Abstract
Background and purpose:
Angiopoietins (Ang) are crucial for new blood vessel formation and exert their effects by acting on the Tie2 receptor. We have recently described a sulindac analogue 2-((1E,Z)-1-benzylidene-5-bromo-2-methyl-1H-inden-3-yl)acetic acid; termed C-18 from now onwards) that inhibits Tie2 receptor activity in kinase assays in vitro. Here, we have assessed the ability of C-18 to inhibit angiogenesis-related properties of endothelial cells and tested its selectivity for the Tie2 receptor.
Experimental approach:
For in vitro experiments human umbilical vein endothelial cells (HUVEC) were used. Proliferation was measured using the MTT assay; migration assays were performed in a modified Boyden chamber and tube-like structure formation was determined on matrigel. The effects of C-18 in vivo were evaluated in the chicken chorioallantoic membrane (CAM).
Key results:
Pre-treatment of HUVEC with C-18 blocked Ang-1-stimulated migration, but also abolished vascular endothelial cell growth factor (VEGF)- and fibroblast growth factor 2-induced responses. Incubation with C-18 inhibited serum-induced proliferation in a concentration-dependent manner; C-18 was, however, without effect on Ang-1-induced survival. In addition, we observed that C-18 did not inhibit ligand-induced receptor phosphorylation of Tie2 or VEGFR2. On the other hand, C-18 blocked activation of members of the mitogen-activated protein kinase family and of the Ser/Thr kinase Akt induced by both VEGF and Ang-1. Furthermore, incubation of CAMs with C-18 led to a dose-dependent inhibition of vascular length.
Conclusions and implications:
C-18 did not act as a Tie2 inhibitor, as originally thought, but rather inhibited growth factor-stimulated signalling pathways that regulate endothelial cell migration and potently reduces neovascularization in vivo.
Keywords: angiogenesis, Tie2, angiopoietin, sulindac, migration, MAPK
Introduction
Angiogenesis is a tightly regulated, multistep process that involves endothelial cell proliferation, migration and organization into capillary structures (Folkman and Shing, 1992; Conway et al., 2001; Carmeliet, 2003). Among the many soluble and matrix-derived angiogenic growth factors and regulators of angiogenesis that contribute to neovascularization, vascular endothelial growth factor (VEGF) and the angiopoietins (Angs) are crucial for new blood vessel formation (Yancopoulos et al., 2000). VEGF acts on the kinase insert domain-containing receptor (KDR) to stimulate growth, migration and permeability of endothelial cells (Ferrara et al., 2003). On the other hand, Ang binds and modulates the activity of the Tie2 receptor (Jones et al., 2001). The best-studied member of the Ang family is Ang-1. Activation of Tie2 by Ang-1 has been linked to promotion of endothelial cell survival, induction of migration and sprouting (Koblizek et al., 1998; Witzenbichler et al., 1998; Papapetropoulos et al., 2000). In addition, Ang-1 stimulates the recruitment of mural cells to the newly formed vessels providing the necessary structural support (Davis et al., 1996; Holash et al., 1999). However, Ang-2 acts as a context-dependent antagonist of Tie2, promoting vessel plasticity (Maisonpierre et al., 1997). Increased Ang-2 levels, lead to destabilization of vascular structures and allow other growth factors, like VEGF, to exert their angiogenic effects (Davis and Yancopoulos, 1999). Upregulation of Ang-2 production in the absence of VEGF is thought to lead to vessel regression.
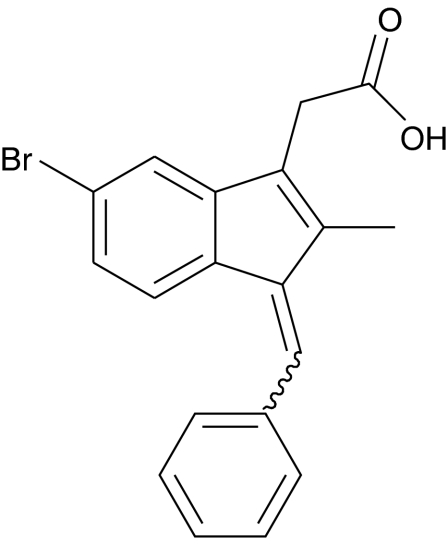
Much of the progress in our understanding of the biology and functions of the Ang/Tie2 system has come from genetic studies (Sato et al., 1995; Suri et al., 1996, 1998; Maisonpierre et al., 1997; Thurston et al., 1999; Gale et al., 2002). Additional strategies for interfering with Ang/Tie2 signalling, which have expanded our knowledge on the role of angiopoietins in new blood vessel formation, include the use of soluble Tie2 (Lin et al., 1997), RNA aptamers (White et al., 2003) and siRNA for the angiopoietins or Tie2 (Daly et al., 2006; Parikh et al., 2006). However, more detailed studies designed to address the contribution of Tie2-regulated pathways in physiology and disease have been hampered by the lack of selective small molecule inhibitors for this receptor tyrosine kinase (RTK). We have recently reported on the synthesis of a series of sulindac analogues that inhibit the enzymatic activity of RTKs for angiogenic growth factors (Gourzoulidou et al., 2005). One of them, 2-((1E,Z)-1-benzylidene-5-bromo-2-methyl-1H-inden-3-yl)acetic acid (C-18; Figure 1), was found to selectively inhibit Tie2 in vitro with an IC50 in the low micromolar range. C-18 was later shown to resensitize retinal vessels in vivo (Hoffmann et al., 2005); this was proposed to result from Tie2 inhibition as administration of soluble Tie2 exerted similar effects. C-18 is, thus, beginning to emerge in the literature as a small molecule inhibitor for Tie2.
