Abstract
PI3Ks (phosphoinositide-3 kinases) produce PIP3 (phosphatidylinositol(3,4,5)-trisphosphate) which mediates signals for cell survival and proliferation. The tumour suppressor PTEN (phosphatase and tensin homologue) dephosphorylates PIP3 and is a key negative regulator of PI3K signalling. Recent research highlighted important roles for PI3K/PTEN in cell polarization and directional cell migration, pointing to a significant role for PTEN in wound healing where spatially organized tissue growth is essential. Lai et al. (in this issue of British Journal of Pharmacology) have moved a step closer in utilizing PTEN for wound healing through pharmacological inhibition. Two vanadium derivative inhibitors targeting PTEN significantly elevated the level of phosphorylated Akt (protein kinase B) and nearly doubled the wound healing rate in monolayer cultures of lung and airway epithelial cells. Damage to airway and lung epithelia underlies a wide spectrum of significant clinical conditions. With further experiments, this promising approach may find potential clinical use in situations where enhanced wound healing of pulmonary and other epithelia is important.
Keywords: wound healing, PTEN, epithelium, Akt/protein kinase B, bisperoxovanadium compounds, PI3 kinase, cell migration
An efficient repair of breaches to the epithelia of lung and airway is critical in many respiratory conditions, such as acute lung injury and asthma, which are common causes of morbidity and mortality (Shimabukuro et al., 2003). It appears that there is a promising new approach to enhance the healing ability of epithelia pharmacologically by targeting the tumour suppressor phosphatase and tensin homologue (PTEN) (Lai et al., 2007, this issue).
The class I family of PI3Ks are activated downstream of membrane receptor tyrosine kinases and G-protein-coupled receptors. Upon activation, PI3Ks phosphorylate position three of the inositol ring of PIP2 (phosphatidylinositol 4,5 bisphosphate) converting PIP2 into PIP3 (phosphatidylinositol 3,4,5 trisphosphate), leading to activation of Akt kinase and other downstream effectors, resulting in enhanced cell proliferation and survival (Engelman et al., 2006) (Figure 1). Akt, or protein kinase B, is a serine/threonine kinase, that comprises three highly homologous members known as PKBα, PKBβ and PKBγ in mammals. Akt contains a pleckstrin homology domain that binds PIP3. Upon binding, Akt translocates to the plasma membrane where it is activated via phosphorylation by upstream kinases including the phosphoinoside-dependent kinase 1. Akt activation plays an important role in diverse cellular processes such as glucose metabolism, cell proliferation, apoptosis, transcription and cell migration. PTEN (phosphatase and tensin homologue deleted on chromosome 10) is a central negative regulator of the PI3K signalling cascade. It was discovered to be a tumour suppressor gene and it is mutated in many advanced cancers. As a phospholipid phosphatase, PTEN antagonizes PI3K by converting PIP3 back to PIP2 by dephosphorylation of PIP3 at the 3-prime position of the inositol ring (Cully et al., 2006). The balance of PI3K and PTEN activities therefore regulates important cellular responses, such as the survival of cardiomyocytes against ischaemia–reperfusion insults (Mocanu and Yellon, 2007). Recent research has demonstrated that spatially regulated PI3K/PTEN signalling underlies cell polarization and directional cell migration (Iijima et al., 2002; Comer and Parent, 2007). This signalling pathway therefore provides not only an activation level signal, but also signals for spatial sensation, which help the cells to define front/back and apical/basal in the context of their milieu.
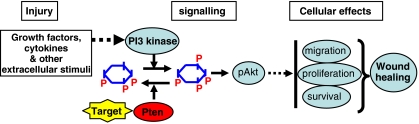
Figure 1.
Pharmacologically targeting PTEN to enhance wound-healing response. Injury induces PI3K signalling, which activates Akt through production of PIP3 and stimulates wound-healing responses, such as cell migration, survival and proliferation. PTEN dephosphorylates the second messenger PIP3 and converts it back to the precursor PIP2, antagonizing the action of PI3K. Targeting PTEN (yellow polygon) with newly developed pharmacological inhibitors is a promising approach to facilitate PI3K signalling and wound healing. Dashed arrows omit many links.
