Abstract
Although much is known about the growth factor changes in ocular tissues during various diseases, little is known about normal aging of the retina. In order to further understand normal aging in the retina, we characterized age-related changes of growth factor expression in three different ages of rat retina. Real time PCR and protein analysis was conducted to investigate steady state mRNA expression and protein levels of VEGF, VEGFR2, PEDF, Ang-1, Tie-2, EphB4 and ephrinB2 in the retina of 8, 22, and 32 month old Brown Norway X Fischer 344 F1 hybrid rats. An increase of VEGF protein levels was found at 32 months compared to 8 and 22 months of age. VEGFR2 protein was found to be increased at 22 and 32 months compared to 8 months. PEDF protein levels were reduced at 22 and 32 months. Tie-2 levels were found to be significantly decreased by 32 months compared to 8 months of age, while ephrinB2 was found to be significantly lower at both 22 and 32 months compared to 8 months of age. The increases found in VEGF and its receptor VEGFR2, with the simultaneous decrease of PEDF protein levels, may stimulate an environment that is well suited for neovascularization in the normal aging retina. Overall, these results suggest that normal aging produces substantial changes in gene expression and protein levels.
Keywords: retina, aging, growth factors
Introduction
Normal aging is associated with slowed responses in a number of aged organs. The elderly show a general decline in wound healing response, a decreased blood flow, and a decrease of angiogenesis in some tissues (Tsuchida 1993; Yazici et al. 2005). While much is known about various ocular diseases such as glaucoma and age-related macular degeneration, little is known about changes that are common to normal aging of the retina.
Unrepairable damage to the human retina begins with the loss of rod cells located inferior to the fovea in mid-adulthood and further degrades in the elderly (Curcio et al. 1993). Although most cellular mechanisms of normal aging in the retina and choroid are unknown, it is clear that rod photoreceptor cells of the retina die much more easily than cone cells (Gao and Hollyfield 1992). A loss of up to 30% of rods in the central retina was seen in normal eyes. With progressing age, cone numbers eventually decline. By the age of 90 years, a 40% reduction in cones has been reported (Bonnel et al. 2003). Due to rod cells being involved in black and white vision, the elderly often have a decreased ability to see at night or in areas of minimal light. The potential reasons for photoreceptor cell death in aging are unknown.
One possibility is that vascular changes may occur with age in the retina and release factors that are not supportive of photoreceptor survival. Vascular changes in the retinas of young and aged Wistar rats have recently been documented. Early aging (3 to 6 months) was characterized by vessel broadening, thickening of the basement membrane, altered length and orientation of desmin filaments in pericytes, and changes in a subset of pre-arteriolar sphincters. The latter stages of aging (22 to 30 months) in the retina included aneurysms, distorted vessels, and loss of capillary blood flow ability. A metabolic uncoupling leads to the reactivation of physiological hypoxia and therefore angiogenesis (Hughes et al. 2006). In addition, recent reports indicate that aging of the retina involves breakdown of the blood-retinal barrier and microglial activation (Chan-Ling et al. 2007). While many degenerative changes have been noted in normal aging, which growth factors are involved and their expression levels are yet to be determined.
This study investigates whether normal aging alters expression of a number of growth factors that are associated with vascular remodeling. Vascular endothelial growth factor (VEGF) is a potent angiogenic, vasopermeability factor mitogen that is expressed by endothelial cells involved in promoting proliferation, migration and vascular formation (Cross and Claesson-Welsh 2001). VEGF shows preferential binding to flt-1 (VEGF-R1) and flk-1/VEGFR-2 (Joussen et al. 2001). Previous work has established that VEGF is expressed on retinal endothelial cells (Kroll and Waltenberger 1998), pericytes (Darland et al. 2003), and glia of the retina (Stone et al. 1995). Binding of VEGF to one of its receptors will lead to the change of endothelial cells from a dormant to an active state. Pigment epithelium-derived factor (PEDF) is a well-known endogenous inhibitor of angiogenesis that has been found in almost all parts of the central nervous system, as well as in multiple areas of the eye (Holekamp et al. 2002). In contrast to VEGF, VEGFR2, and PEDF, which are involved in endothelial cell proliferation, angiopoietin-1 (Ang-1) is known to bind to its receptor, Tie-2, and primarily aid in new vessel stabilization (Hayes et al. 1999; Papapetropoulos et al. 1999). The Eph family of receptor tyrosine kinases and their ligands, the ephrins, have been implicated in many developmental processes including neuronal network formation, guidance of cell migration, and axonal pathfinding (Howard et al. 2003). In addition, these ligands and their receptors have been observed on retinal endothelial cells (Steinle et al. 2003).
Due to changes seen in vascular remodeling observed in the retina during ocular disease states (Churchill et al. 2006; Schlingemann 2004), it is hypothesized that normal aging will induce some changes in growth factor expression. However, without more information on growth factor expression throughout the normal aging process, there is no way to determine when age-related changes occur in the retina. If a change in growth factor expression is found in the normal aging retina, it may suggest that some disease states are merely an acceleration of normal aging.