Figure 1.
Chemical structure of 2-((1E,Z)-1-benzylidene-5-bromo-2-methyl-1H-inden-3-yl)acetic acid (C-18).
The aim of the present study was to characterize this Tie2 inhibitor pharmacologically by determining the ability of C-18 to block Ang-1-stimulated signalling and to evaluate its effects on angiogenesis-related properties of cultured endothelial cells triggered by a variety of growth factors. Moreover, we have evaluated the effects of C-18 on neovascularization in vivo using the chicken chorioallantoic membrane (CAM) assay.
Materials and methods
Endothelial cell culture
Human umbilical vein endothelial cells (HUVECs) were isolated from 3–4 fresh cords and grown on 100-mm dishes in M199 supplemented with 15% fetal calf serum, 50 U ml−1 penicillin and 50 μg ml−1 streptomycin, 50 μg ml−1 gentamycin, 2.5 μg ml−1 amphotericin B, 5 U ml−1 sodium heparin and 150–200 μg ml−1 endothelial cell growth supplement. Cells were used at the first or second passage.
Cell migration
Cells were serum-starved overnight. To inhibit Tie-2, cells were pretreated with C-18 for 15 min prior to trypsinization. After trypsinization, 1 × 105 cells were added to transwells (8 μM pore size) in 100 μl of starvation medium containing C-18. C-18 was also added to the well containing the transwell inserts in 600 μl volume along with the following agents: Ang-1 (250 ng ml−1), Ang-2 (250 ng ml−1), fibroblast growth factor (FGF)-2 (10 ng ml−1), VEGF (50 ng ml−1), BAY 41-2272 (0.1 μM) or vehicle (dimethylsulphoxide (DMSO)). HUVECs were allowed to migrate for 4 h at 37 °C, and after this time, nonmigrated cells at the top of the transwell filter were removed with a cotton swab. The migrated cells were fixed in Carson's solution for at least 30 min at room temperature and then stained in toluidine blue for 20 min at room temperature. Migrated cells were scored in eight random fields and the fold change was determined relative to the number of migrated cells in control wells.
Caspase-3 activity
Human umbilical vein endothelial cells were plated in 12-well cell culture plates and when confluent were switched to serum-free medium. After pretreatment with vehicle or C-18 for 30 min, cells were exposed to Ang-1 (250 ng ml−1) for 24 h. At the end of this incubation period, both floating and adherent cells were collected, and caspase-3 activity was determined by measuring the proteolytic cleavage of the fluorogenic substrate Z-DEVD-AMC. To do so, cells were lysed in a buffer containing 10 mM Tris pH7.5, 100 mM NaCl, 1 mM EDTA and 0.01% Triton X-100. The fluorescence of the cleaved substrate was measured at 380-nm excitation and 469-nm emission, 30 min after the addition of 100 μM substrate to 20–50 μg of protein. Data were expressed in relative fluorescent units after normalization for protein content.
Cell proliferation
Human umbilical vein endothelial cells were seeded in 24-well plates at 6 × 103 cells per cm2 in growth medium. They were then incubated with the indicated concentration of C-18 or vehicle (DMSO) and allowed to proliferate for 48 h. Cell proliferation was measured using the MTT colorimetric assay. In all assays, performed cell viability was >97% as measured by Trypan blue exclusion.
Capillary-like morphogenesis
Formation of endothelial tube-like structures was assessed using growth factor-reduced Matrigel matrix. HUVEC were plated at 15 000 cells per well in 96-well plates that were pre-coated with 45 μl of Matrigel in the presence or absence of C-18 (1 nM). After 24 h of incubation, tube-like structure formation was quantified using image analysis software.
Western blotting
Following treatments cellular proteins were extracted in a buffer containing 1% Triton X-100, 1% SDS, 150 mM NaCl, 50 mM NaF, 1 mM Na3VO4, 0.5% sodium deoxycholate, 1 mM EDTA, 0.1 mM EGTA and protease inhibitors (10 μg ml−1 aprotinin, 10 μg ml−1 pepstatin and 20 mM phenylmethylsulphonyl fluoride (PMSF)). Samples were subjected to SDS–polyacrylamide gel electrophoresis (SDS–PAGE), transferred to a polyvinylidene difluoride membrane and incubated with the indicated primary and appropriate secondary antibodies. Immunoreactive proteins were detected using a chemiluminescent substrate.
Immunoprecipitations
For immunoprecipitation, 250–300 μg of cell lysates were incubated with 2 μg of polyclonal anti-Tie-2 antibody and protein G-conjugated agarose beads overnight at 4 °C. To reduce nonspecific binding, the protein G beads were preincubated with BSA for 1 h at 4 °C and washed twice with lysis buffer. The precipitated proteins were resolved in a 6% gel and transferred to a polyvinylidene difluoride membrane. Membranes were then subjected to western blotting with the appropriate antibodies.
CAM angiogenesis assay
Fertilized White Leghorn chicken eggs were placed in an incubator as soon as embryogenesis started and kept under constant humidity at 37 °C. On day 4, a square window was opened in the shell and then sealed with adhesive tape. On day 9, an O-ring (1 cm2) was placed on the surface of the CAM, and C-18 or vehicle (10−4% DMSO in phosphate-buffered saline) was added inside this restricted area. The indicated dose of C-18 was added on the CAM as a solution of a final volume of 40 μl. After 48 h, CAMs were fixed in Carson's solution (saline-buffered formalin), and angiogenesis was evaluated using image analysis software. For the CAM experiments, 30–50 eggs per group were analysed.
Data analysis
Data are expressed as means±s.e.mean of the indicated number of observations. Statistical comparisons between groups were performed using ANOVA followed by a post hoc test or Student's t-test, as appropriate. Differences were considered significant when P<0.05.
