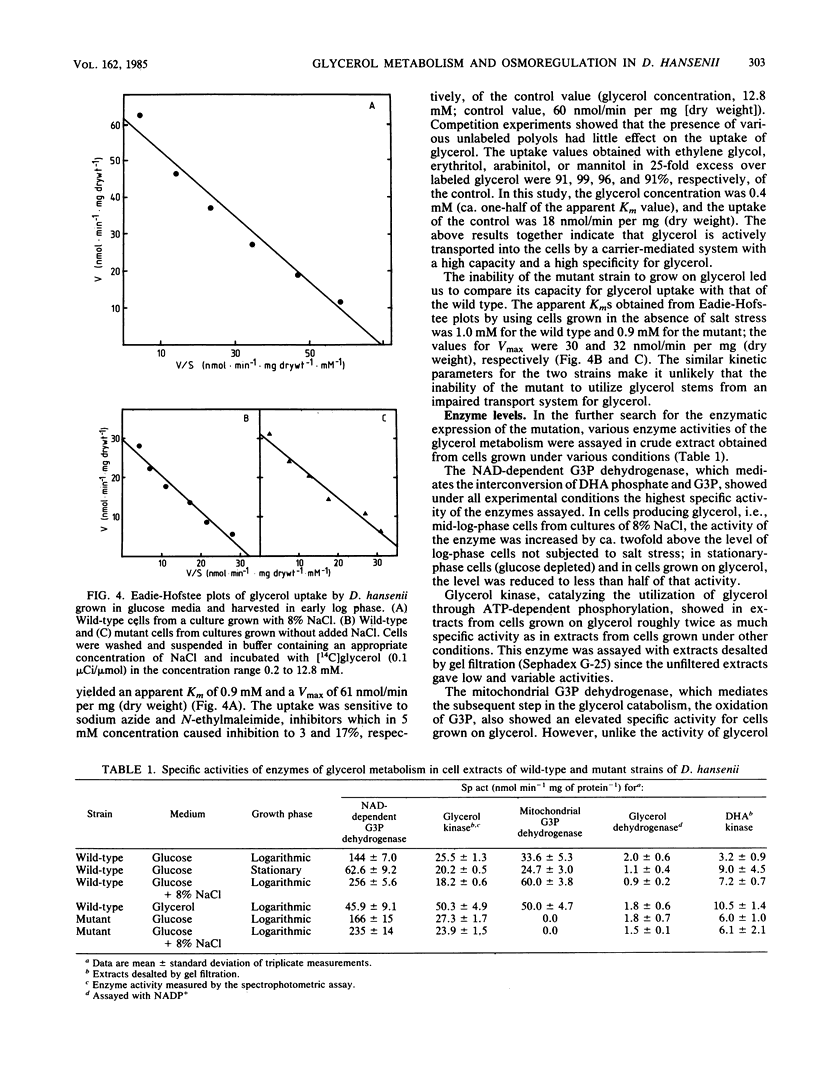
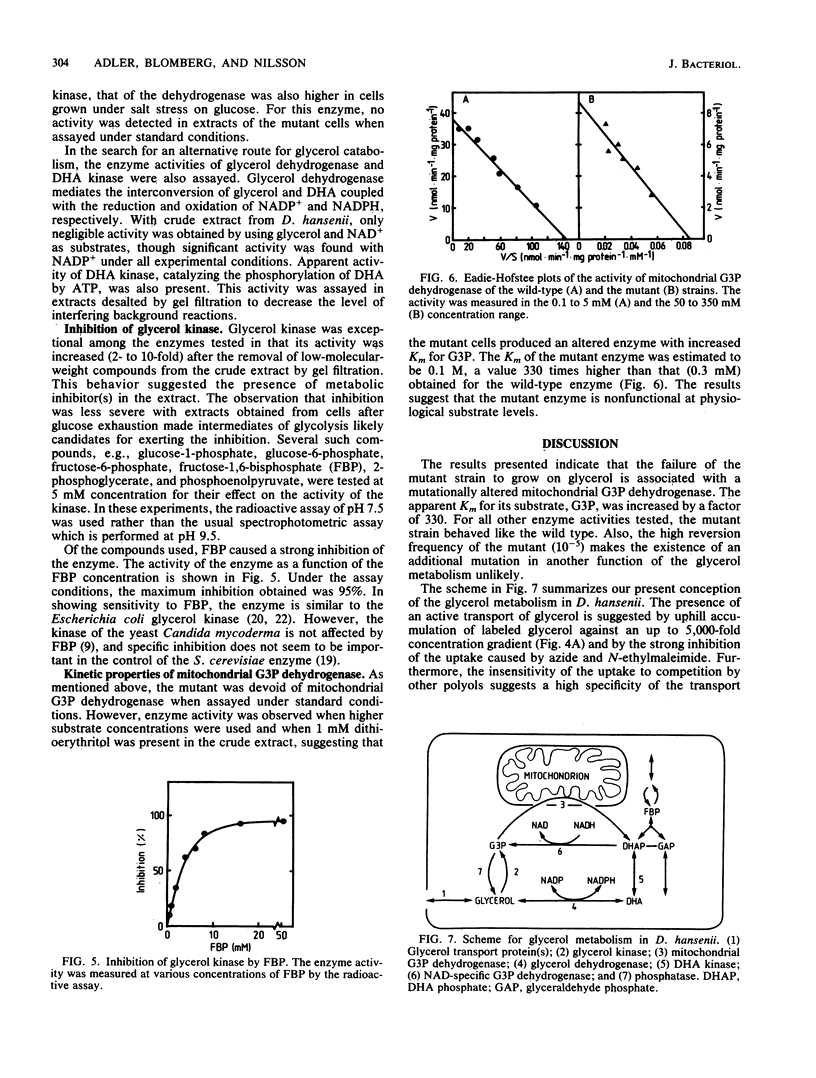
Abstract
A glycerol-nonutilizing mutant of the salt-tolerant yeast Debaryomyces hansenii was isolated. When subjected to salt stress the mutant produced glycerol, and the internal level of glycerol increased linearly in proportion to increases of external salinity as in the wild-type strain. However, at increased salinity the mutant showed a more pronounced decrease of growth rate and growth yield and lost more glycerol to the surrounding medium than did the wild type. Uptake experiments showed glycerol to be accumulated against a strong concentration gradient, and both strains displayed similar kinetic parameters for the uptake of glycerol. An examination of enzyme activities of the glycerol metabolism revealed that the apparent Km of the sn-glycerol 3-phosphate dehydrogenase (EC 1.1.99.5) was increased 330-fold for sn-glycerol 3-phosphate in the mutant. Based on the findings, a scheme for the pathways of glycerol metabolism is suggested.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cozzarelli N. R., Koch J. P., Hayashi S., Lin E. C. Growth stasis by accumulated L-alpha-glycerophosphate in Escherichia coli. J Bacteriol. 1965 Nov;90(5):1325–1329. doi: 10.1128/jb.90.5.1325-1329.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ESTABROOK R. W., SACKTOR B. alpha-Glycerophosphate oxidase of flight muscle mitochondria. J Biol Chem. 1958 Oct;233(4):1014–1019. [PubMed] [Google Scholar]
- Gancedo C., Gancedo J. M., Sols A. Glycerol metabolism in yeasts. Pathways of utilization and production. Eur J Biochem. 1968 Jul;5(2):165–172. doi: 10.1111/j.1432-1033.1968.tb00353.x. [DOI] [PubMed] [Google Scholar]
- Grunnet N., Lundquist F. Kinetics of glycerol kinases from mammalian liver and Candida mycoderma. Eur J Biochem. 1967 Dec;3(1):78–84. doi: 10.1111/j.1432-1033.1967.tb19500.x. [DOI] [PubMed] [Google Scholar]
- Gustafsson L., Norkrans B. On the mechanism of salt tolerance. Production of glycerol and heat during growth of Debaryomyces hansenii. Arch Microbiol. 1976 Nov 2;110(23):177–183. doi: 10.1007/BF00690226. [DOI] [PubMed] [Google Scholar]
