Abstract
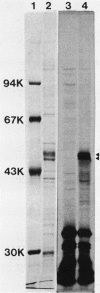
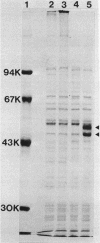
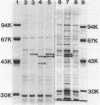
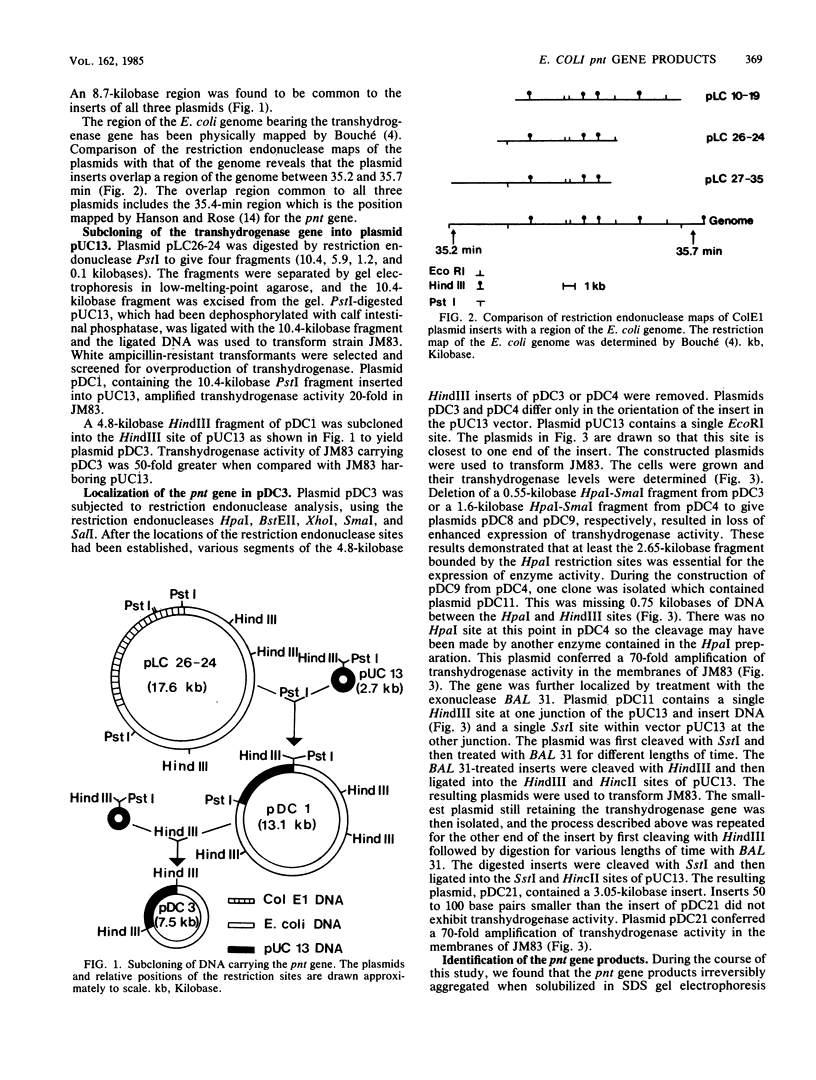
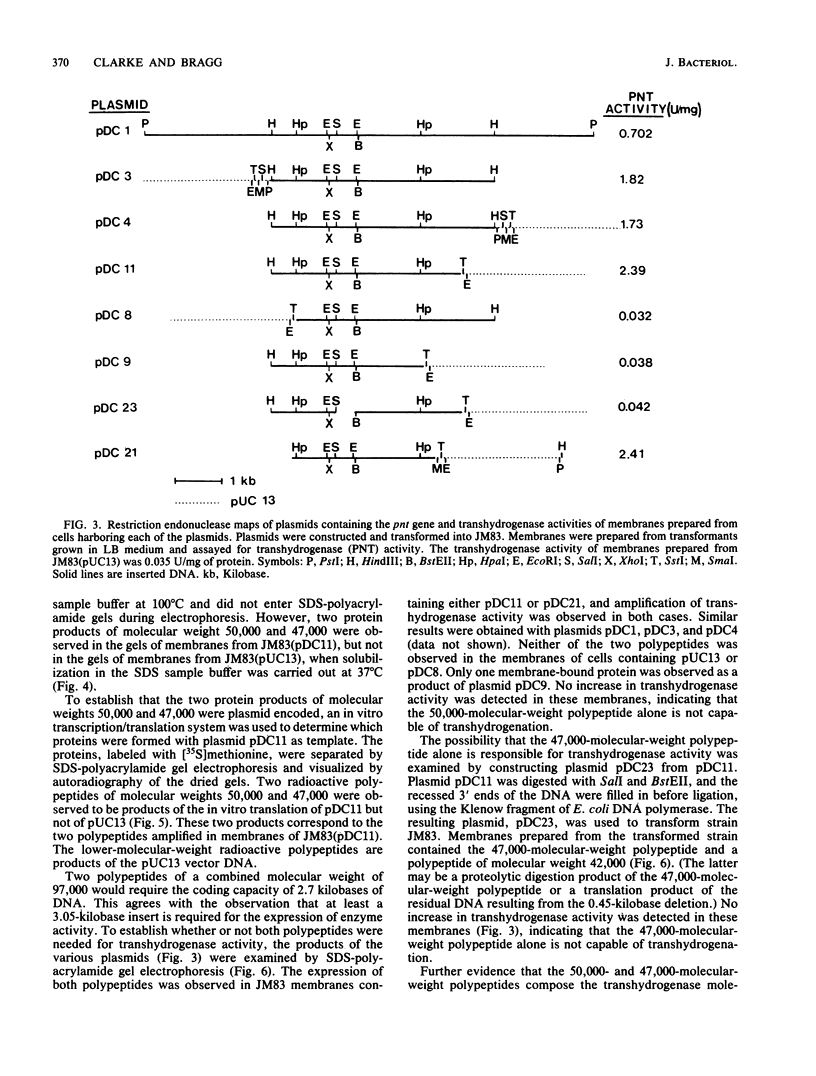
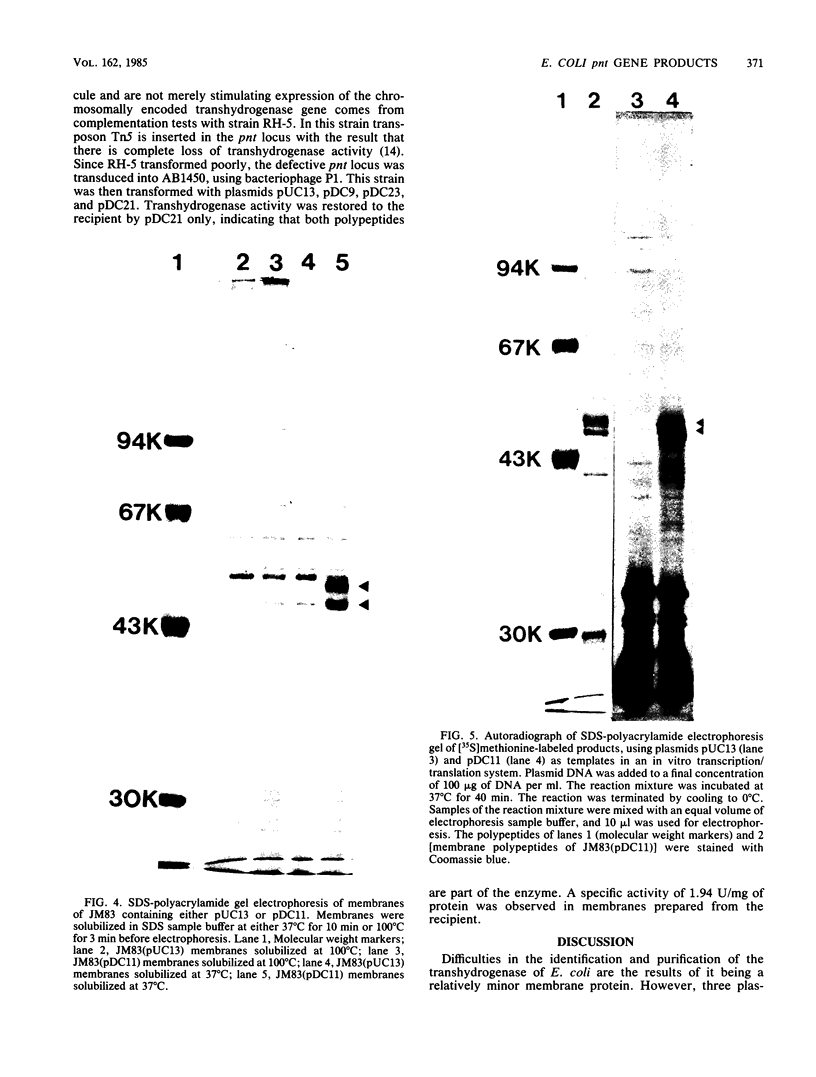
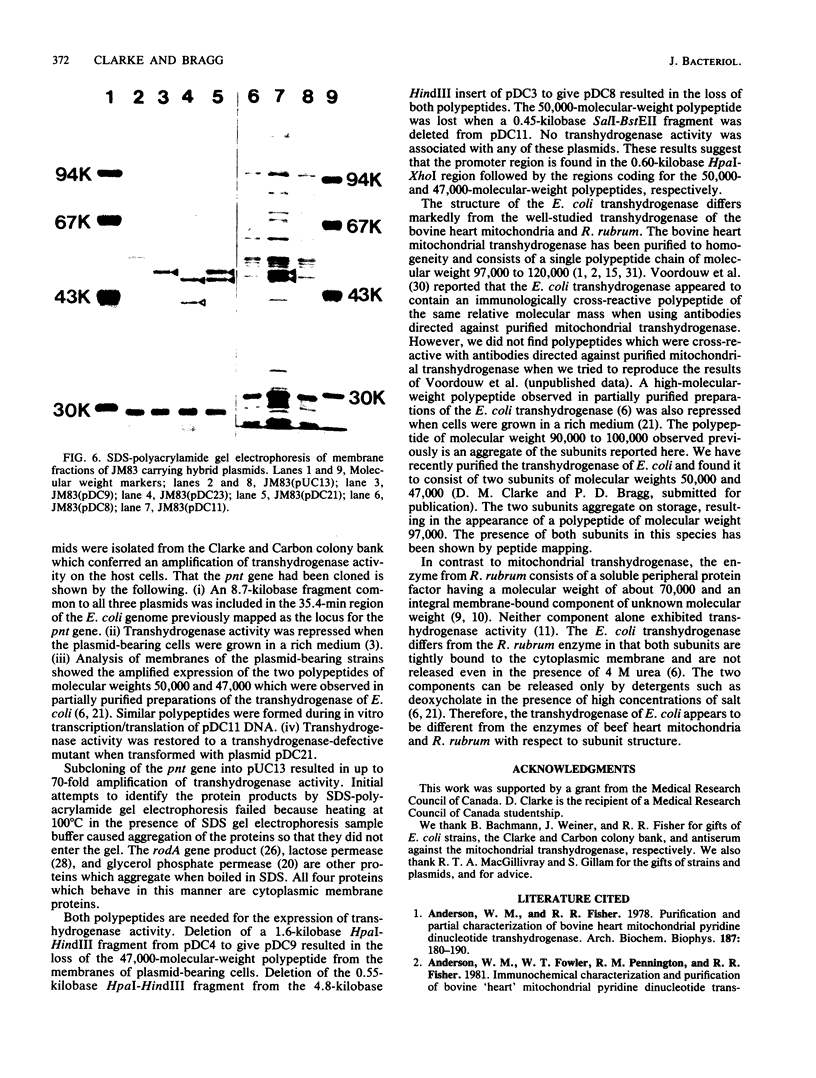
Based on the rationale that Escherichia coli cells harboring plasmids containing the pnt gene would contain elevated levels of enzyme, we have isolated three clones bearing the transhydrogenase gene from the Clarke and Carbon colony bank. The three plasmids were subjected to restriction endonuclease analysis. A 10.4-kilobase restriction fragment which overlapped all three plasmids was cloned into the PstI site of plasmid pUC13. Examination of several deletion derivatives of the resulting plasmid and subsequent treatment with exonuclease BAL 31 revealed that enhanced transhydrogenase expression was localized within a 3.05-kilobase segment. This segment was located at 35.4 min in the E. coli genome. Plasmid pDC21 conferred on its host 70-fold overproduction of transhydrogenase. The protein products of plasmids carrying the pnt gene were examined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis of membranes from cells containing the plasmids. Two polypeptides of molecular weights 50,000 and 47,000 were coded by the 3.05-kilobase fragment of pDC11. Both polypeptides were required for expression of transhydrogenase activity.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson W. M., Fisher R. R. Purification and partial characterization of bovine heart mitochondrial pyridine dinucleotide transhydrogenase. Arch Biochem Biophys. 1978 Apr 15;187(1):180–190. doi: 10.1016/0003-9861(78)90021-8. [DOI] [PubMed] [Google Scholar]