Materials
Cell culture media and serum were obtained from Life Technologies GIBCO-BRL (Paisley, UK). All cell culture plastic ware was purchased from Corning-Costar Inc. (Corning, NY, USA); SuperSignal West Pico chemiluminescent substrate from Pierce Biotechnology (Rockford, IL, USA); DC Protein assay kit, Tween 20 and other immunoblotting reagents were obtained from Bio-Rad Laboratories (Hercules, CA, USA); penicillin and streptomycin from Applichem (Darmstadt, Germany); amphotericin, gentamycin and heparin were purchased from Biochrom AG (Berlin, Germany). All other reagents including BSA, EDTA and protease inhibitors were purchased from Sigma-Aldrich (St Louis, MO, USA). C-18 was synthesized as described by Gourzoulidou et al. (2005). VEGF, Ang-1 and Ang-2 were purchased from R&D (Minneapolis, MN, USA). FGF-2 was from Peprotech (London, UK). The EnzCheck kit for caspase-3 determination was obtained from Molecular Probes (Eugene, OR, USA). The Tie2 antibody was from Santa Cruz Biotechnologies Inc. (Santa Cruz, CA, USA). Extracellular signal regulated kinase (ERK) 1/2, p38, Akt, VEGR2, Tie-2 phospho-specific and total antibodies along with the secondary antibodies were obtained from Cell Signalling Technology (Beverly, MA, USA). Fertilized White Leghorn chicken eggs were obtained from Pindos (Iperos, Greece).
Results
C-18 inhibits Ang-1-induced migration, but has no effect on Ang-1-stimulated survival
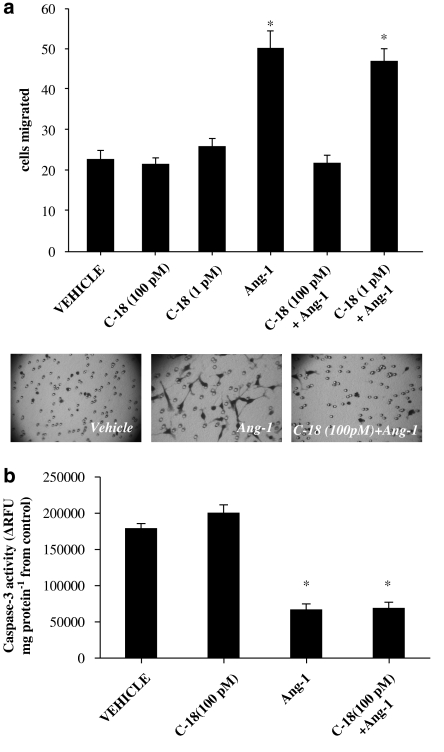
Endothelial migration plays a central part in the process of angiogenesis. In line with its pro-angiogenic profile, we and others have shown that Ang-1 acts as a chemoattractant for endothelial cells (Witzenbichler et al., 1998; Papapetropoulos et al., 1999). To study whether the sulindac analogue (Figure 1) inhibits Ang-1-stimulated migration, HUVEC mobility was evaluated in a modified Boyden chamber assay. C-18 exhibited no effect on basal endothelial migration, but it abolished Ang-1-stimulated migration (Figure 2a). This inhibitory effect of C-18 was observed at 100 pM, a concentration well below its reported IC50 for in vitro kinase assays (Gourzoulidou et al., 2005).
Figure 2.
C-18 inhibits Ang-1-induced human umbilical vein endothelial cell (HUVEC) migration. (a) Cells were placed in transwells and allowed to migrate for 4 h in the presence of C-18 (1 or 100 pM), Ang-1 (250 ng ml−1) or both. Migrated HUVEC were fixed, stained and counted in eight random fields. Data are expressed as means±s.e.mean; n=5; *P<0.05 vs vehicle. Representative photomicrographs of migrated cells at × 100 magnification (bottom). (b) HUVECs were serum-starved, and Ang-1 (250 ng ml−1) was added as a survival factor in the presence or absence of C-18 (100 pM). After 24 h, caspase-3 activity was determined as an index of apoptosis according to the manufacturers' instructions. Data are expressed as means±s.e.mean; n=4; *P<0.05 vs vehicle. Ang, angiopoietin; C-18, 2-((1E,Z)-1-benzylidene-5-bromo-2-methyl-1H-inden-3-yl)acetic acid.
To study whether C-18 in addition to blocking Ang-1-induced migration can also inhibit the pro-survival action of Ang-1, HUVEC were serum-deprived and incubated with Ang-1 in the presence or absence of the sulindac analogue (Figure 2b). In agreement to previous reports, Ang-1 reduced the rate of HUVEC apoptosis as reflected by the inhibition of caspase-3 activity; however, C-18 was unable to reverse the antiapoptotic action of Ang-1.
