Abstract
Background and purpose:
Electrically active atypical smooth muscle cells (ASMCs) within the renal pelvis have long been considered to act as pacemaker cells driving pelviureteric peristalsis. We have investigated the role of Ca2+ entry and uptake into and release from internal stores in the generation of Ca2+ transients and spontaneous transient depolarizations (STDs) in ASMCs.
Experimental approach:
The electrical activity and separately visualized changes in intracellular Ca2+ concentration in typical smooth muscle cells (TSMCs), ASMCs and interstitial cells of Cajal-like cells (ICC-LCs) were recorded using intracellular microelectrodes and a fluorescent Ca2+ indicator, fluo-4.
Results:
In 1 μM nifedipine, high frequency (10–30 min−1) Ca2+ transients and STDs were recorded in ASMCs, while ICC-LCs displayed low frequency (1–3 min−1) Ca2+ transients. All spontaneous electrical activity and Ca2+ transients were blocked upon removal of Ca2+ from the bathing solution, blockade of Ca2+ store uptake with cyclopiazonic acid (CPA) and with 2-aminoethoxy-diphenylborate (2-APB). STD amplitudes were reduced upon removal of the extracellular Na+ or blockade of IP3 dependent Ca2+ store release with neomycin or U73122. Blockade of ryanodine-sensitive Ca2+ release blocked ICC-LC Ca2+ transients but only reduced Ca2+ transient discharge in ASMCs. STDs in ASMCS were also little affected by DIDS, La3+, Gd3+ or by the replacement of extracellular Cl- with isethionate.
Conclusions:
ASMCs generated Ca2+ transients and cation-selective STDs via mechanisms involving Ca2+ release from IP3-dependent Ca2+ stores, STD stimulation of TSMCs was supported by Ca2+ entry through L type Ca2+ channels and Ca2+ release from ryanodine-sensitive stores.
Keywords: pyeloureteric peristalsis; upper urinary tract; smooth muscle, pacemaker mechanisms
Introduction
The mechanisms by which urine is transported from the kidney to the bladder remains little understood. Despite their identification as likely pacemaker cells over 30 years ago, the mechanisms of autorhythmicity in atypical smooth muscle cells (ASMCs) within the renal pelvis have not yet been elucidated. ASMC are located predominately in the proximal regions of the renal pelvis; their distribution decreases with distance from the papilla base such that they are absent in regions more distal to the ureteropelvic junction (UPJ) (Dixon and Gosling, 1973, 1982; Gosling and Dixon, 1974; Lang et al., 1998, 2001; Klemm et al., 1999). We have recently made a systematic study of the spontaneous changes in intracellular Ca2+ concentration and the electrical activity in ASMCs and interstitial cells of Cajal-like cells (ICC-LCs) (Lang et al., 2006, 2007a, 2007b) that have a sparse distribution similar to the distribution of Kit-positive cells in the upper urinary tract of mouse (Pezzone et al., 2003; Lang and Klemm, 2005; Lang et al., 2006) and many other mammals (Metzger et al., 2004, 2005). We reported that nifedipine (1–10 μM)-sensitive action potentials and Ca2+ waves (frequency 6–15 min−1) readily propagate through the renal pelvic wall that consists of a layer of typical smooth muscle cells (TSMCs) (Lang et al., 2007a). High frequency (10–40 min−1) Ca2+ transients and spontaneous transient depolarizations (STDs) are recorded in ASMCs even in the presence of 1 μM nifedipine. In contrast, ICC-LCs display low frequency (1–4 min−1) Ca2+ transients which we speculated arose from the same cells that discharged action potentials with long plateaus (2–5 s) (Lang et al., 2007a). In 1 μM nifedipine, ASMCs or ICC-LCs displayed little synchronicity in their Ca2+ transient discharge suggesting that both cell types may well be acting as ‘point sources' of excitation to the TSMC layer. From their frequency characteristics, we speculated that ASMCs act as primary pacemakers in the renal pelvis while ICC-LCs could take over pacemaking in the absence of the proximal ASMC pacemaker drive (Lang et al., 2006, 2007a, 2007b).
In this study, we have examined the role of external Ca2+ entry and the uptake of Ca2+ into and release from internal stores in the generation of the spontaneous Ca2+ transients and electrical activity in ASMCs in the mouse renal pelvis. We have examined the effects of blockers of both IP3- and ryanodine-sensitive Ca2+ stores as well as the ionic selectivity of the conductance change(s) underlying STDs using standard Na+ and Cl− replacement protocols. We demonstrate that the mechanisms of Ca2+ transient generation in ICC-LCs and ASMCs can be pharmacologically distinguished on the basis of their sensitivity to ryanodine. As such, pyeloureteric ICC-LCs and ASMCs with their unique distribution and pacemaker mechanisms may well provide selective pharmacological targets when considering non-surgical interventions to alleviate hydronephrosis arising from UPJ remodelling during and after ureteric blockade or pyeloplasty.
Methods
All animal procedures were approved by the School of Biomedical Sciences Animal Ethics Committee at Monash University. Conventional Swiss outbred male mice 4–6 weeks of age were killed by cervical dislocation and ex-sanguination and the kidneys and attached ureters removed through an abdominal incision. The kidney was bathed in a bicarbonate-buffered physiological salt solution (PSS). The upper urinary tract, from its point of attachment to the papilla to the UPJ was dissected free of the kidney, opened along its longitudinal axis and loosely pinned out in a dissecting dish with the urothelial layer uppermost.
Intracellular microelectrode and tension recordings
Transverse- or longitudinally orientated strips (2 × 5 mm2) of renal pelvis were dissected free and firmly pinned, urothelial side uppermost, into a silicone resin (Sylgard Dow Corning Corporation, Midland, MI, USA) coated recording chamber. The bath was mounted on an inverted microscope and supperfused with PSS at 3–5 ml min−1 at 35 °C. Electrophysiological recordings were made using glass microelectrodes with resistances of 80–120 mΩ when filled with 1 M KCl. Membrane potential changes were recorded with a high impedance Axoclamp-2 pre-amplifier (Axon Instruments, Molecular Devices, Union City, CA, USA), low-pass filtered at 1 kHz and stored digitally on a personal computer using a Digidata 1200 DMA analog-to-digital interface and Axotape 8 or pClamp 8 software (Axon Instruments) for later analysis.
Measurements of internal Ca2+ concentrations
To visualize changes in the concentration of intracellular calcium ([Ca2+]i) in TSMCs, ASMCs and ICC-LCs, preparations were incubated (30 min) with warmed (36 °C) PSS, until spontaneous muscle contractions occurred. Preparations were then incubated in low Ca2+ PSS ([Ca2+]o=0.1 or 0.5 mM) containing 1–3 μM fluo-4 AM (FluoroPure Molecular Probes, OR, USA) and cremophor EL (0.01%, Sigma) for 45–60 min at 36 °C. Following incubation, the preparations were superfused with dye-free, warmed (36 °C) PSS at a constant flow (about 2 ml min−1) for 30 min. After loading, the recording chamber was mounted on the stage of an inverted fluorescence microscope (IX70, Olympus) equipped with an electron multiplier CCD camera (C9100, Hamamatsu Photonics) and a high-speed scanning polychromatic light source (C7773, Hamamatsu Photonics). Preparations were viewed with a water–immersion × 60 objective (UPlanApo 60, Olympus) and illuminated at 495 nm. The fluorescence emissions were measured through a barrier filter above 515 nm (sampling interval 23–200 ms), using a micro-photoluminescence measurement system (AQUACOSMOS, Hamamatsu Photonics). Relative changes in [Ca2+]i were expressed as the ratio (Ft/F0) of the fluorescence generated by an event at time t (Ft) and the baseline fluorescence at t=0 (F0).