To heal a wound, cells surrounding the wound must survive, proliferate and, importantly, migrate and grow directionally to close the wound. The PI3K/PTEN signalling pathway regulates all of these and may play a role in wound healing (Tsugawa et al., 2003; Pankow et al., 2006; Xu and Yu, 2007). Injury, and ensuing stimuli such as growth factors and ATP, initiate PI3K–Akt signalling. Indeed, this signalling pathway was recently shown to be essential for directional migration of corneal and skin epithelial cells in response to injury, and to physiological electrical signals at wounds (Vanhaesebroeck, 2006; Zhao et al., 2006; Huttenlocher and Horwitz, 2007). Therefore, effective and specific modulation of this pathway could open the door to clinical therapies leading to improved wound healing.
In this issue of the British Journal of Pharmacology, Lai et al. (2007) present an appealing approach to modifying PI3K signalling by inhibition of PTEN. Using two pharmacological agents targeting PTEN, they have achieved specific activation of Akt and significant acceleration of monolayer wound healing in human lung and airway epithelia in vitro.
PTEN has two functional domains. The C2 domain binds membrane lipids, and the phosphatase domain catalyses dephosphorylation of PIP3 to PIP2. The phosphatase domain can also act on polypeptide substrates (Lee et al., 1999). Vanadium and its derivatives interact with the CX5R motif, a highly conserved motif of a large family of cysteine-based phosphatases, leading to reversible inhibition of PTEN. Lai et al. (2007) tested two newly derived bisperoxovanadium compounds: bpV(phen) (potassium bisperoxo (1,10-phenanthroline) oxovanadate) and bpV(pic) (dipotassium bisperoxo (picolinato) oxovanadate). Studies by other groups have shown that these two compounds are more specific inhibitors for PTEN, with IC50s about 10–100 fold lower than for other tyrosine phosphatases (Schmid et al., 2004). The present study demonstrates that both of these compounds, bpV(phen) and bpV(pic), in the range of 0.1–10 μM, resulted in significantly raised levels of phosphorylated Akt, that is elevated PI3K signalling in non-wounded culture. Dose-dependent responses were observed in both primary culture of human airway epithelial cells and a transformed lung epithelial cell line. Well-controlled experiments using cells that do not express PTEN proved that bpV(phen) and bpV(pic) induced an elevation of phosphorylated Akt through PTEN, confirming the specificity of the two compounds. In cells or tissues with PTEN inhibition, injury or other factors that activate PI3K signalling may cause an elevation of pAkt level even further, although this has yet to be tested.
Lai et al. (2007) carried out another important assay: in vitro monolayer wound closure. This is a widely used experimental model for cell polarization and directional cell migration. Although in vivo experiments need to be done, the present results may be highly relevant to wound healing of airway and lung epithelia in vivo, because both types of epithelia are a monolayer in vivo. Scrape wounds were made to the monolayer cultures. Inhibition of PTEN significantly increased (1) wound closure, and (2) cell numbers migrating into the scraped area, and these parameters were enhanced almost twofold when either bpV(phen) or bpV(pic) was used at a concentration of 0.5–2 μM.
Unlike monolayer cultures of other cell types such as fibroblasts, when epithelial cells become completely confluent, they form functional barriers and maintain a tight junction between cells. This increases significantly the trans-epithelial electrical resistance, a reliable index for barrier function recovery. Importantly, bpV(phen) and bpV(pic) also shortened the time for recovery of the trans-epithelial electrical resistance, in addition to accelerating wound closure significantly, as observed with time-lapse video microscopy. The time required to reach 50% of preinjury value was decreased to ∼1.5 days with PTEN inhibition, while control with no PTEN took ∼3 days. PTEN inhibition with these two compounds therefore resulted in a significant acceleration of both morphological and functional recovery of the epithelial monolayer.
