Abstract
The phosphatidylinositol-3-kinase (PI3K) and AKT (Protein Kinase B) signaling pathways play an important role in regulating cell cycle progression and cell survival. In previous studies, we demonstrated that AKT is activated in HTLV-1 transformed cells and that Tax activation of AKT is linked to p53 inhibition and cell survival. In the present study, we extend these observations to identify regulatory pathways affected by AKT in HTLV-1-transformed cells. We demonstrate that inhibition of AKT reduces the level of phosphorylated Bad, an important member of the pro-apoptotic family of proteins. Consistent with the decrease of phosphorylated Bad, cytochrome c is released from the mitochondria and caspase 9 is activated. Pre-treatment of the cells with caspase-9 specific inhibitor z-LEHD-FMK or pan caspase inhibitor Ac-DEVD-CHO prevented LY294002-induced apoptosis. Of interest, p53 siRNA prevents LY294002-induced apoptosis in HTLV-1-transformed cells, suggesting that p53 reactivation is linked to apoptosis. In conclusion, the AKT pathway is involved in targeting multiple proteins which regulate caspase- and p53-dependent apoptosis in HTLV-1-transformed cells. Since AKT inhibitors simultaneously inhibit NF-κB and activate p53, these drugs should be promising candidates for HTLV-1-associated cancer therapy.
Introduction
AKT, also known as protein kinase B (PKB), is a serine/threonine kinase and the primary mediator of PI3K-initiated signaling. AKT and upstream PI3K have a number of substrates that contribute to malignant transformation (Chang, Lee et al., 2003), and have been associated with diverse human cancers including prostate, breast, lung, melanoma and leukemia (Lin, Adam et al., 1999;Fry, 2001;Lin, Bohle et al., 2001;Krasilnikov, Adler et al., 1999;Martinez-Lorenzo, Anel et al., 2000;Nicholson & Anderson, 2002). AKT plays a crucial role in a variety of cellular events such as apoptosis, cell cycle progression and transcriptional regulation (Brazil, Yang et al., 2004). AKT’s ability to prevent apoptosis in some cells is established through phosphorylation and inhibition of pro-apoptotic mediators such as Bad and caspase-9 (Datta, Brunet et al., 1999). In other situations, AKT activates the transcription factor CREB, and the IκB kinase (IKK), a positive regulator of NF-κB, to regulate the expression of genes with anti-apoptotic activity (Fresno Vara, Casado et al., 2004). AKT also affects cell cycle progression by regulating cyclin D function. This is accomplished by phosphorylation of p27 and p21 by AKT. Phosphorylation restricts these proteins to the cytoplasm, effectively segregating the cell cycle inhibitors from CDK-cyclin complexes (Collado, Medema et al., 2000;Zhou, Liao et al., 2001).
Human T cell lymphotrophic virus type 1 (HTLV-1) is the etiologic agent of adult T cell leukemia (ATL) and chronic inflammatory diseases such as HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP) (Poiesz, Ruscetti et al., 1980;Seiki, Hattori et al., 1983;Gessain, Barin et al., 1985;Osame, Izumo et al., 1986). HTLV-1 encodes a 40-kD protein, Tax, which is critical for viral replication, transformation and gene regulation (Akagi, Ono et al., 1995;Grassmann, Dengler et al., 1989;Nerenberg, Hinrichs et al., 1987;Tanaka, Takahashi et al., 1990). Tax interferes with cell growth control pathways through direct interaction with regulatory proteins and regulation of critical transcription pathways including NF-κB, CREB, SRF, E2F and AP-1(Xiao, Cvijic et al., 2001;Lemasson, Thebault et al., 1998;Iwai, Mori et al., 2001;Suzuki, Uchida-Toita et al., 1999). Tax also inhibits the transcription function of the tumor suppressor p53, inhibiting its ability to respond to cellular stress signals (Cereseto, Diella et al., 1996;Mulloy, Kislyakova et al., 1998;Pise-Masison, Mahieux et al., 2001;Pise-Masison, Mahieux et al., 2000;Pise-Masison, Radonovich et al., 1998). Further, we have shown that Tax activates AKT, which is linked to NF-κB activation, p53 inhibition and cell survival (Jeong, Pise-Masison et al., 2005b)
The AKT signaling pathway is believed to contribute to the maintenance of the latent state by suppressing apoptosis and thereby preventing the elimination of virus-infected cells. Our laboratory was the first to demonstrate that AKT is activated in HTLV-1 transformed cells and is phophorylated at S473 and T308 (Jeong, Pise-Masison et al., 2005b). These results were confirmed by Ikezoe et al. who further showed that the upstream mTOR pathway was activated in HTLV-1 transformed cells (Ikezoe, Nishioka et al., 2006). In the present study, we extend these observations to define downstream regulatory pathways which are regulated by AKT in HTLV-1-transformed cells. Our results demonstrated that blocking AKT reduced phosphorylation of Bad, increased cytochrome c release and activated the caspase-9 apoptosis pathway. Of interest, inhibition of p53 through an adenovirus p53 siRNA demonstrated that p53 played an important role in the apoptosis pathway induced by AKT inhibition.
MATERIALS AND METHODS
Cell culture and drug treatment
HTLV-1-transformed C81 cells were maintained in RPMI supplemented with 10% fetal calf serum, 2 mM L-glutamine, and penicillin (100 units/ml)/streptomycin (100 μg/ml). For treatment with LY294002, 5 x 106 cells were cultured in 10 ml of media in 100 mm dishes for the indicated times. Caspase inhibitors z-LEHD-FMK (100 μM) or Ac-DEVD-CHO (100 μM) were added 1 hour prior to addition of LY294002. All drugs were obtained from Calbiochem.
LY294002 and AKT inhibitor II
AKT inhibitor LY294002 is a cell permeable, potent, and specific phosphatidylinositol 3-kinase inhibitor that acts of n the ATP binding site of the enzyme. AKT inhibitor II is a phosphatidylinositol analog that inhibits the activation of AKT without decreasing phosphorylation of upstream PDK-1.
Western blot analysis
Whole cell extracts were prepared by using lysis buffer (50 mM Tris-HCl pH 7.4, 120 mM sodium chloride, 5 mM EDTA, 0.5% Noniodet P-40, 50 mM sodium fluoride, and 0.2 mM sodium vanadate, complete protease inhibitor (Roche Diagnostics) at a cell concentration of 107 cells/ml. The extracts were incubated on ice for 15 min, centrifuged (10,000 x g) at 4ºC for 10 min, and supernatants were collected. Protein concentrations were determined by Bradford assay (Bio-Rad), and 50–100 μg protein was separated by electrophoresis in 4 to 20% Tris-glycine gels (Novex). The proteins were then transferred to PVDF membranes (Immobilon) and western blot analysis performed with indicated antibodies. Phospho-Bad (Ser136), Bad, Bax, phospho-AKT (Ser473), AKT, Caspase-9, p27 and cyclin D1 antibodies were obtained from Cell Signaling. IκBα antibody was obtained from Active Motif. p21 and Cyclin E antibodies were obtained from Santa Cruz Biotechnologies.
Immunofluorescent staining
Following treatment with LY294002 (40μM), C81 cells were placed on lysine coated coverslips, fixed in PBS-buffered 4% paraformaldehyde, and permeabilized in cold methanol. The permeabilized cells were incubated with 10% normal goat serum in PBS for 1 hour, followed by immunostaining with anti-cychrome c antibody (Active Motif) and an Alexa Fluor 488-conjugated anti-mouse IgG antibody. The immunostained cells were mounted in mounting medium containing DAPI (Vectashield, Vector Labs) and were visualized by a Leica confocal microscope.