Materials and Methods
Animals
Male F344 × BN F1 Hybrid rats age 8 months (n=10), 22 months (n=10), and 32 months (n=10) purchased from the National Institute of Aging (NIA) through Harlan were used to determine changes in growth factor activity in the retina with age. This rat strain was used because they show less age-related pathologies and biological variability (Phelan and Austad 1994). Rats were anesthetized using pentabarbitol (150 mg/kg) and the entire globe of each eye was removed. The cornea was cut and the lens and vitreous discarded. The retina was separated from the choroid and placed into tubes for RNA isolation and protein analysis. All procedures were approved by the Institutional Animal Care and Use Committee at Southern Illinois University-Carbondale.
RNA Isolation and Reverse Transcription
RNA isolation through real-time PCR was done as previously described (Smith et al. 2007). Primers specific to growth factors are listed in Table 1.
Table 1. Primers used in real-time PCR experiments.
List of primers used for real-time PCR. GAPDH is used as the housekeeping gene
| Primers | Sequence |
|---|---|
| VEGF | forward ACGAAAGCGCAAGAAATCCC
reverse TTAACTCAAGCTGCCTCGCC |
| VEGFR2 | forward TAGCACGACAGAGACTGTGAGG
reverse TGAGGTGAGAGAGATGGGTAGG |
| Angiopoietin1 | forward CCATGCTTGAGATAGGAACCAG
reverse TTCAAGTCGGGATGTTTGATTT |
| Tie-2 | forward CGGCTTAGTTCTCTGTGGAGTC
reverse GGCATCAGACACAAGAGGTAGG |
| PEDF | forward AAGAGTGCTTCCAGAATTGTG
reverse CCCAGTTGTTAATCTCCTGAAGG |
| Ephrin B2 | forward ATCAAATGGGTCTTTGGAGGGCCTG
reverse CGGAACCGAGGATGTTGTTCCCG |
| Eph B4 | forward CCAAGCTGTGTCCTATGAAG
reverse TAATTTAAGAACCCCTCCAAGC |
| GAPDH | forward TCCACCACCCTGTGCTGTA
reverse ACCACAGTCCATGCCATCAC |
Protein Expression
Western blot analysis was conducted as previously described (Smith, Sharma et al. 2007). Primary antibodies to VEGFR2 (1:500 dilution, Chemicon, Temecula, CA), PEDF (1:200 dilution, Bioproducts MD), Tie-2 (1:100 dilution, Zymed, San Francisco, CA), EphB4 (1:500 dilution, Chemicon), and ephrinB2 (1:500 dilution, Chemicon) were applied overnight at 4°C. Secondary antibodies conjugated to horseradish peroxidase were applied the following day, followed by detection using enhanced chemilluminescence (ECL) (Amersham Biosciences, Little Chalfort, England). Chemilluminescence images were viewed on a Kodak 2000r. Densitometry was conducted by using the data acquisition program, Kodak 1D. Upon completion of chemiluminescence, equal lane loading was checked by Ponceau S Solution (Sigma). Statistical analysis of results from 22 and 32 month old animals were compared to those from the 8 month control with one-way ANOVA tests using student Newman-Keuls post-hoc test in the Prism software program.
ELISA Assays
ELISA assays were used to measure changes in protein levels of VEGF (Quantikine, Minneapolis, MN) and Ang-1 (Ray BioTech, Norcross, GA). ELISA assays for each growth factor were conducted according to manufacturer’s instructions. Analysis was conducted as done for western blots using one-way ANOVA with post-hoc test of student Newman-Keuls. A significance of P<0.05 was accepted.
Results
Examination of real time PCR for VEGF revealed no significant change in steady state mRNA expression with age (Fig. 1A). However, protein levels were significantly upregulated by 32 months vs. 8 months (P<0.05, Fig. 1B), suggesting that VEGF activity is more active in aging.
Figure 1.
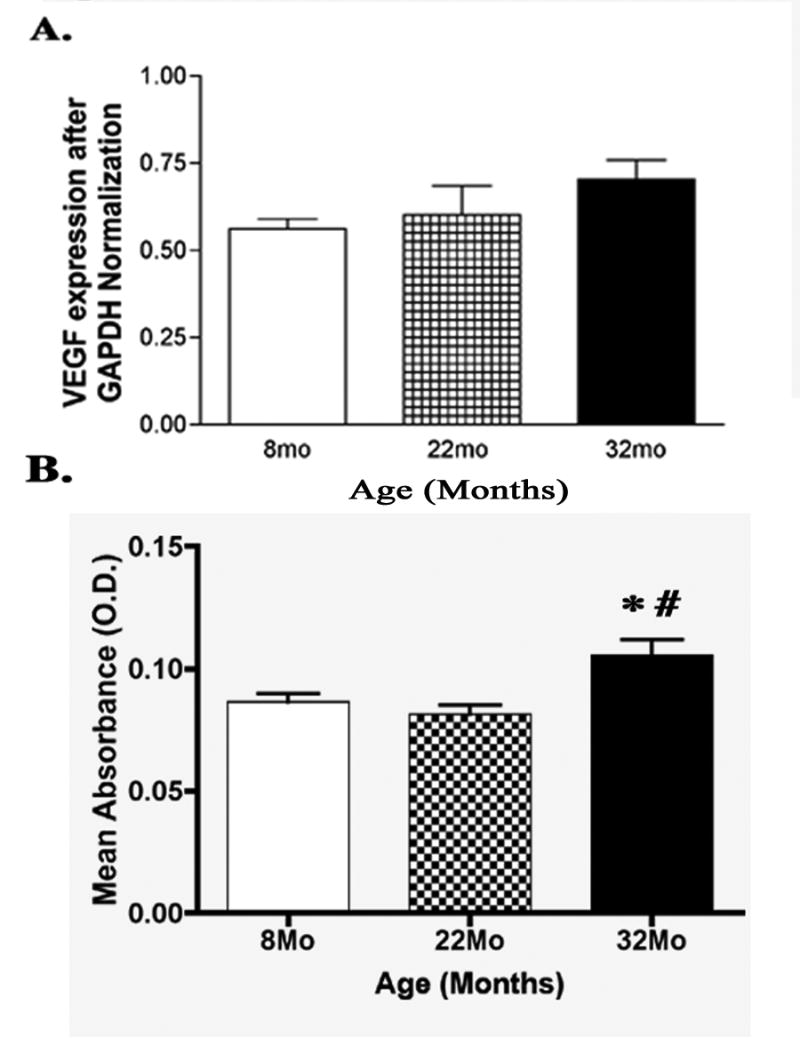
(A) Bar graph of real time PCR steady state mRNA expression of VEGF in the rat retina. One-way ANOVA tests revealed no significant change with age (N=6). (B) ELISA assay analysis comparing VEGF protein levels at 8, 22, and 32 months of age. Protein is significantly upregulated at 32 months compared to both 8 and 22 months of age (N=6, *P<0.05, #P<0.01).