Most of the above conclusions were elegantly confirmed with two independent sets of experiments in this paper (Lai et al. 2007). Overexpression of dominant-negative PTEN and siRNA-mediated suppression of PTEN expression verified that PTEN inhibition elevates the pAkt level and accelerates wound closure in transformed lung epithelial cells. The toxicity and specificity of the two compounds were also carefully evaluated. Satisfyingly, both bpV(phen) and bpV(pic), when used at low concentrations (∼1 μM), did not cause any obvious toxic effects, while the same concentration was effective at inducing a significant elevation of Akt phosphorylation and accelerating wound healing.
Injury to epithelium releases cells from contact inhibition and spatial constraints, ruptures cells at the wound edge, releases growth factors, cytokines, ATP and many other chemicals. Injury may also generate naturally occurring mechanical and electrical signals. All these factors may contribute to the co-ordinated cellular response needed to heal the wound (Block et al., 2004; Zhao et al., 2006; Yin et al., 2007). PI3K is one of the central signalling elements mediating multiple extracellular stimuli into cellular responses. PTEN as the negative regulator counter-balances PI3K activity (Figure 1). We have tested the role of PI3K and PTEN signalling in cornea and skin epithelial wound healing in vitro (Zhao et al., 2006). In monolayer cultures of corneal epithelial cells and skin keratinocytes, inhibition of PI3K with Wortmannin significantly decreased cell migration into the wound. Keratinocytes from mice with the null mutation of PI3Kγ (p110γ−/−) resulted in decreased activation of Akt, while keratinocytes from the conditional knockout of PTEN (pten−/−) showed elevated pAkt upon electric stimulation (Zhao et al., 2006). Delayed wound closure in a monolayer of p110γ−/− keratinocytes was very consistent with an increase in healing rate of keratinocytes from conditional knockout pten−/− mice when subjected to a physiological electrical field.
All the above results tie-in nicely with a previous study demonstrating the importance of PTEN in wound healing of gastric mucosa (Tsugawa et al., 2003). High blood pressure in the hepatic portal vein (blood vessel carrying blood from the digestive tract to the liver) is a clinical condition mainly due to cirrhosis of the liver. Portal hypertensive gastropathy is a severe complication in which the gastric mucosa has an impaired wound-healing response and increased susceptibility to injury by a variety of damaging agents, such as ethanol. Tsugawa et al. (2003) found that gastric mucosa from portal hypertensive rats had an abnormally high level of tumour necrosis factor-α, which led to increased expression of a transcription factor called early growth response factor-1. This transcription factor directly activates PTEN (Virolle et al., 2001). Tsugawa et al. (2003) demonstrated that overexpressed/activated PTEN in gastric mucosa from portal hypertensive rats is responsible for the reduced activation of the PI3K/Akt pathway and impaired healing of injuries in gastric mucosa.
These investigations have focused on the phosphatase function of PTEN to dephosphorylate PIP3 and negatively regulate the PI3K/Akt pathway. However, the PTEN story may not end there: PTEN can also regulate cell migration independently of its lipid phosphatase function, for example through its protein phosphatase activity in chick embryo and glioma cells (Maier et al., 1999; Leslie et al., 2007). More surprisingly, PTEN may inhibit migration of human glioma cells through the C2 domain, which is thought to be a membrane lipid binding domain (Raftopoulou et al., 2004). Suppression of cell proliferation may also be mediated by the C2 domain, independently of phosphatase activities (Okumura et al., 2005). Thus PTEN could also regulate cell migration independently of its lipid phosphatase activities and PI3K pathway. Vanadium compounds bind the phosphatase pocket of PTEN to exert its inhibition, so possible modulation of the C2 domain should also be considered.