Cell viability
Cell viability was determined either by trypan blue staining or the CellTiter-Glo ATP assay. In the trypan blue assay, cells were stained with 0.4% trypan blue solution (Quality Biotech) for one minute. Cells that took up trypan blue were counted as dead cells and expressed as a percentage of the total cell number. Alternatively, cell viability assay was determined using CellTiter-Glo luminescent cell viability assay from Promega using the manufacturer’s instruction. Briefly, 1–2 x 105 cells were cultured in sterile 96 well culture plates in presence of appropriate concentration of LY294002 (20–80 μM) in 100 μl of RPMI media. The plates were then incubated for time indicated. 100 μl of CellTiter-Glo reagent was added to lyse the cells. The contents were mixed in an orbital shaker for 2 min and then incubated at room temperature for 10 min. The luminescence was then recorded in a luminometer with an integration time of 1 second per well. The luminescent signals for the LY294002 treated cells were normalized to the luminescent signal of cells treated with DMSO which was arbitrarily set to 1.
Analysis of caspase-9 activity
Caspase-9 activity was measured by using Caspase Glo-9 assay systems (Promega). Briefly, C81 cells (5 x 106 cells/10 ml) were treated with 40 μM LY294002 for 24 hours. Cells were harvested by centrifugation and supernatants were collected. Samples (100 μl) were gently mixed with Caspase-Glo substrate (100 μl) and the luminescence of each sample was measured by using Luciferase assay system (Promega).
Adenovirus infection of cells
The pAdTrack-si/p53 (Ad-p53 siRNA) construct was kindly provided by Dr. Ling-Jun Zhao (St. Louis University) and pAd-GFP was purchased from Q-Biogene. The recombinant viral genome was linearized with PacI and transfected into 293 cells in a 60-mm dish using PolyFect transfection reagent (Qiagen). Eight days after transfection, the recombinant virus was collected and subjected to one round of amplification by infecting 1.5×106 293 cells, yielding 2 ml of viral stocks. Infection of 293 cells was performed in serum free DMEM. Following viral infection, 293 cells were maintained in DMEM with 10% fetal bovine serum. HTLV-1-transformed C81 cells (1.5×106) in a 60-mm dish were infected with either Ad-p53 siRNA or Ad-GFP in serum-free RPMI media. After 3 hours, cells were resuspended in media containing 10% fetal calf serum. LY294002 (40 μM) was added and incubations carried out at as indicated.
Cell cycle analysis
Adenoviral infected C81 cells with or without LY294002 were harvested and fixed in 70% ethanol. The fixed cells were then stained with propidium iodide (50 μg/ml) after treatment with RNase (5 μg/ml). The stained cells were analyzed for DNA content in FACSCalibur (Becton Dickinson) using the Cell Quest program (Becton Dickinson). The resultant data was analyzed by ModFit LT (Verity Software House) using appropriate gates.
TUNEL assay
To quantify apoptosis, TUNEL assays were performed using the Apo-BrdU TUNEL assay kit (Invitrogen) following manufacturer’s instructions. Briefly LY294002 and AKT inhibitor II treated and control cells were washed with cold PBS, fixed with 1% paraformaldehyde in PBS, followed by fixing with 70% ethanol. The cells were then labeled with BrdUTP using Terminal deoxynucleotide transferase (TdT) at 37°C for 60 min. At the end of the incubation, the cells were rinsed with rinse buffer and stained with Alexa Fluor 488 dye-labeled anti-BrdU antibody for 30 minutes at room temperature in the dark. Finally, the cells were treated with Propidium Iodide/RNase A staining buffer for an additional 30 minutes at room temperature. Immunostained cells were analyzed using FACSCalibur (Becton Dickinson). TUNEL positive cells were quantified with Cell Quest (Becton Dickinson) and FlowJoV8.0 softwares.
RESULTS
Inhibition of AKT by LY294002 and AKT inhibitor II results in cell death in HTLV-1 transformed cells
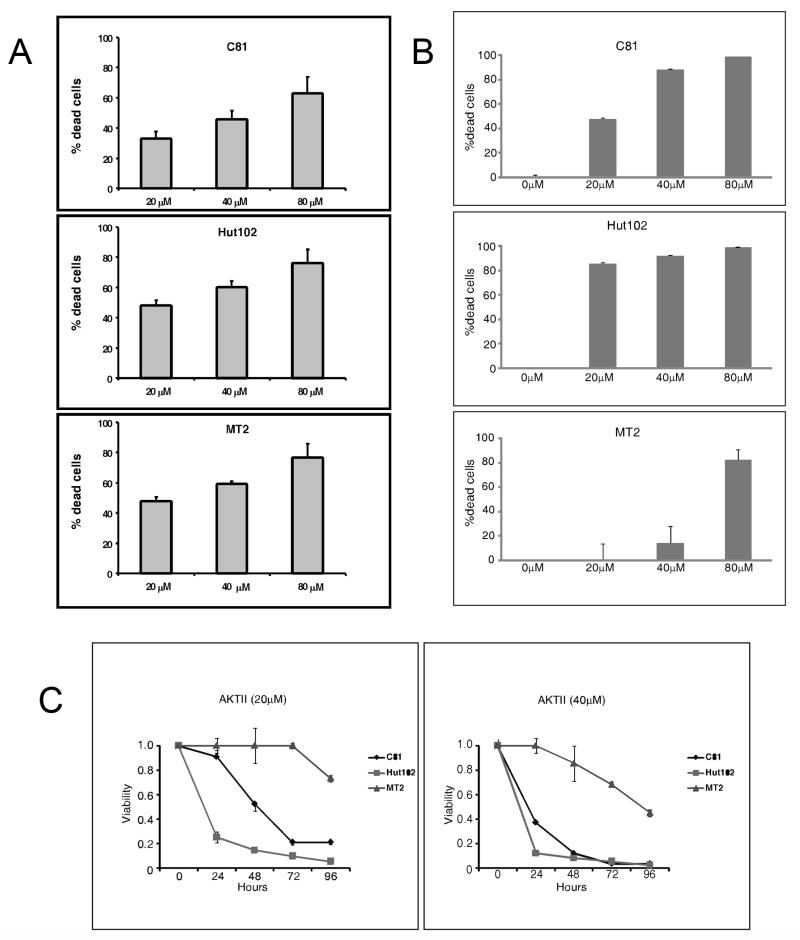
In previous studies, we demonstrated that Tax activates AKT and that treatment of HTLV-1-transformed cells with LY294002 inhibited AKT activity (Jeong, Pise-Masison et al., 2005b). To gain a more complete understanding of the importance of the activated AKT pathway in HTLV-1 transformed cell lines, C81, MT-2 and HUT 102 were cultured with increasing concentrations of the PI3K/AKT inhibitor LY294002. Cells were harvested and analyzed for cell viability using the ATP CellTiter-glo assay. The results presented in Figure 1A demonstrate that upon treatment with LY294002, cell viability decreased in a concentration dependent manner (Figure 1A). MT-2 and Hut102 were the most sensitive to the PI3K/AKT inhibitor, followed by C81 cells. In a parallel set of experiments, we determined that cell death increased with time (data not shown). Thus, a concentration and time dependent cell death response to LY294002 treatment was observed.
Figure 1. Effect of LY294002 on cell viability and cell cycle progression.
(A) C81, MT-2 and HUT 102 cells (105 cells/ml) were treated with 20-, 40-, or 80 μM LY294002 for 72 hours. Cell viability was determined using the CellTiter-Glo ATP assay (Promega). (B) C81, MT-2 and HUT 102 cells (105 cells/ml) were treated with 20-, 40-, or 80 μM AKT inhibitor II for 48 hours. Cell viability was determined using the CellTiter-Glo ATP assay (Promega). (C) C81, MT-2 and HUT 102 cells (105 cells/ml) were treated with 20- or 40 μM AKT inhibtor II for 0, 24, 48, 72 or 96 hours. Cell viability was determined using the CellTiter-Glo ATP assay (Promega).