VEGFR2, the primary receptor of VEGF, was found to have increased protein levels at 22 and 32 months of age compared to 8 months (P<0.05, Fig. 2B), but did not show a significant change in mRNA expression with age. The reason for the discrepancy between gene and protein data is unclear, however, it may be that the mRNA is not stable or requires post-translational modifications that are not detected by the western blot analysis for VEGFR2.
Figure 2.
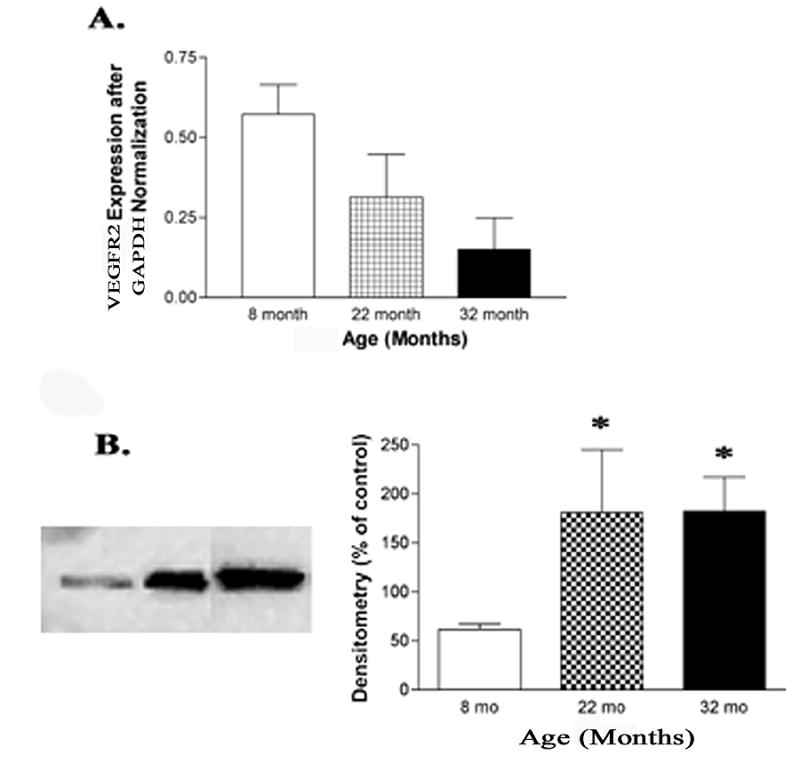
(A) Bar graph of real time PCR steady state mRNA expression of VEGFR2 in the retina found no significant change (N=6). (B) Representative western blot analysis and densitometry found protein expression of VEGFR2 to be significantly increased at 22 months and 32 months of age compared to 8 months of age (N=6, *P<0.05).
Although no significant change of steady state mRNA expression was found with age, western blot analysis revealed a significant decrease of PEDF protein levels at both 22 months and 32 months compared to 8 months of age (P<0.01, Fig. 3B). Since PEDF is normally accepted to be anti-angiogenic, the decrease in protein levels with age may offer clues to observed vascular growth in the retina with age.
Figure 3.

(A) Bar graph of PEDF real time PCR steady state mRNA expression found no significant changes at different ages (N=6). (B) Representative western blot and densitometry analysis found protein levels of PEDF to be significantly decreased at 22 and 32 months compared to 8 months of age (N=5, *P<0.01).
Real time PCR revealed a significant increase of mRNA expression of Ang-1 at 32 months compared to 8 months and 22 months of age of mRNA (P<0.05, Fig. 4A). No change was found in protein activity.
Figure 4.