Solutions and drugs used
The PSS for the electrophysiological experiments was of the following composition (mM): NaCl 120, KCl 5, CaCl2 2.5, MgSO4 1, NaH2PO4 1, NaHCO3 25 and glucose 11, bubbled with a 95% O2:5% CO2 gas mixture to establish a pH of 7.3–7.4. Ca2+ free PSS was created by replacing 2.5 mM Ca2+ with Mg2+, low Na+ PSS and low Cl− PSS were created by replacing 120 mM Na+ with N-methyl-D-glucamine and 120 mM Cl− with isethionate, respectively. The pH of the PSS was then restored to 7.4 with HCl. The concentration of all stock solutions ranged between 0.1 and 10 mM. Most drugs were dissolved in filtered distilled water and diluted with PSS to their final concentrations as indicated. Nifedipine (Sigma, St Louis, MO, USA) was dissolved in absolute ethanol or DMSO. Stock solutions were generally added at 1:1000 dilution. Ethanol and DMSO (0.1%) had no effect on the recorded electrical activity.
Data analysis
STDs and regenerative action potentials were identified for analysis by the creation of unique templates using pClamp 9 software as described previously (Lang et al., 2007a). Various parameters of these individual STDs or action potentials were measured and averaged under each experimental condition: membrane potential, amplitude, half-amplitude duration (½ width), time integral and inter-event interval. Data drawn from a number of similar experiments were then averaged as indicated and presented as mean±s.e.mean. In some experiments variation between experiments was reduced by expressing data in the presence of a drug as a percent of control. N denotes the number of animals, n denotes the number of observations within a single experiment. Paired or unpaired Student's t-test was used for tests of significance; P<0.05 was accepted as statistically significant.
Results
Electrical events underlying contraction
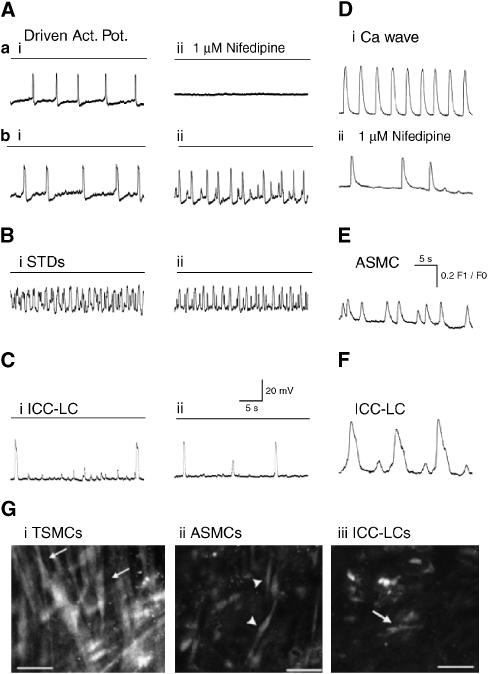
Strips of renal pelvis developed spontaneous contractions within 10 min of being placed in the recording chamber and superfused with PSS at 35 °C. Intracellular microelectrode impalements revealed three patterns of recordings that were often recorded in the same preparation. One pattern was characterized by the firing of regenerative action potentials; the membrane potential between action potentials was mostly quiescent (Figure 1Aai,bi). The time course of these action potentials consisted of an initial spike 30–40 mV in amplitude followed by a plateau 200–800 ms in duration. The plateau was followed by a rapid repolarization, which often produced a small (5–10 mV) after-hyperpolarization which slowly decayed over the following 5–10 s (Figure 1Aai,bi).
Figure 1.
Typical recordings of the effects of nifedipine on the spontaneous electrical and Ca2+ signals in the mouse renal pelvis. (A) In some preparations, regenerative action potentials recorded in typical smooth muscle cells (TSMCs) (Aai) were completely blocked in 1 μM nifedipine (Aaii); dashed line represents 0 mV. In other preparations (Abi–ii), although the time course and amplitude of TSMC action potential were significantly reduced in 1 μM nifedipine; their frequency of discharge often increased. (Bi–ii) High frequency spontaneous transient depolarizations (STDs) were recorded in approximately 50% of impalements and either little affected or more evident (Abii) in 1 μM nifedipine. (Ci–ii) Long plateau action potentials, which did not trigger muscle contraction were occasionally recorded (at frequency of 1–3 min−1) and little affected by 1 μM nifedipine. (Di–ii) Ca2+ waves in TSMC layer were either completely blocked or partially reduced (Di) in 1 μM nifedipine. Ca2+ transients were recorded in spindle-shaped ASMCs (E) and fusiform interstitial cells of Cajal-like cells (ICC-LCs) (F) distinguished by their distinctive discharge frequency and time course were little affected by 1 μM nifedipine. (G) Greyscale fluorescence micrographs of cells displaying Ca2+ transients in TSMC layer (Gi) in the absence of nifedipine and ASMCs (Gii) and ICC-LCs (Giii) in 1 μM nifedipine. Calibration bars represent 30 μm.
After loading with fluo-4, renal pelvis preparations generated spontaneous transients in Ca2+ concentration ([Ca2+]i) (Figure 1Di) which swept across the field of view (Ca2+ waves) and which were accompanied by migrating contractions. The gross morphology of the cells generating the Ca2+ waves was spindle shape and >100 μm in length, resembling TSMCs, viewed previously under the electron microscope (Klemm et al., 1999). These TSMCs formed a loose ‘basket weave' layer within the preparation (Figure 1Gi).
Both driven action potentials and Ca2+ waves displayed a variable sensitivity to 1 μM nifedipine within the same preparation. In many regions of an individual preparation, propagating Ca2+ waves, contractions and TSMC action potentials were completely blocked by 1 μM nifedipine (for >10 min) (Figure 1Aai–ii). However, in other regions within the same tissue, the amplitude and time course of the Ca2+ transients (Figure 1D) and regenerative action potentials (Figure 1Abi–ii) were only reduced in 1 μM nifedipine (Lang et al., 2007a). However, residual action potentials in 1 μM nifedipine were often recorded at a greater frequency when compared with control, and associated with the appearance of small amplitude STDs (Figure 1Abi–ii). Raising the concentration of nifedipine (3 or 10 μM for >2–60 min) blocked the discharge of all TSMC action potentials and their associated contractions (Lang et al., 2007a).
ASMCs
The second pattern of electrical discharge and Ca2+ signalling recorded in the mouse renal pelvis was characterized by the firing of a number of high-frequency STDs of varying amplitude which were either subthreshold or summed and appeared to evoke a regenerative action potential (Figure 1Bi). After fluo-4 loading and fluorescence illumination it was clear that a population of short (<50 μm) spindle-shaped ASMCs discharged Ca2+ transients at frequencies similar to the STD discharge. They formed bundles of similar asynchronously discharging cells, which created a diffuse network not in the same plane of focus as the TMSC layer (Figure 1Gii). Ca2+ transients in ASMCs and large (>10 mV) STDs were little affected by 1–10 μM nifedipine (Figure 1Bii, E). Raising the concentration of nifedipine to 3–10 μM reduced the amplitude, but never blocked the discharge of smaller (<10 mV) STDs thought to be recorded in TSMCs but generated in electrically distant ASMCs (Lang et al., 2007a).
ICC-LCs
The third pattern of electrical events recorded in the mouse renal pelvis was characterized by their very low frequency of discharge (<4 min−1) and their very long plateaus (>1 s) (Figure 1Ci). Paired microelectrode recordings have also established that these long plateau action potentials do not propagate >50 μm. They were not associated with muscle wall contraction, nor affected by 1 μM nifedipine (Figure 1Bii) (Lang et al., 2007a). Unfortunately, these long plateau action potentials were not recorded frequently enough to facilitate an extensive pharmacological study.
Fluorescence imaging of renal pelvis loaded with fluo-4 revealed a population of cells discharging Ca2+ transients in the absence or presence (Figure 1F) of 1 μM nifedipine at frequencies (<6 min−1) similar to the long plateau action potentials. The shape of these cells was variable, being triangular, fusiform (Figure 1Giii), stellate or oval-shaped and they lay within the same plane as the ASMCs. The density of the cells displaying low frequency Ca2+ transients was 1–5 per field of view (135 × 135 μm2) similar to the density of Kit-positive ICC-LCs reported previously (Pezzone et al., 2003; Lang and Klemm, 2005; Lang et al., 2006, 2007a, 2007b). We have speculated that long plateau action potentials and these low frequency long-lasting Ca2+ transients are arising from ICC-LCs (Lang et al., 2007a). Ca2+ transients recorded in ICC-LCs were little affected by 1 μM nifedipine (Figure 1F).