To provide further evidence for the role of AKT in HTLV-1 in cell survival, we analyzed the effect of specific AKT inhibitor II. The inhibitor is a phosphatidylinositol analog that inhibits the activation of AKT without decreasing phosphorylation of upstream kinase PDK-1. C81, Hut102 and MT-2 cells were incubated with 0, 20-, 40- or 80 μM AKT inhibitor II for 48 hours. Cell were harvested and analyzed for cell viability using the ATP CellTiter-glo assay. The results presented in Figure 1B demonstrate that inhibition of AKT lead to a dose dependent increase in cell death. We next determined if cell death was time dependent. C81, Hut102 and MT2 cells were treated with AKT inhibitor II at a concentration of 20- or 40 μM. An aliquot of cells was harvested at 0, 24, 48, 72 and 96 hours and analyzed for cell viability using the ATP CellTiter-glo assay. The results presented in Figure 1C demonstrate that there was a time dependent increase in cell death following treatment with the specific AKT inhibitor. Because of the cost of AKT inhibitor II, all subsequent studies were done with AKT inhibitor LY294002.
Alteration in cell cycle and p27 expression following inhibition of AKT
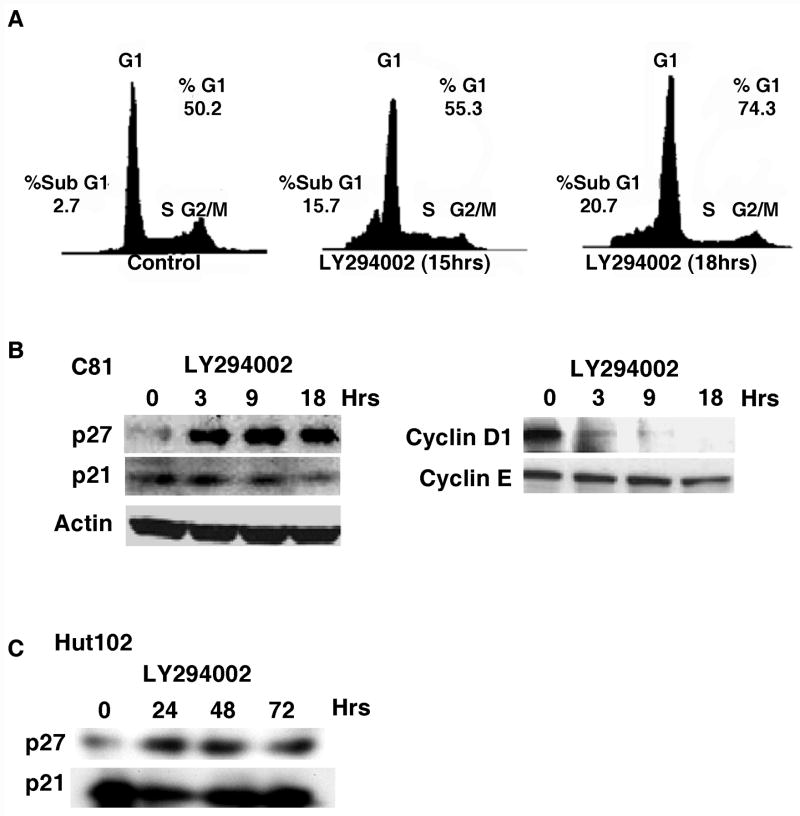
We next analyzed the effect of AKT inhibition on cell cycle distribution by FACS analysis. Cell cycle analysis of the C81, MT-2 and Hut102 cells following treatment with LY294002 demonstrated an accumulation of cells in G1 and an increase in sub-G1 cells (Figure 2A; data not shown). By 18 hours after treatment with LY294002, the percentage of cells in sub-G1 increased from 2.7 to 21%. A similar increase in the percentage of sub-G1 cells was observed in the analysis of MT-2 and Hut102 cells (data not shown). We also noted that the percentage of cells in G1 increased by 18 hours post treatment (Figure 2A).
Figure 2.
(A) C81 cells (5 x 106 cells) were treated with 40 μM LY294002 for 0, 15 or 18 hours. Cells were harvested, fixed in 70% ethanol, treated with RNase and stained with propidium iodide solution and analyzed for cell cycle distribution by flow cytometry. (B/C) C81 or Hut102 cells (5 x 106 cells) were treated with 40 μM LY294002 for times indicated. Cell lysates were prepared and western blot analysis was performed to detect the expression of p27 (Cell Signalling), p21 (Santa Cruz), cyclin D1 (Cell Signalling) and cyclin E (Santa Cruz) as visualized by chemiluminescence.
Consistent with the accumulation of cells in G1, western blot analysis of C81 cell extracts demonstrated that the level of cdk inhibitor p27 increased dramatically, while the level of cyclin D1 decreased (Figure 2B). A similar increase in p27 protein was observed following treatment of Hut102 cells with AKT inhibitor LY294002 (Figure 2C). While the increase in p27 protein is under investigation, the decrease in cyclin D1 expression is likely the result of inhibition of the NF-κB signaling pathway by LY294002 (Joyce, Bouzahzah et al., 1999;Hinz, Krappmann et al., 1999;Guttridge, Albanese et al., 1999) (Jeong, Pise-Masison et al., 2005b). In contrast to these two proteins, the level of p21 and cyclin E remained relatively constant throughout the treatment (Figure 2B). The level of control protein actin remained constant throughout the time course (Figure 2B).
Analysis of Bcl-2, Bad phosphorylation and cytochrome C release following treatment with LY294002
To gain greater insight into the apoptosis pathway induced by LY294002, we analyzed the protein expression of Bcl-2 family members including pro-apoptotic Bad and Bax. Since all the HTLV-1 transformed cell lines had reacted similarly to the AKT inhibitors, we chose C81 cells for a more in depth mechanistic analysis. HTLV-1-transformed C81 cells were treated with LY294002 for 0, 3, 9 or 18 hours and cell extracts were prepared for western blot analysis. As shown in Figure 3A, while the overall level of Bad protein remained constant a significant decrease in the level of phosphorylation of Bad at Ser136 was observed. Consistent with previous results and as a control for these studies, AKT phosphorylation at Ser-473 decreased with time and total AKT was constant (Figure 3A, lower panels) (Jeong, Pise-Masison et al., 2005b). A similar decrease in AKT phosphorylation at Thr-308 was observed in these experiments (data not shown), consistent with previous findings (Jeong, Pise-Masison et al., 2005a).
Figure 3. Effect of LY294002 on Bad phosphorylation and cytochrome c release.
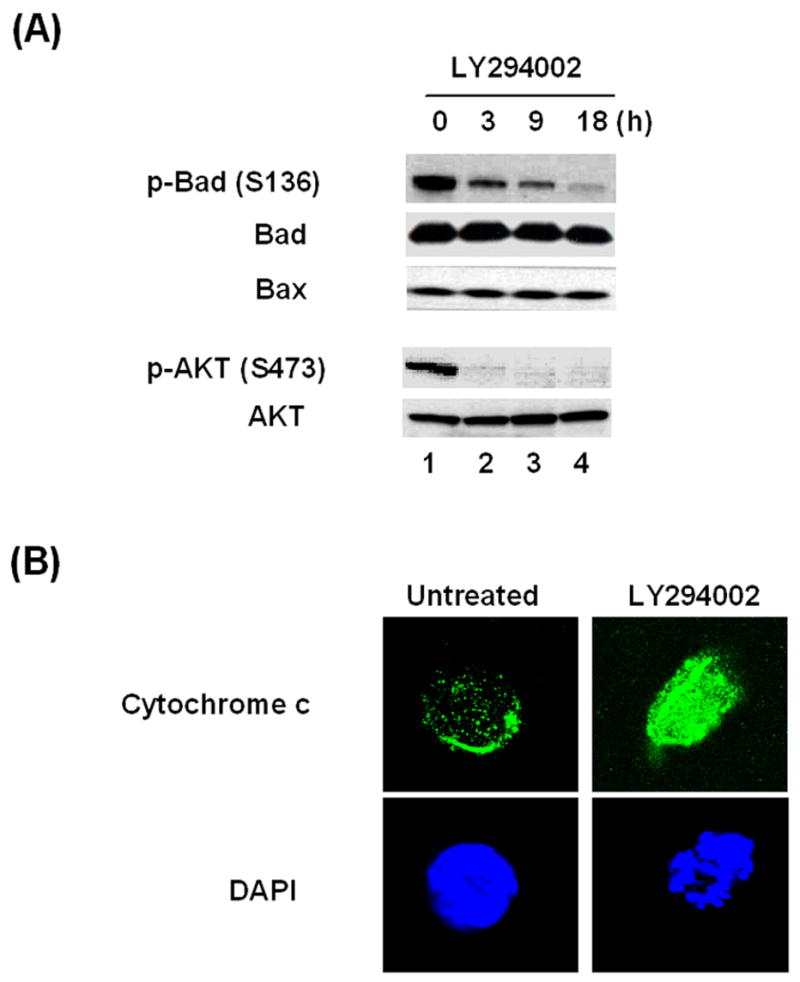
(A) C81 cells (5 x 106 cells) were treated with 40 μM LY294002 for 0, 3, 9 or 18 hours. Cell lysates were prepared and western blot analysis was performed to detect the expression of phospho-Bad (Ser-136), Bad, Bax, phospho-AKT (Ser-473) and AKT (Cell Signaling) as visualized by chemiluminescence. (B) C81 cells were cultured with or without 40 μM LY294002 for 18 hours for analysis of cytochrome c release. Confocal microscopy shows mitochondrial location of cytochrome c (upper panel) and DAPI staining (lower panel) in control cells and cytosolic translocation in LY294002-C81 treated cells.
