Abstract
Background and purpose:
Torsade de pointes (TdP) can be induced by a reduction in cardiac repolarizing capacity. The aim of this study was to assess whether I Ks blockade or enhancement of I Na could potentiate TdP induced by I Kr blockade and to investigate whether short-term variability (STV) or triangulation of action potentials preceded TdP.
Experimental approach:
Experiments were performed in open-chest, pentobarbital-anaesthetized, α1-adrenoceptor-stimulated, male New Zealand White rabbits, which received three consecutive i.v. infusions of either the I Kr blocker E-4031 (1, 3 and 10 nmol kg−1 min−1), the I Ks blocker HMR1556 (25, 75 and 250 nmol kg−1 min−1) or E-4031 and HMR1556 combined. In a second study rabbits received either the same doses of E-4031, the I Na enhancer, ATX-II (0.4, 1.2 and 4.0 nmol kg−1) or both of these drugs. ECGs and epicardial monophasic action potentials were recorded.
Key results:
HMR1556 alone did not cause TdP but increased E-4031-induced TdP from 25 to 80%. ATX-II alone caused TdP in 38% of rabbits, as did E-4031; 75% of rabbits receiving both drugs had TdP. QT intervals were prolonged by all drugs but the extent of QT prolongation was not related to the occurrence of TdP. No changes in STV were detected and triangulation was only increased after TdP occurred.
Conclusions and implications:
Giving modulators of ion channels in combination substantially increased TdP but, in this model, neither STV nor triangulation of action potentials could predict TdP.
Keywords: ATX-II, E-4031, HMR1556, IKr, IKs, INa, K+ channel blockers, proarrhythmia, repolarization reserve, torsade de pointes
Introduction
Torsade de pointes (TdP) is a polymorphic ventricular tachycardia, which is commonly associated with prolongation of the QT interval of the ECG. The electrophysiological mechanisms underlying this arrhythmia are not completely understood, but it has been proposed that TdP can occur when the repolarizing capacity of the cardiac ventricles is reduced (Roden, 1998). In the normal myocardium, several potassium currents contribute to repolarization (and hence the duration of the action potential and the QT interval), and if one current is reduced others can compensate; this is known as repolarization reserve (Roden, 1998; Biliczki et al., 2002). Drug-induced blockade of cardiac potassium channels is a known cause of TdP (Haverkamp et al., 2000) and this may be associated with a reduction in repolarization reserve.
The delayed rectifier potassium current (IK) is the main repolarizing current terminating the ventricular action potential in most species. It is voltage- and time-dependent with a rapidly activating (IKr) and slowly activating (IKs) component (Sanguinetti and Jurkiewicz, 1990). IKr is blocked by drugs such as dofetilide and E-4031 (Sanguinetti and Jurkiewicz, 1990), whereas, HMR1556 is a potent inhibitor of IKs with 1000-fold selectivity for IKs over IKr (Gögelein et al., 2000). In vitro work, which has examined the cardiac electrophysiological effects of these drugs in combination, supports the concept of repolarization reserve, in that, action potential prolongation by IKr blockade is potentiated when combined with an IKs blocker (Varro et al., 2000; Biliczki et al., 2002; Volders et al., 2003; Jost et al., 2005; So et al., 2006). In vivo it has also been shown that HMR 1556 potentiated the QT prolongation induced by dofetilide, in the dog, but this was not associated with the occurrence of arrhythmias or of TdP (Nakashima et al., 2004). The reason for the lack of effect of a reduction in repolarization reserve on the occurrence of TdP in this study may be that Nakashima et al. (2004) did not use an animal model in which drugs that block IKr have been shown to cause TdP.
Increasing inward currents can also contribute to delays in repolarization. DPI 201–106, the plant alkaloid veratridine, and the sea anemone toxin (ATX-II) are compounds that increase sodium current (INa) (Ravens and Himmel, 1999). ATX-II, in particular, has a high potency for cardiac INa (Hanck and Sheets, 2007). In vitro, ATX-II prolonged repolarization and induced arrhythmias (Song et al., 2004; Wu et al., 2004, 2006; Milberg et al., 2005) including TdP (Shimizu and Antzelevitch, 1997). Furthermore, ATX-II potentiated prolongation of the action potential caused by E-4031 in isolated guinea pig hearts (Wu et al., 2004), and potentiated the proarrhythmic actions of several other IKr blockers in isolated rabbit hearts (Wu et al., 2006). However, there is a lack of information on the effects of ATX-II on the induction of TdP in vivo.
In recent years there has been growing interest in the need to identify parameters that can predict the occurrence of TdP. It has been suggested that augmentation of triangulation of the action potential (Hondeghem et al., 2001) and temporal dispersion of repolarization (instability) measured as short-term variability (STV) in the duration of a number of consecutive action potentials (Thomsen et al., 2004), rather than QT prolongation per se, provide the proarrhythmic substrate and precede TdP. These factors have not yet been examined in open-chest, α-adrenoceptor-stimulated anaesthetized rabbits.
The first aim of this study was, therefore, to test the hypothesis that TdP will occur when repolarization reserve is severely limited, either by combined blockade of IKr and IKs or by blockade of IKr in combination with prolongation of INa, in the α1-adrenoceptor-stimulated open-chest pentobarbital-anaesthetized rabbit, which has been well characterized as a model in which drugs that block IKr may cause TdP (Carlsson et al., 1990; Batey and Coker, 2002). A second aim was to assess whether changes in beat-to-beat variability of repolarization or triangulation of the action potential could predict the occurrence of TdP in this model. Some of these results have been presented to the International Society for Heart Research (Michael et al., 2006, 2007).
Methods
Animal preparation
All animal experiments were performed in accordance with the UK Animals (Scientific Procedures) Act 1986 and conducted under the authority of Project Licence number 40/2548. Male New Zealand White rabbits (2.2 to 2.8 kg) were purchased from Harlan (Bicester, UK). Rabbits were anaesthetized by i.v. administration of ∼30 mg kg−1 sodium pentobarbital given 15 min after application of local anaesthetic cream (EMLA 5%) to a marginal ear vein. They were then prepared for the induction of drug-induced arrhythmias as described in detail previously (Batey and Coker, 2002; Farkas and Coker, 2002). ECG limb leads I, II and III were recorded simultaneously along with arterial blood pressure. At certain time points, blood gases, pH and K+ were also measured. Rabbits were ventilated with room air at 38 strokes per min and a stroke volume of 5 to 6 ml kg−1 body weight. After opening the chest, a positive end-expiratory pressure of 0.5 to 1 cm water was applied. In most experiments, an epicardial monophasic action potential (MAP) was recorded from the left ventricle. Details of all equipment and recording conditions have been published previously (Farkas and Coker, 2002), except that in the ATX study a Hugo Sachs Electronik spring-loaded epicardial Ag-AgCl MAP electrode (Linton Instrumentation, Diss, Norfolk, UK) was used to record MAPs.
Experimental protocols
The standard arrhythmia induction protocol consisted of three cycles of drug administration. In each cycle the α-adrenoceptor agonist phenylephrine was infused at a rate of 75 nmol kg−1 min−1 for 15 min, then the dose of phenylephrine was increased to 150 nmol kg−1 min−1 for 3 min followed by further increases to 225 then 300 nmol kg−1 min−1 for 3 min each. Five minutes into the first cycle a concurrent infusion of one or more ion channel modulators was started. At the end of the cycle both infusions were switched off and there was a 10 min drug-free interval. The doses of phenylephrine used in the second and third cycle were the same as in the first cycle, whereas the rate of infusion of ion channel modulator was increased to threefold in the second cycle and tenfold in the third cycle. This drug infusion protocol has been illustrated in previous publications (Batey and Coker, 2002; Farkas and Coker, 2002). For some pilot experiments there were only two cycles of drug infusion with the rate of infusion of E-4031 being the same in both cycles.
In the first main study (HMR study), rabbits were assigned randomly to receive either E-4031 (1, 3 and 10 nmol kg−1 min−1, n=8), HMR1556 (25, 75 and 250 nmol kg−1 min−1, n=7), E-4031 and HMR1556 combined (doses as above, n=10) or vehicle (saline infused at the same rate as E-4031 and polyethylene glycol 400 infused at the same rate as HMR1556, n=4). In a second randomized study (ATX study), rabbits received either E-4031 (1, 3 and 10 nmol kg−1 min−1, n=8), ATX-II (0.4, 1.2 and 4.0 nmol kg−1, n=8) or both drugs (n=8). Each dose of ATX-II was given as a slow i.v. bolus over 1 to 2 min.