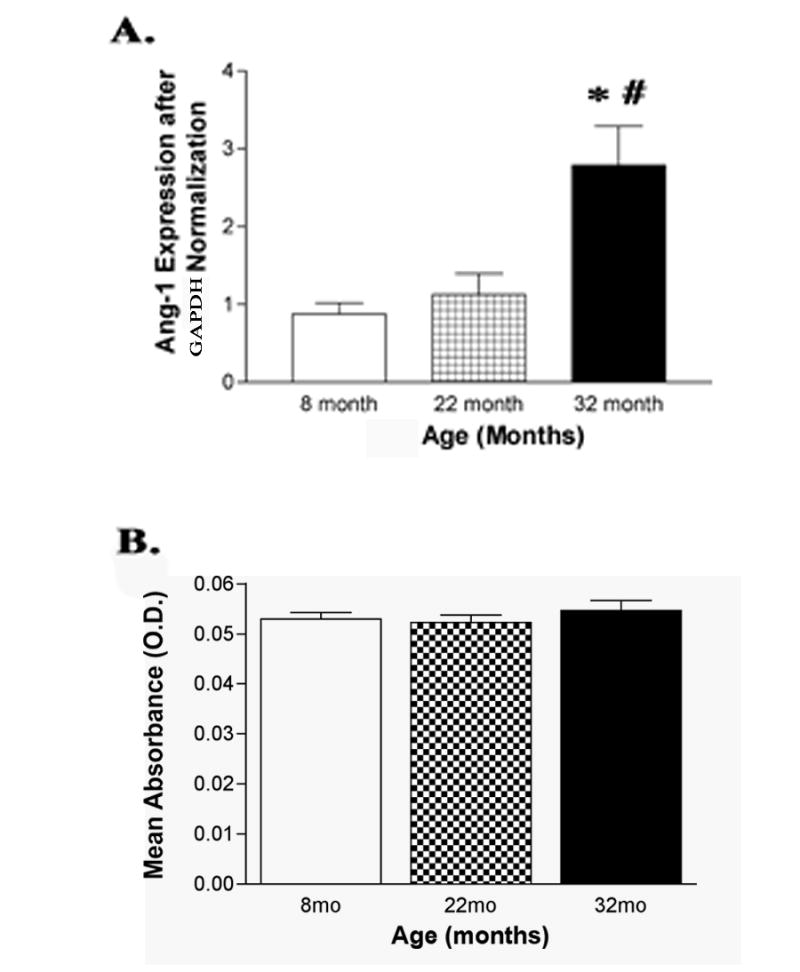
(A) Bar graph of real time steady state PCR mRNA expression of Ang-1 in the rat retina revealed a significant increase at 32 months compared to both 8 and 22 months of age (N=6). (B) ELISA assay analysis of Ang-1 protein concluded that there is no change with age (N=6, *P<0.01).
Steady state mRNA expression of Tie-2 rose to a point of significance by 32 months of age compared to 8 months (P<0.05, Fig. 5A). At the same time, protein levels of Tie-2 fell to a point of significance by 32 months vs. 8 months (P<0.05, Fig. 5B). The decline in Tie-2 protein levels may suggest that neovascularization is not occurring or is not at the stage for stabilization.
Figure 5.

(A) Bar graph of Tie-2 real time PCR steady state mRNA expression representing a significant upregulation at 32 months compared to 8 months of age (N=6, *P<0.05). (B) Representative western blot analysis and densitometry found Tie-2 protein levels to be significantly decreased at 32 months compared to 8 months of age (N=6, *P<0.05).
Steady state mRNA levels of ephrinB2 were not changed in the retina with age. However, protein levels were significantly downregulated at both 22 and 32 months compared to 8 months of age (P<0.01, Fig. 6B).
Figure 6.
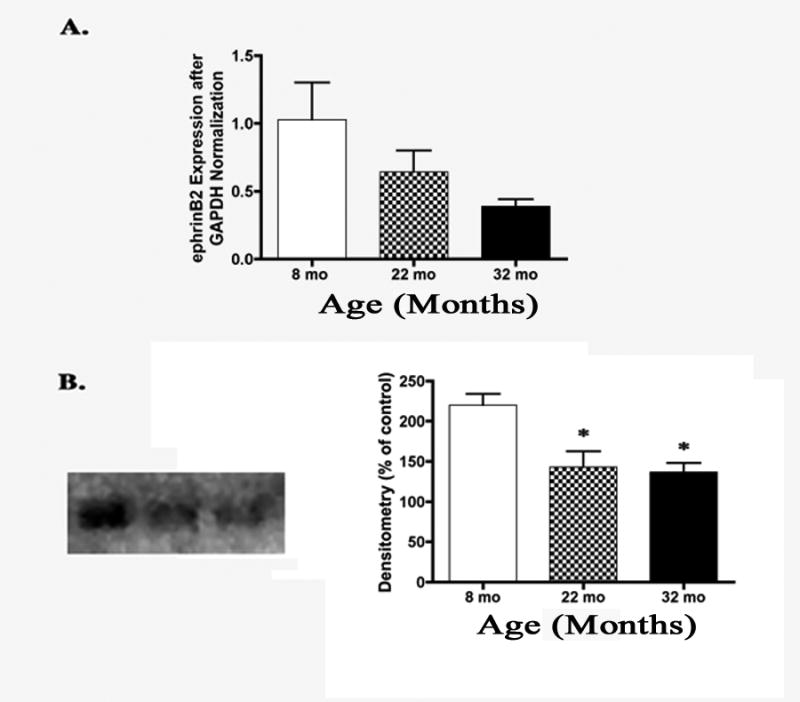
(A) Bar graph of real time PCR steady state mRNA expression of ephrinB2 showed a trend to decrease from 8 months to 32 months of age. However, it did not decrease to a point of significance (N=7). (B) Representative western blot analysis and densitometry showed a significant decrease of ephrinB2 protein levels at 22 months and 32 months compared to 8 months of age (N=6, *P<0.01).