Phosphorylation of Bad at Ser136 inhibits the pro-apoptotic function of the protein by decreasing its interaction with Bcl-xL on the mitochondrial membrane. Given the decrease in Bad phosphorylation observed above, immunofluorescent staining of cytochrome c was conducted with C81 cells in the absence or presence of LY294002. Confocal microscopy revealed that in untreated cells, cytochrome c was localized in a punctate pattern consistent with localization in the mitochondria (Figure 3B, top left). In contrast, after treatment with LY294002, a diffuse distribution of cytochrome c was observed (Figure 3B, top right). The later staining pattern is consistent with the release of cytochrome c from the mitochondria to the cytosol. Control immunofluorescent assays with control IgG revealed no staining of the control or treated cells (data not shown). We further analyzed cytochrome c distribution by Western blot analysis. The results of these studies demonstrated that the level of cytosolic cytochrome c was significantly increased in the LY294002 treated cells (data not shown).
LY294002-induced apoptosis is dependent on activity of caspase-9 in HTLV-1-transformed cells
Release of cytochrome c from the mitochondria is associated with caspase 9 activation (Garrido, Galluzzi et al., 2006). Moreover, AKT also directly inhibits the proteolytic activity of caspase-9 by phosphorylation of the protein at Ser-136 (Cardone, Roy et al., 1998). It was important, therefore, to test whether caspase-9 was activated in the LY294002 treated cells. To test the activation of caspase-9 in LY294002-induced apoptosis, we carried out chemiluminescent assays that specifically measure caspase-9 activity. LY294002 induced caspase-9 activation in a dose dependent manner (Figure 4A). Consistent with these findings, we observed the reduction of full length caspase-9 protein following LY294002 treatment (Figure 4B). Coincident with the dissapearance of full length caspase-9, we observed the appearance of lower molecular weight cleavage products (data not shown). Trypan blue dye exclusion assays confirmed the importance of caspase-9 in the apoptosis pathway (Figure 4C). LY294002 treatment induced apoptosis in a time-dependent manner, with approximately 50% of the cells undergoing apoptosis by 72 hours in this experiment. Pretreatment of the cells with either the caspase-9 specific or pan-specific inhibitor significantly reduced LY294002-induced apoptosis (Figure 4C). Together, the results suggest that LY294002-induced apoptosis involves the Bad/cytochrome c/caspase 9 mitochondria-dependent pathway.
Figure 4. LY294002-induced apoptosis is dependent on caspase-9 activity in HTLV-1-transformed cells.
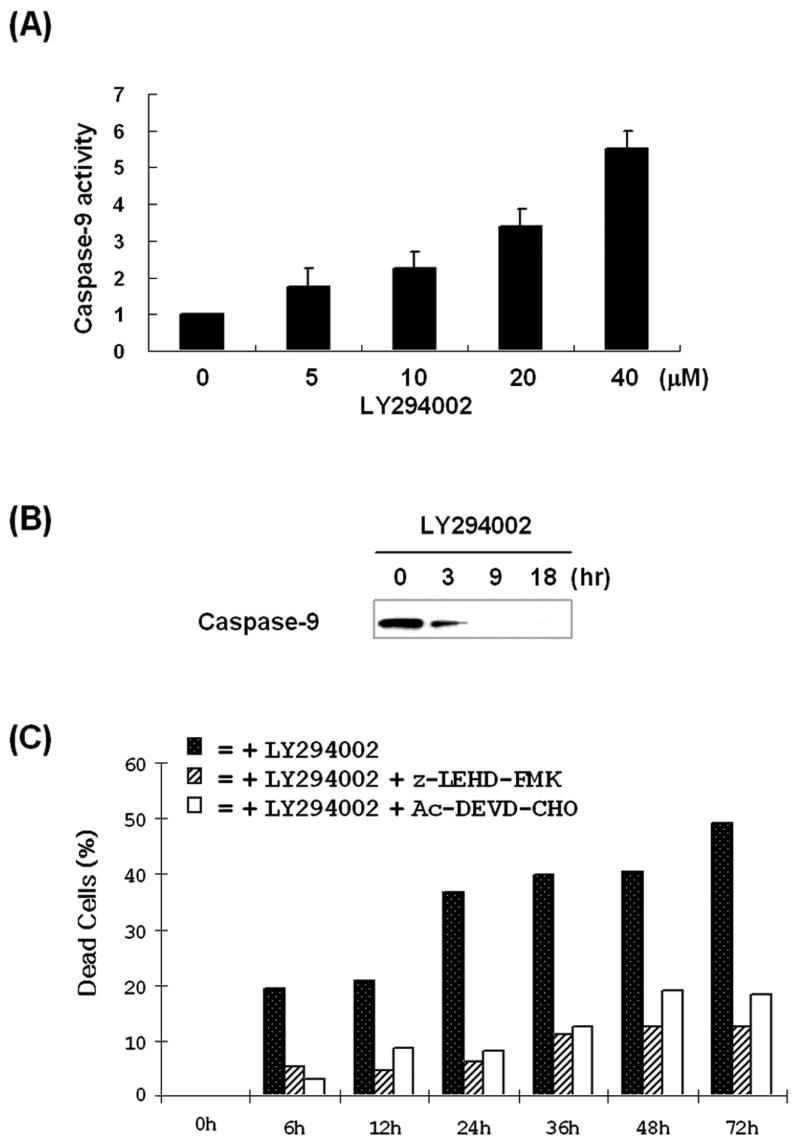
(A) Caspase-9 activity was measured by using Caspase Glo-9 assay systems (Promega). C81 cells (5 x 106 cells) were treated with 40 μM LY294002 for 24 hours and harvested by centrifugation and supernatants were collected. Samples were gently mixed with Caspase-Glo substrate and the luminescence of each sample was measured by using Luciferase assay system. Error bars were calculated using three independent experiments. (B) Lysates were prepared from control or 40 μM LY294002-treated C81 cells and western blot analysis was performed to detect the expression of caspase-9 (Cell Signaling) as visualized by chemiluminescence. (C) C81 cells were treated with caspase inhibitors (100 μM) z-LEHD-FMK or Ac-DEVD-CHO for one hour prior to treatment with 40 μM LY294002 for 0 to 72 hours. Apoptosis was determined by the percentage of trypan blue positive cells.