- Anderson W. M., Fowler W. T., Pennington R. M., Fisher R. R. Immunochemical characterization and purification of bovine heart mitochondrial pyridine dinucleotide transhydrogenase. J Biol Chem. 1981 Feb 25;256(4):1888–1895. [PubMed] [Google Scholar]
- Bouché J. P. Physical map of a 470 x 10(3) base-pair region flanking the terminus of DNA replication in the Escherichia coli K12 genome. J Mol Biol. 1982 Jan 5;154(1):1–20. doi: 10.1016/0022-2836(82)90413-2. [DOI] [PubMed] [Google Scholar]
- Bragg P. D., Davies P. L., Hou C. Function of energy-dependent transhydrogenase in Escherichia coli. Biochem Biophys Res Commun. 1972 Jun 9;47(5):1248–1255. doi: 10.1016/0006-291x(72)90969-2. [DOI] [PubMed] [Google Scholar]
- Burns D. M., Beacham I. R. A method for the ligation of DNA following isolation from low melting temperature agarose. Anal Biochem. 1983 Nov;135(1):48–51. doi: 10.1016/0003-2697(83)90728-5. [DOI] [PubMed] [Google Scholar]
- Clarke L., Carbon J. A colony bank containing synthetic Col El hybrid plasmids representative of the entire E. coli genome. Cell. 1976 Sep;9(1):91–99. doi: 10.1016/0092-8674(76)90055-6. [DOI] [PubMed] [Google Scholar]
- Dontsov A. E., Grinius L. L., Jasaitis A. A., Severina I. I., Skulachev V. P. A study on the mechanism of energy coupling in the redox chain. I. Transhydrogenase: the fourth site of the redox chain energy coupling. J Bioenerg. 1972 Jun;3(3):277–303. doi: 10.1007/BF01515975. [DOI] [PubMed] [Google Scholar]
