Abstract
Background and purpose:
We have shown that endogenous glucocorticoids control neutrophil mobilization in the absence of inflammation. In this study the role of the glucocorticoid receptor (GR) in the physiological control of neutrophil mobilization was investigated, focusing on the specific mechanisms for mature neutrophils in bone marrow, circulating neutrophils and endothelial cells.
Experimental approach:
Male Wistar rats were treated with RU 38486 or adrenalectomized. Cell numbers in bone marrow and circulation were morphologically quantified and expressions of L-selectin determined by flow cytometry. Expressions of P-selectin, E-selectin, PECAM-1, VCAM-1 and ICAM-1 were measured by immunohistochemistry on vessels of cremaster muscle and their mRNA levels quantified in primary cultured endothelial cells. NF-κB activity in neutrophils and endothelium was quantified by EMSA.
Key results:
RU 38486 treatment altered the maturation phases of neutrophilic lineage and reduced expression of L-selectin in mature neutrophils from bone marrow; increased the number of neutrophils in the circulation and elevated the expression of L-selectin in these cells. P-selectin and E-selectin expression in endothelial cells was unchanged by adrenalectomy or RU 38486 treatment. Membrane expressions, mRNA levels of ICAM-1, VCAM-1 and PECAM-1 and NF-κB translocation into the nucleus were higher in the endothelium of adrenalectomized and RU 38486 treated rats.
Conclusions and implications:
Endogenous glucocorticoids, through activation of GR on neutrophils, physiologically control the rolling behaviour of these cells and, by modulating endothelial functions, affect their adhesiveness. The molecular mechanism induced by activated GR is different in each cell, as NF-κB translocation was only altered in endothelial cells.
Keywords: RU 38486, adrenalectomy, neutrophil, endothelial cells, bone marrow, L-selectin, ICAM-1, VCAM-1, PECAM-1, NF-κB
Introduction
Endogenous glucocorticoids modulate several physiological responses, such as glucose metabolism, cardiovascular activity and immune reactions (Wilckens and De Rijk, 1997; Marik and Zaloga, 2002). Indeed, regulation of leukocyte recruitment (Flower et al., 1986; Moraes et al., 1987; Abe et al., 1995; Rovai et al., 1998; Leech et al., 2000), increase in vascular permeability, secretion of cytokines, expression of adhesion molecules, phagocytic and microbicidal activities (Filep et al., 1997; Fassbender et al., 1999; Nakagawa et al., 1999; Torsteinsdottir et al., 1999; Weber et al., 2001, 2004), which occur in the course of an inflammatory or stress injury, are modulated by an increase in the concentration of endogenous glucocorticoids in the plasma. In addition to the effects exerted by endogenous hormones, synthetic hormones are important therapeutic tools for the treatment of inflammatory diseases and as immunosuppressor agents (Rhen and Cidlowski, 2005; Song et al., 2005).
A broad range of anti-inflammatory effects elicited by high concentrations of endogenous glucocorticoids and therapeutic doses of synthetic hormones are mediated through an intracellular receptor, the glucocorticoid cytoplasmic receptor (GR), a member of the steroid hormone receptor superfamily located in the cytoplasm. On binding to the hormone, the GR is released from heat-shock protein 90 and other regulatory proteins, allowing it to gain access to the nucleus. Domains within the N-terminal region of the receptor interact with either negative or positive glucocorticoid response elements (GREs) in promoter regions of genes, with subsequent initiation or repression of transcription (Adcock, 2000; Lu and Cidlowski, 2004). Notwithstanding this classical mechanism of action, it has been suggested that the activated GR has an action directly on nuclear factor-κB (NF-κB) or on activating protein-1, inhibiting their binding to DNA and thus impeding transcription (van der Burg and van der Saag, 1996; Wissink et al., 1998; Reichardt et al., 2001; Lu and Cidlowski, 2004). In addition, a non-genomic mechanism has been proposed as it has been observed that administration of the GR antagonist RU 38486 or of protein synthesis blockers does not alter the reduced phagocytic and microbicidal activities of neutrophils or macrophages evoked by high doses of synthetic steroids (Liu et al., 2005; Long et al., 2005).
Our previous data have shown that endogenous glucocorticoids exert a physiological control on neutrophil mobilization, that is in the absence of an inflammatory response they help to keep neutrophils in circulation, as adrenalectomy or blocking of the GR resulted in increased rolling and adherence of leukocytes to postcapillary venules (Farsky et al., 1995). In addition, endogenous glucocorticoids accelerate neutrophil maturation in the bone marrow and its transference into the peripheral compartment, with consequent neutrophilia (Cavalcanti et al., 2006). The effects of endogenous glucocorticoids are most probably modulated by L-selectin, as adrenalectomy induced a decrease in the expression of L-selectin in mature neutrophils from bone marrow and an increased expression of L-selectin in circulating neutrophils. The expression of L-selectin in these cells is controlled by post-translational mechanisms, as the concentrations of L-selectin mRNA were the same in cells obtained from control and adrenalectomized (ADX) animals (Cavalcanti et al., 2006).
Physiologically, L-selectin has an important role in the mobilization of leukocytes from bone marrow (Takeshita et al., 2004) and in their movement into tissue, being responsible for the rapid onset of the adherence of neutrophils to the endothelium (Petri and Bixel, 2006). Furthermore, our data (Cavalcanti et al., 2006) indicate that endogenous glucocorticoids, by modulating L-selectin expression, maintain mature neutrophils in different compartments during their life span, avoiding the inappropriate mobilization of leukocytes.
The results from the present study reveal the physiological role of endogenous glucocorticoids in the movement of neutrophils in the absence of any injury or stress conditions. The interaction of endogenous glucocorticoids with GR was evaluated in bone marrow, circulating and endothelial cells after treatment with RU 38486. By screening the passage of neutrophils in each phase, we showed that via GR, endogenous glucocorticoids act on neutrophils by modulating their initial contact with the endothelium, whereas they act on the endothelium by controlling the events responsible for the subsequent firm adherence of neutrophils to the vessel wall. In addition, the differential control by the hormones on the expression of adhesion molecules in each microenvironment may involve distinct intracellular mechanisms, as the translocation of NF-κB into the nucleus was only altered in endothelial cells.
Methods
Animals
Male Wistar rats weighing 180–250 g at the beginning of the experiments were used. The animals were fed a standard pellet diet and water ad libitum. Before each experimental procedure, the animals were anaesthetized with sodium pentobarbital (65 mg kg−1, i.p.) to avoid stress. All procedures done to the animals were carried out according to protocols approved by the local Committee for Ethical Surveillance in Animal Experimentation (COBEA) for the proper use and care of experimental animals.
Surgical procedures
The animals were divided into two groups, one consisting of animals subjected to adrenalectomy and the other of control sham-operated (SO) animals. Animals in the adrenalectomy group were subjected to bilateral adrenalectomy in which entrance into the abdominal cavity was achieved through bilateral incisions of skin and muscle. ADX rats were administered isotonic saline solution in addition to water orally. The SO animals were subjected to surgical incisions, handling of the abdominal viscera and closure of the incisions, similar procedures to those applied to the ADX animals. No special postoperative care was required for the SO animals. The animals were used for experimentation 7 days after being operated. Non-manipulated (NM) rats were kept under the same conditions as operated rats.