ECG analysis and arrhythmia diagnosis
ECG intervals were measured manually using on-screen markers and taking the mean of four consecutive complexes. The PR, QRS and QT intervals were measured only in beats originating from the sino-atrial node that were not preceded or followed by ectopic beats or conduction block, in the manner described in Farkas et al. (2004). The QT interval was measured from the onset of the Q wave to where the T wave returns to the isoelectric baseline (including any U wave, if present). In the majority of cases, the end of the T(U) wave was clearly visible, but in some cases the T wave overlapped the P wave of the next heartbeat. In the latter situation, the end of the T(U) wave was estimated by extrapolation to the isoelectric line under the P wave as described previously (Farkas et al., 2004). At some time points ECG intervals could not be measured from all animals because of marked changes in morphology or arrhythmic activity.
Ventricular premature beats (VPBs), bigeminy, salvos and ventricular tachycardia were defined according to the Lambeth Conventions (Walker et al., 1988). TdP was defined as a polymorphic ventricular tachycardia of four or more beats, where twisting of the QRS complex around the isoelectric baseline was visible in at least one ECG lead. It was also accompanied by a decline in arterial blood pressure towards zero with little pulsatile activity. Conduction block, either intraventricular, such as bundle branch block, or atrio-ventricular (AV) block was also identified and quantified.
QT interval correction
QT values were corrected for heart rate using a correction factor derived from baseline heart rate and QT intervals from each animal included in each study. The correction factor was based on that originally described by Carlsson et al. (1993) and subsequently modified by others (Batey and Coker, 2002; Farkas and Coker, 2002, 2003). For the HMR1556 study, the rate corrected QT interval (QTc)=QT−0.655(RR−238) and for the ATX study the formula was QTc=QT−0.556(RR−241) which corrected QT intervals to the mean baseline heart rate observed in that study.
Beat-to-beat variability of repolarization
To assess beat-to-beat variability of QT intervals, values for 30 consecutive beats of sinus origin were measured manually before the start of the experimental protocol, that is, at baseline and immediately before the first VPB. Poincaré plots were produced by plotting each value against the former value. STV was determined as the mean orthogonal distance from the diagonal to the points of the plots and calculated according to the formula described previously (Thomsen et al., 2004; Detre et al., 2005) where STV=∑∣Dn+1−Dn∣/[30 × √2] and D represents QT duration.
Further analysis of variability in repolarization was carried out on the MAP data. The epicardial MAP signal was analysed using Electrophysiology Data Recorder software (version 2.8; John Dempster, University of Strathclyde, Glasgow, UK). The Po-Ne-Mah data files (recorded at 1000 Hz) were imported into the Electrophysiology Data Recorder application, a low pass filter of 100 Hz was applied and any baseline drift was subtracted. MAP durations were measured at 30 and 90% repolarization. From MAP duration at 90% repolarization (APD90), Poincaré plots were constructed and STV calculated as described above at baseline, immediately before the first VPB, before the first episode of TdP (or at an equivalent time point in rabbits that did not have TdP) and during the third cycle of drug administration. Triangulation of the MAP (APD90−APD30), as defined by Hondeghem et al. (2001), was also calculated at these time points. STV and triangulation data have only been presented from experiments in which these measurements could be obtained at all of the first three time points.
Pilot studies: dose selection and study design
It has been reported previously (Bril et al., 1996) that the IKr blocker E-4031 given at rates of 10 and 20 μg kg min−1 (∼20 and 40 nmol kg min−1) to pentobarbital-anaesthetized rabbits caused TdP in 70 and 90% of rabbits respectively. The aim of our preliminary studies was to find a dose of E-4031 that would cause TdP in approximately 20% of rabbits. Two consecutive cycles of administration of the same dose of E-4031 caused TdP in 100, 50, 50 and 20% of phenylephrine-stimulated rabbits which received 24 nmol kg−1 min−1 (n=2), 12 nmol kg−1 min−1 (n=2), 6 nmol kg−1 min−1 (n=4) and 3 nmol kg−1 min−1 (n=5) E-4031 respectively. The dose of 3 nmol kg−1 min−1 E-4031 was therefore chosen to be the middle dose in the sequence of three rising doses used in the combination studies.
As there was no published information on the effects of HMR1556 on TdP in vivo, our preliminary studies in anaesthetized rabbits focused on finding a range of doses that increased QT intervals without having any adverse effects on heart rate or blood pressure. Previous publications indicated that 1 mg kg−1 (∼2.5 μmol kg−1) i.v. HMR1556 increased QT intervals in anaesthetized pigs (Knobloch et al., 2004) and dogs (Nakashima et al., 2004). With consecutive infusions of 25, 75 and 250 nmol kg−1 min−1, there was a 20% increase in the QTc interval with no further increase seen with 750 nmol kg−1 min−1 HMR1556. On the basis of plasma concentrations of HMR1556 measured by Nakashima et al. (2004) in their studies, and extrapolation from in vitro measurements of the potency and selectivity of this drug (Gögelein et al., 2000; Thomas et al., 2003; Volders et al., 2003), infusions of 25, 75 and 250 nmol kg−1 min−1 were selected as doses that should block IKs only.
There is also little published information on the effects of ATX-II in vivo. A bolus dose of 5 μg kg−1 (∼1 nmol kg−1) increased QTc intervals without altering heart rate or blood pressure in halothane-anaesthetized dogs (Miyamoto et al., 2001). Based on this, pilot studies with i.v. bolus doses and infusions of ATX-II in the presence of saline instead of phenylephrine were performed. Infusions of ATX-II increased the QTc interval but also increased heart rate and decreased mean arterial blood pressure by the end of each cycle. In contrast, slow bolus doses of ATX-II (0.4, 1.2 and 4.0 nmol kg−1) had minimal effects on heart rate and blood pressure but did prolong QTc intervals and were therefore selected for the main study.
Statistics
Continuous data were expressed as mean±s.e.mean. One-way ANOVA, with post hoc Tukey's tests, was used to compare haemodynamic and QT interval data at baseline and the 15 min time point in each cycle within and among groups. The General Linear Model with interaction was used to compare differences in profiles between treatment groups. The incidence of arrhythmias and conduction block were compared by Fisher's exact probability test. Kruskal–Wallis tests were used to compare the durations of arrhythmias and conduction block. STV and triangulation were compared with one-way ANOVA (for three or more groups or time points) or using paired Student's t-tests for within group comparisons or unpaired Student's t-tests for between group comparisons. Differences were considered statistically significant when P<0.05.
Drugs
HMR1556 (a gift from Aventis Pharma, Frankfurt, Germany) was dissolved at 3 mg ml−1 in polyethylene glycol 400 each day. Stock solutions of E-4031 (purchased from Wako, Neuss, Germany) in normal saline (0.9% w/v NaCl in distilled H2O) were prepared in advance and stored in aliquots at −20°C, then diluted to 51 μg ml−1 with saline prior to use. ATX-II (purchased from Alomone Laboratories, Jerusalem, Israel) was dissolved at 100 μg ml−1 in distilled water and stored at −20°C, then diluted to 40 μg ml−1 with saline before each experiment. L-Phenylephrine (Sigma, Poole, UK) was dissolved at 1 mg ml−1 in saline and prepared freshly each day.
Results
Effects of HMR1556 and E-4031 alone and in combination on arrhythmias
HMR1556 did not cause TdP itself but potentiated the torsadogenic action of E-4031. TdP was observed in a total of 75% of rabbits receiving both E-4031 and HMR1556 in combination, 25% of rabbits receiving E-4031 alone and in 0% of the rabbits receiving vehicle or HMR1556 alone. In the combination group, most TdP was observed in the second and third cycles (Figure 1a). Another feature noted in the combination group was that there tended to be more episodes of TdP and they lasted longer. Figure 2a illustrates the total duration of all episodes of TdP that occurred during the experimental protocol and emphasizes the highly significant increase in the group receiving the combination of both drugs compared to the others.