LY294002 or SC-514-induced apoptosis is p53-dependent pathway in HTLV-1-transformed cells
p53, through its activity as a transcriptional activator or repressor, functions as a tumor suppressor inducing either cell cycle arrest or apoptosis in response to cellular stress (Oren, 2003;Vousden & Lu, 2002). Previous studies from this laboratory demonstrated that AKT activation plays a critical role in the inhibition of p53 function in HTLV-1-transformed cells as treatment of C81 cells with LY294002 reactivated p53 transcription activity as measured by MDM2 promoter activity (Jeong, Pise-Masison et al., 2005b). Therefore, we tested whether p53 plays a role in the LY294002 induced apoptosis. C81 cells were infected with Ad-GFP or Ad-p53 siRNA in the absence or presence of LY294002. As shown in Figure 5A, infection of the cells with Ad-p53 siRNA significantly reduced p53 expression in the absence or presence of LY294002 (lanes 1, 3, 4). In contrast, infection of cells with the control Ad-GFP virus did not affect p53 expression (lanes 1 and 3). As a control for these studies, we demonstrate that while LY294002 inhibited the phosphorylation of AKT, expression of AKT protein was not significantly affected by infection with the Ad-GFP or Ad-p53 siRNA expressing virus (Figure 5A, middle and bottom panel). These results are consistent with previous studies using the p53 siRNA which demonstrated that the Ad-p53 siRNA specifically inhibits expression of p53, but not other cellular genes (Zhao, Jian et al., 2003). The Ad-GFP virus infection serves as a control for any effect of adenovirus infection that may affect the experiment. Consistent with the results presented above, treatment of the cells with LY294002 decreased the level of phospho-AKT (S473). The decrease in AKT phosphorylation was similar in the Ad-p53 siRNA and Ad-GFP infected cells.
Figure 5. LY294002 or SC-514-induced apoptosis is p53-dependent pathway in HTLV-1-transformed cells.
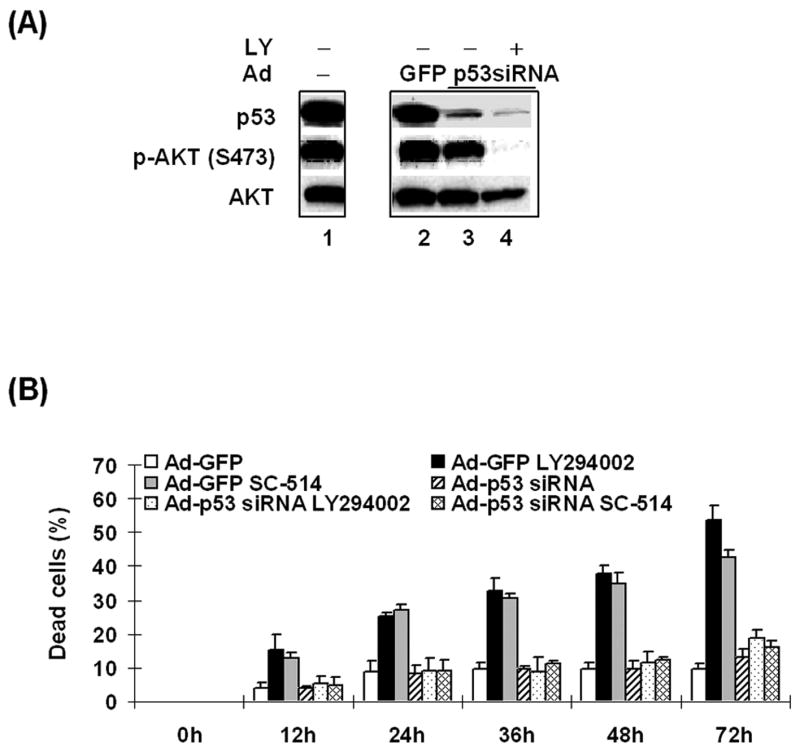
(A) C81 cells were infected with Ad-p53 siRNA or Ad-GFP in the absence or presence of 40 μM LY294002. After 72 hours, cell lysates were prepared and western blot analysis was performed to detect the expression of p53 (Oncogene), phospho-AKT (Ser-473) and AKT (Cell Signaling) as visualized by chemiluminescence. (B) Trypan blue dye exclusion assays were performed to measure apoptosis. C81 cells were infected with Ad-GFP or Ad-p53 siRNA in serum-free media. After 3 hours, cells were harvested and resuspended in serum-containing media and 40 μM LY294002 was added for indicated times.
Lastly, trypan blue assays were used to measure cell viability with Ad-p53 siRNA infected C81 cells in the absence or presence of LY294002 (Figure 5B). In these studies, we also included cells which were treated with IKKβ specific inhibitor SC-514 (Kishore, Sommers et al., 2003). IKKβ was targeted since it is a direct subtrate of AKT and plays an important role in NF-κB activation and inhibition of p53 in HTLV-1-transformed cells (Jeong, Pise-Masison et al., 2005a). The results of these experiments demonstrate that infection of C81 cells with Ad-GFP or Ad-p53 siRNA caused a modest increase in cell death (Figure 5B). Addition of LY294002 or SC-514 to the control Ad-GFP infected cells resulted in a 3–5 fold increase in cell death. In contrast, addition of LY294002 or SC-514 to cells infected with Ad-p53 siRNA did not result in a significant increase in cell death. These results suggest that blocking AKT activation or its downstream target IKKβ leads to the induction of a p53-dependent apoptosis in C81 cells.
DISCUSSION
Activation of the AKT pathway is common in many cancers and contributes to inhibition of apoptosis and therapeutic resistance through multiple mechanisms (Nuutinen, Postila et al., 2006). Consistent with these observations, inhibition of AKT decreases cell survival and potentiates the effects of chemotherapeutic drugs in cancer cells (Clark, West et al., 2002;Asselin, Wang et al., 2001;Asselin, Mills et al., 2001). We and others have shown that AKT is activated in HTLV-1-transformed cells and plays an important role in cell survival. In part, the importance of AKT to cell survival is due to regulation of multiple target pathways through phosphorylation of critical proteins. For example, phosphorylation of Bad by AKT inactivates the proteins ability to induce apoptosis, thus promoting cell survival. Conversely, dephosphorylation of Bad results in targeting of Bad to mitochondrial membranes where Bad interacts with and inactivates anti-apoptotic proteins Bcl-2 and Bcl-xL, inducing apoptosis (Datta, Brunet et al., 1999). We have previously shown that HTLV-1 Tax mediates activation of AKT by mediating phosphorylation at Ser-473 and Thr-308 (Jeong, Pise-Masison et al., 2005b). In the present study, we demonstrate that blocking AKT by LY294002 decreased the phosphorylation of Bad. Since Bad regulates the mitochondrial release of cytochrome c (Yamaguchi, Tamatani et al., 2001), cytochrome c localization was analyzed in C81 cells in the absence or presence of LY294002. Release of cytochrome c was observed both by immunofluorescent staining and western blot analysis (data now shown) of fractionated cell extracts. The best-defined target for cytochrome c is the apoptosome which is a multiprotein complex comprising Apaf-1, cytochrome c and caspase-9 that activates the apoptotic pathway (Zou, Li et al., 1999). In our studies, cleavage of caspase-9 was induced by LY294002 and pre-treatment with a caspase-9 specific or pan caspase inhibitor significantly blocked LY294002-induced apoptosis (Figure 4C). These results suggest that in HTLV-1-transformed cells, LY294002 induces apoptosis which is dependent on the dephosphorylation of Bad and activation of caspase-9.
Recent studies have demonstrated that AKT is also a signaling intermediate upstream of NF-κB dependent survival gene expression. (Datta, Brunet et al., 1999). NF-κB activation requires phosphorylation of IκBα by IκB kinases (IKKs). IκBα phosphorylation targets IκBα for ubiquitination and proteolytic degradation (Baldwin, Jr., 1996;Beg & Baldwin, Jr., 1993), releasing p50–p65 heterodimers to migrate to the nucleus and activate transcription. It has been shown that IKKs are a substrate of AKT and its ability to regulate NF-κB activity may be through direct interaction with IKKs, as AKT can associate with the IKK complex in vivo (Datta, Brunet et al., 1999). In the present study, C81 cells were treated with SC-514, a IKKβ specific inhibitor (Kishore, Sommers et al., 2003). SC-514 induced apoptosis in HTLV-1-transformed cells to a level similar to that obtained with LY294002 treatment. Previous data from our laboratory have showed that LY294002 or SC514 induced p53 dependent transcription. These results and data presented here argue that the AKT/IKKβ pathway plays a critical role in NF-κB activation and cell survival in HTLV-1-transformed cells.