Drug treatment
The glucocorticoid receptor antagonist mifepristone (RU 38486) was dissolved in a mixture of isotonic saline and absolute ethanol (1:1 v/v) and was administered via the intraperitoneal route (10 mg kg−1) every 24 h for 7 days. Control animals received the same volume of the vehicle used to dissolve the drugs, via the same route (vehicle-treated (VT) rats).
Haematological parameters
Animals were anaesthetized with sodium pentobarbital and whole blood samples were obtained via abdominal aorta punctures. EDTA (1 mg ml−1) was used as an anticoagulant. The total number of cells was quantified using a Neubauer chamber. Differential leukocyte counts were performed on blood smears stained with May Grunwald–Giemsa.
Bone marrow cells were obtained by flushing the femoral cavity with 2 ml of Mc Coy's 5A medium with EDTA (1 mg ml−1). The total number of cells was quantified in a Neubauer chamber. Differential counts were performed on the basis of 500 cells per slide in cytocentrifuge smears stained with May Grumwald–Giemsa. The granulocytic lineage of the bone marrow was classified as being either immature, band or mature. The immature class was considered to be promyelocytes, myelocytes and metamyelocytes. Mature cells in the last phase of maturation comprised around 98–99% of neutrophils. In the mononuclear lineage in the bone marrow, lymphoblasts were considered to be immature cells and lymphocytes, plasmocytes and macrophages were considered to be mature cells. In the circulatory compartment, mononuclear cells were comprised of lymphocytes and monocytes.
Flow cytometer
Leukocytes were isolated from blood collected from the abdominal aorta or from the bone marrow to quantify the expression of L-selectin. Leukocytes from the blood were used to evaluate apoptosis and necrosis. Blood was collected using EDTA (2 mg ml−1) as an anticoagulant and bone marrow content was harvested by perfusion of the femur with Hank's balanced salt solution (HBSS) containing 1% BSA and 10% EDTA. Briefly, erythrocytes were lysed by addition of ammonium chloride solution (0.13 M) to both samples, and leukocytes were recovered after washing with HBSS.
To quantify the expression of the adhesion molecule, leukocytes (1 × 106) were incubated in the absence and presence of N-formylmethionyl-leucyl-phenylalanine (FMLP 10−8 M) for 15 min. After being washed, leukocytes were further incubated for 20 min at 4 °C in the dark with 10 μl of L-selectin monoclonal antibody conjugated with FITC (fluorescein isothiocyanate conjugated). To quantify apoptosis and necrosis of circulating cells, leukocytes (1 × 106) were incubated with FITC annexin V (1:500) for 20 min at room temperature in the dark. Subsequently, propidium iodide (50 μg ml−1) was added. Cells obtained from NM animals were irradiated with u.v. (300 J; for 5, 15 or 30 s) and used as a positive control to indicate the efficacy of the assay.
Immediately after incubation with the antibody or protein and dye, the cells were analysed in a FACScalibur flow cytometer (Becton & Dickinson, San Jose, CA, USA). Data from 10 000 cells were obtained and only the morphologically viable leukocytes were considered for analysis. Results of L-selectin expression are presented as fluorescence units and data from apoptosis and necrosis as the percentage of cells positive to annexin V or propidium iodide.
In vitro neutrophil–endothelium adherence
Granulocytic cells-enriched leukocytes
Blood was collected from the abdominal aorta of anaesthetized rats using 2% EDTA. Cell separation was achieved by adding 3 ml of Percoll 56% in sterile phosphate-buffered saline to 5 ml of blood samples. After centrifugation (1000 g, 40 min), the interface containing mainly granulocytes (99% mature neutrophils) was collected and erythrocyte lysis was induced by adding lysis solution (NH4Cl 8.02 g l−1, NaHCO3 0.84 g l−1). Then 106 cells ml−1 were suspended in HBSS with 0.5% BSA and used for the adherence assay.
Endothelial cells
Primary cultures of microvascular endothelial cells were obtained from the cremaster muscle of the rats using the method described by Chen et al. (1995) and modified by Lotufo et al. (2006). Rats were anaesthetized and clotting was avoided by intraperitoneal injection of heparin (1000 U per animal). The animals were then exsanguinated by cutting the bilateral carotid arteries. Subsequently, the cremaster muscles were isolated, cut into cross sections of approximately 2 × 2 mm2, and two of these sections were placed into a flask and cultured in Dulbecco's modified Eagle's medium supplemented with 20% fetal calf serum and 1% gentamycin. After 48 h, the tissues were discarded and the medium was changed. The cells were sub-cultured with 10% pancreatin in phosphate-buffered saline solution and used for an adherence assay on the eighth day, when they achieved confluence. The endothelial cells were identified by adding monoclonal antibody against platelet endothelial cell adhesion molecule-1 (PECAM-1; Lotufo et al., 2006).
Adherence assay
First-passage endothelial cells were plated onto 6.4-mm-diameter wells at 104 cells ml−1 in Dulbecco's modified Eagle's medium supplemented with 20% fetal calf serum. Endothelial cells were washed three times with 100 μl of HBSS, and neutrophils (105cells per 50 μl well) in HBSS containing 0.5% BSA were then added. Endothelial cells and neutrophils were incubated for 5 min (37 °C, 5% CO2). Non-adherent neutrophils were washed away twice with 100 μl of HBSS. A colorimetric tetramethylbenzidine assay was then carried out to detect neutrophils adhering to the monolayers, in which tetramethylbenzidine was used as a peroxidase substrate. Then 50 μl of acetate buffer (0.05 M, pH 5.8), containing 0.25% dodecyltrimethylammonium bromide A as a peroxidase solvent and 25 μl tetramethylbenzidine (16 mM in dimethylsulphoxide), were added and the plate was incubated for 5 min at room temperature. Subsequently, 100 μl of hydrogen peroxidase (final concentration 2.5 nM) was added. After 2 min, the peroxidase reaction was stopped by the addition of 50 μl of sulphuric acid (1 M). The absorbance was determined at 420 nm using an ELISA microplate reader (spectraMAX 250, Molecular Devices). Neutrophil adherence was calculated using a calibration curve obtained by carrying out the peroxidase reaction in wells containing known amounts of neutrophils.