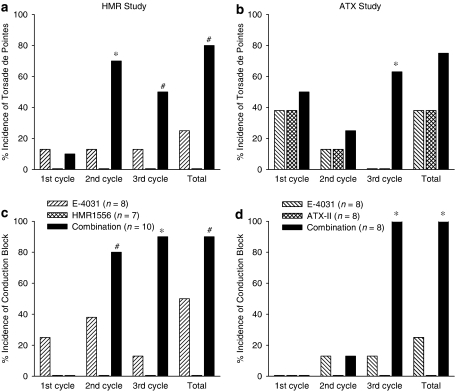
Figure 1.
Incidence of torsade de pointes (a and b) and conduction block (c and d) in the HMR1556 study (a and c) where anaesthetized rabbits received phenylephrine and either E-4031 (1, 3, 10 nmol kg−1 min−1), HMR1556 (25, 75, 250 nmol kg−1 min−1) or the combination of both drugs, and in the ATX study (b and d) where anaesthetized rabbits received phenylephrine and either E-4031 (1, 3, 10 nmol kg−1 min−1), ATX-II (0.4, 1.2, 4.0 nmol kg−1) or the combination of both drugs. *P<0.05 compared to both other groups, #P<0.05 compared to HMR1556 group, Fisher's Exact test. ATX-II, sea anemone toxin.
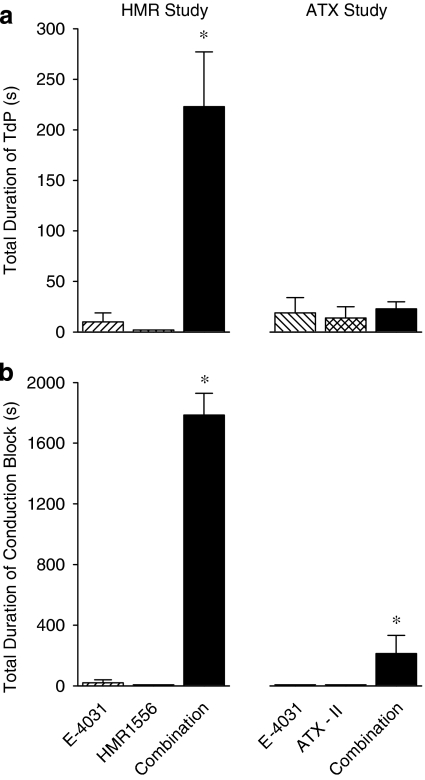
Figure 2.
Total duration of (a) torsade de pointes and (b) conduction block in the HMR1556 study where anaesthetized rabbits received phenylephrine and either E-4031 (1, 3, 10 nmol kg−1 min−1), HMR1556 (25, 75, 250 nmol kg−1 min−1) or the combination of both drugs, and in the ATX study where anaesthetized rabbits received phenylephrine and either E-4031 (1, 3, 10 nmol kg−1 min−1), ATX-II (0.4, 1.2, 4.0 nmol kg−1) or the combination of both drugs. *P<0.05 compared to both other groups, Kruskal–Wallis test. ATX-II, sea anemone toxin.
HMR1556 also potentiated the effects of E-4031 on other arrhythmias, such as salvos, ventricular tachycardia (monomorphic or polymorphic, but not showing the characteristics of TdP) and conduction block. For example, in the third cycle of drug administration, salvos and ventricular tachycardia occurred in 13% of rabbits receiving E-4031 alone, 14% of those receiving HMR1556 alone but in 88% of the rabbits receiving the combination of both drugs. Another rhythm disturbance that was observed frequently in the combination group was conduction block, but none of the rabbits given vehicle or HMR1556 alone had any conduction block (Figure 1c). The conduction block observed with E-4031 consisted of missing QRS complexes, and on average lasted 1 s in duration, whereas conduction block in the combination group was sustained (Figure 2b) with episodes of bundle branch block and 2:1 AV block being observed.
Effects of ATX-II and E-4031 alone and in combination on arrhythmias
ATX-II, administered alone, induced TdP in anaesthetized rabbits, and potentiated the torsadogenic effect of E-4031 during the third cycle of drug administration (Figure 1b). However, when the total incidence of TdP throughout the whole protocol is considered, ATX-II added to, rather than potentiated, the effects of E-4031. TdP was observed in a total of 75% of rabbits receiving ATX-II and E-4031 in combination, 38% of rabbits receiving ATX-II alone and in 38% of rabbits receiving E-4031 alone. With ATX-II alone and E-4031 alone most TdP occurred during the first cycle, whereas TdP was observed in all three cycles with the combination (Figure 1b). The total duration of all episodes of TdP is illustrated in Figure 2a. It is interesting to note that ATX-II did not potentiate the effects of E-4031 on the total duration of TdP.
ATX-II also potentiated the effects of E-4031 on other arrhythmias during the third cycle of drug administration. Salvos were observed in all rabbits given the combination, but only in 25% of rabbits receiving E-4031 alone or ATX-II alone. Ventricular tachycardia was not observed with ATX-II or E-4031 alone during the third cycle, but was seen in all rabbits receiving the combination. Conduction block was never seen with ATX-II alone but was observed in some rabbits that received E-4031 alone and in all rabbits receiving the combination during the third cycle of drug administration (Figure 1d). The low incidence of E-4031-induced conduction block revealed the potentiation of the incidence of conduction block by ATX-II (Figure 1d). As in the HMR1556 study, conduction disturbances observed with E-4031 were brief. In contrast, conduction block that occurred in the presence of the combination of both ATX-II and E-4031 lasted longer (Figure 2b) and included both bundle branch block and 2:1 AV block. Interestingly, however, the total duration of conduction block with the combination of ATX-II and E-4031 was only about a tenth of that seen with combined administration of HMR1556 and E-4031 (Figure 2b).
Effects of HMR1556 and E-4031 alone and in combination on heart rate and QT intervals
In contrast to the effects on arrhythmias, HMR1556 did not potentiate the effects of E-4031 on heart rate or QTc intervals. Heart rate declined over the course of the experimental protocol in all four treatment groups with no significant differences among the groups at the mid-point of each dosing cycle (Figure 3a). Within group analysis, however, revealed that the fall in heart rate reached significance during the first cycle of drug administration in the combination group, during the second cycle in the E-4031 and vehicle groups, but not until the third cycle in the group receiving HMR1556 alone (Figure 3a).
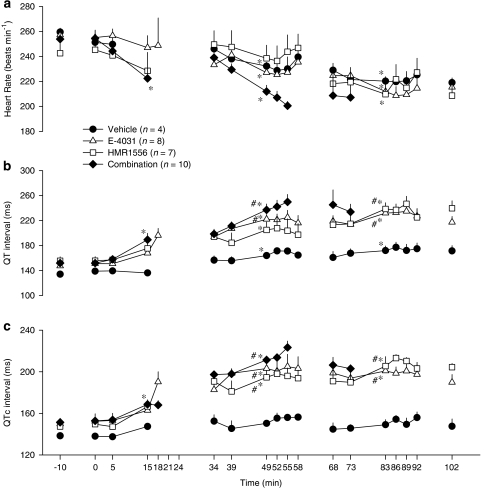
Figure 3.
(a) Heart rate, (b) QT intervals and (c) QTc intervals in anaesthetized rabbits in the HMR study which received phenylephrine in the presence of either E-4031 (1, 3, 10 nmol kg−1 min−1), HMR1556 (25, 75, 250 nmol kg−1 min−1) or the combination of both drugs. Values are expressed as mean±s.e.mean. *P<0.05 compared to baseline (−10 min), #P<0.05 compared to vehicle group, one-way ANOVA. At some time points, there are no data or n is less that the stated values due to frequent arrhythmias which prevented accurate measurement of heart rate and ECG intervals.
QT intervals increased progressively in the drug-treated groups (Figure 3b). In the vehicle group there were statistically significant, but modest, increases in the QT interval during the second and third cycles, but there were no significant differences in the QTc intervals (Figure 3c). In contrast, substantial increases in the QT and QTc interval occurred following administration of E-4031, HMR1556 and the combination of both drugs (Figure 3). Statistical comparison at the mid-point of the second cycle of drug administration indicated no significant differences in QTc intervals among the three groups that received the K+ channels blockers, but the values in all three of these groups were significantly greater than those seen in the vehicle-treated rabbits which only received phenylephrine (Figure 3). When the rabbits that received K+ channels blockers were divided into those that had TdP and those that did not, there was no significant difference in the change in QT intervals from baseline to the mid-point of the second cycle; 52±6 and 47±7% respectively. PR and QRS intervals were 65±2 and 55±1 ms at baseline in the vehicle group and remained similar throughout the course of the experiments. Values in the other groups were not significantly different from those in the vehicle group at any of the measured time points.