C-18 blocks serum-induced proliferation
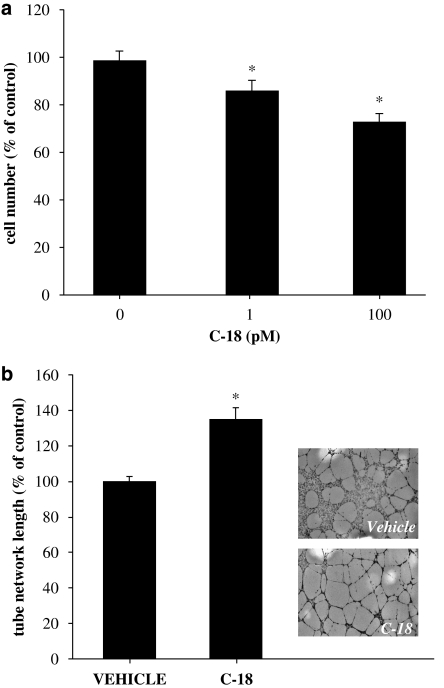
In addition to increased cell motility, endothelial cell proliferation is a prerequisite for new blood vessel formation, and many agents that inhibit neovascularization do so by blocking endothelial cell growth. Although some studies have reported that Ang-1 is mitogenic, in our hands, Ang-1 lacks growth-stimulatory effects (Papapetropoulos et al., 1999). Therefore, to determine the effect of C-18 on proliferation, we studied growth of HUVEC in the presence of serum. Indeed, C-18 reduced HUVEC proliferation reaching a maxima reduction of approximately 30% at 100 pM (Figure 3a). It should be noted that at higher concentrations, C-18 was no longer effective in reducing HUVEC growth, exhibiting a bell-shaped concentration–response curve (data not shown).
Figure 3.
Effect of C-18 in proliferation and capillary morphogenesis. (a) Human umbilical vein endothelial cells (HUVECs) were seeded in 24-well plates, treated with C-18 (1 or 100 pM) or vehicle (dimethylsulphoxide (DMSO)) and allowed to proliferate for 48 h. Cell number was measured using the MTT colorimetric assay. Data are expressed as means±s.e.mean; n=16; *P<0.05 vs control. (b) HUVECs were cultured on growth factor-reduced matrigel in the presence of C-18 (1 nM) or DMSO (control) for 24 h and photographed. Vessel-like network length was determined using image analysis software. Data are expressed as means±s.e.mean; n=6; *P<0.05 vs vehicle. Representative photomicrographs of control and C-18-treated cultures (right). C-18, 2-((1E,Z)-1-benzylidene-5-bromo-2-methyl-1H-inden-3-yl)acetic acid.
Effect of C-18 on matrix-driven capillary morphogenesis
To determine whether C-18 affects the ability of endothelial cells to form tube-like network structures in vitro, we assessed the effects of C-18 on Matrigel-driven network formation (Figure 3b). Contrary to what would be expected for an angiogenesis inhibitor, C-18 increased total length of vessel-like structures.
C-18 inhibits VEGF- and FGF-2-induced migration
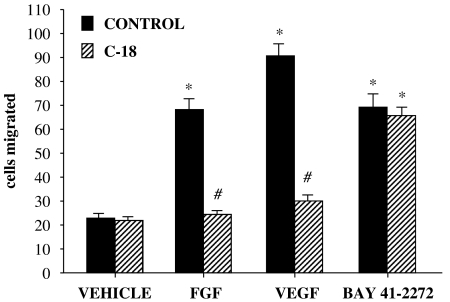
Next, we sought to determine whether this sulindac analogue affects EC motility in response to growth factors other than Ang-1. For these experiments, FGF-2 and VEGF were used; both these agents stimulated a 3.5- and 4.5-fold increase in migration, respectively, under the conditions used (Figure 4). Pretreatment with C-18 (100 pM) abolished the migratory effects of both growth factors, reducing the number of migrating cells to basal levels.
Figure 4.
C-18 inhibits human umbilical vein endothelial cell (HUVEC) migration in response to a variety of growth factors. Cells were placed in transwells and allowed to migrate for 4 h in the presence of fibroblast growth factor (FGF)-2 (10 ng ml−1), vascular endothelial growth factor (VEGF; 50 ng ml−1) or BAY 41-2272 (0.1 μM) with or without C-18 (100 pM). Migrated HUVECs were fixed, stained and counted in eight random fields. Data are expressed as means±s.e.mean; n=5; *P<0.05 vs vehicle; #P<0.05 vs control. C-18, 2-((1E,Z)-1-benzylidene-5-bromo-2-methyl-1H-inden-3-yl)acetic acid.
We and others have previously shown that the VEGF-stimulated migration is mediated by activation of soluble guanylyl cyclase (sGC) and cGMP generation (Papapetropoulos et al., 1997; Ziche et al., 1997). To examine whether C-18 acts upstream or downstream of sGC in inhibiting migration, we determined its ability to interfere with HUVEC migration triggered by BAY 41-2272, an sGC activator (Figure 4). In agreement with our recent work (Pyriochou et al., 2006), treatment of cells with the sCG activator BAY 41-2272 (0.1 μM) resulted in a 3-fold increase in the number of migrating cells. Interestingly, pretreatment with C-18 had no effect on BAY 41-2272-induced migration. These findings suggest that inhibition of VEGF-induced signalling by C-18 occurs upstream of sGC.
Effects of C-18 on receptor tyrosine kinase autophosphorylation and downstream signalling molecules
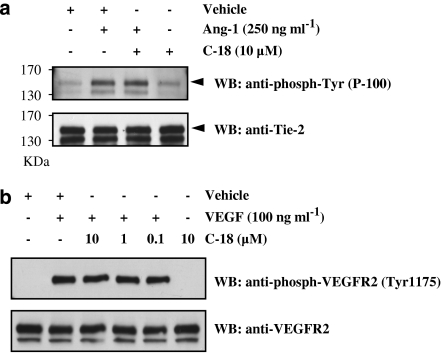
To determine whether C-18 inhibits ligand-induced Tie2 phosphorylation, we exposed HUVEC to Ang-1, immunoprecipitated the Tie2 receptor and blotted the precipitates with a phosphotyrosine antibody (Figure 5a). As expected, Ang-1 promoted Tie2 tyrosine phosphorylation, but concentrations of C-18 up to 10 μM did not block the action of Ang-1, suggesting that C-18 is not a Tie2 inhibitor as previously suggested by in vitro kinase assays. Similarly to what was observed for the Tie2 receptor, C-18 was unable to inhibit phosphorylation of VEGR2 on Tyr1175 triggered by VEGF, suggesting that C-18 does not inhibit the activity of this RTK either (Figure 5b).