Immunohistochemistry
Animals were anaesthetized and their testes were surgically removed. They were immediately frozen in nitrogen-hexan solution, cryosectioned (8 μm thickness) and fixed in cold acetone for 10 min. For a direct immunohistochemistry assay, sections were incubated with 3% H2O2 Superblock solution to block endogenous peroxidase and biotin, respectively. This was followed by an overnight incubation (in a humidified box, 4 °C) with a biotinylated anti-rat ICAM-1 (intercellular cell adhesion molecule-1), PECAM-1 monoclonal antibody, purified anti-rat VCAM-1 (vascular cell adhesion molecule-1) monoclonal antibody, purified anti-rat E-selectin or purified polyclonal P-selectin. Sections were incubated for 60 min with streptavidin conjugated to peroxidase. Colour was developed by the addition of 3,3-diaminobenzidine. Sections were lightly stained in haematoxylin and dehydrated with ethanol and xylene. 3,3-Diaminobenzidine-stained areas of vessel walls were selected and the intensity of colours was quantified using image analyzer software (KS 300, Kontron Eletronik, Carl-Zeiss, Germany). To evaluate the background reaction, all these procedures were also carried out in sections of testes that had been incubated in the absence of antibodies or using goat immunoglobulin G.
Determination of mRNA concentrations
RNA extraction
Total RNA was extracted from endothelial cells cultured using the Trizol reagent method following the manufacturer's instructions. The RNA extraction was carried out in an RNAse-free environment. RNA was quantified by reading the absorbance of the RNA solution obtained at 260 nm.
Reverse transcriptase-PCR
cDNAs were synthesized from 2 μg of total RNA using an oligo(dT)15 primer (20 μg ml−1) after incubation at 70 °C for 5 min in the presence of 2 mM of dNTP mix, ribonuclease inhibitor (20 U) and Moloney murine leukaemia virus reverse transcriptase (200 U) in a final volume of 25 μl in Moloney murine leukaemia virus reverse transcriptase buffer. The reverse transcription occurred by incubation at 42 °C for 60 min. For PCR, the cDNA obtained was incubated with 2.5 U Taq DNA polymerase, 0.4 μM 3′- and 5′-specific primers and 200 μM dNTP mix in buffer-thermophilic DNA polymerase, containing 1.5 mM MgCl2. The primer sequences used were GAPDH, 5′-TATGATGACATCAAGAAGGTGG-3′ (forward) and 5′-CACCACCCTGTTGCTGTA-3′ (reverse); ICAM-1, 5′-CCTCTTGCGAAGACGAGAAC-3′ (forward) and 5′-ACTCGCTCTGGGAACGAATA-3′ (reverse); VCAM-1, 5′-AAGGGGCTACATCCACACTG-3′ (forward) and 5′-ACCGTGCAGTTGACAGTGAC-3′ (reverse); PECAM-1, 5′-TGCAGGAGTCCTTCTCCACT-3′ (forward) and 5′-ACGGTTTGATTCCACTTTGC-3′ (reverse).
Electromobility shift assay
Nuclear protein extracts
Neutrophils from blood and primary cultured endothelial cells were homogenized in 100 μl lysis buffer (10 mM 4-(2-hydroxyethyl)-1-piperazineethanesulphonic acid, pH 7.5, 10 mM KCl, 0.1 mM EDTA, pH 8.0, 10% glycerol, 1.0 mM dithiothreitol, 0.1 mM phenylmethanesulphonylfluoride, 1.0 μg ml−1 leupeptin, 1.0 μg ml−1 pepstatin, 0.08 μg ml−1 aprotinin) and kept for 15 min on ice. After the addition of 10 μl Nonidet-P40 (10%), the samples were vortexed for 10 s and centrifuged (5000 g, 1 min, 4 °C). The resultant pellet was washed with 50 μl lysis buffer and centrifuged (5000 g, 1 min, 4 °C). The nuclear pellet was re-suspended in 20 μl nuclear extract buffer (10 mM 4-(2-hydroxyethyl)-1-piperazineethanesulphonic acid, pH 7.5; 0.5 M KCl; 1 mM EDTA pH 8.0; 10% glycerol, 1 mM dithiothreitol; 0.1 mM phenylmethanesulphonylfluoride; 1.0 μg ml−1 leupeptin, 1.0 μg ml−1 pepstatin, 0.08 μg ml−1 aprotinin). Tubes were kept in a rocking plate (15 min, 4 °C). Samples were then centrifuged (20 000 g, 5 min, 4 °C) and the resulting supernatant (nuclear extract) was aliquoted and stored at −70 °C until used. The protein content was determined by Bradford's method.
Electromobility shift assay
An NF-κB double-strand consensus oligonucleotide probe (5′-AGTTGAGGGGACTTTCCCAGGC-3′) was end-labelled with [γ32P]-ATP (specific activity 3000 Ci mmol−1) in the presence of T4 polynucleotide kinase (10 min, 37 °C; Sigma, St Louis, MO, USA). Unincorporated nucleotides were removed in a Sephadex G25 spin column; 4 μg of nuclear extracts were incubated with gel shift binding buffer (10 mM 4-(2-hydroxyethyl)-1-piperazineethanesulphonic acid pH 7.5; 50 mM KCl; 1.0 mM MgCl2; 0.5 mM EDTA pH 8.0; 0.5 mM dithiothreitol, 4% glycerol and 1.0 μg poly(dI-dC)·poly(dI-dC) in a total volume of 20 μl, for 20 min at room temperature. Each sample was then incubated with 20 000–50 000 c.p.m. of radiolabelled probe (30 min, room temperature). Protein–DNA complexes were resolved by non-denaturing 6% acrylamide/bisacrylamide (37.5:1.0) in 0.25 × Tris-borate/EDTA buffer at 150 V for 1.5 h at room temperature. The gel was vacuum-dried and exposed to XAR-5 film (Kodak Co) for 24–48 h at −70 °C and analysed densitometrically.
Data and statistical analyses
Means and s.e.mean of all data are presented and were compared by Student's t-test or ANOVA. Turkey's Multiple Comparisons or Newman–Keuls test were performed for determining the significance of the differences between experimental conditions. GraphPad Prism 4.0 software (San Diego, CA, USA) was employed. The differences were considered to be significant when P was less than 0.05.
Drugs, chemicals, reagents and other materials
Annexin V protein conjugated with FITC, L-selectin monoclonal antibody conjugated with FITC (anti-rat CD62L), ICAM-1 monoclonal antibody biotinylated (anti-rat CD54), PECAM-1 monoclonal antibody biotinylated (anti-rat CD31), VCAM-1 monoclonal antibody purified (anti-rat CD106), P-selectin polyclonal purified and biotinylated anti-mouse immunoglobulin G secondary antibody were purchased from BD PharMingen Technical (San Diego, CA, USA). Anti-rat E-selectin was obtained from R&D Systems (Minneapolis, MN, USA). RU 38486, N-formylmethionyl-leucyl-phenylalanine; Percoll, Mc Coy's 5A medium, May Grumwald–Giemsa, tetramethylbenzidine, 3,3-diaminobenzidine, Tris-borate/EDTA, T4 polynucleotide kinase, Nonidet-P40, dithiothreitol, phenylmethanesulphonylfluoride, leupeptin, pepstatin, aprotinin, dodecyltrimethylammonium bromide and poly(dI-dC) were purchased from Sigma. Dulbecco's modified Eagle's medium, fetal bovine serum and gentamycin reagent solution were purchased from GIBCO BRL Products (Grand Island, NY, USA). Sodium pentobarbital was purchased from Cristália (São Paulo, Brazil). Heparin (Liquemine) was obtained from Roche (São Paulo, Brazil) and phosphate-buffered saline solution from EMD Chemicals (Darmstadt, Germany). Trizol reagent was purchased from Invitrogen (Grand Island, NY, USA). Oligo(dT)15 primer, ribonuclease inhibitor, Moloney murine leukaemia virus reverse transcriptase, Taq DNA polymerase, dNTP mix were purchased from Promega (Madison, WI, USA). Streptavidin conjugated with goat immunoglobulin G was purchased from Vector Laboratories (Burlingame, CA, USA); 3% H2O2 Superblock solution from Pierce (Rockford, IL, USA); Sephadex G25 spin column from Amersham Bioscience Corporation (CA, USA) and T4 polynucleotide kinase from Sigma. Ammonium chloride from Labsynth Săo Paulo, Brazil.