Inactivation of the tumor suppressor protein p53 plays a critical role in tumorigenesis. p53 functions as an integrator of stress response signals by activating or repressing the transcription of genes that regulate cell cycle progression and/or apoptosis (Appella & Anderson, 2001;Giaccia & Kastan, 1998;Meek, 1999;Prives & Hall, 1999). Over the past several years, it has become evident that p53 and AKT are involved in a complex cross talk that are at the core of the cell control machinery for switching between survival and death. This cross talk is a combination of reciprocally antagonistic pathways emanating from p53 and AKT, which also involve tumor suppressor gene, PTEN, and oncogene, Mdm2 (Wee & Aguda, 2006). We investigated whether p53 plays a role in the regulation of LY294002-mediated apoptosis and cell cycle arrest in HTLV-1-transformed cells. An adenovirus vector expressing a siRNA to p53 was utilized to specifically decrease expression of p53. The results of these studies clearly demonstrate that the Ad-p53 siRNA decreased LY294002-induced apoptosis. Thus, while activation of Bad and caspase-9 occur in response to AKT inhibition, they are not sufficient to induce apoptosis, but must signal through the p53 pathway to induce apoptosis.
In a recent paper, Peloponese et al. suggested that Tax, in the absence of NF-κB activation, can activate activator protein-1 (AP-1) to promote cellular proliferation and survival through the PI3K/AKT pathway (Peloponese, Jr. & Jeang, 2006). These results are not inconsistent with our data, but focus more on the role of Tax-activated AKT in cell proliferation and provide intriguing data that Tax activates AKT though direct interaction with the p85 subunit of PI3K.
Following our original observation that AKT was activated in HTLV-1-transformed cells, Ikezoe et al. (Ikezoe, Nishioka et al., 2006) reported that the PI3K/AKT/mammalian target of rapamycin (mTOR) was activated in HTLV-1 cells. The authors demonstrated that rapamycin, the inhibitor of mTOR, induced growth inhibition and cell cycle arrest. Interestingly, the authors demonstrated that PI3K/AKT inhibitor LY294002 exhibited similar properties, inhibiting cell growth and inducing cell cycle arrest. When rapamycin was combined with LY294002, the ability of rapamycin to induce growth arrest and cause dephosphorylation of p70S6K and 4E-BP-1 was potentiated. It was suggested that the effect of LY294002 was due to its ability to block phosphorylation of AKT at Ser473, which was paradoxically induced by rapamycin.
In the present paper, we demonstrate that in HTLV-1 transformed cells AKT regulates pathways involved in cell cycle and cell viability. AKT phosphorylates or induces the phosphorylation of BAD, decreasing its ability to interact with and inhibit the function of Bcl-xL (Figure 6). AKT also induces NF-κB, which increases expression of Bcl-xL, an inhibitor of apoptosis. AKT regulates cell cycle progression through regulation of p27 and cyclin D1. While AKT likely regulates cyclin D1 expression through NF-κB, its interaction with p27 requires further investigation. Recent studies have focused on drug discovery targeting AKT and its downstream molecules in other human cancers. LY294002 effectively inhibits the growth of many types of tumor cells in vitro and in vivo and combining LY294002 with conventional chemotherapeutic agents may provide a treatment option for drug-resistant cancers. Poor solubility and high toxicity of LY294002 have stimulated the development of derivatives or specific AKT inhibitors including PX-866, IC486068, helenaquinone, perifosine, and PX-316. AKT antagonist API-2 (AKT/PKB signaling inhibitor-2) have been shown to inhibit AKT kinase activity and to induce apoptosis in human cancer cells with high AKT activity (Yang, Dan et al., 2004). The results of this study suggest that these compounds may be considered useful in the treatment of ATL patients.
Figure 6. HTLV-1 Tax activation of AKT regulates cell survival through multiple targeting molecules.
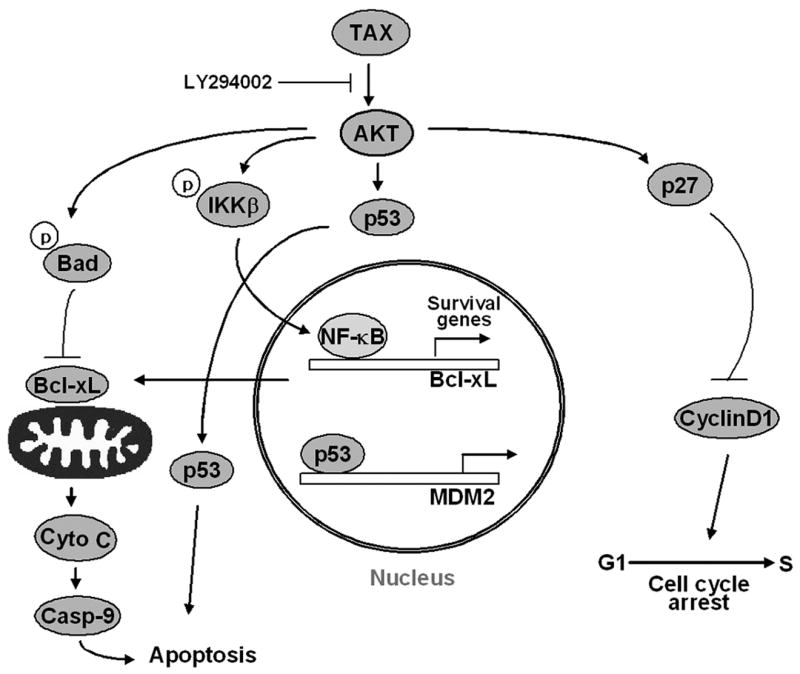
Tax activates AKT in HTLV-1 transformed cells through the PI3 kinase pathway. Blocking AKT function using LY294002 results in decreased phosphorylation of Bad, cytochrome c release, caspase 9 activation and apoptosis. LY294002 treatment also blocks AKT dependent phosphorylation of the IKKs, resulting in a decrease in NF-kB binding and transcription activity. Apoptosis is dependent upon p53 activity as indicated by the ability of a siRNA to p53 to inhibit apoptosis.