The partial and regional blockade of ‘L type' Ca2+ channels in TSMCs with nifedipine (1 μM for >2 min) allowed us to choose areas within each preparation in which Ca2+ waves were absent so that Ca2+ transients in ASMCs and ICC-LCs could be studied in isolation.
Role of Ca2+ entry and store uptake
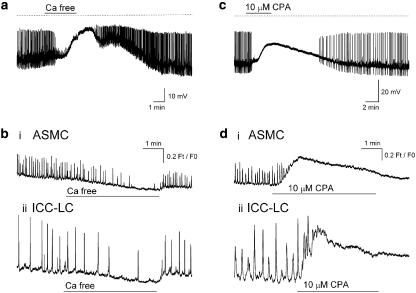
In five experiments, all residual TSMC action potentials (Figure 2a) and STDs recorded in the presence of 1 μM nifedipine were abolished upon exposure to Ca2+ free PSS (for 5–10 min). Blockade of all electrical activity in Ca2+ free PSS was associated with membrane depolarization of 13.5±5.53 mV (Figure 2a). All these effects of Ca2+ free PSS were slowly reversed upon returning to 2.5 mM Ca2+ containing PSS.
Figure 2.
Effects of blocking Ca2+ entry and Ca2+ uptake into internal stores on the spontaneous electrical and Ca2+ signals in the renal pelvis bathed in 1 μM nifedipine. Ca2+ free PSS (a, bi–ii) and 10 μM CPA (c, di–ii) both blocked residual action potential and STD (a, c) discharge and depolarized the membrane some 10–20 mV. Blockade of Ca2+ transients in ASMCs and interstitial cell of Cajal-like cells (ICC-LCs) by Ca2+ free PSS (b) was associated with a reduction of the basal [Ca2+]i of 0.2 Ft/F0, while CPA (10 μM) blockade of Ca2+ transients was associated with a rise in basal [Ca2+]I of 0.3–0.4 Ft/F0 (d).
In seven experiments (N=3), the Ca2+ signals recorded simultaneously in 2–5 ASMCs (Figure 2bi) and 1–2 ICC-LCs (Figure 2bii) within a field of view were mostly blocked after several minutes exposure to Ca2+ free PSS. This blockade was associated with a decrease in basal [Ca]i (Ft/F0 ratio) of 0.21±0.1 (n=21) and 0.23±0.0.5 (n=7) for the ASMCs and ICC-LCs, respectively. The effects of Ca2+ removal on the basal [Ca]i and Ca2+ transient discharge were quickly but incompletely reversible (within <1 min) upon washing with Ca2+ containing PSS.
In 10 preparations bathed in nifedipine (1 μM for >10 min) and displaying spontaneous STDs and (or) residual action potential discharge (Figure 2c), cyclopiazonic acid (CPA 10 μM for 5–20 min), a blocker of the sarcoplasmic endoplasmic reticulum Ca2+-ATPase (SERCA) pump, evoked membrane depolarization of 13.1±0.5 mV. During this period of CPA exposure, the amplitude and frequency of all spontaneous electrical activity decreased until they were completely abolished after 5–10 min (Figure 2c). Application of a hyperpolarizing current to return the membrane potential to near control levels did not reverse these effects of CPA. Upon 10–40 min wash out of CPA, STDs and residual action potential discharge slowly reappeared, albeit at a reduced frequency (Figure 2c).
In six experiments (N=4), basal Ft/F0 ratio of ASMCs (Figure 2di) and ICC-LCs (Figure 2dii) increased to a peak amplitude of 0.34±0.03 (n=15) and 0.44±0.07 (n=10), respectively, in the presence of 10 μM CPA (for 2–5 min). Ca2+ transient discharge in both cell types showed a brief acceleration on the rising phase of the Ft/F0 ratio recorded in CPA, but were then blocked (Figure 2d) and remained blocked even after 20–30 min wash out. After >30 min wash out of CPA, Ca2+ transients in both ASMCs and ICC-LCs slowly reappeared (data not shown).
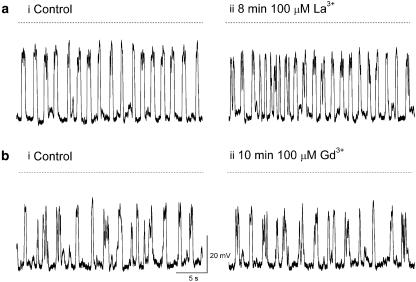
Effects of La3+and Gd3+
The role of Ca2+ entry through other nifedipine-insensitive pathways was investigated using La3+ and Gd3+ (100 μM for 5–10 min), blockers of pacemaker currents in ICC of the mouse intestine (Walker et al., 2002; Chang et al., 2003). Figure 3 is representative of seven experiments in which strips of renal pelvis bathed in 1 μM nifedipine were exposed to 100 μM La3+ (N=7) or Gd3+ (N=7) for 5–10 min. In four of these experiments, the amplitude, ½ width, integral and inter-event interval of discharging STDs were 16.5±6.8 mV, 204±77 ms, 4093±2874 mV ms and 2456±732 ms, respectively, in control PSS. These values were not significantly different (paired t-test) from the equivalent parameters after 5–8 min exposure to 100 μM La3+ containing PSS (16.8±6 mV, 196±64 ms, 4371±2943 mV ms and 2552±920 ms, respectively).
Figure 3.
La3+ (ai–ii) and Gd3+ (bi–ii), blockers of pacemaker currents in intestinal ICCs had little effect on the frequency or time course of spontaneous transient depolarizations recorded in the renal pelvis.
Similarly, 5–10 min exposure to Gd3+ had no significant effect on any of the STD parameters above, being 13.7±4.0 mV, 151±23 ms, 2492±1342 mV ms and 2559±687 ms, respectively, in control PSS and 13.6±5.1 mV, 148±27 ms, 2421±1544 mV ms and 2124±378 ms, respectively, in 100 μM Gd3+ (all P>0.05). In three experiments, residual action potential discharge in 1 μM nifedipine was also not affected by Gd3+ nor La3+ (both 100 μM) (data not shown).
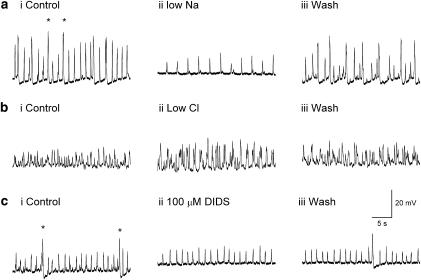
Effects of replacing external Na+ or Cl−
The ionic selectivity of the conductance change(s) underlying STD generation was investigating by replacing >90% of the external Na+ or Cl− with an equimolar concentration of N-methyl-D-glucamine or isethionate, respectively (Koh et al., 2001). In seven experiments, STD amplitudes were either significantly reduced by 43.7±14.3% (N=5, P<0.05) of control (Figure 4aii) or completely blocked (N=2) after 3–4 min exposure to the low Na+ PSS. Low Na+ PSS exposure also evoked membrane depolarization of 11.9±2.9 mV (N=6). No other parameters (½ width integral or inter-event interval) were significantly altered in low Na+ PSS. Any residual action potentials present in 1 μM nifedipine were rapidly blocked when the external Na+ was replaced with its impermeant ion (Figure 4a).
Figure 4.
Effect of monovalent ion replacement and Cl− channel blocker DIDS on spontaneous transient depolarizations (STDs) generation in the renal pelvis bathed in 1 μM nifedipine. (ai–ii) Residual action potentials (*) were abolished and STD amplitudes reduced when 93% of the external Na+ was replaced by N-methyl-D-glucamine (for 3–4 min). STD amplitudes were not significantly affected when 93% of the Cl− concentration was replaced by isethionate (for 3–4 min) (bi–ii) or in the presence of 100 μM DIDS (ci–ii). Residual action potentials (*) were blocked in DIDS (bii). (aiii, biii, ciii) The effects of all treatments were reversible upon washout.