Although no significant change in protein levels of EphB4 was found in the aging retina of rats (Fig. 7B), analysis of real time PCR showed that mRNA expression was found to be significantly upregulated by 22 months, and then significantly downregulated by 32 months as compared to 22 months of age (P<0.05, Fig. 7A). Decreased protein levels of ephrin B2 with no change in Eph B4 receptor protein may indicate that vascular remodeling is not occurring or is not using the ephrin pathway for this remodeling.
Figure 7.
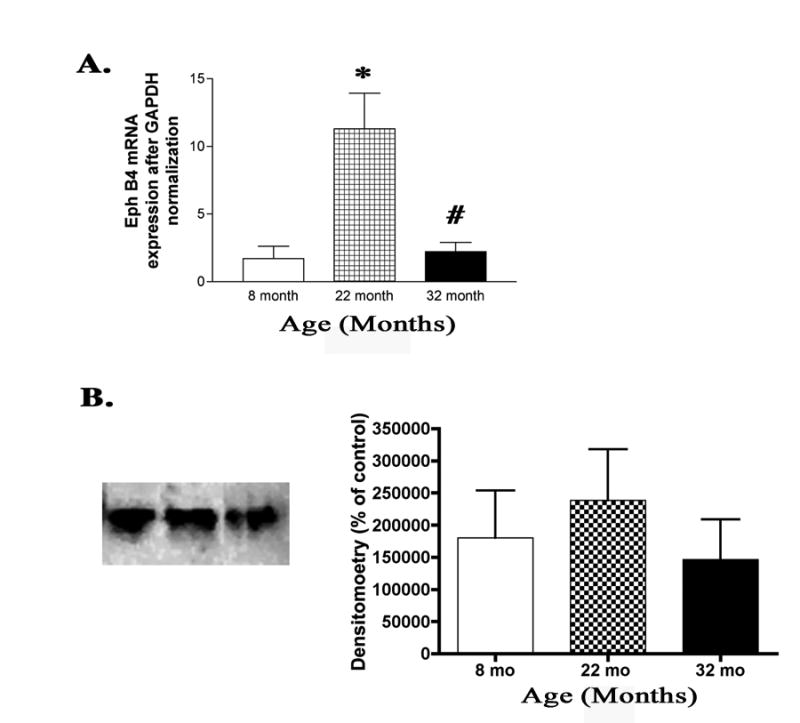
(A) Bar graph of real time PCR steady state mRNA expression of EphB4 found a significant increase at 22 months compared to 8 months. By 32 months of age, steady state mRNA expression had fallen significantly from 22 months to its 8 month expression level (N=4, *P<0.01, #P<0.01). (B) Representative western blot analysis and densitometry showed no significant change in protein levels in the retina as the rats age (N=6).
Discussion
The purpose of the present study was to characterize changes in growth factor expression in the rat retina with normal aging. Many changes in growth factor expression have been documented in various disease states of the eye. Without a greater knowledge of changes in growth factor expression with normal aging, it is difficult to compare with altered growth factor levels observed in the various disease states. It has been found that patients with proliferative retinopathies have increased levels of VEGF in active states of the disease (Adamis et al. 1994; Aiello et al. 1994). The synthesis and secretion of PEDF has been found to decrease with age, and it is known to be downregulated in human and experimental conditions with ocular neovascularization, such as proliferative retinopathy and exudative AMD (Holekamp, Bouck et al. 2002; Marciniak et al. 2006; Ohno-Matsui et al. 2001). It has also been found that Ang-1 and Tie-2 immunoreactivity were present in choroidal neovascular cells from AMD patients (Otani et al. 1999). Little is known about the role of EphB4 and ephrinB2 in aging. It is not known however, what role, if any, changes in growth factors play in normal retinal aging.
VEGF-A, one of the most potent angiogenic factors, has been reported to be responsible for retinal vascular development in localized areas (Curatola et al. 2005). In the present experiment, we found VEGF to have significantly increased protein levels at 32 months compared to 8 months and 22 months of age. This increase of protein level in normal aging could lead to the formation of new, yet leaky vessels that could invade the retinal tissue. This indicates that an increase of VEGF may occur in both normal aging and age-related disease. VEGFR2, the primary receptor for VEGF, is also associated with the formation of new vessels and localized to endothelial cells (Shalaby et al. 1995). It has been found that ischemia will lead to an increase of VEGFR2 in young endothelial nitric oxide synthase-deficient mice, while older animals do not show an increase of VEGFR2 expression, thus showing a decreased ability to synthesize VEGFR2 with age (Qian et al. 2006). In contrast, the results of the present study show that protein levels of VEGFR2 were found to be upregulated at 22 and 32 months compared to 8 months of age. The upregulation of protein levels of VEGFR2 suggests that the normal aging rat retina is capable of vascular remodeling. The combined elevation in expression of VEGF and VEGFR2 found in this study could suggest new vessel growth could occur in the retina with age. This implies that normal aging of the retina may involve vascular growth similar to that observed in AMD in the choroid.