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research. We thank the FACS core facility for flow cytometry analysis and the imaging core facility for using confocal microscopy in CCR, NCI, NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akagi T, Ono H, Shimotohno K. Characterization of T cells immortalized by Tax1 of human T-cell leukemia virus type 1. Blood. 1995;86:4243–4249. [PubMed] [Google Scholar]
- Appella E, Anderson CW. Post-translational modifications and activation of p53 by genotoxic stresses. Eur J Biochem. 2001;268:2764–2772. doi: 10.1046/j.1432-1327.2001.02225.x. [DOI] [PubMed] [Google Scholar]
- Asselin E, Mills GB, Tsang BK. XIAP regulates Akt activity and caspase-3-dependent cleavage during cisplatin-induced apoptosis in human ovarian epithelial cancer cells. Cancer Res. 2001;61:1862–1868. [PubMed] [Google Scholar]
- Asselin E, Wang Y, Tsang BK. X-linked inhibitor of apoptosis protein activates the phosphatidylinositol 3-kinase/Akt pathway in rat granulosa cells during follicular development. Endocrinology. 2001;142:2451–2457. doi: 10.1210/endo.142.6.8080. [DOI] [PubMed] [Google Scholar]
- Baldwin AS., Jr The NF-kappa B and I kappa B proteins: new discoveries and insights. Annu Rev Immunol. 1996;14:649–83. 649–683. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- Beg AA, Baldwin AS., Jr The I kappa B proteins: multifunctional regulators of Rel/NF-kappa B transcription factors. Genes Dev. 1993;7:2064–2070. doi: 10.1101/gad.7.11.2064. [DOI] [PubMed] [Google Scholar]
- Brazil DP, Yang ZZ, Hemmings BA. Advances in protein kinase B signalling: AKTion on multiple fronts. Trends Biochem Sci. 2004;29:233–242. doi: 10.1016/j.tibs.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Cardone MH, Roy N, Stennicke HR, Salvesen GS, Franke TF, Stanbridge E, Frisch S, Reed JC. Regulation of cell death protease caspase-9 by phosphorylation. Science. 1998;282:1318–1321. doi: 10.1126/science.282.5392.1318. [DOI] [PubMed] [Google Scholar]
- Cereseto A, Diella F, Mulloy JC, Cara A, Michieli P, Grassmann R, Franchini G, Klotman ME. p53 functional impairment and high p21waf1/cip1 expression in human T-cell lymphotropic/leukemia virus type I-transformed T cells. Blood. 1996;88:1551–1560. [PubMed] [Google Scholar]
- Chang F, Lee JT, Navolanic PM, Steelman LS, Shelton JG, Blalock WL, Franklin RA, McCubrey JA. Involvement of PI3K/Akt pathway in cell cycle progression, apoptosis, and neoplastic transformation: a target for cancer chemotherapy. Leukemia. 2003;17:590–603. doi: 10.1038/sj.leu.2402824. [DOI] [PubMed] [Google Scholar]
- Clark AS, West K, Streicher S, Dennis PA. Constitutive and inducible Akt activity promotes resistance to chemotherapy, trastuzumab, or tamoxifen in breast cancer cells. Mol Cancer Ther. 2002;1:707–717. [PubMed] [Google Scholar]
- Collado M, Medema RH, Garcia-Cao I, Dubuisson ML, Barradas M, Glassford J, Rivas C, Burgering BM, Serrano M, Lam EW. Inhibition of the phosphoinositide 3-kinase pathway induces a senescence-like arrest mediated by p27Kip1. J Biol Chem. 2000;275:21960–21968. doi: 10.1074/jbc.M000759200. [DOI] [PubMed] [Google Scholar]
- Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three Akts. Genes Dev. 1999;13:2905–2927. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- Fresno Vara JA, Casado E, de Castro J, Cejas P, Belda-Iniesta C, Gonzalez-Baron M. PI3K/Akt signalling pathway and cancer. Cancer Treat Rev. 2004;30:193–204. doi: 10.1016/j.ctrv.2003.07.007. [DOI] [PubMed] [Google Scholar]
- Fry MJ. Phosphoinositide 3-kinase signalling in breast cancer: how big a role might it play? Breast Cancer Res. 2001;3:304–312. doi: 10.1186/bcr312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrido C, Galluzzi L, Brunet M, Puig PE, Didelot C, Kroemer G. Mechanisms of cytochrome c release from mitochondria. Cell Death Differ. 2006;13:1423–1433. doi: 10.1038/sj.cdd.4401950. [DOI] [PubMed] [Google Scholar]
- Gessain A, Barin F, Vernant JC, Gout O, Maurs L, Calender A, de The G. Antibodies to human T-lymphotropic virus type-I in patients with tropical spastic paraparesis. Lancet. 1985;2:407–410. doi: 10.1016/s0140-6736(85)92734-5. [DOI] [PubMed] [Google Scholar]
- Giaccia AJ, Kastan MB. The complexity of p53 modulation: emerging patterns from divergent signals. Genes Dev. 1998;12:2973–2983. doi: 10.1101/gad.12.19.2973. [DOI] [PubMed] [Google Scholar]
- Grassmann R, Dengler C, Muller-Fleckenstein I, Fleckenstein B, McGuire K, Dokhelar MC, Sodroski JG, Haseltine WA. Transformation to continuous growth of primary human T lymphocytes by human T-cell leukemia virus type I X-region genes transduced by a Herpesvirus saimiri vector. Proc Natl Acad Sci USA. 1989;86:3351–3355. doi: 10.1073/pnas.86.9.3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttridge DC, Albanese C, Reuther JY, Pestell RG, Baldwin AS., Jr NF-kappaB controls cell growth and differentiation through transcriptional regulation of cyclin D1. Mol Cell Biol. 1999;19:5785–5799. doi: 10.1128/mcb.19.8.5785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinz M, Krappmann D, Eichten A, Heder A, Scheidereit C, Strauss M. NF-kappaB function in growth control: regulation of cyclin D1 expression and G0/G1-to-S-phase transition. Mol Cell Biol. 1999;19:2690–2698. doi: 10.1128/mcb.19.4.2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikezoe T, Nishioka C, Bandobashi K, Yang Y, Kuwayama Y, Adachi Y, Takeuchi T, Koeffler HP, Taguchi H. Longitudinal inhibition of PI3K/Akt/mTOR signaling by LY294002 and rapamycin induces growth arrest of adult T-cell leukemia cells. Leuk Res. 2006 doi: 10.1016/j.leukres.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Iwai K, Mori N, Oie M, Yamamoto N, Fujii M. Human T-cell leukemia virus type 1 tax protein activates transcription through AP-1 site by inducing DNA binding activity in T cells. Virology. 2001;279:38–46. doi: 10.1006/viro.2000.0669. [DOI] [PubMed] [Google Scholar]
- Jeong SJ, Pise-Masison CA, Radonovich MF, Park HU, Brady JN. A novel NF-kappaB pathway involving IKKbeta and p65/RelA Ser-536 phosphorylation results in p53 Inhibition in the absence of NF-kappaB transcriptional activity. J Biol Chem. 2005a;280:10326–10332. doi: 10.1074/jbc.M412643200. [DOI] [PubMed] [Google Scholar]
- Jeong SJ, Pise-Masison CA, Radonovich MF, Park HU, Brady JN. Activated AKT regulates NF-kappaB activation, p53 inhibition and cell survival in HTLV-1-transformed cells. Oncogene. 2005b;24:6719–6728. doi: 10.1038/sj.onc.1208825. [DOI] [PubMed] [Google Scholar]
- Joyce D, Bouzahzah B, Fu M, Albanese C, D'Amico M, Steer J, Klein JU, Lee RJ, Segall JE, Westwick JK, Der CJ, Pestell RG. Integration of Rac-dependent regulation of cyclin D1 transcription through a nuclear factor-kappaB-dependent pathway. J Biol Chem. 1999;274:25245–25249. doi: 10.1074/jbc.274.36.25245. [DOI] [PubMed] [Google Scholar]
- Kishore N, Sommers C, Mathialagan S, Guzova J, Yao M, Hauser S, Huynh K, Bonar S, Mielke C, Albee L, Weier R, Graneto M, Hanau C, Perry T, Tripp CS. A selective IKK-2 inhibitor blocks NF-kappa B-dependent gene expression in interleukin-1 beta-stimulated synovial fibroblasts. J Biol Chem. 2003;278:32861–32871. doi: 10.1074/jbc.M211439200. [DOI] [PubMed] [Google Scholar]