Results
Role of GR in the control of neutrophil mobilization from bone marrow
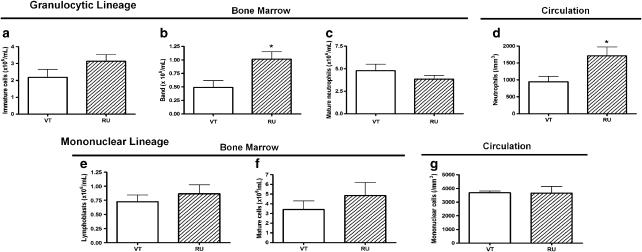
RU 38486 treatment affected the number of cells from granulocytic lineage in the bone marrow compartment, represented as a shunting line to the left in the neutrophilic sector. No alteration in the number of cells in the last phase of maturation (mature neutrophils) was observed, probably due to their enhanced migration to the peripheral compartment, as corroborated by neutrophilia. No change of lymph/mononuclear lineage cell counts was noted at any phase of bone marrow maturation and circulation (Figure 1).
Figure 1.
Effects of RU 38486 on the number of cells in the bone marrow and in the circulation. Vehicle-treated (VT) or RU 38486 was administered for 7 days (10 mg kg−1, i.p., every 24 h) and cells were collected 24 h after last doses. (a) Number of immature, (b) band and (c) mature neutrophils from bone marrow; (d) neutrophils in the circulation; (e) immature, (f) mature mononuclear cells from bone marrow; (g) mononuclear cells in the circulation. Data are expressed as mean±s.e.mean values obtained in six animals for each group. *P<0.01 vs VT.
Endogenous glucocorticoids do not interfere with the apoptosis or necrosis of circulating neutrophils
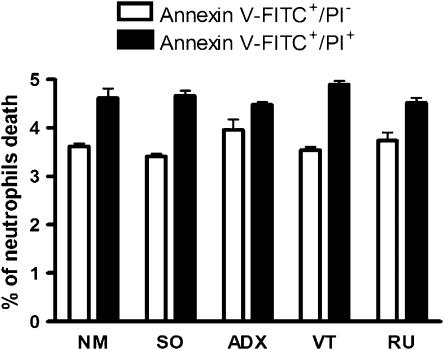
To investigate whether the neutrophilia detected in ADX (24) and RU 38486-treated rats was dependent on a reduction of apoptosis or necrosis of circulating neutrophils, phosphatidylserine exposure was quantified by annexin V binding and necrosis was determined by propidium iodide. Equivalent numbers of neutrophils positive to annexin V and to propidium iodide were found in cells from ADX or RU 38486-treated rats and their respective controls (Figure 2).
Figure 2.
Effects of adrenalectomy and RU 38486 on the apoptosis and necrosis of circulating neutrophils. Circulating neutrophils were collected from non-manipulated (NM), adrenalectomized (ADX), sham-operated (SO), vehicle-treated (VT) or RU 38486-treated rats (RU). RU 38486 was administered for 7 days (10 mg kg−1, i.p., every 24 h). Cells were collected 24 h after the last dose of RU 38486 or 7 days after surgery. Data were obtained by blood flow cytometry assay after incubation with annexin V/fluorescein isothiocyanate conjugated (FITC) or propidium iodide (PI).
Endogenous glucocorticoids modulate rolling behaviour by acting on GR of neutrophils
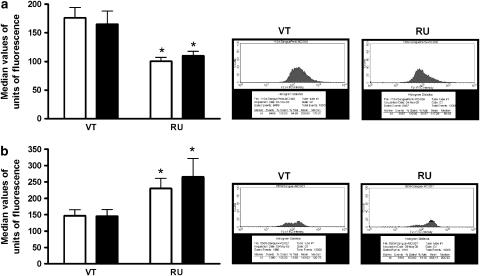
Data presented in Figure 3 show that RU 38486 treatment induced a decrease in the expression of L-selectin in bone marrow neutrophils (Figure 3a) and an increase in circulating neutrophils (Figure 3b), when compared to the expression of L-selectin in the same cells collected from VT rats. The effects did not reflect an alteration in the number of cells expressing the molecules, but rather the number of molecules per cell, as shown in the flow cytometer diagrams. This pattern of L-selectin expression was similar to that detected in ADX animals (Cavalcanti et al., 2006).
Figure 3.
Effects of RU 38486 on the expression of L-selectin in cells from the bone marrow or peripheral blood. Vehicle-treated (VT) or RU 38486 was administered for 7 days (10 mg kg−1, i.p., every 24 h) and cells were collected 24 h after the last dose. (a) Represents L-selectin expression on mature neutrophils from bone marrow and (b) represents L-selectin expression on neutrophils from circulating blood. Black columns indicate L-selectin expression after FMLP incubation (10−8M; 15min). Data are expressed as mean±s.e.mean of values obtained in seven animals in VT or RU 38486-treated animals. *P<0.05 vs respective controls.
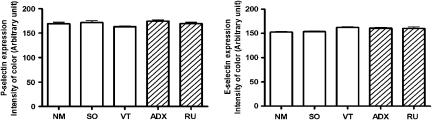
The expression of P- or E-selectin in endothelial cells from postcapillary venules obtained from the cremaster muscle was quantified by immunohistochemistry assays. The results demonstrate that the expression of these molecules was the same in tissues collected from ADX, RU 38486-treated rats and their respective controls, SO, NM and VT rats (Figure 4).
Figure 4.
Effect of adrenalectomy and RU 38486 treatment on expression of P- or E-selectin on endothelial cell membranes. Vehicle-treated (VT) or RU 38486 was administered for 7 days (10 mg kg−1, i.p., every 24 h) and cremaster muscle was collected 24 h after last doses or 7 days after adrenalectomy or sham surgery. The expressions of P- or E-selectin were quantified by immunohistochemistry. Results are expressed as the mean±s.e.mean of values obtained from three animals in each group. Assays were performed in quadruplicate. NM, non-manipulated; SO, sham-operated; VT, vehicle treated; ADX, adrenalectomized; RU, RU 38486-treated rats.