In contrast, STD parameters (data not shown) were not significantly altered after 3–4 min exposure to low Cl− PSS applied to the same seven preparations exposed to low Na+ PSS (Figure 4bi–ii). This lack of effect of Cl− free PSS was confirmed by examining the effects of the chloride channel blocker 4,4′-diisothiocyanostilbene-2,2′-disulphonic acid (DIDS 100 μM) in five experiments (Figure 4c). The amplitude, ½ width, integral and inter-event interval of the STDs in control PSS (Figure 4ai) were 9.7±1.1 mV, 131±13.9, 1474±106 mV ms and 1877±217 ms, respectively, in control PSS and not significantly different when measured after 4–6 min exposure to 100 μM DIDS (8.3±1.2 mV, 120±13.4, 1237±220 mV ms and 2068±219 ms, respectively) (all P>0.05).
Role of IP3-dependent Ca2+ stores
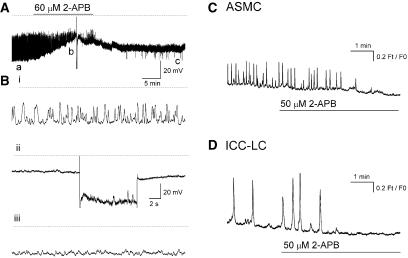
The role of IP3-dependent Ca2+ release channels were investigated using 2-aminoethoxy-diphenylborate (2-APB 60 μM for 30–60 min) and U73122 (10 μM for 5–10 min) or neomycin (4 mM for 5–0 min), inhibitors of IP3 formation in smooth muscle. 2-APB (60 μM for >10 min) decreased the amplitude and frequency of STD or residual regenerative action potential discharge in six preparations (in 1 μM nifedipine) in a manner associated with a membrane depolarization of 20.7±2.1 mV (Figure 5A). In two of these six preparations, 60 μM 2-APB abolished STD discharge completely in a manner that was not reversed when a hyperpolarizing current was applied to return the membrane to control potentials (Figures 5A and Bii). 2-APB (50 μM for >3 min) completely abolished the Ca2+ transients in 52 ASMCs (Figure 5C) sampled in 20 fields of view (N=3) as well as all Ca2+ transients in 5 ICC-LCs followed over the same time (Figure 5D).
Figure 5.
Effect of blocking IP3 receptors with 2-aminoethoxy-diphenylborate (2-APB) on spontaneous transient depolarization (STD) discharge (A) and Ca2+ transients in ASMCs (C) and interstitial cells of Cajal-like cells (ICC-LCs) (D) in the renal pelvis bathed in 1 μM nifedipine. 2-APB (60 μM) blocked STD discharge in a manner associated with a membrane depolarization of 20 mV. (Bi–iii) Sections of trace indicated by a–c in (A) displayed on an expanded time base for better comparison. STDs remain blocked even when the membrane was repolarized upon injection of a constant hyperpolarizing current (Bii). Ca2+ transients in ASMCs (C) and ICC-LCs (D) were blocked in 50 μM 2-APB.
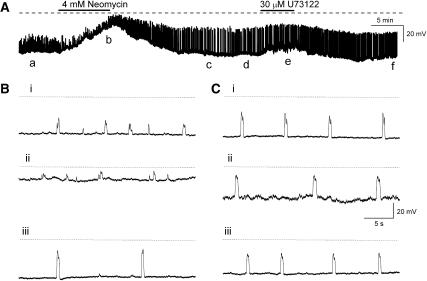
Neomycin (4 mM for 5–10 min, N=7) and U73122 (30 μM for 5–10 min, N=5) had less pronounced effects on STD discharge when compared with 2-APB. In three of seven experiments, neomycin completely blocked STD discharge in a manner that resembled the effects of 2-APB. In the remaining four experiments (Figures 6A and B), neomycin (4 mM for 5–10 min) depolarized the membrane 19±5.4 mV (N=4) which significantly reduced STD amplitude and their frequency of discharge. In the example illustrated in Figures 6A and B, STDs (n=51) were 13.2±0.5 mV in amplitude and discharged at an inter-event interval of 3598±211 ms in control PSS, compared with 7.7±0.65 mV and 17 512±3801 ms (n=9, both P<0.05), respectively, in neomycin. Figures 6A and C also illustrate that U73122 (30 μM for 5 min) generally evoked a smaller membrane depolarization (of 3.9±2.3 mV, N=5) compared with neomycin. STD amplitudes were also significantly reduced from 13.3±0.3 mV (n=38) in control PSS to 12.1±0.3 mV (n=18, P<0.05) in U73122. However, their inter-event interval was not significantly affected by U73122 (3374±239 ms in control and 4476±431 ms in U73122, P>0.05).
Figure 6.
(A) Effects of blockade of PLC with neomycin (4 mM for 5 min) (A, B) or U73122 (30 μM for 5 min) (A, C) on spontaneous transient depolarization amplitude and discharge frequency were associated with a membrane depolarization of 4–20 mV. (Bi–iii) Sections of trace indicated by a–c in (A) displayed on an expanded time base while (Ci–iii) illustrates the sections of trace denoted by (Ad–f).
Role of ryanodine-sensitive Ca2+ stores
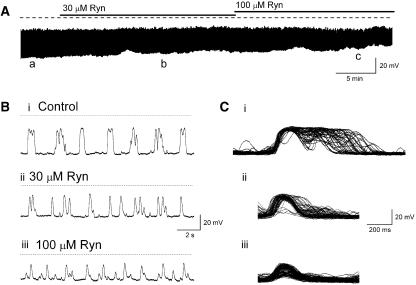
The role of ryanodine-sensitive Ca2+ stores in the initiation of STDs and Ca2+ transients in ASMCs and ICC-LCs was examined using ryanodine and caffeine, modulators of ryanodine receptor Ca2+ release channels. In six preparations, ryanodine (30 and 100 μM) produced a concentration-dependent reduction of the amplitude and time course but not blockade of STDs recorded in 1 μM nifedipine. Figure 7 is representative of one of these experiments and illustrates that ryanodine reduced the number of near synchronous STDS so that their amplitudes and ½ widths decreased from 24.3±0.8 mV and 194±7.2 ms (n=114) in control PSS to 14.7±0.4 mV and 145±1.9 ms (n=169), respectively, in 100 μM ryanodine (for >20 min) (both P<0.05). Mean inter-event intervals also decreased in a concentration-dependent manner from 2495±171 in control PSS to 1068± 31 ms in 100 μM ryanodine (both P<0.05).
Figure 7.
(A) Blockade of ryanodine-sensitive Ca2+ release produced a concentration-dependent reduction in the synchronistic behaviour of spontaneous transient depolarizations (STD) discharge resulting in a reduction in STD amplitude and ½ width, but an increase in their frequency. (Bi–iii) Sections of trace indicated by a–c in A were displayed on an expanded time base. Superimposing STDs recorded in control PSS (Ci), 30 μM (Cii) or 100 μM (Ciii) ryanodine reveals changes in time course and synchronicity in ryanodine.
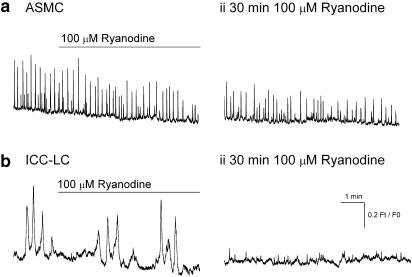
The amplitude, ½ width, integral and inter-event interval of the spontaneous Ca2+ transients in six ASMCs sampled in three tissues were 0.22±0.06 Ft/F0, 782±370 ms, 219±83 Ft/F0 ms and 12 276±2893 ms were not significantly altered after 30 min exposure to 100 μM ryanodine (0.16±0.03 Ft/F0, 888±180 ms, 156±66 Ft/F0 ms and 23 314±75 ms; all P> 0.05 N=3) (Figure 8ai,ii). In contrast, the Ca2+ transients in the 1–2 ICC-LCs followed at the same time in each field of view were completely abolished >10 min exposure to 100 μM ryanodine (Figure 8bi,ii).