Effects of ATX-II and E-4031 alone and in combination on heart rate and QT intervals
Heart rate declined progressively over the course of the experimental protocol in all groups, falling significantly from baseline with E-4031 alone and the combination during the second cycle, but not until the third cycle with ATX-II alone. At the mid-points of the second and third cycles, heart rates were significantly lower in rabbits receiving the combination treatment compared with ATX-II alone (Figure 4a).
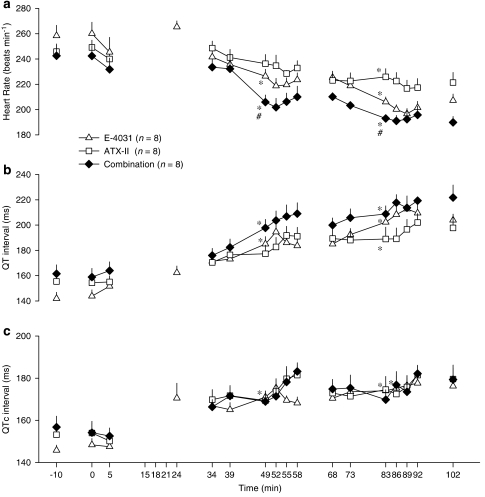
Figure 4.
(a) Heart rate, (b) QT intervals and (c) QTc intervals in anaesthetized rabbits in the ATX study which received phenylephrine in the presence of either E-4031 (1, 3, 10 nmol kg−1 min−1), ATX-II (0.4, 1.2, 4.0 nmol kg−1) or the combination of both drugs. Values are expressed as mean±s.e.mean. *P<0.05 compared to baseline (−10 min), #P<0.05 compared to ATX-II group, one-way ANOVA. At some time points, there are no data or n is less that the stated values due to frequent arrhythmias which prevented accurate measurement of heart rate and ECG intervals. ATX-II, sea anemone toxin.
ATX-II did not potentiate the effects of E-4031 on QT or QTc intervals. Although QT intervals increased progressively and were significantly prolonged from baseline in all groups by the third cycle, there were no differences between groups at the mid-point of the second or third cycles (Figure 4b). The QT-prolonging effects of these drugs were diminished when intervals were rate-corrected. For example, in the second cycle, the QTc interval was only increased significantly from baseline in the E-4031 group. There were no significant differences in QTc intervals among the groups (Figure 4c). When the rabbits were divided into those that had TdP and those that did not, the change in QT intervals from baseline to the mid-point of the second cycle, 23±5 and 24±5% respectively, was similar.
PR intervals increased in all three groups as the experimental protocol progressed; from 60±2 ms at baseline to 68±3 ms by the mid-point of the third cycle in the ATX-II group, 60±2 to 72±3 ms in the E-4031 group and 57±3 to 76±4 ms in the combination group, but there were no differences among the groups at either time point. Comparing the same time points, QRS intervals only increased in the groups receiving ATX-II alone and in combination with E-4031; from 52±2 to 61±3 ms and from 56±3 to 70±3 ms respectively. Again, there were no significant differences among the groups at either time point.
Effects of HMR1556 and E-4031 alone and in combination on blood pressure, blood gases and K+
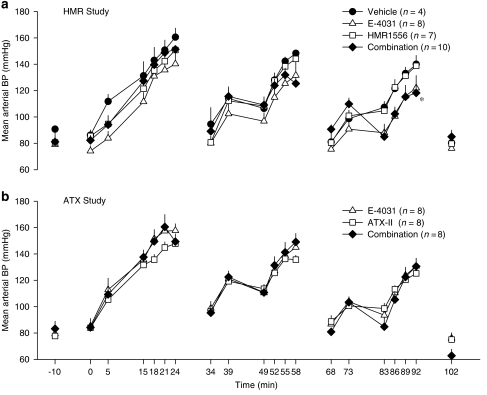
Phenylephrine dose-dependently increased mean arterial blood pressure in all three drug administration cycles, with recovery to baseline values when the phenylephrine infusion was stopped (Figure 5a). There appeared to be some attenuation of the phenylephrine-induced increase in mean blood pressure in the middle of the second and third cycles although there were no significant differences among the groups at these time points. Detailed statistical analysis of each cycle as a whole revealed that in the third cycle giving both drugs in combination gave rise to a significantly different profile of mean arterial blood pressure values than those in the other treatment groups (Figure 5a). This latter effect is probably a consequence of the persistent conduction block reducing heart rate and therefore cardiac output.
Figure 5.
Mean arterial blood pressure (BP) in (a) the HMR1556 study where anaesthetized rabbits received phenylephrine and either E-4031 (1, 3, 10 nmol kg−1 min−1), HMR1556 (25, 75, 250 nmol kg−1 min−1) or the combination of both drugs, and in (b) the ATX study where anaesthetized rabbits received phenylephrine and either E-4031 (1, 3, 10 nmol kg−1 min−1), ATX-II (0.4, 1.2, 4.0 nmol kg−1) or the combination of both drugs. Values are expressed as mean±s.e.mean. *P<0.05 for the profile of all the values in the third cycle in the combination group compared to the other groups, General Linear Model with interaction. To aid clarity, symbols for the within group statistical analyses have been omitted. At baseline and at the mid-point of each cycle there were no significant differences among groups, one-way ANOVA. ATX-II, sea anemone toxin.
Baseline blood gas, pH and K+ values were PO2=83±2 mm Hg, PCO2=31±1 mm Hg, pH 7.50±0.01 units, K+=1.79±0.05 mmol l−1 (n=29). During the course of the experiments there were no significant changes in PO2 or PCO2 and no differences in blood gas values among the groups at any time point. In the group that received the combination of E-4031 and HMR1556, blood K+ values were 1.81±0.08, 2.14±0.19, 3.12±0.37* and 4.03±0.29* mmol l−1 at baseline, end of the first cycle, end of the second cycle and end of the third cycle, respectively (*P<0.05 compared to baseline). The corresponding pH values were 7.50±0.02, 7.40±0.01*, 7.30±0.01* and 7.20±0.02* units (*P<0.05 compared to baseline). Similar changes in pH and K+ occurred in the other groups but there were no significant differences among the groups at any time point.
Effects of ATX-II and E-4031 alone and in combination on blood pressure, blood gases and K+
The changes in mean arterial blood pressure throughout the course of the experimental protocol in the ATX study were similar to those seen in the HMR study. Phenylephrine increased blood pressure in all three cycles and there were no differences among the groups at any time point (Figure 5b).
In the ATX study, baseline blood gas, pH and K+ values were PO2=85±2 mm Hg, PCO2=34±1 mm Hg, pH 7.49±0.01 units,K+=1.93±0.08 mmol l−1 (n=24). No significant changes in PO2 or PCO2 occurred, but pH decreased and K+ increased in each group as the protocol progressed. The pattern and magnitude of these changes in K+ and pH were similar to those seen in the HMR1556 study and there were no significant differences among the groups at any time point.
Effects of ion channel modulators on variability of repolarization
In the HMR1556 study, beat-to-beat variability of repolarization was assessed initially by calculating STV from Poincaré plots of QT intervals at baseline and immediately before the first premature beat in each rabbit that received one or more K+ channel blockers. These values were 3.6±0.3 and 3.9±0.3 ms in the E-4031 group, 3.7±0.5 and 3.8±0.3 ms in the HMR1556 group and 3.6±0.3 and 4.1±0.7 ms in the group given both drugs. There were no significant differences either within or between groups. When the data were sorted by whether or not TdP occurred, the STV values were 3.6±0.3 ms at baseline and 3.9±0.2 ms immediately before the first VPB in rabbits that did not have TdP (n=15). The corresponding values in the rabbits that did have TdP (n=8) were 3.6±0.3 and 4.1±0.7 ms. Again, there were no significant differences in these values either within or between those that had TdP and those that did not.