Figure 5.
C-18 does not inhibit receptor tyrosine kinase (RTK) phosphorylation. (a) Human umbilical vein endothelial cells (HUVECs) were incubated with Ang-1 (250 μg ml−1) for 5 min in the presence or absence of C-18 (10 μM; 30 min pretreatment). Cells were then lysed and the Tie2 receptor precipitated; after SDS–polyacrylamide gel electrophoresis (SDS–PAGE) and transfer, membranes were blotted with a phosphotyrosine Ab or a Tie2 antibody. Similar results were obtained when C-18 was used at 1 nM. (b) HUVECs were incubated with vascular endothelial growth factor (VEGF; 100 μg ml−1) for 2 min with or without pretreatment of the indicated C-18 concentration. Cells lysates were analysed using total or phospho-specific (Tyr-1175) VEGFR2 antibodies. Blots shown are representative of experiments repeated twice with similar results. Ang, angiopoietin; C-18, 2-((1E,Z)-1-benzylidene-5-bromo-2-methyl-1H-inden-3-yl)acetic acid.
Inhibition of angiogenic signalling by C-18
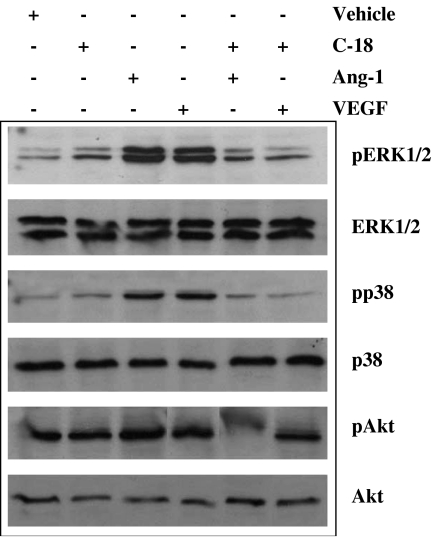
Ang-1 and VEGF use many common signalling cascades including phosphoinositide-3 kinase/Akt and MAP kinase pathways. To investigate whether C-18 inhibits the above-mentioned angiogenesis-related cascades, we pretreated cells with C-18 and used phospho-specific antibodies to probe the activation status. Both VEGF and Ang-1 increased the phosphorylation of residues critical for activation of ERK1/2, p38 and Akt (Figure 6). In agreement with its ability to inhibit biological responses to VEGF and Ang-1, C-18 blocked the effects of both growth factors on ERK1/2, p38 and Akt activation.
Figure 6.
C-18 inhibits Ang-1- and vascular endothelial growth factor (VEGF)-induced mitogen-activated protein kinase (MAPK) and Akt activation. Serum-starved human umbilical vein endothelial cells (HUVECs) were pretreated with C-18 (1 nM) for 15 min and incubated with Ang-1 (250 ng ml−1) or VEGF (50 ng ml−1) for an additional 20 min. Consequently, cells were lysed and samples were subjected to SDS–polyacrylamide gel electrophoresis (SDS–PAGE) and western blotting using antibodies that specifically recognize the phosphorylated or the total forms of the kinases. Blots shown are representative of experiments repeated at least twice with similar results. Ang, angiopoietin; C-18, 2-((1E,Z)-1-benzylidene-5-bromo-2-methyl-1H-inden-3-yl)acetic acid.
In vivo anti-angiogenic properties of C-18
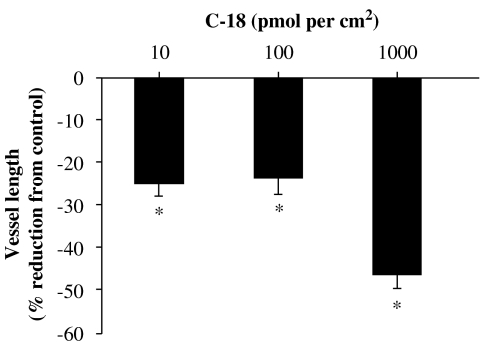
To investigate if C-18 exhibits anti-angiogenic properties in vivo, we tested this compound in the CAM model. Different doses of C-18 were applied in restricted areas of the chorioallantoic membranes of fertilized eggs, and the length of the vascular network was assessed using image analysis software (Figure 7). Treatment with increasing concentrations of C-18 resulted in a dose-dependent reduction of vessel length, reaching approximately a 50% reduction at 100 pmol per egg, making C-18 one of the most effective inhibitors in this system tested in our laboratory.
Figure 7.
C-18 inhibits neovessel formation in vivo. C-18 or vehicle (dimethylsulphoxide (DMSO) in phosphate-buffered saline) was added to restricted areas of the chorioallantoic membrane (CAM) of fertilized eggs, as described in Materials and methods. After 48 h, CAMs were fixed and vessel length evaluated using image analysis software. Data are expressed as means±s.e.mean; n=30–50 eggs per group; *P<0.05 vs control. C-18, 2-((1E,Z)-1-benzylidene-5-bromo-2-methyl-1H-inden-3-yl)acetic acid.
Discussion
We have previously demonstrated that sulindac analogues inhibit angiogenesis-relevant RTKs (Gourzoulidou et al., 2005). Among the analogues tested, a compound, which we refer to as C-18, inhibited RTK Tie2 in vitro.