Figure 8.
Effects of ryanodine (100 μM) on Ca2+ transients recorded in single ASMCs (ai–ii) and interstitial cells of Cajal-like cells (ICC-LCs) (bi–ii) of the renal pelvis bathed in 1 μM nifedipine. In comparison to ICC-LCs (bii), Ca2+ transients in ASMCs (aii) were only partially reduced even after 30–60 min exposure to ryanodine.
Effects of caffeine
In the presence of 1 μM nifedipine, caffeine (1 mM for 2–6min) hyperpolarized all preparations of the renal pelvis by 10.3±3.2 mV (N=11). Any residual action potentials and contractions were also blocked by 1 mM caffeine (Figure 9Ai). The effects of caffeine on cells displaying STDs were variable. In four preparations the conductance increase during the hyperpolarization evoked by caffeine was sufficiently great to decrease the amplitude of all STDs (Figure 9Ai), while in another four preparations the hyperpolarizations appeared to increase the amplitude and decrease the frequency of recorded STDs (Figure 9Aii). In the absence of caffeine, membrane hyperpolarization, evoked by passing a hyperpolarizing current, to levels similar to those evoked by caffeine increased STD amplitudes. This is consistent with the notion that membrane hyperpolarization increased the driving force for the conductance change underlying STD discharge. Conversely, repolarization of the membrane potential to control levels during the peak of the caffeine-evoked hyperpolarization revealed small STDs that were not always apparent at the negative potentials firing and firing at a frequency less than control (Figures 10A and Bi,ii).
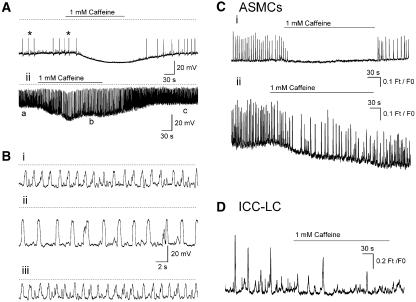
Figure 9.
Caffeine (1 mM) rapidly blocked residual action potential discharge (*) in all tissues bathed in 1 μM nifedipine and hyperpolarized the membrane some 10–20 mV. Spontaneous transient depolarization amplitudes were either reduced (in 50% of preparations) (Ai) or increased in a manner associated with a decrease in their discharge frequency (Aii). (Bi–iii) Sections of trace indicated by a–c in (Aii) displayed on an expanded time base. Caffeine (1 mM 2–4 min) either blocked completely (Ci) or reduced the frequency of discharge of Ca2+ transients in ASMCs (Cii) and interstitial cells of Cajal-like cells (ICC-LCs) (D).
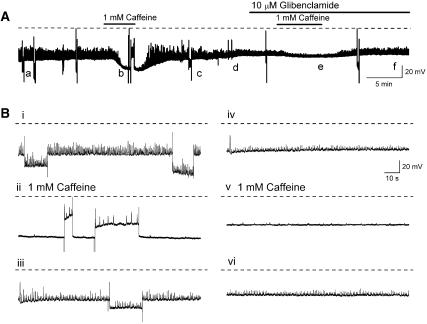
Figure 10.
(A) Hyperpolarizing effects of caffeine (1 mM for 3–5 min) were prevented by the KATP channel blocker gliblenclamide (10 μM). (Bi–vi) Sections of trace indicated by a–f in (A) displayed on an expanded time base for better comparison. It can be seen that membrane hyperpolarization evoked by passing a constant current did not mimic the effects of caffeine (1 mM for 3–5 min) on spontaneous transient depolarisation (STD) amplitude (Bi, iii). (Bii) Repolarizing the membrane to control potentials in the presence of 1 mM caffeine revealed the presence of small amplitude STDs that were not apparent at the more negative potentials. Exposure to 10 μM glibenclamide reduced the hyperpolarizing action of caffeine but did not reduce the caffeine-evoked inhibition of STD discharge (A, Biv-vi).
In fluro-4 loaded preparations, caffeine (1 mM for 2–4 min) blocked Ca2+ transient discharge in approximately 50% (11 of 23 cells) of ASMCs and (6 of 11) ICC-LCs in nine fields of view (N=3). It was not possible to analyse changes in the parameters of the Ca2+ transients in ICC-LCs, which were not completely blocked in the presence of 1 mM caffeine (Figure 9D), owing to their low frequency of discharge and the relatively short maximum period (8 min) of sampling in these experiments. However, in four of the ASMCs that still displayed Ca2+ transients in caffeine (1 mM for 2–3 min), the inter-event intervals between Ca2+ transients was significantly increased 327±70% (n=4 P<0.05) without any other parameters being significantly affected (Figure 9Cii).
The hyperpolarization to caffeine (1 mM for 2–5 min, N=5) was readily reduced by the selective blocker of KATP channels, glibenclamide (10 μM for >2–10 min N=4) (Figures 10A and Bii). In the presence of glibenclamide it was evident that caffeine still abolished any residual-driven action potential discharge and reduced STD amplitudes and their frequency of discharge (Figures 10A and Biv-vi).
Discussion
STDs and Ca2+ transients in ASMCs
The data presented here represents the first examination of the mechanisms of autorhythmicity in two distinct populations of spontaneously active cells within the mouse renal pelvis that are likely to be responsible for triggering the migrating regenerative action potentials, Ca2+ waves and muscle contractions underlying pelviureteric peristalsis. High-frequency STDs and Ca2+ transients were recorded in short ASMCs, which formed randomly orientated bundles that were not in the same plane of focus as the TSMC layer. These STDs were little affected by the Cl− channel blocker, DIDS or the removal of 93% of the Cl− concentration from the PSS. Instead, STDs were reduced or blocked completely when the extracellular Na+ was mostly replaced with N-methyl-D-glucamine, suggesting that these spontaneous events were arising from the opening of cationic selective channels. However, blockers of cationic pacemaker currents in cultured intestinal ICC (Chang et al., 2003) were without affect suggesting that STDs in the renal pelvis were arising from the opening of cationic channels different from the TRP-like channels thought to be responsible for intestinal ICC pacemaking (Walker et al., 2002).
The presence of Kit-positive ICC-LCs has been demonstrated in the mouse renal pelvis both immunohistochemically (Pezzone et al., 2003; Lang and Klemm, 2005; Lang et al., 2006, 2007a) while spontaneously active interstitial cells have been demonstrated electrophysiologically after enzymatic dispersal (Lang et al., 2007b). In the present experiments, low frequency Ca2+ transients were recorded in a population of fusiform cells that were sparsely distributed in each field of view, consistent with the sparse distribution of Kit-positive ICC-LCs in the mouse renal pelvis and the infrequently recorded slowly discharging action potentials with long plateaus. This infrequency of impalement of ICC-LCs, or of the cells electrically close to an ICC-LC, did not allow us to examine the effects of our modifiers of Ca2+ mobilization on their electrical behaviour. However, it was possible to examine the effects of these agents on large low frequency Ca2+ transients in ICC-LCs at the same time as our examination of the Ca2+ transients in ASMCs.
Although the relative insensitivity of the ureteric system to nifedipine has been described previously in the guinea pig, (Lang, 1990; Meini et al., 1995; Santicioli and Maggi, 1998) the regional variability of this insensitivity within the mouse renal pelvis was unexpected. However, this variability was particularly advantageous as it allowed us to examine both the fundamental processes of autorhythmicity of ASMCs and ICC-LCs, as well as the mechanisms by which these cells then communicate their pacemaker signals to the TSMC layer. In 1 μM nifedipine, STDs, presumably recorded in a TSMC bundle but arising from a number of neighbouring ASMCs, were often seen to discharge near synchronously and trigger a residual TSMC action potential. This notion of some sort of synchronization of multiple ‘point sources' of excitation by a number of ASMCs is supported by our recent demonstration that, although Ca2+ transient discharge in ASMCs (in 1 μM nifedipine) is mostly asynchronous, STD and Ca2+-transient discharge was blocked by the putative gap junction uncouplers, 18 β-glycyrrhetinic acid and carbenoxolone (Lang et al., 2007a).