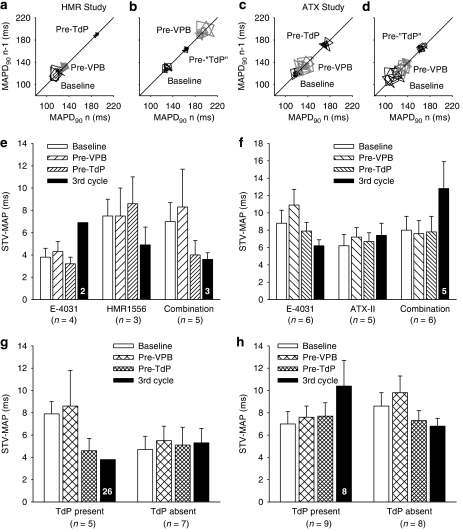
Further assessment of variability of repolarization focused on analysis of data obtained from measurements of epicardial MAPs. Figures 6a–d illustrate Poincaré plots of APD90 from rabbits with and without TdP in both the HMR1556 and ATX studies. The STV of APD90 was calculated at baseline, immediately before the first VPB, before the first episode of TdP (or at equivalent time points in those that did not have TdP) and during the third cycle of drug administration. In the HMR1556 study, neither of the K+ channel blockers altered STV when given alone or in combination (Figure 6e). Similarly, in the ATX study, neither E-4031 nor ATX-II altered STV. Combined administration of ATX-II and E-4031 did appear to increase STV in the third cycle but this apparent change was not significantly different (Figure 6f). Even when the data were plotted by whether or not TdP occurred there were no significant differences either within or between the groups in either the HMR1556 study (Figure 6g) or the ATX study (Figure 6h).
Figure 6.
(a–d) Examples of Poincaré plots of monophasic action potential (MAP) duration at 90% repolarization (MAPD90) at baseline, immediately prior to the first ventricular premature beat (Pre-VPB) and (a and c) before the first episode of torsade de pointes (Pre-TdP) or (b and d) at similar time points in rabbits that did not have TdP (Pre-‘TdP'). (e–h) Mean±s.e.mean values of short-term variability in MAPD90 (STV-MAP) by treatment in (e) the HMR study and (f) the ATX study or by the presence or absence of TdP in (g) the HMR study and (h) the ATX study. Numbers in columns indicate n-values where these are less than the group value.
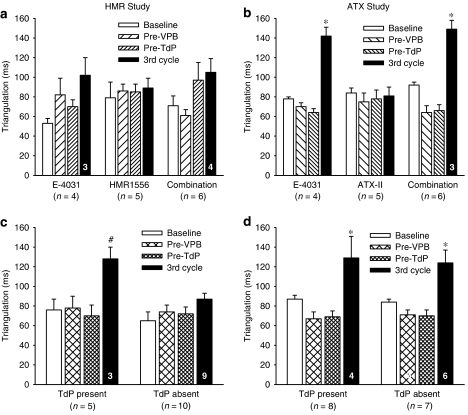
Possible changes in action potential morphology were assessed by calculating triangulation of the MAP (APD90−APD30) at the same time points as STV had been measured. In the HMR1556 study, no clear pattern of changes was observed within each drug treatment group (Figure 7a), although E-4031 tended to increase triangulation by the final time point. However, in the ATX study, E-4031 clearly increased triangulation in the third cycle when given alone or in combination with ATX-II (Figure 7b). Sorting the data from each study by whether or not TdP occurred produced differing results. In the HMR1556 study, triangulation was only increased in the third cycle in rabbits that had TdP (Figure 7c), whereas in the ATX study triangulation increased in the third cycle in rabbits in which TdP was present and in those in which it was absent (Figure 7d). It is important to note, however, that there were no changes in triangulation before TdP occurred, irrespective of how the data were plotted.
Figure 7.
Triangulation (MAPD90–MAPD30) displayed by treatment in (a) the HMR study and (b) the ATX study or by the presence or absence of TdP in (c) the HMR1556 study and (d) the ATX study. Values are mean±s.e.mean. *P<0.05 compared to all other time points, #P<0.05 compared to Pre-TdP, one-way ANOVA. Numbers in columns indicate n-values where these are less than the group value.
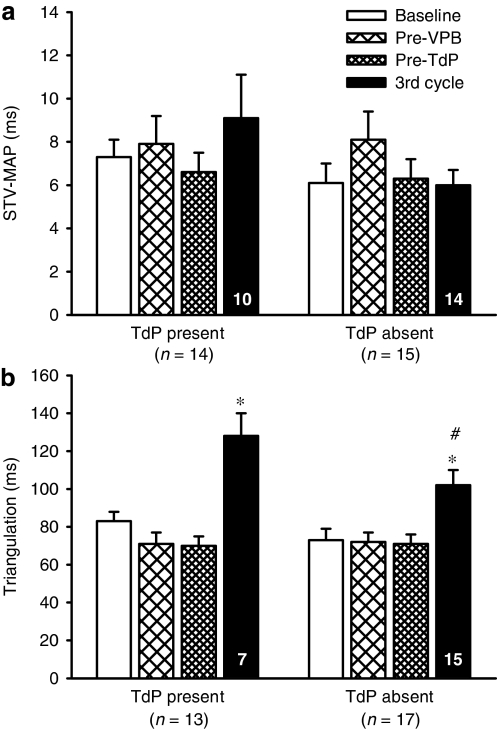
STV and triangulation data from both studies were pooled to increase the power of the comparisons between rabbits that had TdP and those that did not. This pooling of data shows clearly that in this rabbit model no significant changes in STV occurred before the first VPB, before TdP or even in the third cycle of drug administration, in rabbits with TdP or in those without TdP (Figure 8a). In contrast, there were significant increases in triangulation but these were only seen towards the end of the experimental protocol (Figure 8b). Although there was greater triangulation at this time point in the rabbits that had TdP compared to those that did not have TdP, a significant increase did still occur in the latter group.
Figure 8.
Pooled data from both the HMR and ATX studies illustrating (a) short-term variability in MAPD90 (STV-MAP) and (b) triangulation of the action potential in rabbits with TdP and those in which TdP was absent. Values are mean±s.e.mean. *P<0.05 compared to all other time points, one-way ANOVA, #P<0.05 compared to TdP present, unpaired t-test. Numbers in columns indicate n-values where these are less than the group value.
Discussion
The major novel findings of the present work are that (1) HMR1556 caused more potentiation of the torsadogenic action of E-4031 than ATX-II, at the doses studied; (2) ATX-II induced TdP in vivo and increased the proarrhythmic actions of E-4031; (3) neither changes in STV of repolarization nor triangulation of the action potential could predict the occurrence of TdP in open-chest, α1-adrenoceptor-stimulated anaesthetized rabbits.
Comparison of potentiation of the effects of IKr blockade by blocking IKs or prolonging INa
The IKs blocker, HMR1556, potentiated the torsadogenic action of the IKr blocker, E-4031, substantially increasing both the incidence and the duration of TdP. HMR1556 on its own prolonged repolarization but did not induce TdP. Prior to publication of our abstract on the HMR1556 study (Michael et al., 2006), the in vivo cardiac electrophysiological effects of HMR1556 had only been reported in dog (Volders et al., 2003; Nakashima et al., 2004) and pig models (Knobloch et al., 2004). A marked prolongation of the QT interval was observed in these species but HMR1556 was not associated with the induction of TdP. A recently published paper, however, demonstrated that HMR1556 potentiated TdP induced by dofetilide in anaesthetized rabbits (Lengyel et al., 2007). The effects on the incidence of TdP reported by Lengyel et al. (2007) are very similar to those reported here. HMR1556 itself did not cause TdP but increased the incidence of dofetilide-induced TdP from approximately 30 to 70%. In the present study, we have shown that HMR1556 also potentiated the total duration of episodes of E-4031-induced TdP.
A different pattern of changes was seen when ATX-II was combined with E-4031. First, unlike HMR1556, ATX-II given alone did cause TdP. Second, when the total incidence of TdP throughout the experimental protocol is considered, the effects of ATX-II and E-4031 were additive, whereas the interaction between HMR1556 and E-4031 was synergistic. Third, ATX-II did not increase the duration of TdP; in fact the duration of TdP with the combination of ATX-II and E-4031 was less than additive. Previously, it has been reported that ATX-II potentiated the proarrhythmic actions of IKr blockers in vitro (Wu et al., 2006). The present work has demonstrated clearly that ATX-II can also cause TdP in vivo and potentiate some of the proarrhythmic actions of E-4031. In the third cycle of drug administration, ATX-II potentiated E-4031-induced salvos, VT, conduction block and TdP. At this time point, these arrhythmias were either absent or only occurred in one or two rabbits given either ATX-II or E-4031 alone. Thus the capacity to see substantial potentiation of these arrhythmias existed. In their studies in isolated, AV-blocked, rabbit hearts, Wu et al. (2006) found that ATX-II given at a concentration which did not cause VT itself, significantly increased the incidence of VT induced by IKr blockers. It is possible that the interaction of ATX-II and E-4031 on the total incidence of TdP in our experiments appeared to be additive because each drug on its own already induced TdP in 38% of rabbits and thus the capacity to see significant potentiation was limited. However, this reasoning cannot be applied to the data on the duration of TdP, as unlike HMR1556, ATX-II did not increase the duration of TdP induced by E-4031. This suggests that there may be differences in the mechanisms by which episodes of TdP are sustained by drugs that prolong INa compared to those that block IK.