Endogenous glucocorticoids modulate neutrophil adherence by acting on GR of endothelial cells
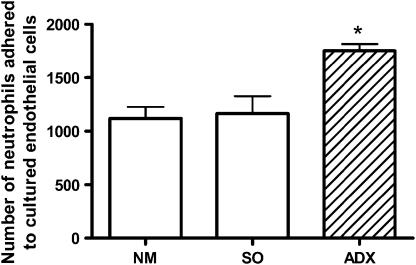
When we evaluated the in vitro ability of the endothelial cells obtained from ADX rats to adhere to circulating neutrophils collected from NM animals, it was found that a higher number of neutrophils adhered to endothelial cells obtained from ADX animals than to endothelial cells collected from NM or SO animals (Figure 5). Our previous results showed that neutrophils from ADX rats presented impaired adherence to endothelial cells obtained from NM rats (Cavalcanti et al., 2006).
Figure 5.
Effect of adrenalectomy on in vitro neutrophil adherence to endothelium. Primary cultured endothelial cells were obtained from cremaster muscle of adrenalectomized (ADX), non-manipulated (NM) or sham-operated (SO) rats, and neutrophils were collected from circulating blood of NM rats. Tissues were collected 7 days after surgery. Data are expressed as mean±s.e.mean of four experiments, done in duplicate. *P<0.05 vs controls.
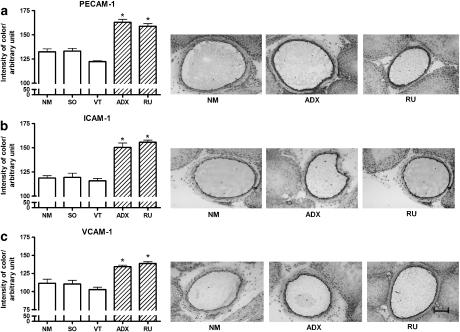
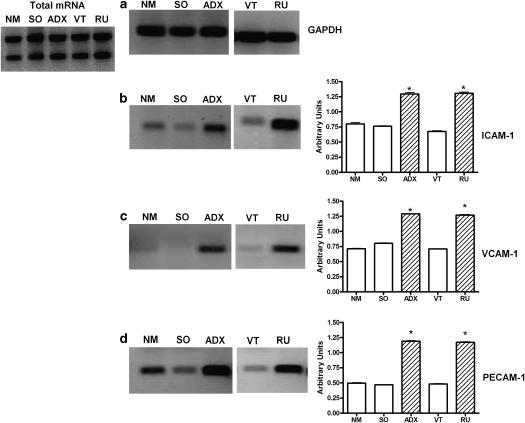
The expression in endothelial cells of adhesion molecules responsible for the firm adherence of neutrophils to the endothelium was quantified in postcapillary venules of the cremaster muscle obtained from ADX and RU 38486-treated rats and their respective controls, SO, NM and VT rats. The results show that adrenalectomy and RU 38486 treatment increased the expression of PECAM-1, ICAM-1 and VCAM-1 in the endothelial cell membrane (Figure 6). Additionally, the concentrations of mRNA of each adhesion molecule were found to be significantly increased in primary cultured cells obtained from ADX and RU 38486-treated rats when compared to that of cells from the control animals (Figure 7).
Figure 6.
Effects of adrenalectomy or RU 38486 treatment on expression of adhesion molecules on endothelial cell membranes. Vehicle-treated (VT) or RU 38486 was administered for 7 days (10 mg kg−1, i.p., every 24 h). Cremaster muscle was collected 24 h after the last doses of vehicle or RU 38486 or 7 days after adrenalectomy or sham surgery. Data and immunohistochemistry images were obtained from non-manipulated (NM), sham-operated (SO), Vehicle (VT), adrenalectomized (ADX) or RU 38486-treated rats. a=PECAM; b=ICAM-1; c=VCAM=1. Results are expressed as the mean±s.e.mean of three assays performed in quadruplicate. Bar=10 μm. *P<0.05 vs controls.
Figure 7.
Effects of adrenalectomy or RU 38486 treatment on gene expression of adhesion molecules in endothelial cells. Vehicle-treated (VT) or RU 38486 was administered for 7 days (10 mg kg−1, i.p., every 24 h). Cremaster muscle was collected 24 h after the last doses of vehicle or RU 38486 or 7 days after adrenalectomy or sham surgery. Reverse transcriptase-PCR assays were carried out on primary cultured endothelial cells obtained from cremaster muscle of non-manipulated (NM), sham-operated (SO), adrenalectomized (ADX), vehicle-treated (VT) or RU 38486-treated rats. Image represent agarose gel electrophoresis of GAPDH (a); ICAM-1 (b); VCAM-1 (c); PECAM-1 (d). Results are expressed as the mean±s.e.mean of cells collected from four animals in each group. *P<0.05 vs controls.
β2 integrin is a molecule expressed in neutrophils during their firm adherence to the endothelium (Petri and Bixel, 2006). Its expression in circulating neutrophils from RU 38486-treated rats was comparable to that found in cells collected from NM or VT rats (data not shown).
Participation of NF-κB in the actions of endogenous glucocorticoids on neutrophils and endothelial cells
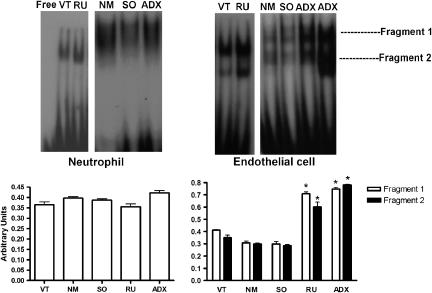
To investigate the possible mechanisms of action that endogenous glucocorticoids have on neutrophils and endothelial cells, NF-κB translocation into the cell nucleus was investigated. The results presented in Figure 7 show that the translocation of the transcription factor into the nucleus of neutrophils obtained from ADX and RU 38486-treated rats and their respective controls were similar. Conversely, translocation of the transcription factor into the nucleus was markedly enhanced in cultured cells collected from ADX and RU 38486-treated rats when compared to endothelial cells obtained from control animals (Figure 8).
Figure 8.
Effects of adrenalectomy or RU 38486 treatment on NF-κB translocation into the nucleus of circulating neutrophils or primary cultured endothelial cells. Vehicle-treated (VT) or RU 38486 was administered for 7 days (10 mg kg−1, i.p., every 24 h). Cremaster muscle or neutrophils were collected 24 h after the last doses of vehicle or RU 38486 or 7 days after adrenalectomy or sham surgery. Data represent gels and values obtained from gel shift assay of cells collected from non-manipulated (NM), sham-operated (SO), adrenalectomized (ADX), VT or RU 38486-treated rats. Results are expressed as the mean±s.e.mean of cells collected from three animals in each group. *P<0.05 vs controls.
Discussion
The results presented here show that endogenous glucocorticoids have an important role in the physiological control of neutrophil mobilization displaying different actions on neutrophils and endothelial cells, and that the control of the expression of adhesion molecules in each cell microenvironment may involve distinct intracellular mechanisms.