Role of Ca2+
All spontaneous electrical activity and Ca2+ transients in both ASMCs and ICC-LCs were blocked upon removal of Ca2+ from the bathing solution or upon blockade of SERCA with CPA (Lang et al., 2002b). Interestingly, although these agents caused membrane depolarization of some 10–20 mV (Figure 2) in the intact renal pelvis, they evoked opposite effects on basal [Ca2+]i in ASMCs and ICC-LCs. Ca2+ free PSS reduced global Ca2+ levels some 0.2 Ft/F0 while CPA transiently raised Ca2+ levels 0.3–0.4 Ft/F0 before decaying towards control levels even in the continued presence of CPA (Figures 2b and d). This difference may well arise from changes in the function of the plasma membrane Ca2+ ATPase (PMCA) in the presence of Ca2+ free PSS versus CPA. It has recently been demonstrated that the phosphatase activity of red blood cell PMCA is maximal in the absence of Ca2+ and this activity is highly dependent on the Mg2+ concentration (Mazzitelli and Adamo, 2007). A similar modulation of PMCA Ca2+ extrusion in our Ca2+ free PSS which contains a raised Mg2+ concentration (3.5 mM) could well be responsible for the fall in basal [Ca2+]i occurring in our experiments.
The membrane depolarization evoked by the fall of basal [Ca2+]i in Ca2+ free PSS is presumably arising from a reduction of a Ca2+-activated membrane conductance for K+ or from a cationic conductance activated upon lowering [Ca2+]i (Walker et al., 2002). The lack of effect of DIDS, La3+ or Gd3+ on the membrane potential suggests that Cl− or cationic channels are not involved in its development. The mostly likely K+ channels closed during a fall of basal [Ca2+]i would be large conductance Ca2+-activated K+ (BKCa) channels expressed in TSMCs but not ICC-LCs of the mouse UPJ (Lang et al., 2007b). This notion was confirmed by our recent observation that renal pelvis strips exposed to the selective BKCa channel blocker, iberiotoxin (100 nM for 10 min N=4), displayed a membrane depolarization of 5–10 mV which did not block STD discharge (MA Tonta and RJ Lang unpublished observations).
If ASMCs, TSMCs and ICC-LCs in the mouse renal pelvis are equally endowed with endoplasmic reticulum, as demonstrated in the guinea pig and rat renal pelvis (Lang et al., 1998, 2001; Klemm et al., 1999), blockade of SERCA would be expected to result in a rise of basal [Ca2+]i in all three cell types. The association of a rise in [Ca2+]i and the generation of cation-selective STDs suggests that the membrane depolarization in CPA may be arising from the activation of a Ca2+-activated cation conductance in the ASMCs which passively conducts into the TSMC layer. However, rat ureteric myocytes have been demonstrated to express Ca2+-activated Cl− channels (Smith et al., 2002) which, if present in mouse TSMCs, could also contribute to this membrane depolarization in CPA.
Ca2+ stores in the renal pelvis
Intestinal ICCs, urogenital ICC-LCs and many smooth muscles have all been demonstrated to display abrupt global rises in internal Ca2+, which are achieved by the entry of Ca2+ upon the opening of Ca2+-permeable channels or via the release from internal stores via Ca2+ release channels coupled to ryanodine or IP3 receptors. Both receptor populations are sensitive to Ca2+ so that Ca2+ entry and (or) Ca2+ release via one receptor population can stimulate the Ca2+ release from the other receptor population via Ca2+-induced Ca2+ release (CICR) mechanisms. In many, but not all smooth muscles, the pool of Ca2+ within ryanodine- and IP3-sensitive store appears to be functionally coupled via the colocalization of their receptors within the sarcoplasmic reticulum membrane (Boittin et al., 1998, 1999). The smooth muscle cells of the ureter are unique in that they display species dependence in their expression of only one functioning Ca2+ store. In the rat ureter only IP3 receptor sensitive Ca2+ stores can be detected (Burdyga et al., 1998; Boittin et al., 2000), while in the guinea-pig ureter only the ryanodine sensitive store appears to be functional (Burdyga et al., 1998).
The blockade of stomach slow wave activity in knockout mice lacking Type 1 IP3 receptors has established the essential role of IP3-sensitive Ca2+ release channels in intestinal autorhythmicity (Suzuki et al., 2000). Blockers of IP3 receptor function (2-APB), phospholipase C (PLC) and IP3 formation (neomycin, xestospongin C) and binding (heparin) have all been shown to block or reduce electrical oscillations in gastrointestinal (Van Helden et al., 2000; Ward et al., 2000; Aoyama et al., 2004; Liu et al., 2005) and urogenital (Sergeant et al., 2001; Lang et al., 2002b) preparations. In the present experiments, Ca2+ transient discharge in both ASMCs and ICC-LCs were blocked by 2-APB. Neither cell type displayed a rise in basal [Ca2+]i even though the membrane depolarized some 10 mV suggesting that 2-APB was not having a non-specific blocking action on SERCA in a manner similar to CPA (Gordienko and Bolton, 2002).
Blockers of PLC, neomycin and U73122, only reduced STD discharge and depolarized the membrane some 5–20 mV. Previously, it has been well established that the tonic production of prostaglandins and release of tachykinins from intrinsic sensory nerves are essential in the maintenance of both the amplitude and frequency of the spontaneous contractions underlying pelviureteric peristalsis (Lang et al., 2002a; Weiss et al., 2006). In the presence of this constant intrinsic excitatory PLC drive, it could well be that 10–20 min exposure to neomycin or U73122 was not sufficiently long to reduce IP3 levels to affect the rhythmic activity of ICC-LCs and ASMCs as effectively as directly blocking IP3 receptors with 2-APB. Alternatively, 2-APB has been suggested to deplete Ca2+ stores by blocking store-operated channels without affecting IP3-dependent Ca2+ release (Gregory et al., 2001). However, this seems unlikely as intestinal TRP channels blockers, La3+ or Gd3+, had little effect in the present experiments.
The function of ryanodine-sensitive Ca2+ stores in intestinal and urogenital pacemaking remains controversial. Blockade of ryanodine receptors, using blocker (ryanodine, tetracaine) concentrations thought to block ryanodine-sensitive Ca2+ release channels without inducing store depletion (Sutko et al., 1997) abolishes spontaneous electrical and Ca2+ signalling in ICC within cultured intestinal explants (Aoyama et al., 2004; Liu et al., 2005), ICC-LCs in the urethra (Johnston et al., 2005; McHale et al., 2006; Hashitani and Suzuki, 2007) and corporal tissue of the guinea-pig penis (Hashitani and Suzuki, 2004), but not the spontaneous electrical activity in freshly prepared portions of the small intestine (Malysz et al., 2001) or guinea-pig renal pelvis (Lang et al., 2002b). In the present experiments, ryanodine blocked Ca2+-transient discharge in ICC-LCs within several minutes. In contrast, ASMCs Ca2+ transients in the same preparation were only slightly reduced in ryanodine (30–100 μM for 30–60 min). This reduction of ASMCs Ca2+ transients in ryanodine resembled the reduction but not blocked of STD amplitudes with ryanodine and higher concentrations of nifedipine.
The present experiments suggest that ryanodine receptors and CICR mechanisms in ICC-LCs may well represent the fundamental mechanism underlying the integration of multiple pacemaker signals from ASMCs before the stimulation of TSMC action potential discharge (Lang et al., 1998; Klemm et al., 1999). However, we are yet to demonstrate that such a mechanism is resident in single ICC-LCs. Nor have we been able to demonstrate any synchronicity between ASMCs and ICC-LCs in mouse UPJs when bathed in 1 μM nifedipine (Lang et al., 2007a). As many smooth muscles have been demonstrated to possess ryanodine receptors intimately involved in facilitating the propagation of Ca2+ waves and action potentials (Gordienko et al., 1998; Burdyga and Wray, 2005), it seems more likely that ryanodine-sensitive Ca2+ release in UPJ TSMCs act as the point of integration of the pacemaker drive from both ASMCs and ICC-LCs.