Influence of the extent of QT prolongation, hypokalaemia and conduction block
In contrast to results from rabbit perfused heart experiments, where IKs blockers given alone did not increase action potential duration (So et al., 2006) or QTc interval (Lengyel et al., 2001), HMR1556 did prolong QT and QTc intervals in our in vivo experiments. This can be explained by the presence of intact sympathetic innervation in vivo and indicates that the rabbit, like dog and man (Volders et al., 2003; Jost et al., 2005), requires sympathetic influence to reveal the important contribution of IKs to repolarization reserve. This in vivo sympathetic drive may, however, limit the ability to see potentiation of repolarization. Previous in vitro studies on combined IKr and IKs blockade have shown synergistic increases in prolongation of repolarization, supporting the concept of repolarization reserve (Varro et al., 2000; Biliczki et al., 2002; Volders et al., 2003; Jost et al., 2005; So et al., 2006). Similar findings have also been reported with combined INa enhancement and IKr block (Wu et al., 2006). Such changes were not seen in the present in vivo studies. There were no significant differences in QT interval, QTc interval or APD90 among the groups receiving ion channel modulators in either the HMR or ATX studies. In their recent studies in conscious dogs, Lengyel et al. (2007) reported additive rather than synergistic effects of combined IKr and IKs blockade on QTc prolongation. A recent commentary (Thomsen, 2007) on that paper suggested that the presence of adrenergic stimulation in vivo could explain why supra-additive effects on QT prolongation were not seen.
The lack of further prolongation of QT or QTc intervals with drug combinations in the present studies supports the hypothesis that there is no correlation between the extent of QT prolongation and the occurrence of TdP. It could be argued, however, that there was limited capacity to see potentiation of QT prolongation. In the HMR study, QTc intervals were increased substantially by about 50%, which may be about the maximum increase that can be seen with these drugs before the development of persistent conduction block prevented measurement of ECG intervals. However, in the ATX study, the changes in QTc intervals were just under 25% with either ATX-II or E-4031 alone, but there was no further increase when both drugs were given together. Thus, despite there being capacity to see a further increase, it did not occur. Looking at the results overall, one group (HMR1556 alone) had a substantial increase in QTc intervals but no TdP, whereas the combination groups in both studies had similar incidences of TdP (75 and 80%) but differing extents of QTc prolongation (25 and 50%).
Hypokalaemia is a risk factor for the development of TdP, particularly with drugs that block IKr as their potency is increased when extracellular K+ concentrations are low (Yang and Roden, 1996). In the present study, blood K+ concentrations were low (∼2 mmol l−1) at baseline. Low K+ concentrations have been reported previously in pentobarbital-anaesthetized rabbits (Barrett et al., 1997; Barrett and Walker, 1998; Lightbown et al., 2001; Farkas and Coker, 2002; Gil et al., 2004). As discussed in our previous publications (Lightbown et al., 2001; Farkas and Coker, 2002), the reduction of blood K+ concentrations is a consequence of anaesthesia and is influenced by ventilation status as there is a correlation between PCO2 and K+. As there were no differences in K+ concentrations among the groups of rabbits that received E-4031, HMR1556, ATX-II or combinations, variations in the extent of hypokalaemia cannot explain the differences in the occurrence of TdP. The gradual rise in K+ that occurred with time in all the rabbit groups also occurred previously in similar studies (Farkas and Coker, 2002, 2003). It was probably a consequence of continued administration of phenylephrine, as α-adrenoceptor agonists have been reported to cause K+ release in anaesthetized rabbits (Coats, 1985).
Potentiation of conduction block was seen in both the HMR and ATX studies. Intraventricular block can develop as a consequence of extremely delayed repolarization, and 2:1 AV block can occur when the refractory period of the ventricular muscle is longer than the sino-atrial cycle length (Farkas et al., 2004). As well as the anaesthetized α-adrenoceptor-stimulated rabbit model (Carlsson et al., 1990; Batey and Coker, 2002), rabbit isolated perfused heart models with acute AV block (Eckardt et al., 1998; Barrett et al., 2001) have been used to study TdP. It may be that conduction block is a predisposing factor to TdP. However, ATX-II given alone did not cause any conduction block but did induce TdP. Thus conduction block per se is not essential for TdP to occur.
Predictors of TdP: STV of repolarization, action potential triangulation
The present results indicate that TdP cannot be predicted by increases in STV of the QT interval or epicardial APD90 in this particular rabbit model. A lack of predictive value for STV of QT intervals has been reported previously in the chronic AV blocked dog model of TdP (Thomsen et al., 2004), but increased STV of left ventricular monophasic APD90 was found to be an excellent predictor of TdP in that study and others (Thomsen et al., 2004, 2006, 2007; Detre et al., 2005). Recently, increased STV of QT intervals has been reported to predict TdP in conscious dogs and to ‘parallel the increase in the incidence of TdP in anaesthetized rabbits' challenged with combined IKr and IKs blockade (Lengyel et al., 2007). Despite finding similar potentiation of TdP with HMR1556+E-4031 in the present studies as Lengyel et al. (2007) achieved with HMR1556+dofetilide, no increases in STV were detected even when STV of APD90 was calculated.
These divergent results raise the important question of how they may have occurred. There are two major differences between our rabbit model and that of Lengyel et al. (2007). First, our rabbits are open-chest and mechanically ventilated; second, they received phenylephrine. Our rabbits had lower mean arterial blood pressures (∼80 vs ∼100 mm Hg) and lower heart rates (∼240 vs ∼285 beats per min) at baseline, probably as a consequence of the deeper anaesthesia needed to perform a thoracotomy. This could have suppressed autonomic influences on the heart in our studies, which may be important in beat-to-beat changes in repolarization. The reduced heart rate per se is unlikely to be a factor as it has been shown in the chronic AV blocked dog model that it is the remodelling process rather than bradycardia which leads to increases in STV (Thomsen et al., 2007). Alternatively, the continued administration of phenylephrine in our model may have an influence. A recent abstract suggests that QT variability could not predict TdP induced by phenylephrine+dofetilide in closed-chest anaesthetized rabbits (Orosz et al., 2007). This agrees with our findings in open-chest anaesthetized rabbits.
Triangulation of the MAP was seen in these studies but only during the third cycle of drug administration. No changes were detected before the occurrence of VPBs or TdP. Neither HMR1556 nor ATX-II given alone caused any triangulation but ATX-II did cause TdP. It is clear from the present results that there is no association between triangulation and TdP in this model. There is, however, good evidence from in vitro studies that triangulation precedes TdP (Hondeghem et al., 2001, 2003; Milberg et al., 2005). Perhaps the much lower heart rates in perfused hearts in vitro allow greater development of triangulation at earlier time points. In rabbit Purkinje fibres, triangulation (and instability calculated in a similar manner to STV) induced by an IKr blocker was much greater at a lower stimulation rate (Lu et al., 2006).
Limitations
In the present study, MAPs were only recorded from the epicardium of the left ventricle. It is possible that this area does not exhibit as much variability in repolarization as some other parts of the heart. For example, in the rabbit perfused left ventricular wedge preparation, QT prolonging drugs had greater effects on endocardium than epicardium (Liu et al., 2006). Thus we cannot exclude the possibility, that in our in vivo model, changes in STV or triangulation of the action potential might have been detected from endocardial MAPs. It should also be noted that the present studies have focused on examining temporal dispersion of repolarization through measurement of STV. Although it has been suggested recently that neither QT dispersion nor changes in Tpeak–Tend of the QT interval predicted TdP in anaesthetized α1-adrenoceptor-stimulated rabbits (Orosz et al., 2007), we cannot exclude the possibility that spatial dispersion of repolarization may predict TdP in our model.