In the present study, this putative Tie2 inhibitor abolished Ang-1 stimulated HUVEC migration at 100 pM, at a concentration well below its reported IC50 for Tie2 activity inhibition (1 μM) (Gourzoulidou et al., 2005), suggesting that C-18 is either actively taken up by cells leading to intracellular accumulation of this drug, or C-18 inhibits another target(s) necessary for migration at lower concentrations than the one needed to inhibit Tie2. To directly test the ability of C-18 to block activation of Tie2 by its native ligand, HUVECs were exposed to Ang-1, and tyrosine phosphorylation was studied in Tie2 immunoprecipitates. Phosphorylation of Tyr-992, -1002 and -1008 (human sequence) is known to be crucial for Ang-1-stimulated signalling and actions (Ward and Dumont, 2002). Indeed, incubation of HUVEC with Ang-1 promoted Tie2 phosphotyrosine content; however, C-18 when used at 1 nM or 10 μM failed to block the action of Ang-1. This finding is in conflict with our previous report that C-18 inhibits Tie2 activity in in vitro kinase assays (Gourzoulidou et al., 2005) and can be explained by the fact that (1) during the in vitro kinase assays, we utilized a recombinant truncated form of the receptor and (2) basal (not agonist-stimulated) activity of the Tie2 was determined.
Serum-deprivation of HUVEC triggers apoptosis that can be monitored by measuring caspase-3 activity. In line with previous reports, Ang-1 reduced the increase in caspase-3 activity brought about by serum withdrawal (Harfouche et al., 2002); however, C-18 did not reverse the effect of Ang-1 on caspase-3 activity. The lack of effect of C-18 at the receptor level is in agreement with the finding that C-18 does not block the antiapoptotic action of Ang-1.
To better characterize the effects of C-18 on migration, we determined the ability of this sulindac analogue to inhibit migration in response to VEGF and FGF-2. Indeed, pretreatment with C-18 abolished the migratory properties to both growth factors, suggesting that this agent inhibits a common, RTK-stimulated, signalling pathway involved in cell motility. To determine the level at which C-18 exerts its inhibitory effects, we concentrated on VEGF-stimulated migration and signalling. Cells were exposed to VEGF with or without C-18 pretreatment and the phosphorylation status of Tyr-1175 of VEGFR2 was determined, as this residue is critical in mediating migratory responses (Ferrara et al., 2003). VEGFR2 phosphorylation was induced by its cognate ligand, and this response was not modified by C-18, suggesting that C-18 acts downstream of VEGFR2 to block cell motility. We and others have previously shown that VEGF-stimulated migration depends on NO/cGMP signalling, as NO synthase and sGC inhibitors block VEGF-induced motility (Ziche et al., 1997; Pyriochou et al., 2006). Pretreatment with C-18 did not alter the migratory response to the sGC activator BAY 41-2272, indicating that C-18 targets a molecule(s) downstream of VEGFR2, but upstream of sGC.
To further study the mechanism of C-18 action, we incubated cells with either Ang-1 or VEGF and tested the ability of the sulindac analogue to inhibit activation of cascades relevant to angiogenesis. In line with previous reports, both growth factors stimulated ERK1/2 and p38 mitogen-activated protein kinase (MAPK) phosphorylation, as well as Akt activation (Jones et al., 2001; Harfouche et al., 2003; Tsigkos et al., 2003). C-18 inhibited activation of all three pathways tested. In agreement with our results, other non-steroidal anti-inflammatory drugs including indomethacin and sulindac metabolites at high micromolar concentrations have been show to inhibit ERK1/2 and p38 activation (Jones et al., 1999; Rice et al., 2001).
Having established that C-18 blocks growth factor-induced migration, we next sought to determine its effects on other angiogenesis-related properties of endothelial cells, namely growth and tube-like structure formation. Most agents that inhibit angiogenic responses also block endothelial cell proliferation; indeed, C-18 blocked serum-induced endothelial proliferation. However, unexpectedly for an angiogenesis inhibitor, the sulindac analogue stimulated tube-like structure formation on matrigel. To evaluate the in vivo properties of this compound, we tested C-18 in the CAM model. C-18 exerted a strong anti-angiogenic activity, reaching a 50% decrease in vascular length, making this compound one of the most potent and effective used in our system. The differential effect of C-18 on capillary morphogenesis in vitro (matrigel) vs in vivo (CAM) can be easily explained, as matrigel-stimulated tube-like network formation on matrigel is regulated by extracellular matrix and involves only endothelial cells, while neovascularization in the CAM depends on multiple soluble and matrix-derived stimuli and involves more cell types. It should also be noted that, sulindac sulphone, an oxidized sulindac analogue that does not inhibit cyclo-oxygenase, was also reported to block angiogenesis in the CAM (Elwich-Flis et al., 2003).
In summary, our findings suggest that C-18 is not a direct Tie2 or VEGFR2 inhibitor in vivo, but expresses antimitogenic and anti-migratory properties against key angiogenic growth factors. The observations that C-18 is capable of inhibiting activation of ERK1/2, p38 and Akt suggest that this sulindac analogue interferes with signalling molecule(s) downstream of RTK, but upstream of MAPK. This observation is in line with the finding that some sulindac analogues inhibit Ras-regulated pathways (Muller et al., 2004; Waldmann et al., 2004). Although the exact mechanism of action of C-18 remains elusive, we have shown that this agent is a potent inhibitor of angiogenesis in vivo, suggesting that sulindac analogues could be investigated for their usefulness in anti-angiogenesis therapies.
Acknowledgments
This study was supported by funds from the Greek Ministry of Education and the Thorax Foundation.