Effects of caffeine
Low concentrations of caffeine (<3 mM) have been used to inhibit spontaneous electrical activity in a number of smooth muscles (Hashitani and Edwards, 1999; van Helden et al., 2000; Lang et al., 2002b)) via mechanisms that have yet to be fully elucidated. In the present experiments, the most noticeable effect evoked by caffeine was the hyperpolarization recorded with intracellular microelectrodes and the significant reduction in Ca2+-transient discharge in both ASMCs and ICC-LCs. Although, STD frequencies were reduced by caffeine, their amplitudes could increase or decrease depending on the relative increase in driving force and ‘shunting' during the membrane conductance increase which evoked the hyperpolarization. These confounding effects of the membrane hyperpolarization were avoided if preparations were bathed in glibenclamide (Figure 10Biv–vi). Under these conditions STD amplitudes can still be seen to be reduced by caffeine. We have preliminary evidence that this caffeine-induced hyperpolarization and reduction in STD amplitudes can be mimicked by forskolin and IBMX as has been demonstrated in the murine small intestine (Malysz et al., 2001), but not by sodium nitroprusside, suggesting that cAMP and the activation of protein kinase A signalling pathways are involved. These mechanisms remain to be elucidated.
In conclusion, it is very likely ASMCs and ICC-LCs, are both influencing the spontaneous electrical and contractile activity of the UPJ. It is clear that the mechanisms of STD generation in ASMCs display a unique insensitivity to nifedipine and ryanodine (Malysz et al., 2001) when compared with the mechanisms of autorythmicity of neighbouring UPJ ICC-LCs, ICC-LCs in the urethra (Hashitani and Suzuki, 2007) and ICC in cultured intestinal explants (Aoyama et al., 2004). Our results suggest that ryanodine-sensitive Ca2+ release is required to synchronize, but not to initiate STD discharge. This synchronization of STDs into large enough pacemaker events that can evoke action potential discharge and contraction in the TSMC layer also appears to be supported by Ca2+ entry through L type Ca2+ channels.
Acknowledgments
This work was supported in part by the National Health & Medical Research Council Australia (HCP), the Australian Research Council (RJL & HCP) and by the Grant-in-Aids from Japan Society for the Promotion of Science to HH (No.17390443).
Abbreviations
- 2-APB
2-aminoethoxy-diphenylborate
- ASMC
atypical smooth muscle cell
- BKCa
large conductance Ca2+-activated K+ channels
- [Ca2+]i
cytosolic free calcium concentration
- CICR
Ca2+-induced Ca2+ release
- CPA
cyclopiazonic acid
- DIDS
4,4′-diisothiocyanostilbene-2′2′-disulphonic acid
- Ft/F0
ratio of fluorescence generated at time t (Ft) divided by the baseline fluorescence at t=0 (F0).
- ICC
interstitial cells of Cajal
- ICC-LC
ICC-like cell
- IP3
inositol trisphosphate
- PLC
phospholipase C
- PMCA
plasma membrane Ca2+ ATPase
- PSS
physiological salt solution
- SERCA
sarcoplasmic endoplasmic reticulum Ca2+ATPase
- STDs
spontaneous transient depolarizations
- TSMC
typical smooth muscle cell
- UPJ
ureteropelvic junction
Conflict of interest
The authors state no conflict of interest.
References
- Aoyama M, Yamada A, Wang J, Ohya S, Furuzono S, Goto T, et al. Requirement of ryanodine receptors for pacemaker Ca2+ activity in ICC and HEK293 cells. J Cell Sc. 2004;117:2813–2825. doi: 10.1242/jcs.01136. [DOI] [PubMed] [Google Scholar]
- Boittin FX, Coussin F, Macrez N, Mironneau C, Mironneau J. Inositol 1,4,5-trisphosphate- and ryanodine-sensitive Ca2+ release channel dependent Ca2+ signalling in rat portal vein myocytes. Cell Calcium. 1998;23:303–311. doi: 10.1016/s0143-4160(98)90026-4. [DOI] [PubMed] [Google Scholar]
- Boittin FX, Coussin F, Morel JL, Halet G, Macrez N, Mironneau J. Ca2+ signals mediated by Ins(1,4,5)P3-gated channels in rat ureteric myocytes. Biochem J. 2000;349:323–332. doi: 10.1042/0264-6021:3490323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boittin FX, Macrez N, Halet G, Mironneau J. Norepinephrine-induced Ca2+ waves depend on InsP3 and ryanodine receptor activation in vascular myocytes. Am J Physiol-Cell Physiol. 1999;277:C139–C151. doi: 10.1152/ajpcell.1999.277.1.C139. [DOI] [PubMed] [Google Scholar]
- Burdyga T, Wray S. Action potential refractory period in ureter smooth muscle is set by Ca sparks and BK channels. Nature. 2005;436:559–562. doi: 10.1038/nature03834. [DOI] [PubMed] [Google Scholar]
- Burdyga TV, Taggart MJ, Crichton C, Smith GL, Wray S. The mechanism of Ca2+ release from the SR of permeabilised guinea-pig and rat ureteric smooth muscle. Biochim Biophys Acta. 1998;1402:109–114. doi: 10.1016/s0167-4889(97)00149-3. [DOI] [PubMed] [Google Scholar]
- Chang SJ, Cho ET, Heo GS, Park CG, Kim MW, Chang IY, et al. Characterization of pacemaking currents in cultured interstitial cells of Cajal from mice small intestine. Korean J Gastroenterol. 2003;42:121–127. [PubMed] [Google Scholar]
- Dixon JS, Gosling JA. The fine structure of pacemaker cells in the pig renal calices. Anat Res. 1973;175:139–153. doi: 10.1002/ar.1091750203. [DOI] [PubMed] [Google Scholar]
- Dixon JS, Gosling JA. The musculature of the human renal calices, pelvis and upper ureter. J Anat. 1982;135:129–137. [PMC free article] [PubMed] [Google Scholar]
- Gordienko DV, Bolton TB. Crosstalk between ryanodine receptors and IP3 receptors as a factor shaping spontaneous Ca2+-release events in rabbit portal vein myocytes. J Physiol. 2002;542:743–762. doi: 10.1113/jphysiol.2001.015966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordienko DV, Bolton TB, Cannell MB. Variability in spontaneous subcellular calcium release in guinea-pig ileum smooth muscle cells. J Physiol. 1998;507:707–720. doi: 10.1111/j.1469-7793.1998.707bs.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosling JA, Dixon JS. Species variation in the location of upper urinary tract pacemaker cells. Invest Urol. 1974;11:418–423. [PubMed] [Google Scholar]
- Gregory RB, Rychkov G, Barritt GJ. Evidence that 2-aminoethyl diphenylborate is a novel inhibitor of store-operated Ca2+ channels in liver cells, and acts through a mechanism which does not involve inositol trisphosphate receptors. BiochemJ. 2001;354:285–290. doi: 10.1042/0264-6021:3540285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashitani H, Edwards FR. Spontaneous and neurally activated depolarizations in smooth muscle cells of the guinea-pig urethra. J Physiol. 1999;514:459–470. doi: 10.1111/j.1469-7793.1999.459ae.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashitani H, Suzuki H. Identification of interstitial cells of Cajal in corporal tissues of the guinea-pig penis. Brit J Pharmacol. 2004;141:199–204. doi: 10.1038/sj.bjp.0705622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashitani H, Suzuki H. Properties of spontaneous Ca2+ transients recorded from interstitial cells of Cajal-like cells (ICC-LCs) of the rabbit urethra in situ. J Physiol. 2007;583:505–519. doi: 10.1113/jphysiol.2007.136697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston L, Sergeant GP, Hollywood MA, Thornbury KD, McHale NG. Calcium oscillations in interstitial cells of the rabbit urethra. J Physiol. 