- Fisher R. J., Sanadi D. R. Energy-linked nicotinamide adenine dinucleotide transhydrogenase in membrane particles from Escherchia coli. Biochim Biophys Acta. 1971 Aug 6;245(1):34–41. doi: 10.1016/0005-2728(71)90005-3. [DOI] [PubMed] [Google Scholar]
- Fisher R. R., Guillory R. J. A soluble factor related to the energy-linked transhydrogenase reaction of Rhodospirillum rubrum chromatophores. J Biol Chem. 1969 Feb 10;244(3):1078–1079. [PubMed] [Google Scholar]
- Fisher R. R., Guillory R. J. Partial resolution of energy-linked reactions in Rhodospirillum rubrum chromatophores. FEBS Lett. 1969 Apr;3(1):27–30. doi: 10.1016/0014-5793(69)80087-6. [DOI] [PubMed] [Google Scholar]
- Fisher R. R., Guillory R. J. Resolution of enzymes catalyzing energy-linked transhydrogenation. 3. Preparation and properties of Rhodospirillum rubrum transhydrogenase factor. J Biol Chem. 1971 Aug 10;246(15):4687–4693. [PubMed] [Google Scholar]
- Grinius L. L., Jasaitis A. A., Kadziauskas Y. P., Liberman E. A., Skulachev V. P., Topali V. P., Tsofina L. M., Vladimirova M. A. Conversion of biomembrane-produced energy into electric form. I. Submitochondrial particles. Biochim Biophys Acta. 1970 Aug 4;216(1):1–12. doi: 10.1016/0005-2728(70)90153-2. [DOI] [PubMed] [Google Scholar]