PEDF, an endogenous inhibitor of angiogenesis, is known to increase the growth and survival of photoreceptor cells of the retina as well as protect the immature neuron cells from apoptosis (Marciniak, Butwicka et al. 2006). Our findings indicate that although there is no change in steady state mRNA expression of PEDF, there is a decrease in protein level in the rat retina at both 22 months and 32 months compared to 8 months of age. Without the protective effects of PEDF, photoreceptor cells may have a difficult time surviving in the elderly retina. Work has been done to indicate that aging of the retina is associated with a decrease in photoreceptor numbers, as well as ERG wave amplitudes (Militante and Lombardini 2004). Furthermore, the synthesis and secretion of PEDF has been found to be more effective in young human retinoblastoma cells and decreases during aging (Marciniak, Butwicka et al. 2006). Since PEDF often changes in the opposite direction of VEGF (Holekamp, Bouck et al. 2002), PEDF may contribute to maintaining the equilibrium of neovascularization in ocular tissues. The decrease of PEDF protein levels found to occur with age, along with a simultaneous increase of VEGF and VEGFR2 expression, could favor neovascularization. While the loss of photoreceptor due to the decrease in PEDF should decrease the oxygen demand in the retina, the morphological changes occurring in the aged retina may still activate a “physiological hypoxia (Chan-Ling et al. 1995; Hughes, Gardiner et al. 2006),” which would increase both VEGF and VEGFR2. In this way, the potential for increased angiogenesis may occur in the elderly eye and could be caused by two different factors. It is possible that the vessel development experienced with age is due not only to the retina’s increased ability to create new vessels, but also to the retina’s decreased ability to stop new vessel escalation. Decreases of PEDF observed in this study may lead to both an increase in photoreceptor cell death and indirectly to increase vascular remodeling.
New blood vessels formed from VEGF and VEGFR2 expression in local areas are completed by Ang-1 and Tie-2 stabilization. Due to Tie-2 and Ang-1 being involved in vessel stabilization, these factors are not expressed in vast quantities until the latter stages of angiogenesis. Ang-1 induced activation of Tie-2 results in the remodeling and stabilization of vessels by inducing endothelial cells to associate with their matrix and recruit pericytes to support the vessels (Kanda et al. 2005). It has been found in one study that Ang-1 and Tie-2 immunoreactivity were present in choroidal neovascular cells from AMD patients (Otani, Takagi et al. 1999). The increased expression found in this study of both Ang-1 and Tie-2 steady state mRNA expression without increased protein levels of Ang-1 suggests that the Ang-1 mRNA is not stable enough to translate into protein. The decreased protein levels of Tie-2 at 32 months suggests that there is likely less recruitment of mural cells to aid in stabilization in the normal aging process. This again points to a more volatile vasculature in normal aging retina.
Findings of Ephs and ephrins have shown them to be involved in the developmental process of the retinogeniculate map (Cang et al. 2005). Other research also suggests a role of maintenance and cell migration of laminar boundaries (Vidovic and Marotte 2003). Although no changes were found in protein levels, EphB4 mRNA expression was found to be significantly high at 22 months compared to 8 months, then significantly decreased by 32 months. We believe that this lack of translation from mRNA to protein is due to the mRNA not being stable, or the mRNA is degraded before protein can be made. Protein for the ligand of EphB4, ephrinB2, was found to be significantly decreased at both 22 and 32 months compared to 8 months of age. This decreased expression of ephrinB2 suggests that the retina is not attempting to increase vessel growth through mechanisms mediated by ephrinB2.
Our hypothesis that growth factor expression does change with age in the rat retina has been supported. The findings of this study suggest that the normal aging retina is a tissue that may produce an environment well suited for neovascularization much like that of the choroid in AMD. Further investigations into the regulation of growth factor expression with age should be conducted to better understand the difference between normal aging and disease states. In addition, more work is needed to explain the discrepancy between gene expression and protein levels of the various growth factors in age. It is unclear whether the altered mRNA expression cannot be translated to protein or is degraded. Alternatively, it may be something in the cellular milieu that leads to increased protein levels without increased mRNA expression.
Overall, increases found in VEGF and VEGFR2 protein levels suggest that vascular remodeling is possible during normal aging of the rat retina. The simultaneous decrease in PEDF expression with age further supports an unstable retinal vasculature with normal aging. The decrease in protein levels of PEDF with age could also be a mechanism the loss of photoreceptor cells seen in the elderly. Without proper stabilization from Ang-1 and Tie-2, new leaking vessels may bleed throughout the retina, thus contributing to a diminished vision found in the elderly. In conclusion, it appears that normal aging is associated with substantial changes in growth factor levels in the retina, and these changes may be associated with the morphological changes observed in aging.