2005;565:449–461. doi: 10.1113/jphysiol.2004.078097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemm MF, Exintaris B, Lang RJ. Identification of the cells underlying pacemaker activity in the guinea- pig upper urinary tract. J Physiol. 1999;519:867–884. doi: 10.1111/j.1469-7793.1999.0867n.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh SD, Monaghan K, Ro S, Mason HS, Kenyon JL, Sanders KM. Novel voltage-dependent non-selective cation conductance in murine colonic myocytes. J Physiol. 2001;533:341–355. doi: 10.1111/j.1469-7793.2001.0341a.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang RJ. The whole-cell Ca2+ channel current in single smooth muscle cells of the guinea-pig ureter. J Physiol. 1990;423:453–473. doi: 10.1113/jphysiol.1990.sp018033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang RJ, Davidson ME, Exintaris B. Pyeloureteral motility and ureteral peristalsis: essential role of sensory nerves and endogenous prostaglandins. Exp Physiol. 2002a;87:129–146. doi: 10.1113/eph8702290. [DOI] [PubMed] [Google Scholar]
- Lang RJ, Exintaris B, Teele ME, Harvey J, Klemm MF. Electrical basis of peristalsis in the mammalian upper urinary tract. Clinl & Exp Pharmacol & Physiol. 1998;25:310–321. doi: 10.1111/j.1440-1681.1998.tb02357.x. [DOI] [PubMed] [Google Scholar]
- Lang RJ, Hashitani H, Keller S, Takano H, Mulholland EL, Fukuta H, et al. Modulators of internal Ca2+ stores and the spontaneous electrical and contractile activity of the guinea-pig renal pelvis. Brit J Pharmacol. 2002b;135:1363–1374. doi: 10.1038/sj.bjp.0704609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang RJ, Hashitani H, Tonta MA, Suzuki H, Parkington HC. Spontaneous electrical and Ca2+ signals in typical and atypical smooth muscle cells and interstitial cell of Cajal-like cells of mouse renal pelvis. J Physiol. 2007a;583:1049–1068. doi: 10.1113/jphysiol.2007.137034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang RJ, Klemm MF. Interstitial cell of Cajal-like cells in the upper urinary tract. J Cell Mol Med. 2005;9:543–556. doi: 10.1111/j.1582-4934.2005.tb00487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang RJ, Takano H, Davidson ME, Suzuki H, Klemm MF. Characterization of the spontaneous electrical and contractile activity of smooth muscle cells in the rat upper urinary tract. J Urol. 2001;166:329–334. [PubMed] [Google Scholar]
- Lang RJ, Tonta MA, Zoltkowski BZ, Meeker WE, Wendt I, Parkington HC. Pyeloureteric peristalsis: role of atypical smooth muscle cells and interstitial cells of Cajal-like cells as pacemakers. J Physiol. 2006;576:695–705. doi: 10.1113/jphysiol.2006.116855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang RJ, Zoltkowski BZ, Hammer JM, Meeker WF, Wendt I. Electrical characterization of interstitial cells of Cajal-like cells and smooth muscle cells isolated from the mouse ureteropelvic junction. J Urol. 2007b;177:1573–1580. doi: 10.1016/j.juro.2006.11.073. [DOI] [PubMed] [Google Scholar]
- Liu HN, Ohya S, Furuzono S, Wang J, Imaizumi Y, Nakayama S. Co-contribution of IP3R and Ca2+ influx pathways to pacemaker Ca2+ activity in stomach ICC. J Biol Rhythms. 2005;20:15–26. doi: 10.1177/0748730404269572. [DOI] [PubMed] [Google Scholar]
- Malysz J, Donnelly G, Huizinga JD. Regulation of slow wave frequency by IP3-sensitive calcium release in the murine small intestine. Am J Physiol. 2001;280:G439–G448. doi: 10.1152/ajpgi.2001.280.3.G439. [DOI] [PubMed] [Google Scholar]
- Mazzitelli LR, Adamo HP. The phosphatase activity of the plasma membrane Ca2+ pump. Activation by acidic lipids in the absence of Ca2+ increases the apparent affinity for Mg2+ Biochem Biophys Acta. 2007;1768:1777–1783. doi: 10.1016/j.bbamem.2007.04.019. [DOI] [PubMed] [Google Scholar]
- McHale N, Hollywood M, Sergeant G, Thornbury K. Origin of spontaneous rhythmicity in smooth muscle. J Physiol. 2006;570:23–28. doi: 10.1113/jphysiol.2005.098376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meini S, Santicioli P, Maggi CA. Propagation of impulses in the guinea-pig ureter and its blockade by calcitonin gene-related peptide (CGRP) Naunyn-Schmiedebergs Arch Pharmacol. 1995;351:79–86. doi: 10.1007/BF00169067. [DOI] [PubMed] [Google Scholar]
- Metzger R, Schuster T, Till H, Franke FE, Dietz HG. Cajal-like cells in the upper urinary tract: comparative study in various species. Pediatr Surg Int. 2005;21:169–174. doi: 10.1007/s00383-004-1314-4. [DOI] [PubMed] [Google Scholar]
- Metzger R, Schuster T, Till H, Stehr M, Franke FE, Dietz HG. Cajal-like cells in the human upper urinary tract. J Urol. 2004;172:769–772. doi: 10.1097/01.ju.0000130571.15243.59. [DOI] [PubMed] [Google Scholar]
- Pezzone MA, Watkins SC, Alber SM, King WE, de Groat WC, Chancellor MB, et al. Identification of c-kit-positive cells in the mouse ureter: the interstitial cells of Cajal of the urinary tract. Am J Physiol-Renal Physiol. 2003;284:F925–F929. doi: 10.1152/ajprenal.00138.2002. [DOI] [PubMed] [Google Scholar]
- Santicioli P, Maggi CA. Myogenic and neurogenic factors in the control of pyeloureteral motility and ureteral peristalsis. Pharmacol Rev. 1998;50:683–722. [PubMed] [Google Scholar]
- Sergeant GP, Hollywood MA, McCloskey KD, McHale NG, Thornbury KD. Role of IP3 in modulation of spontaneous activity in pacemaker cells of rabbit urethra. Am J Physiol. 2001;280:C1349–C1356. doi: 10.1152/ajpcell.2001.280.5.C1349. [DOI] [PubMed] [Google Scholar]
- Smith RD, Borisova L, Wray S, Burdyga T. Characterisation of the ionic currents in freshly isolated rat ureter smooth muscle cells: evidence for species-dependent currents. Pflugers Archiv-Eur J Physiol. 2002;445:444–453. doi: 10.1007/s00424-002-0941-7. [DOI] [PubMed] [Google Scholar]
- Sutko JL, Airey JA, Welch W, Ruest L. The pharmacology of ryanodine and related compounds. Pharmacol Rev. 1997;49:53–98. [PubMed] [Google Scholar]
- Suzuki H, Takano H, Yamamoto Y, Komuro T, Saito M, Kato K, et al. Properties of gastric smooth muscles obtained from mice, which lack inositol trisphosphate receptor. J Physiol. 2000;525:105–111. doi: 10.1111/j.1469-7793.2000.00105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Helden DF, Imtiaz MS, Nurgaliyeva K, von der WP, Dosen PJ. Role of calcium stores and membrane voltage in the generation of slow wave action potentials in guinea-pig gastric pylorus. J Physiol. 2000;524:245–265. doi: 10.1111/j.1469-7793.2000.00245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker RL, Koh SD, Sergeant GP, Sanders KM, Horowitz B. TRPC4 currents have properties similar to the pacemaker current in interstitial cells of Cajal. Am J Physiol-Cell Physiol. 2002;283:C1637–C1645. doi: 10.1152/ajpcell.00266.2002. [DOI] [PubMed] [Google Scholar]
- Ward SM, Ordog T, Koh SD, Baker SA, Jun JY, Amberg G, et al. Pacemaking in interstitial cells of Cajal depends upon calcium handling by endoplasmic reticulum and mitochondria. J Physiol. 2000;525:355–361. doi: 10.1111/j.1469-7793.2000.t01-1-00355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss RM, Tamarkin FJ, Wheeler MA. Pacemaker activity in the upper urinary tract. J Smooth Muscle Res. 2006;42:103–115. doi: 10.1540/jsmr.42.103. [DOI] [PubMed] [Google Scholar]