- Hanson R. L., Rose C. Effects of an insertion mutation in a locus affecting pyridine nucleotide transhydrogenase (pnt::Tn5) on the growth of Escherichia coli. J Bacteriol. 1980 Jan;141(1):401–404. doi: 10.1128/jb.141.1.401-404.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höjeberg B., Rydström J. Purification and molecular properties of reconstitutively active nicotinamide nucleotide transhydrogenase from beef heart mitochondria. Biochem Biophys Res Commun. 1977 Oct 24;78(4):1183–1190. doi: 10.1016/0006-291x(77)91418-8. [DOI] [PubMed] [Google Scholar]
- Katz L., Kingsbury D. T., Helinski D. R. Stimulation by cyclic adenosine monophosphate of plasmid deoxyribonucleic acid replication and catabolite repression of the plasmid deoxyribonucleic acid-protein relaxation complex. J Bacteriol. 1973 May;114(2):577–591. doi: 10.1128/jb.114.2.577-591.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz L., Williams P. H., Sato S., Leavitt R. W., Helinski D. R. Purification and characterization of covalently closed replicative intermediates of ColEl DNA from Escherichia coli. Biochemistry. 1977 Apr 19;16(8):1677–1683. doi: 10.1021/bi00627a024. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Larson T. J., Schumacher G., Boos W. Identification of the glpT-encoded sn-glycerol-3-phosphate permease of Escherichia coli, an oligomeric integral membrane protein. J Bacteriol. 1982 Dec;152(3):1008–1021. doi: 10.1128/jb.152.3.1008-1021.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang A., Houghton R. L. Structural aspects of the membrane-bound Escherichia colipyridine nucleotide transhydrogenase (EC 1.6.1.1). FEBS Lett. 1980 Jan 14;109(2):185–188. doi: 10.1016/0014-5793(80)81082-9. [DOI] [PubMed] [Google Scholar]