- Krasilnikov M, Adler V, Fuchs SY, Dong Z, Haimovitz-Friedman A, Herlyn M, Ronai Z. Contribution of phosphatidylinositol 3-kinase to radiation resistance in human melanoma cells. Mol Carcinog. 1999;24:64–69. doi: 10.1002/(sici)1098-2744(199901)24:1<64::aid-mc9>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Lemasson I, Thebault S, Sardet C, Devaux C, Mesnard JM. Activation of E2F-mediated transcription by human T-cell leukemia virus type I Tax protein in a p16(INK4A)-negative T-cell line. J Biol Chem. 1998;273:23598–23604. doi: 10.1074/jbc.273.36.23598. [DOI] [PubMed] [Google Scholar]
- Lin J, Adam RM, Santiestevan E, Freeman MR. The phosphatidylinositol 3′-kinase pathway is a dominant growth factor-activated cell survival pathway in LNCaP human prostate carcinoma cells. Cancer Res. 1999;59:2891–2897. [PubMed] [Google Scholar]
- Lin X, Bohle AS, Dohrmann P, Leuschner I, Schulz A, Kremer B, Fandrich F. Overexpression of phosphatidylinositol 3-kinase in human lung cancer. Langenbecks Arch Surg. 2001;386:293–301. doi: 10.1007/s004230100203. [DOI] [PubMed] [Google Scholar]
- Martinez-Lorenzo MJ, Anel A, Monleon I, Sierra JJ, Pineiro A, Naval J, Alava MA. Tyrosine phosphorylation of the p85 subunit of phosphatidylinositol 3-kinase correlates with high proliferation rates in sublines derived from the Jurkat leukemia. Int J Biochem Cell Biol. 2000;32:435–445. doi: 10.1016/s1357-2725(99)00142-9. [DOI] [PubMed] [Google Scholar]
- Meek DW. Mechanisms of switching on p53: a role for covalent modification? Oncogene. 1999;18:7666–7675. doi: 10.1038/sj.onc.1202951. [DOI] [PubMed] [Google Scholar]
- Mulloy JC, Kislyakova T, Cereseto A, Casareto L, LoMonico A, Fullen J, Lorenzi MV, Cara A, Nicot C, Giam C, Franchini G. Human T-cell lymphotropic/leukemia virus type 1 Tax abrogates p53-induced cell cycle arrest and apoptosis through its CREB/ATF functional domain. J Virol. 1998;72:8852–8860. doi: 10.1128/jvi.72.11.8852-8860.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nerenberg M, Hinrichs SH, Reynolds RK, Khoury G, Jay G. The tat gene of human T-lymphotropic virus type 1 induces mesenchymal tumors in transgenic mice. Science. 1987;237:1324–1329. doi: 10.1126/science.2888190. [DOI] [PubMed] [Google Scholar]
- Nicholson KM, Anderson NG. The protein kinase B/Akt signalling pathway in human malignancy. Cell Signal. 2002;14:381–395. doi: 10.1016/s0898-6568(01)00271-6. [DOI] [PubMed] [Google Scholar]
- Nuutinen U, Postila V, Matto M, Eeva J, Ropponen A, Eray M, Riikonen P, Pelkonen J. Inhibition of PI3-kinase-Akt pathway enhances dexamethasone-induced apoptosis in a human follicular lymphoma cell line. Exp Cell Res. 2006;312:322–330. doi: 10.1016/j.yexcr.2005.10.023. [DOI] [PubMed] [Google Scholar]
- Oren M. Decision making by p53: life, death and cancer. Cell Death Differ. 2003;10:431–442. doi: 10.1038/sj.cdd.4401183. [DOI] [PubMed] [Google Scholar]
- Osame M, Izumo S, Igata A, Matsumoto M, Matsumoto T, Sonoda S, Tara M, Shibata Y. Blood transfusion and HTLV-I associated myelopathy. Lancet. 1986;2:104–105. doi: 10.1016/s0140-6736(86)91636-3. [DOI] [PubMed] [Google Scholar]
- Peloponese JM, Jr, Jeang KT. Role for Akt/protein kinase B and activator protein-1 in cellular proliferation induced by the human T-cell leukemia virus type 1 tax oncoprotein. J Biol Chem. 2006;281:8927–8938. doi: 10.1074/jbc.M510598200. [DOI] [PubMed] [Google Scholar]
- Pise-Masison CA, Mahieux R, Jiang H, Ashcroft M, Radonovich M, Duvall J, Guillerm C, Brady JN. Inactivation of p53 by human T-cell lymphotropic virus type 1 Tax requires activation of the NF-kappaB pathway and is dependent on p53 phosphorylation. Mol Cell Biol. 2000;20:3377–3386. doi: 10.1128/mcb.20.10.3377-3386.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pise-Masison CA, Mahieux R, Radonovich M, Jiang H, Brady JN. Human T-lymphotropic virus type I Tax protein utilizes distinct pathways for p53 inhibition that are cell type-dependent. J Biol Chem. 2001;276:200–205. doi: 10.1074/jbc.M005601200. [DOI] [PubMed] [Google Scholar]
- Pise-Masison CA, Radonovich M, Sakaguchi K, Appella E, Brady JN. Phosphorylation of p53: a novel pathway for p53 inactivation in human T-cell lymphotropic virus type 1-transformed cells. J Virol. 1998;72:6348–6355. doi: 10.1128/jvi.72.8.6348-6355.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poiesz BJ, Ruscetti FW, Gazdar AF, Bunn PA, Minna JD, Gallo RC. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci USA. 1980;77:7415–7419. doi: 10.1073/pnas.77.12.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prives C, Hall PA. The p53 pathway. J Pathol. 1999;187:112–126. doi: 10.1002/(SICI)1096-9896(199901)187:1<112::AID-PATH250>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Seiki M, Hattori S, Hirayama Y, Yoshida M. Human adult T-cell leukemia virus: complete nucleotide sequence of the provirus genome integrated in leukemia cell DNA. Proc Natl Acad Sci USA. 1983;80:3618–3622. doi: 10.1073/pnas.80.12.3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T, Uchida-Toita M, Yoshida M. Tax protein of HTLV-1 inhibits CBP/p300-mediated transcription by interfering with recruitment of CBP/p300 onto DNA element of E-box or p53 binding site. Oncogene. 1999;18:4137–4143. doi: 10.1038/sj.onc.1202766. [DOI] [PubMed] [Google Scholar]
- Tanaka A, Takahashi C, Yamaoka S, Nosaka T, Maki M, Hatanaka M. Oncogenic transformation by the tax gene of human T-cell leukemia virus type I in vitro. Proc Natl Acad Sci USA. 1990;87:1071–1075. doi: 10.1073/pnas.87.3.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vousden KH, Lu X. Live or let die: the cell's response to p53. Nat Rev Cancer. 2002;2:594–604. doi: 10.1038/nrc864. [DOI] [PubMed] [Google Scholar]
- Wee KB, Aguda BD. Akt versus p53 in a Network of Oncogenes and Tumor Suppressor Genes Regulating Cell Survival and Death. Biophysical Journal. 2006;91:857–865. doi: 10.1529/biophysj.105.077693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao G, Cvijic ME, Fong A, Harhaj EW, Uhlik MT, Waterfield M, Sun SC. Retroviral oncoprotein Tax induces processing of NF-kappaB2/p100 in T cells: evidence for the involvement of IKKalpha. EMBO J. 2001;20:6805–6815. doi: 10.1093/emboj/20.23.6805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi A, Tamatani M, Matsuzaki H, Namikawa K, Kiyama H, Vitek MP, Mitsuda N, Tohyama M. Akt activation protects hippocampal neurons from apoptosis by inhibiting transcriptional activity of p53. J Biol Chem. 2001;276:5256–5264. doi: 10.1074/jbc.M008552200. [DOI] [PubMed] [Google Scholar]
- Yang L, Dan HC, Sun M, Liu Q, Sun XM, Feldman RI, Hamilton AD, Polokoff M, Nicosia SV, Herlyn M, Sebti SM, Cheng JQ. Akt/protein kinase B signaling inhibitor-2, a selective small molecule inhibitor of Akt signaling with antitumor activity in cancer cells overexpressing Akt. Cancer Res. 2004;64:4394–4399. doi: 10.1158/0008-5472.CAN-04-0343. [DOI] [PubMed] [Google Scholar]
- Zhao LJ, Jian H, Zhu H. Specific gene inhibition by adenovirus-mediated expression of small interfering RNA. Gene. 2003;316:137–141. doi: 10.1016/s0378-1119(03)00750-9. [DOI] [PubMed] [Google Scholar]
- Zhou BP, Liao Y, Xia W, Spohn B, Lee MH, Hung MC. Cytoplasmic localization of p21Cip1/WAF1 by Akt-induced phosphorylation in HER-2/neu-overexpressing cells. Nat Cell Biol. 2001;3:245–252. doi: 10.1038/35060032. [DOI] [PubMed] [Google Scholar]
- Zou H, Li Y, Liu X, Wang X. An APAF-1. cytochrome c multimeric complex is a functional apoptosome that activates procaspase-9. J Biol Chem. 1999;274:11549–11556. doi: 10.1074/jbc.274.17.11549. [DOI] [PubMed] [Google Scholar]