Total counts and relative differential proportions of blood leukocytes provide an important representation of the state of activation of the immune system, and of the pattern of distribution of leukocytes in the body. Cell–cell and cell–stromal contact, mediated by adhesion molecules on cell surfaces, are responsible for the maintenance of leukocytes in the bone marrow and peripheral compartments (van Eeden et al., 1997; Bauer et al., 2001). L-selectin is involved in these processes, as its expression is evident in all phases of granulocyte maturation and peaks in mature neutrophils (Lund-Johansen and Terstappen, 1993; Takeshita et al., 2004). In addition, it mediates the rolling behaviour of neutrophils in peripheral blood (Petri and Bixel, 2006).
Our previous results obtained in ADX animals (Cavalcanti et al., 2006) have now been corroborated by the results obtained in RU 38486-treated animals and indicate that secreted glucocorticoids regulate, via GR, different phases of neutrophil mobilization. It is likely that high concentrations of endogenous glucocorticoids control the number of leukocytes in circulation in stress conditions as leukocytosis is one of the characteristics of hormone therapy (Harris et al., 1995; Liles et al., 1997; Nakagawa et al., 1998; Weber et al., 2001, 2004). The mechanism involved in these conditions seems to be related to a change in the longevity of the cell in the circulation induced by altering apoptosis (Chang et al., 2004; Madsen-Bouterse et al., 2006) or by impairing the synthesis and expression of L-selectin (Nakagawa et al., 1999; Weber et al., 2001, 2004). Our data suggest that endogenous glucocorticoids act as a selective modulator of the delivery of granulocyte cells from the bone marrow, as ADX (Cavalcanti et al., 2006) and RU 38486-treated rats presented an increased rate of neutrophil maturation in the bone marrow with consequent neutrophilia, but with no effects on the lymphocyte/mononuclear cell lineages. Also, our data do not support the possibility that endogenous glucocorticoids affect the death of circulating neutrophils. Nevertheless, modifications in the expression of L-selectin may be an important control target of endogenous glucocorticoids, as hormone deficiency (Cavalcanti et al., 2006) or blockage of GR caused a decrease and increase in L-selectin expression in bone marrow and peripheral neutrophils, respectively, that was not dependent on pre-translational mechanisms (Cavalcanti et al., 2006). Next, we hypothesized that these hormones regulate L-selectin shedding. The effect of exogenous glucocorticoids on L-selectin expression by post-translational mechanisms has already been implicated by observations that annexin-1, a protein indicated as a mediator of the anti-inflammatory effects of glucocorticoids, hinders the migration of neutrophils to inflammatory sites (Perretti and Ahluwalia, 2000) by inducing L-selectin enzymatic cleavage from the surface of neutrophils (Strausbaugh and Rosen, 2001; De Coupade et al., 2003). However, recent data have shown that the action of annexin-1 on L-selectin shedding is dependent on concentrations of the protein and its bioactive peptide Ac2-26, and on the state of neutrophil activation (Hayhoe et al., 2006). Conversely, higher doses of annexin-1 caused an upregulation of L-selectin expression in human neutrophils (Hayhoe et al., 2006). From these findings and our results, it is probable that a differential molecular control by physiological and therapeutic doses of hormones occurs during healthy and stress-related conditions.
Furthermore, our data show that the expressions of P- and E-selectin in endothelial cells were not altered by a reduction in endogenous glucocorticoids levels. As selectins are responsible for the rolling behaviour of leukocytes (Sperandio, 2006), we suggest that endogenous glucocorticoids physiologically regulate the rolling behaviour by acting mainly on neutrophils.
Conversely, the role that endogenous glucocorticoids play in the firm adherence of leukocytes to the endothelium, the subsequent step to rolling during leukocyte transference into tissue, may reflect their actions on endothelium. This hypothesis is supported by our previous data demonstrating that neutrophils from ADX animals had a smaller than normal capacity to adhere to endothelial cells in vitro and that the expression of β2 integrin was normal in both basal and FMLP-stimulated conditions. Also, in the present study, β2 integrin expression was not altered in cells obtained from RU 38486-treated rats compared to controls (data not shown). Furthermore, the ability of endothelial cells from ADX animals to adhere to neutrophils in vitro was enhanced and the synthesis and membrane expression of ICAM-1, VCAM-1 or PECAM-1 in the endothelium of both ADX or RU 38486-treated animals were increased. Moreover, these data indicate the differential actions of endogenous glucocorticoids on neutrophils and endothelial cells during the firm adhesion process, and point to the long-lasting effects of endogenous glucocorticoids, as in vitro adherence and adhesion molecule mRNA concentrations were measured in primary cultured cells. Inhibition of the expression of endothelial adhesion molecules has been demonstrated previously after the administration of these hormones, at doses used in hormonal therapy (Tailor et al., 1999; Pitzalis et al., 2002). This is the first time that endogenous glucocorticoids have been shown to be involved in these physiological control mechanisms and the synthesis and expression of immunoglobulins, and indicates that the action of endogenous glucocorticoids on endothelial cells functions as an important physical barrier that contributes to the cessation of inappropriate mobilization of neutrophils from the circulation.
Repression of many pro-inflammatory genes via the inhibition of transcription factors, including NF-κB and activating protein-1, has been shown to be an important mechanism of the anti-inflammatory actions of endogenous and exogenous glucocorticoids (Pitzalis et al., 2002; Hermoso and Cidlowski, 2003). As the synthesis of adhesion molecules investigated here is mediated by NF-κB, the translocation of this factor into the nucleus was assessed. While NF-κB translocation was markedly enhanced in endothelial cells obtained from ADX and RU 38486-treated rats, no alteration was detected in neutrophils from either group of animals. Hence, in addition to corroborating the mechanism of action of exogenous glucocorticoids in the expression of adhesion molecules in endothelial cells (Pitzalis et al., 2002), our data show that these hormones are involved in the control of neutrophil mobilization, and that the expression of L-selectin on neutrophils is not dependent on NF-κB transcription.
Results from recent studies have led to the awareness that endogenous biochemical routes must be promptly activated to defend the host and then inactivated when resolution is achieved (Serhan et al., 2007). Here, by studying neutrophil mobilization, we highlight the physiological role secreted glucocorticoids play in a host's defence mechanism and disclose the effects of each component of this important system, which needs to respond promptly to any aggression but must not be activated inappropriately. Also, it is now clear that the fine adjustment induced by endogenous glucocorticoids on the mobilization of neutrophils may differ from that evoked by hormone therapy.
Acknowledgments
The authors thank Erika Cecon, Jorge M Ferreira and Marco Aurélio Ramirez Vignolo for technical assistance. This work was supported by FAPESP Grant no. 03/09410-5. RPM and SHPF are fellows of the Conselho Nacional de Pesquisa e Tecnologia (CNPq). DMHC is a FAPESP graduate fellow (05/59753-1). FAPESP is Fundaçáo de Amparo à Pesquisa do Estado de Săo Paulo.