Acknowledgments
This work was supported by NIA R01AG027827A to JJS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adamis AP, Miller JW, Bernal MT, D’Amico DJ, Folkman J, Yeo TK, Yeo KT. Increased vascular endothelial growth factor levels in the vitreous of eyes with proliferative diabetic retinopathy. Am J Ophthalmol. 1994;118(4):445–50. doi: 10.1016/s0002-9394(14)75794-0. [DOI] [PubMed] [Google Scholar]
- Aiello LP, Avery RL, Arrigg PG, Keyt BA, Jampel HD, Shah ST, Pasquale LR, Thieme H, Iwamoto MA, Park JE. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994;331(22):1480–1487. doi: 10.1056/NEJM199412013312203. [DOI] [PubMed] [Google Scholar]
- Bonnel S, Mohand-Said S, Sahel JA. The aging of the retina. Exp Gerontol. 2003;38(8):825–31. doi: 10.1016/s0531-5565(03)00093-7. [DOI] [PubMed] [Google Scholar]
- Cang J, Kaneko M, Yamada J, Woods G, Stryker MP, Feldheim DA. Ephrin-as guide the formation of functional maps in the visual cortex. Neuron. 2005;48(4):577–89. doi: 10.1016/j.neuron.2005.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan-Ling T, Gock B, Stone J. The effect of oxygen on vasoformative cell division. Evidence that ‘physiological hypoxia’ is the stimulus for normal retinal vasculogenesis. Invest Ophthalmol Vis Sci. 1995;36(7):1201–14. [PubMed] [Google Scholar]
- Chan-Ling T, Hughes S, Baxter L, Rosinova E, McGregor I, Morcos Y, van Nieuwenhuyzen P, Hu P. Inflammation and breakdown of the blood-retinal barrier during ‘physiological aging’ in the rat retina: a model for CNS aging. Microcirculation. 2007;14(1):63–76. doi: 10.1080/10739680601073451. [DOI] [PubMed] [Google Scholar]
- Churchill AJ, Carter JG, Lovell HC, Ramsden C, Turner SJ, Yeung A, Escardo J, Atan D. VEGF polymorphisms are associated with neovascular age-related macular degeneration. Hum Mol Genet. 2006;15(19):2955–61. doi: 10.1093/hmg/ddl238. [DOI] [PubMed] [Google Scholar]
- Cross MJ, Claesson-Welsh L. FGF and VEGF function in angiogenesis: signalling pathways, biological responses and therapeutic inhibition. Trends Pharmacol Sci. 2001;22(4):201–7. doi: 10.1016/s0165-6147(00)01676-x. [DOI] [PubMed] [Google Scholar]
- Curatola AM, Moscatelli D, Norris A, Hendricks-Munoz K. Retinal blood vessels develop in response to local VEGF-A signals in the absence of blood flow. Exp Eye Res. 2005;81(2):147–58. doi: 10.1016/j.exer.2005.06.001. [DOI] [PubMed] [Google Scholar]
- Curcio CA, Millican CL, Allen KA, Kalina RE. Aging of the human photoreceptor mosaic: evidence for selective vulnerability of rods in central retina. Invest Ophthalmol Vis Sci. 1993;34(12):3278–96. [PubMed] [Google Scholar]
- Darland DC, Massingham LJ, Smith SR, Piek E, Saint-Geniez M, D’Amore PA. Pericyte production of cell-associated VEGF is differentiation-dependent and is associated with endothelial survival. Dev Biol. 2003;264(1):275–88. doi: 10.1016/j.ydbio.2003.08.015. [DOI] [PubMed] [Google Scholar]
- Gao H, Hollyfield JG. Aging of the human retina. Differential loss of neurons and retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 1992;33(1):1–17. [PubMed] [Google Scholar]
- Hayes AJ, Huang WQ, Mallah J, Yang D, Lippman ME, Li LY. Angiopoietin-1 and its receptor Tie-2 participate in the regulation of capillary-like tubule formation and survival of endothelial cells. Microvasc Res. 1999;58(3):224–237. doi: 10.1006/mvre.1999.2179. [DOI] [PubMed] [Google Scholar]
- Holekamp NM, Bouck N, Volpert O. Pigment epithelium-derived factor is deficient in the vitreous of patients with choroidal neovascularization due to age-related macular degeneration. Am J Ophthalmol. 2002;134(2):220–7. doi: 10.1016/s0002-9394(02)01549-0. [DOI] [PubMed] [Google Scholar]
- Howard MA, Rodenas-Ruano A, Henkemeyer M, Martin GK, Lonsbury-Martin BL, Liebl DJ. Eph receptor deficiencies lead to altered cochlear function. Hear Res. 2003;178(12):118–30. doi: 10.1016/s0378-5955(03)00068-6. [DOI] [PubMed] [Google Scholar]
- Hughes S, Gardiner T, Hu P, Baxter L, Rosinova E, Chan-Ling T. Altered pericyte-endothelial relations in the rat retina during aging: implications for vessel stability. Neurobiol Aging. 2006;27(12):1838–47. doi: 10.1016/j.neurobiolaging.2005.10.021. [DOI] [PubMed] [Google Scholar]
- Joussen AM, Lemmen KD, Kirchhof B. Diabetic maculopathy. Etiological mechanisms and possible treatment approaches. Ophthalmologe. 2001;98(9):908–18. doi: 10.1007/s003470170073. quiz 919, 921. [DOI] [PubMed] [Google Scholar]