- Klingenberg M. Localization of the glycerol-phosphate dehydrogenase in the outer phase of the mitochondrial inner membrane. Eur J Biochem. 1970 Apr;13(2):247–252. doi: 10.1111/j.1432-1033.1970.tb00924.x. [DOI] [PubMed] [Google Scholar]
- LIN E. C., KOCH J. P., CHUSED T. M., JORGENSEN S. E. Utilization of L-alpha-glycerophosphate by Escherichia coli without hydrolysis. Proc Natl Acad Sci U S A. 1962 Dec 15;48:2145–2150. doi: 10.1073/pnas.48.12.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIN E. C., MAGASANIK B. The activation of glycerol dehydrogenase from Aerobacter aerogenes by monovalent cations. J Biol Chem. 1960 Jun;235:1820–1823. [PubMed] [Google Scholar]
- Lin E. C. Glycerol dissimilation and its regulation in bacteria. Annu Rev Microbiol. 1976;30:535–578. doi: 10.1146/annurev.mi.30.100176.002535. [DOI] [PubMed] [Google Scholar]
- Meredith S. A., Romano A. H. Uptake and phosphorylation of 2-deoxy-D-glucose by wild type and respiration-deficient bakers' yeast. Biochim Biophys Acta. 1977 May 26;497(3):745–759. doi: 10.1016/0304-4165(77)90295-1. [DOI] [PubMed] [Google Scholar]
- Newsholme E. A., Robinson J., Taylor K. A radiochemical enzymatic activity assay for glycerol kinase and hexokinase. Biochim Biophys Acta. 1967 Mar 15;132(2):338–346. doi: 10.1016/0005-2744(67)90153-2. [DOI] [PubMed] [Google Scholar]
- Norkrans B., Kylin A. Regulation of the potassium to sodium ratio and of the osmotic potential in relation to salt tolerance in yeasts. J Bacteriol. 1969 Nov;100(2):836–845. doi: 10.1128/jb.100.2.836-845.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprague G. F., Cronan J. E. Isolation and characterization of Saccharomyces cerevisiae mutants defective in glycerol catabolism. J Bacteriol. 1977 Mar;129(3):1335–1342. doi: 10.1128/jb.129.3.1335-1342.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorner J. W., Paulus H. Catalytic and allosteric properties of glycerol kinase from Escherichia coli. J Biol Chem. 1973 Jun 10;248(11):3922–3932. [PubMed] [Google Scholar]
- Zwaig N., Lin E. C. Feedback inhibition of glycerol kinase, a catabolic enzyme in Escherichia coli. Science. 1966 Aug 12;153(3737):755–757. doi: 10.1126/science.153.3737.755. [DOI] [PubMed] [Google Scholar]