Abbreviations
- Ang
angiopoietin
- C-18
2-((1E,Z)-1-benzylidene-5-bromo-2-methyl-1H-inden-3-yl)acetic acid
- CAM
chicken chorioallantoic membrane
- ERK
extracellular signal regulated kinase
- FGF
fibroblast growth factor
- HUVEC
human umbilical vein endothelial cell
- MAPK
mitogen-activated protein kinase
- RTK
receptor tyrosine kinase
- sGC
soluble guanylyl cyclase
- SNP
sodium nitroprusside
- VEGF
vascular endothelial growth factor
Conflict of interest
The authors state no conflict of interest.
References
- Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9:653–660. doi: 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- Conway EM, Collen D, Carmeliet P. Molecular mechanisms of blood vessel growth. Cardiovasc Res. 2001;49:507–521. doi: 10.1016/s0008-6363(00)00281-9. [DOI] [PubMed] [Google Scholar]
- Daly C, Pasnikowski E, Burova E, Wong V, Aldrich TH, Griffiths J, et al. Angiopoietin-2 functions as an autocrine protective factor in stressed endothelial cells. Proc Natl Acad Sci USA. 2006;103:15491–15496. doi: 10.1073/pnas.0607538103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis S, Aldrich TH, Jones PF, Acheson A, Compton DL, Jain V, et al. Isolation of angiopoietin-1, a ligand for the TIE2 receptor, by secretion-trap expression cloning. Cell. 1996;87:1161–1169. doi: 10.1016/s0092-8674(00)81812-7. [DOI] [PubMed] [Google Scholar]
- Davis S, Yancopoulos GD. The angiopoietins: yin and yang in angiogenesis. Curr Top Microbiol Immunol. 1999;237:173–185. doi: 10.1007/978-3-642-59953-8_9. [DOI] [PubMed] [Google Scholar]
- Elwich-Flis S, Soltysiak-Pawluczuk D, Splawinski J. Anti-angiogenic and apoptotic effects of metabolites of sulindac on chick embryo chorioallantoic membrane. Hybrid Hybridomics. 2003;22:55–60. doi: 10.1089/153685903321538099. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Gerber H-P, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- Folkman J, Shing Y. Angiogenesis. J Biol Chem. 1992;267:10931–10934. [PubMed] [Google Scholar]
- Gale NW, Thurston G, Hackett SF, Renard R, Wang Q, McClain J, et al. Angiopoietin-2 is required for postnatal angiogenesis and lymphatic patterning, and only the latter role is rescued by Angiopoietin-1. Dev Cell. 2002;3:411–423. doi: 10.1016/s1534-5807(02)00217-4. [DOI] [PubMed] [Google Scholar]
- Gourzoulidou E, Carpintero M, Baumhof P, Giannis A, Waldmann H. Inhibition of angiogenesis-relevant receptor tyrosine kinases by sulindac analogues. Chembiochem. 2005;6:527–531. doi: 10.1002/cbic.200400192. [DOI] [PubMed] [Google Scholar]
- Harfouche R, Gratton J-P, Yancopoulos GD, Noseda M, Karsan A, Hussain SNA. Angiopoietin-1 activates both anti- and proapoptotic mitogen-activated protein kinases. FASEB J. 2003;17:1523–1525. doi: 10.1096/fj.02-0698fje. [DOI] [PubMed] [Google Scholar]
- Harfouche R, Hassessian HM, Guo Y, Faivre V, Srikant CB, Yancopoulos GD, et al. Mechanisms which mediate the antiapoptotic effects of Angiopoietin-1 on endothelial cells. Microvasc Res. 2002;64:135–147. doi: 10.1006/mvre.2002.2421. [DOI] [PubMed] [Google Scholar]
- Hoffmann J, Feng Y, vom Hagen F, Hillenbrand A, Lin J, Erber R, et al. Endothelial survival factors and spatial completion, but not pericyte coverage of retinal capillaries, determine vessel plasticity. FASEB J. 2005;19:2035–2036. doi: 10.1096/fj.04-2109fje. [DOI] [PubMed] [Google Scholar]
- Holash J, Wiegand SJ, Yancopoulos DG. New model of tumor angiogenesis: dynamic balance between vessel regression and growth mediated by angiopoietins and VEGF. Oncogene. 1999;18:5356–5362. doi: 10.1038/sj.onc.1203035. [DOI] [PubMed] [Google Scholar]
- Jones MK, Wang H, Peskar BM, Levin E, Itani RM, Sarfeh IJ, et al. Inhibition of angiogenesis by nonsteroidal anti-inflammatory drugs: insight into mechanisms and implications for cancer growth and ulcer healing. Nat Med. 1999;5:1418–1423. doi: 10.1038/70995. [DOI] [PubMed] [Google Scholar]
- Jones N, Iljin K, Dumont D, Alitalo K. Tie receptors: new modulators of angiogenic and lymphangiogenic responses. Nat Rev Mol Cell Biol. 2001;2:257–267. doi: 10.1038/35067005. [DOI] [PubMed] [Google Scholar]
- Koblizek TI, Weiss C, Yancopoulos GD, Deutsch U, Risau W. Angiopoietin-1 induces sprouting angiogenesis in vitro. Curr Biol. 1998;8:529–532. doi: 10.1016/s0960-9822(98)70205-2. [DOI] [PubMed] [Google Scholar]
- Lin P, Polverini P, Dewhirst M, Shan S, Rao PS, Peters K. Inhibition of tumor angiogenesis using a soluble receptor establishes a role for Tie2 in pathologic vascular growth. J Clin Invest. 1997;100:2072–2078. doi: 10.1172/JCI119740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisonpierre PC, Suri C, Jones PF, Bartunkova S, Wiegand SJ, Radziejewski C, et al. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science. 1997;277:55–60. doi: 10.1126/science.277.5322.55. [DOI] [PubMed] [Google Scholar]