In summary, PTEN appears to be a good therapeutic target to enhance epithelial wound healing. Pharmacological approaches to inhibit PTEN may provide a beneficial outcome as PTEN suppression can be controlled in time and in space relatively easily through topical application. The two drugs tested by Lai et al. (2007) offer exciting opportunities for further experiments, especially on epithelial wounds in vivo. Perhaps, this can be done in conjunction with PI3K activators. In addition, it would be interesting to elucidate the effects of PTEN inhibition on wound healing in stratified epithelia of skin and cornea. The mechanism of the effects on proliferation and migration of lipid phosphatase, protein phosphatase and C2 domain need to be investigated further. At the same time, new derivatives with higher potency and specificity add to the battery of PTEN inhibitors (Rosivatz et al., 2006, 2007), bringing the hope of clinical use closer to fruition.
Acknowledgments
I am grateful to the Wellcome Trust for continuous support, and to the Royal Society, London, the Royal Society of Edinburgh and Medical Research Scotland for support of my international collaboration. Dr Brian Reid's help with English expression is gratefully acknowledged.
Abbreviations
- Akt
protein kinase B
- pAkt
phosphorylated Akt
- bpV(phen), potassium bisperoxo (1
10-phenanthroline) oxovanadate
- bpV(pic)
dipotassium bisperoxo (picolinato) oxovanadate
- PI3K
phosphoinositide 3-OH kinase
- PIP2
phosphatidylinositol(4,5)-bisphosphate, PtdIns(4,5)P2
- PIP3
phosphatidylinositol (3,4,5)-trisphosphate, PtdIns(3,4,5)P3
- PTEN
phosphatase and tensin homologue deleted on chromosome 10
- siRNA
small interfering RNA
References
- Block ER, Matela AR, SundarRaj N, Iszkula ER, Klarlund JK. Wounding induces motility in sheets of corneal epithelial cells through loss of spatial constraints: role of heparin-binding epidermal growth factor-like growth factor signaling. J Biol Chem. 2004;279:24307–24312. doi: 10.1074/jbc.M401058200. [DOI] [PubMed] [Google Scholar]
- Comer FI, Parent CA. Phosphoinositides specify polarity during epithelial organ development. Cell. 2007;128:239–240. doi: 10.1016/j.cell.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Cully M, You H, Levine AJ, Mak TW. Beyond PTEN mutations: the PI3K pathway as an integrator of multiple inputs during tumorigenesis. Nat Rev Cancer. 2006;6:184–192. doi: 10.1038/nrc1819. [DOI] [PubMed] [Google Scholar]
- Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet. 2006;7:606–619. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- Huttenlocher A, Horwitz AR. Wound healing with electric potential. N Engl J Med. 2007;356:303–304. doi: 10.1056/NEJMcibr066496. [DOI] [PubMed] [Google Scholar]
- Iijima M, Huang YE, Devreotes P. Temporal and spatial regulation of chemotaxis. Dev Cell. 2002;3:469–478. doi: 10.1016/s1534-5807(02)00292-7. [DOI] [PubMed] [Google Scholar]
- Lai JP, Dalton JT, Knoell DL.Phosphatase and Tensin homolog deleted on chromosome Ten (PTEN) as a Molecular target in lung epithelial wound repair Br J Pharmacol 20071521172–1184.(this issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JO, Yang H, Georgescu MM, Di Cristofano A, Maehama T, Shi Y, et al. Crystal structure of the PTEN tumor suppressor: implications for its phosphoinositide phosphatase activity and membrane association. Cell. 1999;99:323–334. doi: 10.1016/s0092-8674(00)81663-3. [DOI] [PubMed] [Google Scholar]