Abbreviations
- ADX
adrenalectomized
- FITC
fluorescein isothiocyanate conjugated
- GR
glucocorticoid cytoplasmic receptor
- HBSS
Hank's balanced salt solution
- ICAM-1
intercellular cell adhesion molecule-1
- NF-κB
nuclear factor-κB
- NM rats
non-manipulated rats
- PECAM-1
platelet endothelial cell adhesion molecule
- SO rats
sham-operated rats
- VCAM-1
vascular cell adhesion molecule-1
- VT rats
vehicle-treated rats
Conflict of interest
The authors state no conflict of interest.
References
- Abe R, Shimosegawa T, Kimura K, Abe T, Kashimura J, Koizumi M, et al. The role of endogenous glucocorticoids in rat experimental models of acute pancreatitis. Gastroenterology. 1995;109:933–943. doi: 10.1016/0016-5085(95)90404-2. [DOI] [PubMed] [Google Scholar]
- Adcock IM. Molecular mechanisms of glucocorticosteroid actions. Pulm Pharmacol Ther. 2000;13:115–126. doi: 10.1006/pupt.2000.0243. [DOI] [PubMed] [Google Scholar]
- Bauer ME, Perks P, Lightman SL, Shanks N. Are adhesion molecules involved in stress-induced changes in lymphocyte distribution. Life Sci. 2001;69:1167–1179. doi: 10.1016/s0024-3205(01)01200-0. [DOI] [PubMed] [Google Scholar]
- Cavalcanti DM, Lotufo CM, Borelli P, Tavassi AM, Pereira AL, Markus RP, et al. Adrenal deficiency alters mechanisms of neutrophil mobilization. Mol Cell Endocrinol. 2006;249:32–39. doi: 10.1016/j.mce.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Chang LC, Madsen SA, Toelboell T, Weber PS, Burton JL. Effects of glucocorticoids on Fas gene expression in bovine blood neutrophils. J Endocrinol. 2004;183:569–583. doi: 10.1677/joe.1.05822. [DOI] [PubMed] [Google Scholar]
- Chen SF, Fei X, Li SH. A new simple method for isolation of microvascular endothelial cells avoiding both chemical and mechanical injuries. Microvasc Res. 1995;50:119–128. doi: 10.1006/mvre.1995.1044. [DOI] [PubMed] [Google Scholar]
- De Coupade C, Solito E, Levine JD. Dexamethasone enhances interaction of endogenous annexin-1 with L-selectin and triggers shedding of L-selectin in the monocytic cell line U-937. Br J Pharmacol. 2003;140:133–145. doi: 10.1038/sj.bjp.0705413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farsky SP, Sannomiya P, Garcia-Leme J. Secreted endogenous glucocorticoids regulate leukocyte–endothelial interactions in inflammation. A direct vital microscopic study. J Leukoc Biol. 1995;57:379–386. doi: 10.1002/jlb.57.3.379. [DOI] [PubMed] [Google Scholar]
- Fassbender K, Kaptur S, Becker P, Groschel J, Schmidt R, Hennerici M. Inverse association between endogenous glucocorticoid secretion and L-selectin (CD62L) expression in trauma patients. Life Sci. 1999;65:2471–2480. doi: 10.1016/s0024-3205(99)00513-5. [DOI] [PubMed] [Google Scholar]
- Filep JG, Delalandre A, Payette Y, Foldes-Filep E. Glucocorticoid receptor regulates expression of L-selectin and CD11/CD18 on human neutrophils. Circulation. 1997;96:295–301. doi: 10.1161/01.cir.96.1.295. [DOI] [PubMed] [Google Scholar]
- Flower RJ, Parente L, Persico P, Salmon JA. A comparison of the acute inflammatory response in adrenalectomised and sham-operated rats. Br J Pharmacol. 1986;87:57–62. doi: 10.1111/j.1476-5381.1986.tb10156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JG, Flower RJ, Perretti M. Endogenous corticosteroids mediate the neutrophilia caused by platelet-activating factor in the mouse. Eur J Pharmacol. 1995;283:9–18. doi: 10.1016/0014-2999(95)00274-o. [DOI] [PubMed] [Google Scholar]
- Hayhoe RP, Kamal AM, Solito E, Flower RJ, Cooper D, Perretti M. Annexin 1 and its bioactive peptide inhibit neutrophil–endothelium interactions under flow: indication of distinct receptor involvement. Blood. 2006;107:2123–2130. doi: 10.1182/blood-2005-08-3099. [DOI] [PubMed] [Google Scholar]
- Hermoso MA, Cidlowski JA. Putting the brake on inflammatory responses: the role of glucocorticoids. IUBMB Life. 2003;55:497–504. doi: 10.1080/15216540310001642072. [DOI] [PubMed] [Google Scholar]
- Leech M, Huang XR, Morand EF, Holdsworth SR. Endogenous glucocorticoids modulate experimental anti glomerular basement membrane glomerulonephritis. Clin Exp Immunol. 2000;119:161–168. doi: 10.1046/j.1365-2249.2000.01086.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liles WC, Huang JE, Llewellyn C, SenGupta D, Price TH, Dale DC. A comparative trial of granulocyte-colony-stimulating factor and dexamethasone, separately and in combination, for the mobilization of neutrophils in the peripheral blood of normal volunteers. Transfusion. 1997;37:182–187. doi: 10.1046/j.1537-2995.1997.37297203521.x. [DOI] [PubMed] [Google Scholar]
- Liu L, Wang YX, Zhou J, Long F, Sun HW, Liu Y, et al. Rapid non-genomic inhibitory effects of glucocorticoids on human neutrophil degranulation. Inflamm Res. 2005;54:37–41. doi: 10.1007/s00011-004-1320-y. [DOI] [PubMed] [Google Scholar]
- Long F, Wang YX, Liu L, Zhou J, Cui RY, Jiang CL. Rapid nongenomic inhibitory effects of glucocorticoids on phagocytosis and superoxide anion production by macrophages. Steroids. 2005;70:55–61. doi: 10.1016/j.steroids.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Lotufo CM, Yamashita CE, Farsky SH, Markus RP. Melatonin effect on endothelial cells reduces vascular permeability increase induced by leukotriene B4. Eur J Pharmacol. 2006;534:258–263. doi: 10.1016/j.ejphar.2006.01.050. [DOI] [PubMed] [Google Scholar]
- Lu NZ, Cidlowski JA. The origin and functions of multiple human glucocorticoid receptor isoforms. Ann NY Acad Sci. 2004;1024:102–123. doi: 10.1196/annals.1321.008. [DOI] [PubMed] [Google Scholar]
- Lund-Johansen F, Terstappen LW. Differential surface expression of cell adhesion molecules during granulocyte maturation. J Leukoc Biol. 1993;54:47–55. doi: 10.1002/jlb.54.1.47. [DOI] [PubMed] [Google Scholar]