- Muller O, Gourzoulidou E, Carpintero M, Karaguni IM, Langerak A, Herrmann C, et al. Identification of potent Ras signaling inhibitors by pathway-selective phenotype-based screening. Angew Chem Int Ed Engl. 2004;43:450–454. doi: 10.1002/anie.200352587. [DOI] [PubMed] [Google Scholar]
- Papapetropoulos A, Fulton D, Mahboubi K, Kalb RG, O'Connor DS, Li F, et al. Angiopoietin-1 inhibits endothelial cell apoptosis via the Akt/survivin pathway. J Biol Chem. 2000;275:9102–9105. doi: 10.1074/jbc.275.13.9102. [DOI] [PubMed] [Google Scholar]
- Papapetropoulos A, Garcia-Cardena G, Dengler TJ, Maisonpierre PC, Yancopoulos GD, Sessa WC. Direct actions of angiopoietin-1 on human endothelium: evidence for network stabilization, cell survival, and interaction with other angiogenic growth factors. Lab Invest. 1999;79:213–223. [PubMed] [Google Scholar]
- Papapetropoulos A, Garcia-Cardena G, Madri JA, Sessa WC. Nitric oxide production contributes to the angiogenic properties of vascular endothelial growth factor in human endothelial cells. J Clin Invest. 1997;100:3131–3139. doi: 10.1172/JCI119868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh SM, Mammoto T, Schultz A, Yuan H-T, Christiani D, Karumanchi SA, et al. Excess circulating angiopoietin-2 may contribute to pulmonary vascular leak in sepsis in humans PLoS Med 20063e46doi:10.1371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyriochou A, Beis D, Koika V, Potytarchou C, Papadimitriou E, Zhou Z, et al. Soluble guanylyl cyclase activation promotes angiogenesis. J Pharmacol Exp Ther. 2006;319:663–671. doi: 10.1124/jpet.106.108878. [DOI] [PubMed] [Google Scholar]
- Rice PL, Goldberg RJ, Ray EC, Driggers LJ, Ahnen DJ. Inhibition of extracellular signal-regulated kinase 1/2 phosphorylation and induction of apoptosis by sulindac metabolites. Cancer Res. 2001;61:1541–1547. [PubMed] [Google Scholar]
- Sato TN, Tozawa Y, Deutsch U, Wolburg-Buchholz K, Fujiwara Y, Gendron-Maguire M, et al. Distinct roles of the receptor tyrosine kinases Tie-1 and Tie-2 in blood vessel formation. Nature. 1995;376:70–74. doi: 10.1038/376070a0. [DOI] [PubMed] [Google Scholar]
- Suri C, Jones PF, Patan S, Bartunkova S, Maisonpierre PC, Davis S, et al. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell. 1996;87:1171–1180. doi: 10.1016/s0092-8674(00)81813-9. [DOI] [PubMed] [Google Scholar]
- Suri C, McClain J, Thurston G, McDonald DM, Zhou H, Oldmixon EH, et al. Increased vascularization in mice overexpressing angiopoietin-1. Science. 1998;282:468–471. doi: 10.1126/science.282.5388.468. [DOI] [PubMed] [Google Scholar]
- Thurston G, Suri C, Smith K, McClain J, Sato TN, Yancopoulos GD, et al. Leakage-resistant blood vessels in mice transgenically overexpressing angiopoietin-1. Science. 1999;286:2511–2514. doi: 10.1126/science.286.5449.2511. [DOI] [PubMed] [Google Scholar]
- Tsigkos S, Koutsilieris M, Papapetropoulos A. Angiopoietins in angiogenesis and beyond. Expert Opin Investig Drugs. 2003;12:933–941. doi: 10.1517/13543784.12.6.933. [DOI] [PubMed] [Google Scholar]
- Waldmann H, Karaguni I, Carpintero M, Gourzoulidou E, Herrmann C, Brockmann C, et al. Sulindac-derived Ras pathway inhibitors target the Ras–Raf interaction and downstream effectors in the Ras pathway. Angew Chem Int Ed Engl. 2004;43:454–458. doi: 10.1002/anie.200353089. [DOI] [PubMed] [Google Scholar]
- Ward NL, Dumont DJ. The angiopoietins and Tie2/Tek: adding to the complexity of cardiovascular development. Sem Cell Develop Biol. 2002;13:19–27. doi: 10.1006/scdb.2001.0288. [DOI] [PubMed] [Google Scholar]
- White RR, Shan S, Rusconi CP, Shetty G, Dewhirst MW, Kontos CD, et al. Inhibition of rat corneal angiogenesis by a nuclease-resistant RNA aptamer specific for angiopoietin-2. Proc Natl Acad Sci USA. 2003;100:5028–5033. doi: 10.1073/pnas.0831159100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witzenbichler B, Maisonpierre PC, Jones P, Yancopoulos GD, Isner JM. Chemotactic properties of angiopoietin-1 and -2, ligands for the endothelial-specific receptor tyrosine kinase Tie2. J Biol Chem. 1998;273:18514–18521. doi: 10.1074/jbc.273.29.18514. [DOI] [PubMed] [Google Scholar]
- Yancopoulos GD, Davis S, Gale NW, Rudge JS, Wiegand SJ, Holash J. Vascular-specific growth factors and blood vessel formation. Nature. 2000;407:242–248. doi: 10.1038/35025215. [DOI] [PubMed] [Google Scholar]
- Ziche M, Morbidelli L, Choudhuri R, Zhang HT, Donnini S, Granger HJ, et al. Nitric oxide synthase lies downstream from vascular endothelial growth factor-induced but not basic fibroblast growth factor-induced angiogenesis. J Clin Invest. 1997;99:2625–2634. doi: 10.1172/JCI119451. [DOI] [PMC free article] [PubMed] [Google Scholar]