- Leslie NR, Yang X, Downes CP, Weijer CJ. PtdIns(3,4,5)P(3)-dependent and -independent roles for PTEN in the control of cell migration. Curr Biol. 2007;17:115–125. doi: 10.1016/j.cub.2006.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier D, Jones G, Li X, Schonthal AH, Gratzl O, Van Meir EG, et al. The PTEN lipid phosphatase domain is not required to inhibit invasion of glioma cells. Cancer Res. 1999;59:5479–5482. [PubMed] [Google Scholar]
- Mocanu MM, Yellon DM. PTEN, the Achilles' heel of myocardial ischaemia/reperfusion injury. Br J Pharmacol. 2007;150:833–838. doi: 10.1038/sj.bjp.0707155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okumura K, Zhao M, Depinho RA, Furnari FB, Cavenee WK. Cellular transformation by the MSP58 oncogene is inhibited by its physical interaction with the PTEN tumor suppressor. Proc Natl Acad Sci USA. 2005;102:2703–2706. doi: 10.1073/pnas.0409370102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankow S, Bamberger C, Klippel A, Werner S. Regulation of epidermal homeostasis and repair by phosphoinositide 3-kinase. J Cell Sci. 2006;119:4033–4046. doi: 10.1242/jcs.03175. [DOI] [PubMed] [Google Scholar]
- Raftopoulou M, Etienne-Manneville S, Self A, Nicholls S, Hall A. Regulation of cell migration by the C2 domain of the tumor suppressor PTEN. Science. 2004;303:1179–1181. doi: 10.1126/science.1092089. [DOI] [PubMed] [Google Scholar]
- Rosivatz E. Inhibiting PTEN. Biochem Soc Trans. 2007;35:257–259. doi: 10.1042/BST0350257. [DOI] [PubMed] [Google Scholar]
- Rosivatz E, Matthews JG, McDonald NQ, Mulet X, Ho KK, Lossi N, et al. A small molecule inhibitor for phosphatase and tensin homologue deleted on chromosome 10 (PTEN) ACS Chem Biol. 2006;1:780–790. doi: 10.1021/cb600352f. [DOI] [PubMed] [Google Scholar]
- Schmid AC, Byrne RD, Vilar R, Woscholski R. Bisperoxovanadium compounds are potent PTEN inhibitors. FEBS Lett. 2004;566:35–38. doi: 10.1016/j.febslet.2004.03.102. [DOI] [PubMed] [Google Scholar]
- Shimabukuro DW, Sawa T, Gropper MA. Injury and repair in lung and airways. Crit Care Med. 2003;31:S524–S531. doi: 10.1097/01.CCM.0000081437.06466.B3. [DOI] [PubMed] [Google Scholar]
- Tsugawa K, Jones MK, Akahoshi T, Moon WS, Maehara Y, Hashizume M, et al. Abnormal PTEN expression in portal hypertensive gastric mucosa: a key to impaired PI 3-kinase/Akt activation and delayed injury healing. FASEB J. 2003;17:2316–2318. doi: 10.1096/fj.02-1107fje. [DOI] [PubMed] [Google Scholar]
- Vanhaesebroeck B. Charging the batteries to heal wounds through PI3K. Nat Chem Biol. 2006;2:453–455. doi: 10.1038/nchembio0906-453. [DOI] [PubMed] [Google Scholar]
- Virolle T, Adamson ED, Baron V, Birle D, Mercola D, Mustelin T, et al. The Egr-1 transcription factor directly activates PTEN during irradiation-induced signalling. Nat Cell Biol. 2001;3:1124–1128. doi: 10.1038/ncb1201-1124. [DOI] [PubMed] [Google Scholar]
- Xu KP, Yu FS. Cross talk between c-Met and epidermal growth factor receptor during retinal pigment epithelial wound healing. Invest Ophthalmol Vis Sci. 2007;48:2242–2248. doi: 10.1167/iovs.06-0560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin J, Xu K, Zhang J, Kumar A, Yu FS. Wound-induced ATP release and EGF receptor activation in epithelial cells. J Cell Sci. 2007;120:815–825. doi: 10.1242/jcs.03389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M, Song B, Pu J, Wada T, Reid B, Tai G, et al. Electrical signals control wound healing through phosphatidylinositol-3-OH kinase-gamma and PTEN. Nature. 2006;442:457–460. doi: 10.1038/nature04925. [DOI] [PubMed] [Google Scholar]