Conclusions
These studies have demonstrated that TdP induced by IKr blockade can be potentiated by prolonging INa or blocking IKs. The latter approach was more effective. In the particular model used here, the open-chest, α-adrenoceptor-stimulated, anaesthetized rabbit, neither changes in STV of repolarization nor triangulation of the action potential could predict the occurrence of TdP. It is important that further studies are performed to assess the predictive power of STV of QT intervals, APD90 and triangulation of the action potential in in vivo models of TdP.
Acknowledgments
This work was funded by the British Heart Foundation (FS/03/118). We thank Professor Heinz Gögelein, Aventis Pharma Deutschland GmbH for arranging the gift of HMR1556.
Abbreviations
- APD90
action potential duration at 90% repolarization
- ATX-II
sea anemone toxin
- AV
atrio-ventricular
- IK
delayed rectifier potassium current
- IKr
rapidly activating delayed rectifier potassium current
- IKs
slowly activating delayed rectifier potassium current
- INa
sodium current
- MAP
monophasic action potential
- STV
short-term variability
- TdP
torsade de pointes
Conflict of interest
The authors state no conflict of interest.
References
- Barrett TD, Hennan JK, Fischbach PS, O'Neill BP, Driscoll EM, Jr, Lucchesi BR. Tedisamil and dofetilide-induced torsades de pointes, rate and potassium dependence. Br J Pharmacol. 2001;132:1493–1500. doi: 10.1038/sj.bjp.0703967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett TD, MacLeod BA, Walker MJ. A model of myocardial ischemia for the simultaneous assessment of electrophysiological changes and arrhythmias in intact rabbits. J Pharmacol Toxicol Methods. 1997;37:27–36. doi: 10.1016/s1056-8719(96)00145-1. [DOI] [PubMed] [Google Scholar]
- Barrett TD, Walker MJ. Glibenclamide does not prevent action potential shortening induced by ischemia in anesthetized rabbits but reduces ischemia-induced arrhythmias. J Mol Cell Cardiol. 1998;30:999–1008. doi: 10.1006/jmcc.1998.0664. [DOI] [PubMed] [Google Scholar]
- Batey AJ, Coker SJ. Proarrhythmic potential of halofantrine, terfenadine and clofilium in a modified in vivo model of torsade de pointes. Br J Pharmacol. 2002;135:1003–1012. doi: 10.1038/sj.bjp.0704550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biliczki P, Virag L, Iost N, Papp JG, Varro A. Interaction of different potassium channels in cardiac repolarization in dog ventricular preparations: role of repolarization reserve. Br J Pharmacol. 2002;137:361–368. doi: 10.1038/sj.bjp.0704881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bril A, Gout B, Bonhomme M, Landais L, Faivre JF, Linee P, et al. Combined potassium and calcium channel blocking activities as a basis for antiarrhythmic efficacy with low proarrhythmic risk: experimental profile of BRL-32872. J Pharmacol Exp Ther. 1996;276:637–646. [PubMed] [Google Scholar]
- Carlsson L, Abrahamsson C, Andersson B, Duker G, Schiller-Linhardt G. Proarrhythmic effects of the class III agent almokalant: importance of infusion rate, QT dispersion, and early afterdepolarisations. Cardiovasc Res. 1993;27:2186–2193. doi: 10.1093/cvr/27.12.2186. [DOI] [PubMed] [Google Scholar]
- Carlsson L, Almgren O, Duker G. QTU-prolongation and torsades de pointes induced by putative class III antiarrhythmic agents in the rabbit: etiology and interventions. J Cardiovasc Pharmacol. 1990;16:276–285. doi: 10.1097/00005344-199008000-00014. [DOI] [PubMed] [Google Scholar]
- Coats RA. The effects of adrenoceptor agonists and antagonists on plasma potassium concentration in anaesthetized guinea-pigs, rabbits and rats. Br J Pharmacol. 1985;86:827–836. doi: 10.1111/j.1476-5381.1985.tb11104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detre E, Thomsen MB, Beekman JD, Petersen KU, Vos MA. Decreasing the infusion rate reduces the proarrhythmic risk of NS-7: confirming the relevance of short-term variability of repolarisation in predicting drug-induced torsades de pointes. Br J Pharmacol. 2005;145:397–404. doi: 10.1038/sj.bjp.0706203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckardt L, Haverkamp W, Mertens H, Johna R, Clague JR, Borggrefe M, et al. Drug-related torsades de pointes in the isolated rabbit heart: comparison of clofilium, d,l-sotalol, and erythromycin. J Cardiovasc Pharmacol. 1998;32:425–434. doi: 10.1097/00005344-199809000-00013. [DOI] [PubMed] [Google Scholar]
- Farkas A, Batey AJ, Coker SJ. How to measure electrocardiographic QT interval in the anaesthetized rabbit. J Pharmacol Toxicol Methods. 2004;50:175–185. doi: 10.1016/j.vascn.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Farkas A, Coker SJ. Limited induction of torsade de pointes by terikalant and erythromycin in an in vivo model. Eur J Pharmacol. 2002;449:143–153. doi: 10.1016/s0014-2999(02)01992-1. [DOI] [PubMed] [Google Scholar]
- Farkas A, Coker SJ. Prevention of clofilium-induced torsade de pointes by prostaglandin E2 does not involve ATP-dependent K+ channels. Eur J Pharmacol. 2003;472:189–196. doi: 10.1016/s0014-2999(03)01910-1. [DOI] [PubMed] [Google Scholar]
- Gil AG, Silvan G, Illera M, Illera JC. The effects of anesthesia on the clinical chemistry of New Zealand White rabbits. Contemp Top Lab Anim Sci. 2004;43:25–29. [PubMed] [Google Scholar]
- Gögelein H, Bruggemann A, Gerlach U, Brendel J, Busch AE. Inhibition of IKs channels by HMR 1556. Naunyn Schmiedebergs Arch Pharmacol. 2000;362:480–488. doi: 10.1007/s002100000284. [DOI] [PubMed] [Google Scholar]
- Hanck DA, Sheets MF. Site-3 toxins and cardiac sodium channels. Toxicon. 2007;49:181–193. doi: 10.1016/j.toxicon.2006.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haverkamp W, Breithardt G, Camm AJ, Janse MJ, Rosen MR, Antzelevitch C, et al. The potential for QT prolongation and pro-arrhythmia by non-anti-arrhythmic drugs: clinical and regulatory implications Report on a Policy Conference of the European Society of Cardiology. Cardiovasc Res. 2000;47:219–233. doi: 10.1016/s0008-6363(00)00119-x. [DOI] [PubMed] [Google Scholar]
- Hondeghem LM, Carlsson L, Duker G. Instability and triangulation of the action potential predict serious proarrhythmia, but action potential duration prolongation is antiarrhythmic. Circulation. 2001;103:2004–2013. doi: 10.1161/01.cir.103.15.2004. [DOI] [PubMed] [Google Scholar]
- Hondeghem LM, Lu HR, van Rossem K, De Clerck F. Detection of proarrhythmia in the female rabbit heart: blinded validation. J Cardiovasc Electrophysiol. 2003;14:287–294. doi: 10.1046/j.1540-8167.2003.02466.x. [DOI] [PubMed] [Google Scholar]
- Jost N, Virag L, Bitay M, Takacs J, Lengyel C, Biliczki P, et al. Restricting excessive cardiac action potential and QT prolongation: a vital role for IKs in human ventricular muscle. Circulation. 2005;112:1392–1399. doi: 10.1161/CIRCULATIONAHA.105.550111. [DOI] [PubMed] [Google Scholar]
- Knobloch K, Brendel J, Rosenstein B, Bleich M, Busch AE, Wirth KJ. Atrial-selective antiarrhythmic actions of novel Ikur vs Ikr, Iks, and IKAch class Ic drugs and beta blockers in pigs. Med Sci Monit. 2004;10:BR221–BR228. [PubMed] [Google Scholar]