- Kanda S, Miyata Y, Mochizuki Y, Matsuyama T, Kanetake H. Angiopoietin 1 is mitogenic for cultured endothelial cells. Cancer Res. 2005;65(15):6820–7. doi: 10.1158/0008-5472.CAN-05-0522. [DOI] [PubMed] [Google Scholar]
- Kroll J, Waltenberger J. VEGF-A induces expression of eNOS and iNOS in endothelial cells via VEGF receptor-2 (KDR) Biochem Biophys Res Commun. 1998;252(3):743–746. doi: 10.1006/bbrc.1998.9719. [DOI] [PubMed] [Google Scholar]
- Marciniak K, Butwicka A, Nowak JZ. PEDF: an endogenous factor displaying potent neuroprotective, neurotrophic, and antiangiogenic activity. Postepy Hig Med Dosw (Online) 2006:60387–96. [PubMed] [Google Scholar]
- Militante J, Lombardini JB. Age-related retinal degeneration in animal models of aging: possible involvement of taurine deficiency and oxidative stress. Neurochem Res. 2004;29(1):151–60. doi: 10.1023/b:nere.0000010444.97959.1b. [DOI] [PubMed] [Google Scholar]
- Ohno-Matsui K, Morita I, Tombran-Tink J, Mrazek D, Onodera M, Uetama T, Hayano M, Murota SI, Mochizuki M. Novel mechanism for age-related macular degeneration: an equilibrium shift between the angiogenesis factors VEGF and PEDF. J Cell Physiol. 2001;189(3):323–33. doi: 10.1002/jcp.10026. [DOI] [PubMed] [Google Scholar]
- Otani A, Takagi H, Oh H, Koyama S, Matsumura M, Honda Y. Expressions of angiopoietins and Tie2 in human choroidal neovascular membranes. Invest Ophthalmol Vis Sci. 1999;40(9):1912–1920. [PubMed] [Google Scholar]
- Papapetropoulos A, Garcia-Cardena G, Dengler TJ, Maisonpierre PC, Yancopoulos GD, Sessa WC. Direct actions of angiopoietin-1 on human endothelium: evidence for network stabilization, cell survival, and interaction with other angiogenic growth factors. Lab Invest. 1999;79(2):213–23. [PubMed] [Google Scholar]
- Phelan JP, Austad SN. Selecting animal models of human aging: inbred strains often exhibit less biological uniformity than F1 hybrids. J Gerontol. 1994;49(1):B1–11. doi: 10.1093/geronj/49.1.b1. [DOI] [PubMed] [Google Scholar]
- Qian HS, de Resende MM, Beausejour C, Huw LY, Liu P, Rubanyi GM, Kauser K. Age-dependent acceleration of ischemic injury in endothelial nitric oxide synthase-deficient mice: potential role of impaired VEGF receptor 2 expression. J Cardiovasc Pharmacol. 2006;47(4):587–93. doi: 10.1097/01.fjc.0000211736.55583.5c. [DOI] [PubMed] [Google Scholar]
- Schlingemann RO. Role of growth factors and the wound healing response in age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 2004;242(1):91–101. doi: 10.1007/s00417-003-0828-0. [DOI] [PubMed] [Google Scholar]
- Shalaby F, Rossant J, Yamaguchi TP, Gertsenstein M, Wu XF, Breitman ML, Schuh AC. Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature. 1995;376(6535):62–66. doi: 10.1038/376062a0. [DOI] [PubMed] [Google Scholar]
- Smith CP, Sharma S, Steinle JJ. Age-related changes in sympathetic neurotransmission in rat retina and choroid. Exp Eye Res. 2007;84(1):75–81. doi: 10.1016/j.exer.2006.08.018. [DOI] [PubMed] [Google Scholar]
- Steinle JJ, Meininger CJ, Chowdhury U, Wu G, Granger HJ. Role of ephrin B2 in human retinal endothelial cell proliferation and migration. Cell Signal. 2003;15(11):1011–7. doi: 10.1016/s0898-6568(03)00072-x. [DOI] [PubMed] [Google Scholar]
- Stone J, Itin A, Alon T, Pe’er J, Gnessin H, Chan-Ling T, Keshet E. Development of retinal vasculature is mediated by hypoxia-induced vascular endothelial growth factor (VEGF) expression by neuroglia. J Neurosci. 1995;15(7 Pt 1):4738–47. doi: 10.1523/JNEUROSCI.15-07-04738.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchida Y. The effect of aging and arteriosclerosis on human skin blood flow. J Dermatol Sci. 1993;5(3):175–81. doi: 10.1016/0923-1811(93)90764-g. [DOI] [PubMed] [Google Scholar]
- Vidovic M, Marotte LR. Analysis of EphB receptors and their ligands in the developing retinocollicular system of the wallaby reveals dynamic patterns of expression in the retina. Eur J Neurosci. 2003;18(6):1549–58. doi: 10.1046/j.1460-9568.2003.02882.x. [DOI] [PubMed] [Google Scholar]
- Yazici B, Erdogmus B, Tugay A. Cerebral blood flow measurements of the extracranial carotid and vertebral arteries with Doppler ultrasonography in healthy adults. Diagn Interv Radiol. 2005;11(4):195–8. [PubMed] [Google Scholar]