- Schwartz S. A., Helinski D. R. Purification and characterization of colicin E1. J Biol Chem. 1971 Oct 25;246(20):6318–6327. [PubMed] [Google Scholar]
- Skulachev V. P. Electric fields in coupling membranes. FEBS Lett. 1970 Dec 18;11(5):301–308. doi: 10.1016/0014-5793(70)80554-3. [DOI] [PubMed] [Google Scholar]
- Stoker N. G., Pratt J. M., Spratt B. G. Identification of the rodA gene product of Escherichia coli. J Bacteriol. 1983 Aug;155(2):854–859. doi: 10.1128/jb.155.2.854-859.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teather R. M., Müller-Hill B., Abrutsch U., Aichele G., Overath P. Amplification of the lactose carrier protein in Escherichia coli using a plasmid vector. Mol Gen Genet. 1978 Feb 27;159(3):239–248. doi: 10.1007/BF00268260. [DOI] [PubMed] [Google Scholar]
- Voordouw G., van der Vies S. M., Themmen A. P. Why are two different types of pyridine nucleotide transhydrogenase found in living organisms? Eur J Biochem. 1983 Apr 5;131(3):527–533. doi: 10.1111/j.1432-1033.1983.tb07293.x. [DOI] [PubMed] [Google Scholar]
- Wu L. N., Pennington R. M., Everett T. D., Fisher R. R. An improved method for the purification of bovine heart mitochondrial transhydrogenase. J Biol Chem. 1982 Apr 25;257(8):4052–4055. [PubMed] [Google Scholar]
- van de Stadt R. J., Nieuwenhuis F. J., van Dam K. On the reversibility of the energy-linked transhydrogenase. Biochim Biophys Acta. 1971 Apr 6;234(1):173–176. doi: 10.1016/0005-2728(71)90143-5. [DOI] [PubMed] [Google Scholar]