- Lengyel C, Iost N, Virag L, Varro A, Lathrop DA, Papp JG. Pharmacological block of the slow component of the outward delayed rectifier current (IKs) fails to lengthen rabbit ventricular muscle QT(c) and action potential duration. Br J Pharmacol. 2001;132:101–110. doi: 10.1038/sj.bjp.0703777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengyel C, Varro A, Tabori K, Papp JG, Baczko I. Combined pharmacological block of IKr and IKs increases short-term QT interval variability and provokes torsades de pointes. Br J Pharmacol. 2007;151:941–951. doi: 10.1038/sj.bjp.0707297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lightbown ID, Lambert JP, Edwards G, Coker SJ. Potentiation of halofantrine-induced QTc prolongation by mefloquine: correlation with blood concentrations of halofantrine. Br J Pharmacol. 2001;132:197–204. doi: 10.1038/sj.bjp.0703823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Brown BS, Wu Y, Antzelevitch C, Kowey PR, Yan G-X. Blinded validation of the isolated arterially perfused rabbit ventricular wedge in preclinical assessment of drug-induced proarrhythmias. Heart Rhythm. 2006;3:948–956. doi: 10.1016/j.hrthm.2006.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu HR, Vlaminckx E, Van De Water A, Gallacher DJ. Calmodulin antagonist W-7 prevents sparfloxacin-induced early afterdepolarizations (EADs) in isolated rabbit Purkinje fibers: importance of beat-to-beat instability of the repolarization. J Cardiovasc Electrophysiol. 2006;17:415–422. doi: 10.1111/j.1540-8167.2006.00420.x. [DOI] [PubMed] [Google Scholar]
- Michael G, Kane KA, Coker SJ. Repolarisation reserve and the development of torsade de pointes in models of long QT syndromes 1 and 2. J Mol Cell Cardiol. 2006;40:985. [Google Scholar]
- Michael G, Kane KA, Coker SJ. The effect of ATX-II on torsade de pointes induced by E-4031 in vivo. J Mol Cell Cardiol. 2007;42:S7–S8. [Google Scholar]
- Milberg P, Reinsch N, Wasmer K, Monnig G, Stypmann J, Osada N, et al. Transmural dispersion of repolarization as a key factor of arrhythmogenicity in a novel intact heart model of LQT3. Cardiovasc Res. 2005;65:397–404. doi: 10.1016/j.cardiores.2004.10.016. [DOI] [PubMed] [Google Scholar]
- Miyamoto S, Zhu BM, Aye NN, Hashimoto K. Slowing Na+ channel inactivation prolongs QT interval and aggravates adrenaline-induced arrhythmias. Jpn J Pharmacol. 2001;86:114–119. doi: 10.1254/jjp.86.114. [DOI] [PubMed] [Google Scholar]
- Nakashima H, Gerlach U, Schmidt D, Nattel S. In vivo electrophysiological effects of a selective slow delayed-rectifier potassium channel blocker in anesthetized dogs: potential insights into class III actions. Cardiovasc Res. 2004;61:705–714. doi: 10.1016/j.cardiores.2003.12.016. [DOI] [PubMed] [Google Scholar]
- Orosz S, Farkas A, Makra P, Csik N, Lepran I, Rudas L, et al. Repolarization-related ECG parameters do not predict the proarrhythmic activity of dofetilide. J Mol Cell Cardiol. 2007;42:S6. [Google Scholar]
- Ravens U, Himmel HM. Drugs preventing Na+ and Ca2+ overload. Pharmacol Res. 1999;39:167–174. doi: 10.1006/phrs.1998.0416. [DOI] [PubMed] [Google Scholar]
- Roden DM. Taking the ‘idio' out of ‘idiosyncratic': predicting torsades de pointes. Pacing Clin Electrophysiol. 1998;21:1029–1034. doi: 10.1111/j.1540-8159.1998.tb00148.x. [DOI] [PubMed] [Google Scholar]
- Sanguinetti M, Jurkiewicz N. Two components of cardiac delayed rectifier K+ current. Differential sensitivity to block by class III antiarrhythmic agents. J Gen Physiol. 1990;96:195–215. doi: 10.1085/jgp.96.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu W, Antzelevitch C. Sodium channel block with mexiletine is effective in reducing dispersion of repolarization and preventing torsade des pointes in LQT2 and LQT3 models of the long-QT syndrome. Circulation. 1997;96:2038–2047. doi: 10.1161/01.cir.96.6.2038. [DOI] [PubMed] [Google Scholar]
- So PP-S, Hu X-D, Backx PH, Puglisi JL, Dorian P. Blockade of IKs by HMR 1556 increases the reverse rate-dependence of refractoriness prolongation by dofetilide in isolated rabbit ventricles. Br J Pharmacol. 2006;148:255–263. doi: 10.1038/sj.bjp.0706721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y, Shryock JC, Wu L, Belardinelli L. Antagonism by ranolazine of the pro-arrhythmic effects of increasing late INa in guinea pig ventricular myocytes. J Cardiovasc Pharmacol. 2004;44:192–199. doi: 10.1097/00005344-200408000-00008. [DOI] [PubMed] [Google Scholar]
- Thomas GP, Gerlach U, Antzelevitch C. HMR 1556, a potent and selective blocker of slowly activating delayed rectifier potassium current. J Cardiovasc Pharmacol. 2003;41:140–147. doi: 10.1097/00005344-200301000-00018. [DOI] [PubMed] [Google Scholar]
- Thomsen MB. Double pharmacological challenge on repolarization opens new avenues for drug safety research. Br J Pharmacol. 2007;151:909–911. doi: 10.1038/sj.bjp.0707299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen MB, Oros A, Schoenmakers M, van Opstal JM, Maas JN, Beekman JDM, et al. Proarrhythmic electrical remodelling is associated with increased beat-to-beat variability of repolarisation. Cardiovasc Res. 2007;73:521–530. doi: 10.1016/j.cardiores.2006.11.025. [DOI] [PubMed] [Google Scholar]
- Thomsen MB, Verduyn SC, Stengl M, Beekman JDM, de Pater G, van Opstal J, et al. Increased short-term variability of repolarization predicts d-sotalol-induced torsades de pointes in dogs. Circulation. 2004;110:2453–2459. doi: 10.1161/01.CIR.0000145162.64183.C8. [DOI] [PubMed] [Google Scholar]
- Thomsen MB, Volders PGA, Beekman JDM, Matz J, Vos MA. Beat-to-beat variability of repolarization determines proarrhythmic outcome in dogs susceptible to drug-induced torsades de pointes. J Am Coll Cardiol. 2006;48:1268–1276. doi: 10.1016/j.jacc.2006.05.048. [DOI] [PubMed] [Google Scholar]
- Varro A, Balati B, Iost N, Takacs J, Virag L, Lathrop DA, et al. The role of the delayed rectifier component IKs in dog ventricular muscle and Purkinje fibre repolarization. J Physiol (London) 2000;523:67–81. doi: 10.1111/j.1469-7793.2000.00067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volders PG, Stengl M, van Opstal JM, Gerlach U, Spatjens RL, Beekman JD, et al. Probing the contribution of IKs to canine ventricular repolarization: key role for β-adrenergic receptor stimulation. Circulation. 2003;107:2753–2760. doi: 10.1161/01.CIR.0000068344.54010.B3. [DOI] [PubMed] [Google Scholar]
- Walker MJA, Curtis MJ, Hearse DJ, Campbell RWF, Janse MJ, Yellon DM, et al. The Lambeth Conventions—guidelines for the study of arrhythmias in ischemia, infarction, and reperfusion. Cardiovasc Res. 1988;22:447–455. doi: 10.1093/cvr/22.7.447. [DOI] [PubMed] [Google Scholar]
- Wu L, Shryock JC, Song Y, Belardinelli L. An increase in late sodium current potentiates the proarrhythmic activities of low-risk QT-prolonging drugs in female rabbit hearts. J Pharmacol Exp Ther. 2006;316:718–726. doi: 10.1124/jpet.105.094862. [DOI] [PubMed] [Google Scholar]
- Wu L, Shryock JC, Song Y, Li Y, Antzelevitch C, Belardinelli L. Antiarrhythmic effects of ranolazine in a guinea pig in vitro model of long-QT syndrome. J Pharmacol Exp Ther. 2004;310:599–605. doi: 10.1124/jpet.104.066100. [DOI] [PubMed] [Google Scholar]
- Yang T, Roden DM. Extracellular potassium modulation of drug block of Ikr. Implications for torsade de pointes and reverse use-dependence. Circulation. 1996;93:407–411. doi: 10.1161/01.cir.93.3.407. [DOI] [PubMed] [Google Scholar]