- Madsen-Bouterse SA, Rosa GJ, Burton JL. Glucocorticoid modulation of Bcl-2 family members A1 and Bak during delayed spontaneous apoptosis of bovine blood neutrophils. Endocrinology. 2006;147:3826–3834. doi: 10.1210/en.2006-0142. [DOI] [PubMed] [Google Scholar]
- Marik PE, Zaloga GP. Adrenal insufficiency in the critically ill: a new look at an old problem. Chest. 2002;122:1784–1796. doi: 10.1378/chest.122.5.1784. [DOI] [PubMed] [Google Scholar]
- Moraes FR, Bechara GH, Moraes JR. Effect of alloxan diabetes and adrenalectomy on carrageenin-induced pleurisy in the rat. Braz J Med Biol Res. 1987;20:47–53. [PubMed] [Google Scholar]
- Nakagawa M, Bondy GP, Waisman D, Minshall D, Hogg JC, van Eeden SF. The effect of glucocorticoids on the expression of L-selectin on polymorphonuclear leukocyte. Blood. 1999;93:2730–2737. [PubMed] [Google Scholar]
- Nakagawa M, Terashima T, D'yachkova Y, Bondy GP, Hogg JC, vanEeden SF. Glucocorticoid-induced granulocytosis: contribution of marrow release and demargination of intravascular granulocytes. Circulation. 1998;98:2307–2313. doi: 10.1161/01.cir.98.21.2307. [DOI] [PubMed] [Google Scholar]
- Perretti M, Ahluwalia A. The microcirculation and inflammation: site of action for glucocorticoids. Microcirculation. 2000;7:147–161. [PubMed] [Google Scholar]
- Petri B, Bixel MG. Molecular events during leukocyte diapedesis. FEBS J. 2006;273:4399–4407. doi: 10.1111/j.1742-4658.2006.05439.x. [DOI] [PubMed] [Google Scholar]
- Pitzalis C, Pipitone N, Perretti M. Regulation of leukocyte–endothelial interactions by glucocorticoids. Ann NY Acad Sci. 2002;966:108–118. doi: 10.1111/j.1749-6632.2002.tb04208.x. [DOI] [PubMed] [Google Scholar]
- Reichardt HM, Tuckermann JP, Gottlicher M, Vujic M, Weih F, Angel P, et al. Repression of inflammatory responses in the absence of DNA binding by the glucocorticoid receptor. EMBO J. 2001;20:7168–7173. doi: 10.1093/emboj/20.24.7168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhen T, Cidlowski JA. Antiinflammatory action of glucocorticoids––new mechanisms for old drugs. N Engl J Med. 2005;353:1711–1723. doi: 10.1056/NEJMra050541. [DOI] [PubMed] [Google Scholar]
- Rovai LE, Herschman HR, Smith JB. The murine neutrophil-chemoattractant chemokines LIX, KC, and MIP-2 have distinct induction kinetics, tissue distributions, and tissue-specific sensitivities to glucocorticoid regulation in endotoxemia. J Leukoc Biol. 1998;64:494–502. doi: 10.1002/jlb.64.4.494. [DOI] [PubMed] [Google Scholar]
- Serhan CN, Brain SD, Buckley CD, Gilroy DW, Haslett C, O'Neill LA, et al. Resolution of inflammation: state of the art, definitions and terms. FASEB J. 2007;21:325–332. doi: 10.1096/fj.06-7227rev. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song IH, Gold R, Straub RH, Burmester GR, Buttgereit F. New glucocorticoids on the horizon: repress, don't activate. J Rheumatol. 2005;32:1199–1207. [PubMed] [Google Scholar]
- Sperandio M. Selectins and glycosyltransferases in leukocyte rolling in vivo. FEBS J. 2006;273:4377–4389. doi: 10.1111/j.1742-4658.2006.05437.x. [DOI] [PubMed] [Google Scholar]
- Strausbaugh HJ, Rosen SD. A potential role for annexin 1 as a physiologic mediator of glucocorticoid-induced L-selectin shedding from myeloid cells. J Immunol. 2001;166:6294–6300. doi: 10.4049/jimmunol.166.10.6294. [DOI] [PubMed] [Google Scholar]
- Tailor A, Tomlinson A, Salas A, Panés J, Granger DN, Flower RJ, et al. Dexamethasone inhibition of leucocyte adhesion to rat mesenteric postcapillary venules: role of intercellular adhesion molecule 1 and KC. Gut. 1999;45:705–712. doi: 10.1136/gut.45.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeshita K, Bacon KB, Gantner F. Critical role of L-selectin and histamine H4 receptor in zymosan-induced neutrophil recruitment from the bone marrow: comparison with carrageenan. J Pharmacol Exp Ther. 2004;310:272–280. doi: 10.1124/jpet.103.063776. [DOI] [PubMed] [Google Scholar]
- Torsteinsdottir I, Arvidson NG, Hallgren R, Hakansson L. Enhanced expression of integrins and CD66b on peripheral blood neutrophils and eosinophils in patients with rheumatoid arthritis, and the effect of glucocorticoids. Scand J Immunol. 1999;50:433–439. doi: 10.1046/j.1365-3083.1999.00602.x. [DOI] [PubMed] [Google Scholar]
- van der Burg B, van der Saag PT. Nuclear factor-kappa-B/steroid hormone receptor interactions as a functional basis of anti-inflammatory action of steroids in reproductive organs. Mol Hum Reprod. 1996;2:433–438. doi: 10.1093/molehr/2.6.433. [DOI] [PubMed] [Google Scholar]
- van Eeden SF, Bicknell S, Walker BA, Hogg JC. Polymorphonuclear leukocytes L-selectin expression decreases as they age in circulation. Am J Physiol. 1997;272:H401–H408. doi: 10.1152/ajpheart.1997.272.1.H401. [DOI] [PubMed] [Google Scholar]
- Weber PS, Madsen SA, Smith GW, Ireland JJ, Burton JL. Pre-translational regulation of neutrophil L-selectin in glucocorticoid challenged cattle. Vet Immunol Immunopathol. 2001;83:213–240. doi: 10.1016/s0165-2427(01)00381-6. [DOI] [PubMed] [Google Scholar]
- Weber PS, Toelboell T, Chang LC, Tirrell JD, Saama PM, Smith GW, et al. Mechanisms of glucocorticoid-induced down regulation of neutrophil L-selectin in cattle: evidence for effects at the gene-expression level and primarily on blood neutrophils. J Leukoc Biol. 2004;75:815–827. doi: 10.1189/jlb.1003505. [DOI] [PubMed] [Google Scholar]
- Wilckens T, De Rijk R. Glucocorticoids and immune function: unknown dimensions and new frontiers. Immunol Today. 1997;18:418–424. doi: 10.1016/s0167-5699(97)01111-0. [DOI] [PubMed] [Google Scholar]
- Wissink S, van Heerde EC, vand der Burg B, van der Saag PT. A dual mechanism mediates repression of NF-kappaB activity by glucocorticoids. Mol Endocrinol. 1998;12:355–363. doi: 10.1210/mend.12.3.0081. [DOI] [PubMed] [Google Scholar]